Abstract
Background/Purpose:
Most community-acquired urinary tract infections (UTIs) are usually treated empirically. The knowledge of antibiotic resistance patterns of the microorganisms causing UTI is essential for defining the empirical treatment.
Objective:
The aim of the present study is to determine the distribution of bacterial strains isolated from lower UTIs and their resistance patterns against commonly used antimicrobial agents and treatment results in female patients.
Subjects and Methods:
This is a retrospective analysis of medical case records of 90 female patients with lower UTI for a period of 4 years from January 2013 to December 2016 in a tertiary care hospital in the Trakya region of Turkey.
Results:
The most common causative agent was Escherichia coli (66.6% of cases) followed by Klebsiella pneumoniae (16.6%). Fosfomycin was the most active agent against E. coli (resistant isolates: 5.5%), followed by nitrofurantoin (resistant isolates: 7.4%). Extended-spectrum beta-lactamases (ESBLs) production was observed in 29 (32.2%) isolates (22 in E. coli, 6 in K. pneumoniae, and 1 in Enterobacter spp.). The antimicrobial resistance rates among ESBL-producing E. coli isolates for trimethoprim-sulfamethoxazole, ciprofloxacin, fosfomycin, and nitrofurantoin were 77.7%, 72.7%, 13.6%, and 18.2%, respectively (P < 0.05). The estimated microbiological eradication rates for nitrofurantoin and fosfomycin were 89.7% and 83.8%, respectively.
Conclusions:
The results of the present study indicate that nitrofurantoin and fosfomycin may be considered for empirical therapy of lower UTIs in Trakya region of Turkey.
Keywords: Antimicrobial resistance, etiology, fosfomycin, nitrofurantoin, urinary tract infection
INTRODUCTION
Urinary tract infections (UTIs) are among the most common infectious diseases occurring in either the community or health-care setting. Uncomplicated UTIs typically occur in the healthy premenopausal, nonpregnant women with no history suggestive of an abnormal urinary tract.[1] Complicated UTIs may occur in both women and men and in any age groups and are frequently associated with either structural or functional urinary tract abnormalities. Most infections occur in the lower urinary tract. These infections are common in women than in men.[1,2,3,4] The microbial spectrum of UTIs consists mainly of Escherichia coli, with occasional other species of Enterobacteriaceae such as Proteus mirabilis and Klebsiella pneumoniae and other bacteria such as Staphylococcus saprophyticus. Other Gram-negative and Gram-positive species are rarely isolated in UTIs.[5,6]
Antimicrobial resistance, especially in Gram-negative bacteria (GNB) causing UTIs, both cystitis and pyelonephritis, is increasing. This makes empirical treatment of these infections difficult. Knowledge of the antimicrobial resistance patterns of uropathogens in specific geographical locations is an important factor for choosing an appropriate empirical antimicrobial treatment.[6,7] The aim of the present study is to determine the distribution of bacterial strains isolated from lower UTIs and their resistance patterns against commonly used antimicrobial agents, and treatment results in female patients.
SUBJECTS AND METHODS
This is a retrospective analysis of medical case records of 90 female patients with lower UTI between January 2013 and December 2016 at the university hospital in Tekirdag, Turkey. Women were classified to have acute uncomplicated UTI if they had a structurally normal urinary tract and symptoms of cystitis. Urine cultures were done in our laboratory of clinical microbiology according to standard techniques. Antimicrobial susceptibility tests were performed using the Kirby-Bauer disk diffusion method and interpretation of these tests was according to the guidelines proposed by the National Committee for Clinical Laboratory Standards M100-S25. Extended-spectrum beta-lactamases (ESBLs) production was detected by double-disk synergy test. The Chi-square test was used to compare the data between the groups. P < 0.05 was considered statistically significant.
This retrospective study was deemed exempt from written informed consent by the Namik Kemal University Hospital Review Board because it used only retrospective, deidentified patient data.
RESULTS
A total of 90 women were included and their mean age was 55.5 years (range 16–78). In majority of patients, urolithiasis (20%) was associated with lower UTI. Diabetes mellitus (15.6%) was the second leading risk factor causing UTI. Other risk factors were neurogenic bladder (7.8%), malignancy (colon cancer and endometrial cancer) (7.8%), current corticosteroid use (5.55%), urinary tract anomaly (4.4%), chronic renal failure (2.2%), renal cyst (1.1%), hydronephrosis (1.1%), and urinary procedure (1.1%); 33.3% of patients had no risk factors.
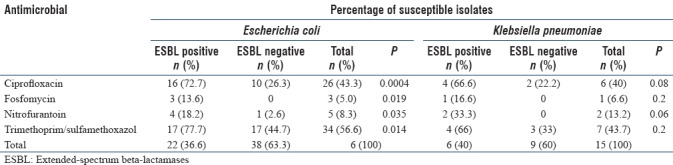
Urinalysis showed nitrite positivity in 40 (44.4%), leukocyte esterase positivity in 84 (93.3%), and leukocyte positivity on microscope in 87 (96.6%). The most common causative agent was E. coli (66.6% of cases) followed by K. pneumoniae (16.6%) and others such as Enterobacter spp. (7.7%), Enterococcus spp. (3.3%), Pseudomonas aeruginosa (1.2%), Citrobacter freundii (1.2%), S. saprophyticus (1.2%). The other two microorganisms were Candida spp. (Candida glabrata and Candida krusei). The distribution of the causative agents is shown in Table 1. Resistance to ampicillin (86.6%), trimethoprim-sulfamethoxazole (TMP-SMX) (47.7%), and ciprofloxacin (33.3%) was significant in all isolates. Fosfomycin was the most active agent against E. coli (resistant isolates: 5%), followed by nitrofurantoin (resistant isolates: 8.3%). ESBL production was observed in 29 (32.2%) isolates (22 E. coli, 6 K. pneumoniae, and 1 Enterobacter spp.). ESBL production was 36.6% and 40% against E. coli and K. pneumoniae, respectively. The antimicrobial resistance rates among ESBL-producing E. coli isolates for TMP-SMX, ciprofloxacin, fosfomycin, and nitrofurantoin were 77.7%, 72.7%, 13.6%, and 18.2%, respectively (P < 0.05). The antibiotic susceptibility profile of E. coli and K. pneumoniae isolates is summarized in Table 2. After the microbiological specimens were taken, the agents prescribed for lower UTIs were nitrofurantoin (43.3%), fosfomycin (41.1%), ceftriaxone (14.5%), and ciprofloxacin (1.1%). The estimated microbiological eradication rates for nitrofurantoin and fosfomycin were 89.7% and 83.8%, respectively. Twelve (92.3%) patients were treated with ceftriaxone successfully. One patient was treated with ciprofloxacin successfully. Overall cure rate was 87.7%.
Table 1.
Distribution of urinary tract infections pathogens isolated from urine cultures

Table 2.
Antibiotic susceptibility patterns of extended-spectrum beta-lactamases producing and nonproducing isolates of Escherichia coli and Klebsiella pneumoniae
DISCUSSION
UTIs are among the most common prevalent infections in clinical practice. These infections occur in every age and both genders. According to the demographic data, it is more frequent in women. Anatomical and physical factors such as a shorter urethra predispose women to UTI. Some conditions such as surgery, pregnancy, advanced age, diabetes mellitus, kidney stones, impairment of the immune system, neurogenic bladder, and urinary tract abnormalities may increase the risk of developing UTIs.[4] In majority of our patients (20%), urolithiasis and diabetes mellitus (15.6%) were associated with lower UTI.
E. coli is the most common community-acquired UTI pathogen and is responsible for 75%–95% of cases. Proteus mirabilis, K. pneumoniae, and S. saprophyticus are other frequently observed species. One study from the United States and European hospitals reported that the most frequently isolated pathogens were E. coli (63.3%/71·3% in USA/EU), Klebsiella spp. (16.7%/11·2%), and Proteus mirabilis (6.4%/5.0%).[8] In the current study, E. coli was the most frequently isolated urinary pathogen (63.7%), followed by K. pneumoniae (18.7%).
The prevalence of multidrug-resistant Enterobacteriaceae is being increasingly reported in UTIs. Common pathogens in community-acquired UTI have a high resistance to widely used antibiotics. The increasing resistance of ampicillin and TMP-SMX to E. coli has been reported in studies from Turkey and other countries. According to various studies, the lowest observed resistance rates in the lower UTIs were reported for fosfomycin, nitrofurantoin, and mecillinam (prodrug pivmecillinam).[6] Fluoroquinolones have high resistance rates among multidrug-resistant uropathogens and are being strongly discouraged as first-line agents for UTIs.[7] The activity of fosfomycin and nitrofurantoin has been reported high for most cases of multidrug-resistant E. coli UTIs.[5,9,10] One study reported that amikacin and nitrofurantoin were the most effective antibiotics in community-acquired UTIs.[11] Seo et al.[12] reported that the resistance rate of fosfomycin and nitrofurantoin did not differ significantly between the ciprofloxacin-resistant and ciprofloxacin-susceptible isolates. Marchisio et al.[13] reported that 21% of the isolates were resistant to three or more tested antibiotics families. In a study involving high prevalence of multidrug-resistant Enterobacteriaceae isolated from outpatient urine samples, more than 90% of the urinary isolates were reported to be resistant to sulfonamides.[14]
UTIs caused by ESBL-producing bacteria have become an emerging problem limiting therapeutic options. Especially these microorganisms tend to be multidrug resistant. According to the various studies, there is an increasing trend over time. Stefaniuk et al.[15] reported that K. pneumoniae was the pathogen most frequently associated with ESBL production with 46.2% of all K. pneumoniae isolates being positive. As expected, ESBL-producing urinary pathogens were significantly more often resistant to non-beta-lactam antibiotics, and 27% of the isolates were classified as multidrug resistant. However, the majority of ESBL-GNB isolates causing UTIs/bacteriuria was susceptible to carbapenems (100%) and nitrofurantoin (84%).[16] In a study that compared antimicrobial susceptibilities of urinary ESBL-producing E. coli and ESBL-producing K. pneumoniae to fosfomycin and nitrofurantoin in a teaching hospital in Taiwan, fosfomycin had good susceptibility to ESBL-producing E. coli (95.5%), including inhospital-acquired isolates, but lower antimicrobial activity against ESBL-producing K. pneumoniae (57.6%). TMP-SMX had the highest resistance rate to ESBL-producing isolates.[17] Resistance rates to nitrofurantoin in ESBL-nonproducing and ESBL-producing E. coli were reported to be 6.6% and 23.2%, respectively, from a tertiary care educational hospital in Turkey.[18] In our study, ESBL production was observed in 29 (32.2%) isolates (22 E. coli and 6 K. pneumoniae, 1 Enterobacter spp.).
The current study showed a significant resistance among the Enterobacteriaceae family to commonly available antibiotics. Fosfomycin was the most active agent against E. coli (resistant isolates: 5%), followed by nitrofurantoin (resistant isolates: 8.3%). Resistance rates to nitrofurantoin in ESBL-nonproducing and ESBL-producing E. coli were 2.6% and 18.2%, respectively. The antimicrobial resistance rates among ESBL-producing isolates for fosfomycin and nitrofurantoin were 13.7% and 20.7%, respectively. Comparing with ESBL-nonproducing isolates, ESBL-producing isolates were associated with significantly lower susceptibility of fosfomycin (0% vs. 13.7%), nitrofurantoin (3.27% vs. 20.7%), TMP-SMX (38.3% vs. 72.4%), and ciprofloxacin (19.6% vs. 68.9%) (P < 0.05).
TMP-SMX should be used as first-line therapy because of its low cost and efficacy for uncomplicated UTIs in women unless the prevalence of resistance to these agents among uropathogens in the community is >10%–20%.[6] Other options include a 7-day course of nitrofurantoin or a single dose of fosfomycin. Nitrofurantoin is a member of a group of synthetic nitrofuran derivative. This antibiotic is an important treatment option for uncomplicated UTIs in the current era of increasing fluoroquinolone and TMP-SMX resistance among uropathogens. It is active against most common uropathogens, but most Proteus species, Serratia marcescens, and Pseudomonas aeruginosa are naturally resistant. Nitrofurantoin is also active against most strains of multidrug-resistant Gram-negative bacilli, including most ESBL-producing strains. Studies indicate a clinical cure rate with nitrofurantoin of 88%–92% and a microbiologic cure rate of 81%–92%. Despite availability and use of nitrofurantoin for more than 5 decades, the prevalence of nitrofurantoin resistance among common uropathogens remains low because it has a low resistance potential. Nitrofurantoin appears to have good clinical and microbiological efficacy for UTI caused by common uropathogens.[19,20]
Fosfomycin is a phosphoric acid derivative used only for the treatment of uncomplicated UTIs. It is administered as a 1-time 3 g oral dose. Fosfomycin's spectrum of activity includes E. coli, Enterococci, and Serratia, Enterobacter, Citrobacter, and Klebsiella species but does not cover S. saprophyticus. According to the recent analysis reports, a fosfomycin resistance rate is low in community-acquired E. coli UTI. The development of resistance to fosfomycin appears to be more frequent both in vitro and in clinical studies for non-E. coli Enterobacteriaceae in comparison with E. coli.[19,21,22,23] In one study, the clinical remission rate was 83% in the fosfomycin group.[21] In our study, the estimated microbiological eradication rates for nitrofurantoin and fosfomycin were 89.7% and 83.8%, respectively. Twelve (92.3%) patients were treated with ceftriaxone successfully. One patient was treated with ciprofloxacin successfully. Overall cure rate was 87.7%.
CONCLUSIONS
The results of the present study indicate that we had a high percentage of ESBL-producing isolates. Nitrofurantoin and fosfomycin may be considered for empirical therapy of lower UTIs in our region. According to the results of this study, TMP-SMX is not recommended as the first choice antibiotic because of the high resistance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gupta K, Grigoryan L, Trautner B. Urinary tract infection. Ann Intern Med. 2017;167:ITC49–64. doi: 10.7326/AITC201710030. [DOI] [PubMed] [Google Scholar]
- 2.Pallett A, Hand K. Complicated urinary tract infections: Practical solutions for the treatment of multiresistant gram-negative bacteria. J Antimicrob Chemother. 2010;65(Suppl 3):iii25–33. doi: 10.1093/jac/dkq298. [DOI] [PubMed] [Google Scholar]
- 3.Nicolle L. Complicated urinary tract infections in adults. Can J Infect Dis Med Microbiol. 2005;16:349–60. doi: 10.1155/2005/385768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenberger P, Hooton TM. Complicated urinary tract infections. Curr Infect Dis Rep. 2008;10:499–504. doi: 10.1007/s11908-008-0081-0. [DOI] [PubMed] [Google Scholar]
- 5.Tandogdu Z, Wagenlehner FM. Global epidemiology of urinary tract infections. Curr Opin Infect Dis. 2016;29:73–9. doi: 10.1097/QCO.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 6.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the e treatment of acute noncomplicated cystitis and pyelonephritis in women: A 2010 update by the infectious diseases society of America and European society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:E103–20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 7.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 8.Sader HS, Flamm RK, Jones RN. Frequency of occurrence and antimicrobial susceptibility of gram-negative bacteremia isolates in patients with urinary tract infection: Results from United States and European hospitals (2009-2011) J Chemother. 2014;26:133–8. doi: 10.1179/1973947813Y.0000000121. [DOI] [PubMed] [Google Scholar]
- 9.Walker E, Lyman A, Gupta K, Mahoney MV, Snyder GM, Hirsch EB, et al. Clinical management of an increasing threat: Outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960–5. doi: 10.1093/cid/ciw396. [DOI] [PubMed] [Google Scholar]
- 10.Meier S, Weber R, Zbinden R, Ruef C, Hasse B. Extended-spectrum β-lactamase-producing gram-negative pathogens in community-acquired urinary tract infections: An increasing challenge for antimicrobial therapy. Infection. 2011;39:333–40. doi: 10.1007/s15010-011-0132-6. [DOI] [PubMed] [Google Scholar]
- 11.Bours PH, Polak R, Hoepelman AI, Delgado E, Jarquin A, Matute AJ, et al. Increasing resistance in community-acquired urinary tract infections in latin America, five years after the implementation of national therapeutic guidelines. Int J Infect Dis. 2010;14:e770–4. doi: 10.1016/j.ijid.2010.02.2264. [DOI] [PubMed] [Google Scholar]
- 12.Seo MR, Kim SJ, Kim Y, Kim J, Choi TY, Kang JO, et al. Susceptibility of Escherichia coli from community-acquired urinary tract infection to fosfomycin, nitrofurantoin, and temocillin in Korea. J Korean Med Sci. 2014;29:1178–81. doi: 10.3346/jkms.2014.29.8.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchisio M, Porto A, Joris R, Rico M, Baroni MR, Di Conza J. Susceptibility to beta-lactams and quinolones of Enterobacteriaceae isolated from urinary tract infections in outpatients. Braz J Microbiol. 2015;46:1155–9. doi: 10.1590/S1517-838246420140880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leski TA, Taitt CR, Bangura U, Stockelman MG, Ansumana R, Cooper WH, 3rd, et al. High prevalence of multidrug resistant Enterobacteriaceae isolated from outpatient urine samples but not the hospital environment in Bo, Sierra Leone. BMC Infect Dis. 2016;16:167. doi: 10.1186/s12879-016-1495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefaniuk E, Suchocka U, Bosacka K, Hryniewicz W. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur J Clin Microbiol Infect Dis. 2016;35:1363–9. doi: 10.1007/s10096-016-2673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olafsson M, Kristinsson KG, Sigurdsson JA. Urinary tract infections, antibiotic resistance and sales of antimicrobial drugs – An observational study of uncomplicated urinary tract infections in Icelandic women. Scand J Prim Health Care. 2000;18:35–8. doi: 10.1080/02813430050202532. [DOI] [PubMed] [Google Scholar]
- 17.Liu HY, Lin HC, Lin YC, Yu SH, Wu WH, Lee YJ, et al. Antimicrobial susceptibilities of urinary extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae to fosfomycin and nitrofurantoin in a teaching hospital in Taiwan. J Microbiol Immunol Infect. 2011;44:364–8. doi: 10.1016/j.jmii.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Tasbakan MI, Pullukcu H, Sipahi OR, Yamazhan T, Ulusoy S. Nitrofurantoin in the treatment of extended-spectrum ß-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554–6. doi: 10.1016/j.ijantimicag.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Horton JM. 8th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2015. Urinary tract agents. Nitrofurantoin, fosfomycin, and methenamin. Mandell, Douglass and Bennett's Principles and Practice of Infectious Diseases; pp. 447–51. [Google Scholar]
- 20.Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW, et al. Nitrofurantoin revisited: A systematic review and meta-analysis of controlled trials. J Antimicrob Chemother. 2015;70:2456–64. doi: 10.1093/jac/dkv147. [DOI] [PubMed] [Google Scholar]
- 21.Ceran N, Mert D, Yuksel Kocdogan F, Erdem I, Adalati R, Ozyurek S, et al. A randomized comparative study of single-dose fosfomycin and 5-day ciprofloxacin in female patients with uncomplicated lower urinary tract infections. J Infect Chemother. 2010;29:424–30. doi: 10.1007/s10156-010-0079-z. [DOI] [PubMed] [Google Scholar]
- 22.Matthews PC, Barrett LK, Warren S, Stoesser N, Snelling M, Scarborough M, et al. Oral fosfomycin for treatment of urinary tract infection: A retrospective cohort study. BMC Infect Dis. 2016;16:556. doi: 10.1186/s12879-016-1888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karageorgopoulos DE, Wang R, Yu XH, Falagas ME. Fosfomycin: Evaluation of the published evidence on the emergence of antimicrobial resistance in gram-negative pathogens. J Antimicrob Chemother. 2012;67:255–68. doi: 10.1093/jac/dkr466. [DOI] [PubMed] [Google Scholar]


