Abstract
Background:
Developing countries like India are heavily burdened with multidrug resistant tuberculosis (MDR-TB).
Materials and Methods:
A retrospective study was carried out at the directly observed treatment short course chemotherapy plus site in our tertiary care center (All India Institute of Medical Sciences, New Delhi) where records of all patients enrolled between 2009 and 2013 were reviewed. The aim of this study was to calculate the frequency of predefined outcomes in these patients receiving standardized treatment for MDR-TB.
Results:
Out of a total of 819 patients, the frequency of outcomes in these patients was as follows: Cured (n = 415, 52%), default (n = 199, 24%), death (n = 130, 16%), switched to category V (n = 27, 3%), transferred out (n = 12, 1%), treatment failure (n = 13, 1%), and treatment completed (n = 23, 3%).
Conclusion:
The modest cure rate in concordance with other Indian studies highlights the need for continuing efforts to fight the menace of MDR-TB.
Keywords: Directly observed treatment short course chemotherapy-plus, Programmatic Management of Drug-resistant Tuberculosis, Mycobacterium tuberculosis
INTRODUCTION
Globally, tuberculosis is an important disease of public health importance. Its impact is even higher in resource-limited countries which are heavily burdened with overcrowding and lack of medical facilities. The problem is further complicated by the high prevalence of resistance in Mycobacterium tuberculosis to first-line antitubercular drugs. The Revised National Tuberculosis Control Programme (RNTCP) under the programmatic management of drug-resistant tuberculosis or directly observed treatment short course chemotherapy plus (DOTS-Plus) strategy has adopted Category IV regimen as the standard of care for the treatment of multidrug-resistant tuberculosis (MDR-TB) since 2007. The success of any national program can only be ascertained by studying the outcomes of treatment regimes in patients with MDR-TB enrolled in different DOTS-plus sites of the country. Therefore, the aim of this study was to find the treatment outcomes in patients with MDR-TB at our DOTS-plus site.
MATERIALS AND METHODS
A retrospective study was carried out during which records of all patients who were registered at the DOTS-plus site in our tertiary care center (All India Institute of Medical Sciences, New Delhi) between 2009 and 2013 were reviewed. MDR-TB was defined as resistance to rifampicin and isoniazid detected by either culture and drug susceptibility testing (Solid-Lowenstein-Jensen media or liquid - mycobacteria growth indicator tube 960) [Figures 1 and 2] or Genotype MTBDR assay (Hain Life science GmbH, Nehren, Germany). The number of patients primarily diagnosed by either of the methods was recorded. Those who were diagnosed as extensively drug-resistant tuberculosis were excluded from the analysis. Additional drug susceptibilities for ethambutol, streptomycin, kanamycin, ofloxacin, and ethionamide were recorded, wherever available. The standardized treatment regimen (Category IV) of RNTCP was used for the treatment of MDR-TB cases. The six drugs that were used during the intensive phase were kanamycin, levofloxacin, ethionamide, cycloserine, pyrazinamide, and ethambutol. After 6–9 months of intensive phase, levofloxacin, ethionamide, ethambutol, and cycloserine were continued for 18 months. The baseline demographic characteristics of all the patients were recorded. The patients were categorized into predefined treatment outcomes. The cure was defined as a patient who has completed treatment and has at least five consecutive negative results in the past 12–15 months. In case of positivity in the last three-quarters, if the positive culture was followed by three negative cultures (at least 30 days apart), they were still considered as cured. The patients were categorized as treatment failure if two or more of the cultures turned out to be positive in the final 12–15 months or even one of the final three cultures were positive. In cases, where no bacteriological results were available, they were categorized into treatment completed after 24 months of treatment. Treatment default was considered to be the outcome if the treatment was interrupted for 2 or more months. The other outcomes that were defined according to the RNTCP guidelines were as follows: Death, Transfer out (to another DOTS-plus site) and switched to Category V treatment.
Figure 1.

Negative mycobacteria growth indicator tube 960 tube after 6 weeks of incubation
Figure 2.

Positive mycobacteria growth indicator tube 960 tube after 3 weeks of incubation
Data analysis
The frequency of different baseline characteristics and outcomes were expressed as a percentage. Their 95% confidence intervals (CIs) were calculated. The data were analyzed with Stata version 12.1 (StataCorp LLC, College Station, Texas, USA).
RESULTS
A total of 819 patients were registered at our DOTS-plus site from 2009 to 2013. Of these, 329 patients (40.2%, 95% CI - 36.9–43.6) were female and 490 patients (59.8%, 95% CI - 56.4–63.1) were male. The mean age at the time of presentation was 30.6 years (standard deviation: 13). The year wise distribution from 2009 to 2013 was as follows: 75, 71, 100, 275, and 298, respectively. A total of 626 patients (76.4%, 95%CI - 73.4–79.2) received Category II treatment before MDR regimen was started, whereas 105 (12.8%, 95%CI - 10.7–15.3) received Category I. A total of eight patients were already on Category IV and were transferred in. A total of 26 patients (3.2%, 95% CI - 2.2–4.6) received individualized or incorrect regimens from private practitioners. A total of 54 patients (6.6%, 95% CI - 5.1–8.5) either did not receive antitubercular therapy (ATT) or information was not available about their past ATT regimen. The primary method for diagnosis of MDR was by using solid media (213, 26%, 95% CI - 23.1–29.1), Liquid media (67, 8.2%, 95% CI - 6.5–10.3) and Genotype MTBDR assay (539, 65.8%, 95% CI - 62.5–69). Additional drug susceptibility data were available only for selected patients. Ethambutol was sensitive in 121/288 (42%, 95% CI - 36.4–47.8) while it was resistant in 167/288 (58%, 95% CI - 52.2–63.5). Streptomycin was sensitive in 67/289 (23.2%, 95% CI - 18.7–28.4) individuals while it was resistant in 222/289 (76.8%, 95% CI - 71.6–81.3) individuals. Ofloxacin was resistant in 45/57 (78.9%, 95% CI - 66.6–87.7) individuals while it was sensitive in 12/57 (21%, 95% CI - 12.3–33.4). Kanamycin was resistant in 13/60 (21.7%, 95% CI - 13–33.7) while it was sensitive in 47/60 (78.3%, 95% CI - 66.2–87). Ethionamide was sensitive in 4/13 (30.8%, 95% CI - 12.3–58) while it was resistant in 9/13 (69.2%, 95% CI - 42–87.6) [Figure 3]. HIV was negative in 794/812 (97.8, 95% CI - 96.5–98.6) individuals while it was positive in 18/812 (2.2%, 95% CI - 1.4–3.5) individuals. 22/303 (7.3%, 95% CI - 4.8–10.8) individuals were diabetic while 281/303 (92.7%, 95% CI - 89.2–95.2) were nondiabetic. The frequency of predefined outcomes in these patients was as follows: cured (n = 415, 52%, 95% CI - 47.2–54), default (n = 199, 24%, 95% CI - 21.5–27.3), death (n = 130, 16%, 95% CI - 13.5–18.5), switched to category V (n = 27, 3%, 95% CI - 2.3–4.8), transferred out (n = 12, 1%, 95% CI - 0.8–2.6), treatment failure (n = 13, 1%, 95% CI - 0.9–2.7) [Figure 4]. A total of only 23 patients (3%, 95% CI - 1.9–4.2) were extra-pulmonary MDR-TB and so proof of cure could not be ascertained at the end of the treatment course. These patients were classified as treatment completed.
Figure 3.
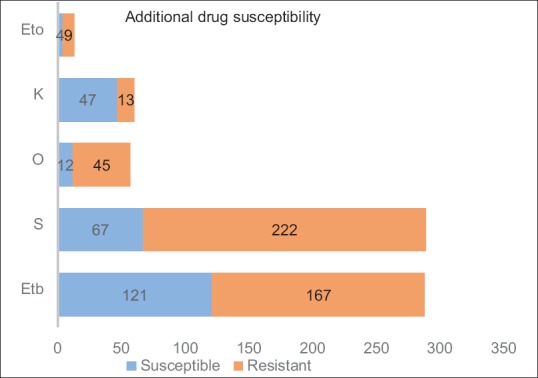
Phenotypic drug susceptibility of ethambutol (Etb), streptomycin (S), ofloxacin (O), kanamycin (K) and ethionamide (Eto)
Figure 4.
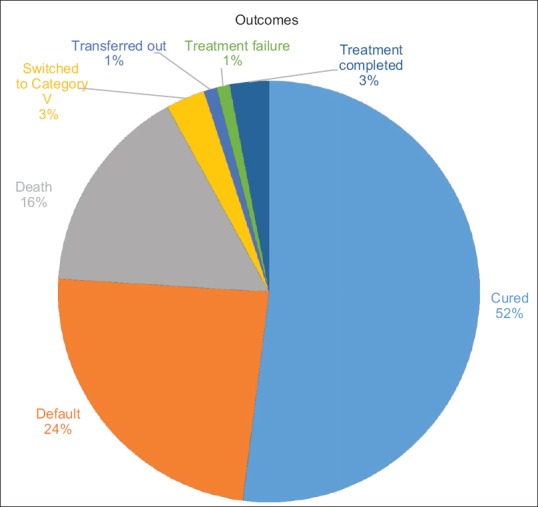
Treatment outcomes of multidrug-resistant tuberculosis patients
DISCUSSION
According to the Global tuberculosis report, 2013, the incidence of MDR-TB in new cases was 3.6% while that in retreatment cases was 20.2%.[1] A very high proportion of these MDR-TB cases were from India. The incidence of MDR-TB in India is around 1%–3% in newly diagnosed cases.[2,3] In most studies from India and abroad, MDR-TB was more commonly seen in patients who had a history of previous treatment.[4,5,6] In concordance with these studies, majority of our patients had received previous antitubercular treatment. Although base line immune status (HIV and diabetes) was recorded for most of the patients, it has been seen in systematic reviews that treatment outcomes are not significantly affected by them.[7] Successful treatment outcomes or cure in other Indian studies ranged from 33% to 61%.[8,9,10,11,12] The cure rate of MDR treatment from other parts of the world ranges from 44% to 77%.[13,14,15,16,17] The percentage of patients succumbing to death during treatment varies from 8% to 30% in Indian settings.[8,9,10,11,12] The percentage of defaulters in various Indian centers differs from 8% to 21%.[8,9,10,11,12] The failure rate in the patients on treatment ranges from 6% to 13% in the Indian subcontinent.[9,10,11,12] As evident from our review, there was considerable variation in the treatment outcomes in different studies. It was found in a meta-analysis that the duration of treatment and whether the therapy was directly observed or not, was very important in determining the treatment outcomes. Furthermore, it was seen that individualized treatment regimens were slightly better than standardized treatment regimens.[18] The patients received long duration of directly observed therapy, but due to the lack of abundant laboratories with drug susceptibility testing capabilities, we still follow standardized treatment regimens under the national program. Most of the findings in our study were consistent with other published Indian studies. Even though our cure rate of 52% was comparable to the cure rates reported from other national and international studies, it is still far behind the current goals. We also had a very high percentage of patients who defaulted (24%) or died (16%) during treatment. The high percentage of failure rates in the treatment of MDR-TB is primarily due to the long duration of treatment and side effects associated with the second-line drugs. There is a lot of research going on toward the development of shorter, simpler, and alloral regimes. The STREAM trial Stage 1 has shown promising results.[19] WHO has given a conditional recommendation for short regimes but their applicability in Indian settings is a question for debate.[20]
Limitation of the study
The retrospective study design was associated with its inherent limitations. The drug resistance pattern to other drugs and previous treatment history was not available for all the patients.
CONCLUSION
This study points out the menace of MDR-TB in a high burden country like India. Although, the national program has been somewhat successful in implementing the DOTS-plus strategy, the high death and default rates needs to be addressed further. Continued programmatic efforts are required to tackle the challenge that MDR-TB is.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2013. World Health Organization. 2013:303. [Google Scholar]
- 2.Chopra KK. Drug resistance among TB cases and its clinical implications. Indian J Tuberc. 2015;62:151–5. doi: 10.1016/j.ijtb.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Singla N, Singla R, Fernandes S, Behera D. Post treatment sequelae of multi-drug resistant tuberculosis patients. Indian J Tuberc. 2009;56:206–12. [PubMed] [Google Scholar]
- 4.Gupta A, Nagaraja MR, Kumari P, Singh G, Raman R, Singh SK, et al. Association of MDR-TB isolates with clinical characteristics of patients from Northern region of India. Indian J Med Microbiol. 2014;32:270–6. doi: 10.4103/0255-0857.136561. [DOI] [PubMed] [Google Scholar]
- 5.Murase Y, Maeda S, Yamada H, Ohkado A, Chikamatsu K, Mizuno K, et al. Clonal expansion of multidrug-resistant and extensively drug-resistant tuberculosis, Japan. Emerg Infect Dis. 2010;16:948–54. doi: 10.3201/eid1606.091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quy HT, Cobelens FG, Lan NT, Buu TN, Lambregts CS, Borgdorff MW, et al. Treatment outcomes by drug resistance and HIV status among tuberculosis patients in Ho Chi Minh city, Vietnam. Int J Tuberc Lung Dis. 2006;10:45–51. [PubMed] [Google Scholar]
- 7.Isaakidis P, Casas EC, Das M, Tseretopoulou X, Ntzani EE, Ford N, et al. Treatment outcomes for HIV and MDR-TB co-infected adults and children: Systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19:969–78. doi: 10.5588/ijtld.15.0123. [DOI] [PubMed] [Google Scholar]
- 8.Singla R, Sarin R, Khalid UK, Mathuria K, Singla N, Jaiswal A, et al. Seven-year DOTS-plus pilot experience in India: Results, constraints and issues. Int J Tuberc Lung Dis. 2009;13:976–81. [PubMed] [Google Scholar]
- 9.Jain K, Desai M, Solanki R, Dikshit RK. Treatment outcome of standardized regimen in patients with multidrug resistant tuberculosis. J Pharmacol Pharmacother. 2014;5:145–9. doi: 10.4103/0976-500X.130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph P, Desai VB, Mohan NS, Fredrick JS, Ramachandran R, Raman B, et al. Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, India. Indian J Med Res. 2011;133:529–34. [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav AK, Mehrotra AK, Agnihotri SP, Swami S. Study of factors influencing response and outcome of Cat-IV regimen in MDRTB patients. Indian J Tuberc. 2016;63:255–61. doi: 10.1016/j.ijtb.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Patel SV, Nimavat KB, Alpesh PB, Shukla LK, Shringarpure KS, Mehta KG, et al. Treatment outcome among cases of multidrug-resistant tuberculosis (MDR TB) in Western India: A prospective study. J Infect Public Health. 2016;9:478–84. doi: 10.1016/j.jiph.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Liu CH, Li L, Chen Z, Wang Q, Hu YL, Zhu B, et al. Characteristics and treatment outcomes of patients with MDR and XDR tuberculosis in a TB referral hospital in Beijing: A 13-year experience. PLoS One. 2011;6:e19399. doi: 10.1371/journal.pone.0019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SK, Lee WC, Lee DH, Mitnick CD, Han L, Seung KJ, et al. Self-administered, standardized regimens for multidrug-resistant tuberculosis in South Korea. Int J Tuberc Lung Dis. 2004;8:361–8. [PubMed] [Google Scholar]
- 15.Chiang CY, Enarson DA, Yu MC, Bai KJ, Huang RM, Hsu CJ, et al. Outcome of pulmonary multidrug-resistant tuberculosis: A 6-yr follow-up study. Eur Respir J. 2006;28:980–5. doi: 10.1183/09031936.06.00125705. [DOI] [PubMed] [Google Scholar]
- 16.Shin SS, Pasechnikov AD, Gelmanova IY, Peremitin GG, Strelis AK, Mishustin S, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis. 2006;10:402–8. [PubMed] [Google Scholar]
- 17.Van Deun A, Salim MA, Das AP, Bastian I, Portaels F. Results of a standardised regimen for multidrug-resistant tuberculosis in Bangladesh. Int J Tuberc Lung Dis. 2004;8:560–7. [PubMed] [Google Scholar]
- 18.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: Systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–61. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 19.Moodley R, Godec TR STREAM Trial Team. Short-course treatment for multidrug-resistant tuberculosis: The STREAM trials. Eur Respir Rev. 2016;25:29–35. doi: 10.1183/16000617.0080-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Last accessed on 2017 Sep 25]. Available from: http://www.who.int/tb/Short_MDR_regimen_factsheet.pdf .


