Abstract
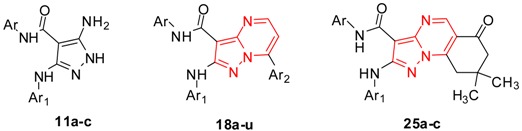
A series of N-aryl-7-aryl-pyrazolo[1,5-a]pyrimidines 18a–u and N-aryl-pyrazolo[1,5-a]quinazolines 25a–c were designed and synthesized via the reaction of 5-aminopyrazoles 11a–c with enaminones 12a–g or 19, respectively. The new compounds were screened for their in vitro antitumor activity toward liver (HepG-2) and breast (MCF-7) human cancer cells using 3-[4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide MTT assay. From the results, it was found that all compounds showed dose-dependent cytotoxic activities against both HepG-2 and MCF-7 cells. Two compounds 18o and 18a were selected for further investigations. Cell cycle analysis of liver (HepG-2) cells treated with 18o and breast (MCF-7) cells treated with 18a showed cell cycle arrest at G2/M phase and pro-apoptotic activity as indicated by annexin V-FITC staining.
Keywords: pyrazolopyrimidines, pyrazoloquinazolines, synthesis, antitumor activity, cell cycle analysis
1. Introduction
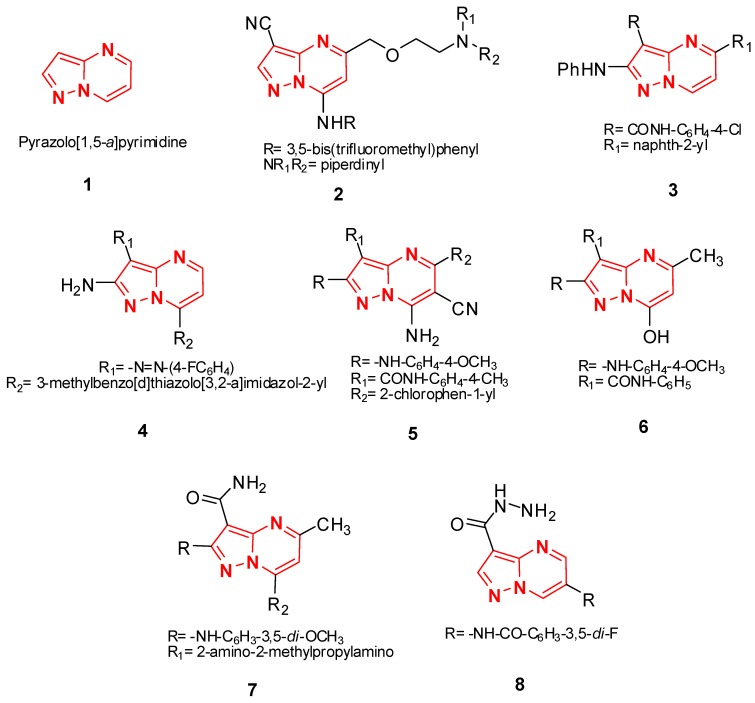
Pyrazolo[1,5-a]pyrimidine ring 1 and its derivatives occupy a unique place in medicinal chemistry due to its various pharmacological activities [1,2,3,4,5,6] especially antitumor properties [7,8,9]. In 2006, Li et al. synthesized compound 2 which exhibited significant in vitro antitumor activity against Bel-7402 (liver) and HT-1080 (fibrosarcoma) cell lines [10]. In 2009, Ahmed et al., prepared compound 3 which was more effective and exhibited cytotoxicity against HCT116 (colon) and HeLa (cervix) cell lines [11]. In 2010, Abdel-Aziz and co-workers have described a facile synthesis of compound 4 which exhibited promising in vitro antitumor activity against CaCo-2 (colon) and BHK (normal fibroblast) cell lines [12]. Furthermore, we have reported the synthesis of compounds 5 and 6 in high yield by treating 5-aminopyrazole with 2-(2-chlorobenzylidene)malononitrile and ethyl acetoacetate, respectively, these compounds show good antitumor activities against HCT-116 and HepG2 cells [13,14] (Figure 1).
Figure 1.
Structures of the antitumor activity compounds 1–6 and enzymes inhibitors 7–8.
In addition, pyrazolo[1,5-a]pyrimidine derivatives have been reported as potent enzymes inhibitors [15,16,17]. Mukaiyama et al., have prepared compound 7 which exhibited potent inhibitory activity against c-Src Kinase and good CNS penetration [18]. Very recently, Kumar et al., have prepared pyrazolo[1,5-a]pyrimidine carboxamide 8 which showed good aurora kinase A and B activity [19] (Figure 1).
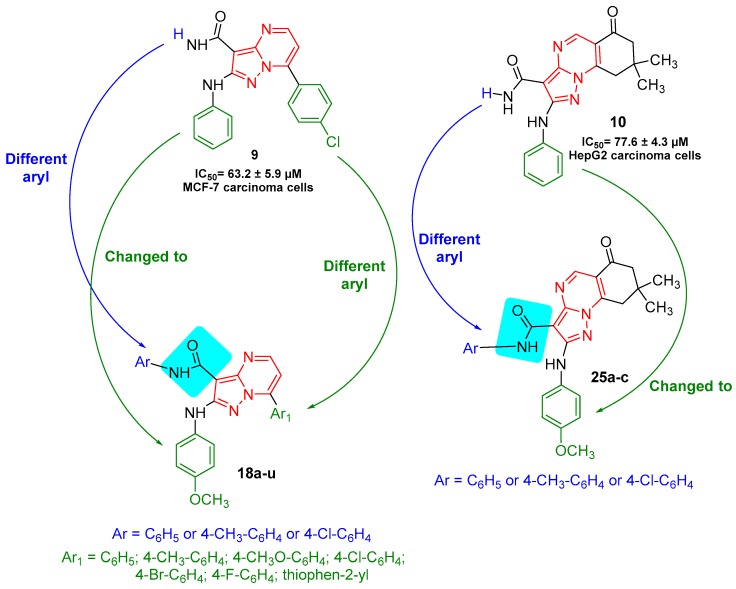
In continuation of our research program [20,21,22,23,24,25,26,27] and following the potent biological activity results against MCF-7 and HepG2 carcinoma cells which were obtained from our synthesized compounds such as 7-(4-chlorophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine (9, IC50 = 63.2 ± 5.9 µM) and 2-(phenylamino)-pyrazolo[1,5-a]quinazoline (10, IC50 = 77.6 ± 4.3 µM) compared to doxorubicin [28]. In this work, we have planned to modify the pyrazolo[1,5-a]pyrimidine 9 and pyrazolo[1,5-a]quinazoline 10 to obtain N-aryl-7-aryl-pyrazolo[1,5-a]pyrimidines 18a–u and N-aryl-pyrazolo[1,5-a]quinazolines 25a–c, respectively, incorporating different aryl groups (blue and green) into the structures to evaluate their in vitro antitumor activities against HepG-2 and MCF-7 human cells to find novel and potent antitumor compounds (Figure 2).
Figure 2.
Design of novel N-aryl-pyrazolo[1,5-a]pyrimidines 18a–u and N-aryl-pyrazolo[1,5-a]quinazolines 25a–c-based amide linkages.
2. Results and Discussion
2.1. Chemistry
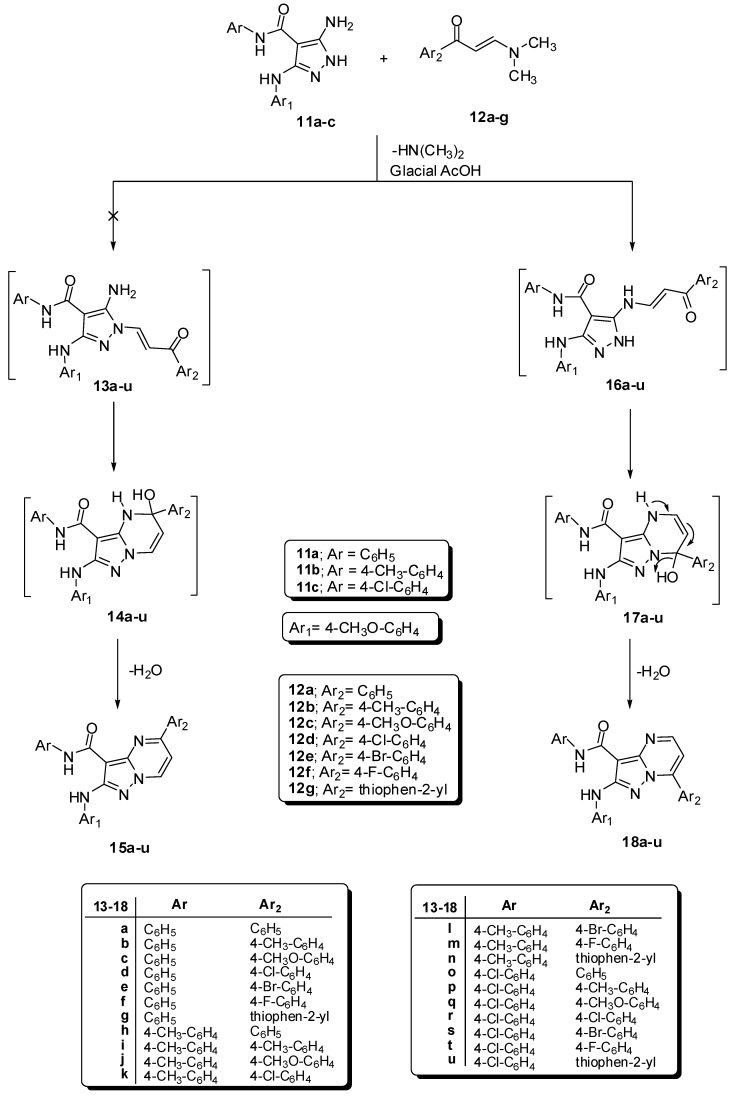
The syntheses oftarget compounds 18a–u and 25a–c are illustrated in Scheme 1 and Scheme 2. The starting materials, 5-amino-N-aryl-1H-pyrazole-4-carboxamides 11a–c were synthesized according to our previous work [29]. Reaction of compounds 11a–c with 1-(aryl)-3-(dimethylamino)prop-2-en-1-ones 12a–g in glacial acetic acid furnished one isolable product 5-aryl-pyrazolo[1,5-a]pyrimidines 15a–u or 7-aryl-pyrazolo[1,5-a]pyrimidines 18a–u. As depicted in Scheme 1, the final products were confirmed by the spectral analysis.
Scheme 1.
Synthesis of 7-aryl-pyrazolo[1,5-a]pyrimidines 18a–u.
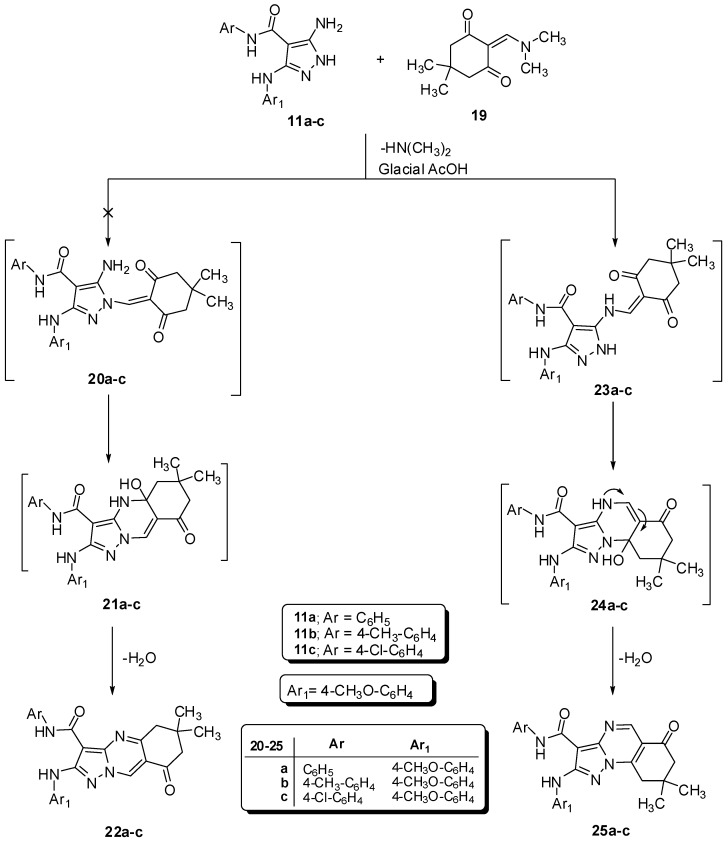
Scheme 2.
Synthesis of N-aryl-2-(arylamino)-pyrazolo[1,5-a]quinazolines 25a–c.
The 1H-NMR spectrum (CDCl3, δ ppm) exhibited, in each case 18c or 15c, characteristic two doublets of the pyrimidine protons at 6.87 (1H, H-6) and at 8.40 (1H, H-5) (each with J = 8.4 Hz) and four signals at 3.80, 3.91, 9.36 and 10.02 due to 2OCH3 and 2NH, respectively. The 13C-NMR spectrum (CDCl3, δ ppm), in each case 18c or 15c, also characterized by signals of –OCH3, –OCH3, C3-pyrazolopyrimidine, C6-pyrazolopyrimidine, C5-pyrazolopyrimidine and C=O at 55.53, 55.60, 87.45, 106.43, 157.52 and 163.21, respectively.
Although, 1H- and 13C-NMR spectra cannot differentiate between 15a–u and 18a–u, 1H-15N HMBC spectrum used for differentiating between the two isomers. The 1H-15N HMBC spectrum of the final product shows the most important correlated coupling between the proton H-5 of pyrazolopyrimidine (1H, at 8.40 ppm) with N-4 of pyrimidine (15N, at 255 ppm) 2J (H-5, N-4) gave absolute confirmation for the structure of 18a–u and conclude 15a–u (cf. Supporting Information). Also, the structure of 18a–u was supported by X-ray crystallography of similar analogs and products [12].
In addition, N-aryl-2-(arylamino)-pyrazolo[1,5-a]quinazolines 25a–c were formed by the condensation of 11a–c with 2-((dimethylamino)methylene)-5,5-dimethylcyclohexane-1,3-dione (19) in a glacial AcOH (Scheme 2) while pyrazolo[1,5-a]quinazolines 22a–c were not formed. The spectral analysis of the products supported the structures of 25a–c.
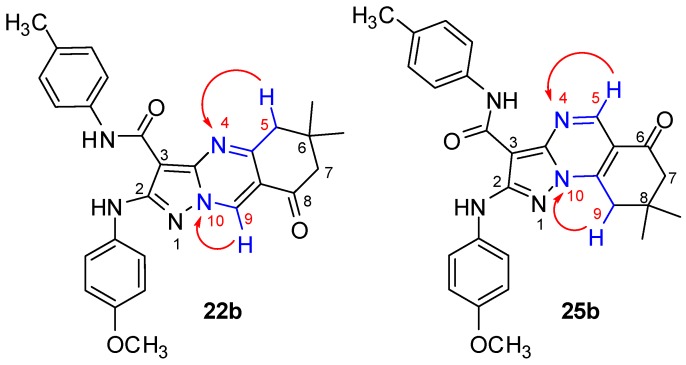
The mass spectrum of 25b confirmed the molecular formula C27H27N5O3 (469.53) {MS (m/z, %): 469 (M+, 93.88)}. The 1H-NMR (CDCl3, 400 MHz, δ ppm) spectrum of 25b was characterized by sharp signals of 2CH3, CH2 and CH2 groups of the dimedone at 1.19, 2.52 and 3.22, respectively. The OCH3 group, H-5 proton of quinazoline and 2NH protons appeared as singlet signals at 3.81, 8.90, 9.41 and 9.72, respectively. The aromatic protons of 4-methoxyphenylamino ring appeared as two doublets at 6.91 (2H) and 7.58 (2H) with the coupling constant J = 9.0 Hz and the four aromatic protons of N-(4-methylphenyl) ring appeared as two doublets at 7.16 (J = 8.2 Hz) and 7.54 (J = 8.3 Hz). Also, the 13C-NMR (CDCl3, 100 MHz, δ ppm) spectrum showed characteristic signals at 28.70 corresponding to 2CH3, at 32.65 for a C8 (quinazoline), two signals at 37.52 and 50.99 corresponding to 2CH2 and signal at 194.01 due to C=O (quinazoline). The 1H-15N HMBC spectrum showed that the two most important correlated coupling which gave absolute and unique confirmation for the structure of 25a–c, the first was between the proton H-5 of quinazoline (1H, at 8.90 ppm) with N-4 of quinazoline (15N, at 260 ppm) 2J (H-5, N-4) and the second was between the CH2 of quinazoline (1H, at 3.22 ppm) with N-10 of quinazoline (15N, at 216 ppm) 3J (H-9, N-10) (cf. Supporting Information) (Figure 3).
Figure 3.
Diagnostic correlations in the 1H-15N HMBC spectrum for the two isomers 22b and 25b.
If the compound 22b was obtained, its 1H-15N HMBC spectrum would have exhibited that correlated coupling between the proton H-9 of quinazoline with N-10 of quinazoline 2J (H-9, N-10) and correlated coupling between the CH2 of quinazoline with N-4 of quinazoline 3J (H-5, N-4), but, these two correlated coupling were not detected in 1H-15N HMBC spectrum (Figure 3). Furthermore, X-ray diffraction of similar analogs added unequivocal evidence for the structures of 25a–c and confirmed the reaction mechanism [30].
2.2. In Vitro Cytotoxic Activity
For evaluation of in vitro cytotoxic activity of compounds {5-aminopyrazoles 11a–c, pyrazolo[1,5-a]pyrimidines 18a–u and pyrazolo[1,5-a]quinazolines 25a–c} against liver (HepG-2) and breast (MCF-7) human carcinoma cell lines, MTT assay was used [31,32,33]. Doxorubicin® was used as a reference cytotoxic compound. The results were expressed as growth inhibitory concentration (IC50) values (Table 1).
Table 1.
The IC50 (µM) values of compounds 11a–c, 18a–u and 25a–c using MTT assay against two human carcinoma cell lines (HepG-2 and MCF-7).
| Compounds | Ar | Ar1 | Ar1 | IC50 (µM) | |
|---|---|---|---|---|---|
| HepG-2 | MCF-7 | ||||
| 11a | C6H5 | 4-CH3O-C6H4 | - | 81.3 ± 4.1 | 65.5 ± 4.3 |
| 11b | 4-CH3-C6H4 | 4-CH3O-C6H4 | - | 86.2 ± 4.5 | 69.2 ± 3.9 |
| 11c | 4-Cl-C6H4 | 4-CH3O-C6H4 | - | 94.8 ± 6.5 | 69.1 ± 3.7 |
| 18a | C6H5 | 4-CH3O-C6H4 | C6H5 | 85.4 ± 5.1 | 63.1 ± 3.1 * |
| 18b | C6H5 | 4-CH3O-C6H4 | 4-CH3-C6H4 | 90.9 ± 6.5 | 64.9 ± 3.1 |
| 18c | C6H5 | 4-CH3O-C6H4 | 4-CH3O-C6H4 | 75.9 ± 5.3 | 64.3 ± 4.2 |
| 18d | C6H5 | 4-CH3O-C6H4 | 4-Cl-C6H4 | 77.1 ± 4.2 | 65.1 ± 2.8 |
| 18e | C6H5 | 4-CH3O-C6H4 | 4-Br-C6H4 | 81.2 ± 5.5 | 68.1 ± 4.0 |
| 18f | C6H5 | 4-CH3O-C6H4 | 4-F-C6H4 | 92.8 ± 6.7 | 66.1 ± 2.9 |
| 18g | C6H5 | 4-CH3O-C6H4 | thiophen-2-yl | 91.1 ± 6.4 | 69.2 ± 3.2 |
| 18h | 4-CH3-C6H4 | 4-CH3O-C6H4 | C6H5 | 73.2 ± 3.2 | 66.8 ± 2.6 |
| 18i | 4-CH3-C6H4 | 4-CH3O-C6H4 | 4-CH3-C6H4 | 83.3 ± 4.3 | 67.7 ± 2.7 |
| 18j | 4-CH3-C6H4 | 4-CH3O-C6H4 | 4-CH3O-C6H4 | 77.4 ± 2.9 | 64.3 ± 3.1 |
| 18k | 4-CH3-C6H4 | 4-CH3O-C6H4 | 4-Cl-C6H4 | 74.0 ± 3.1 | 66.8 ± 3.9 |
| 18l | 4-CH3-C6H4 | 4-CH3O-C6H4 | 4-Br-C6H4 | 78.7 ± 5.1 | 66.7 ± 3.2 |
| 18m | 4-CH3-C6H4 | 4-CH3O-C6H4 | 4-F-C6H4 | 80.3 ± 3.9 | 66.2 ± 3.8 |
| 18n | 4-CH3-C6H4 | 4-CH3O-C6H4 | thiophen-2-yl | 82.5 ± 5.7 | 65.9 ± 3.1 |
| 18o | 4-Cl-C6H4 | 4-CH3O-C6H4 | C6H5 | 72.2 ± 3.8 * | 64.7 ± 1.9 |
| 18p | 4-Cl-C6H4 | 4-CH3O-C6H4 | 4-CH3-C6H4 | 87.8 ± 5.4 | 67.1 ± 2.1 |
| 18q | 4-Cl-C6H4 | 4-CH3O-C6H4 | 4-CH3O-C6H4 | 72.8 ± 3.9 | 65.5 ± 2.1 |
| 18r | 4-Cl-C6H4 | 4-CH3O-C6H4 | 4-Cl-C6H4 | 73.0 ± 1.9 | 65.9 ± 2.6 |
| 18s | 4-Cl-C6H4 | 4-CH3O-C6H4 | 4-Br-C6H4 | 78.2 ± 3.2 | 66.8 ± 5.0 |
| 18t | 4-Cl-C6H4 | 4-CH3O-C6H4 | 4-F-C6H4 | 78.7 ± 4.7 | 67.0 ± 1.8 |
| 18u | 4-Cl-C6H4 | 4-CH3O-C6H4 | thiophen-2-yl | 83.1 ± 5.1 | 64.5 ± 2.9 |
| 25a | C6H5 | 4-CH3O-C6H4 | - | 87.9 ± 6.0 | 68.9 ± 4.2 |
| 25b | 4-CH3-C6H4 | 4-CH3O-C6H4 | - | 81.9 ± 5.9 | 66.2 ± 2.9 |
| 25c | 4-Cl-C6H4 | 4-CH3O-C6H4 | - | 79.5 ± 4.8 | 66.5 ± 3.1 |
| Doxorubicin | - | - | - | 80.9 ± 2.1 | 65.6 ± 4.2 |
* The most potent compound and selected for further experiments.
From the results of in vitro cytotoxic activity, it was found that most of the prepared compounds displayed comparable IC50 values against liver (HepG-2) and breast (MCF-7) cancer cell lines compared to positive control.
For HepG-2 cancer cells, most of the tested compounds did not show any significant difference compared to the positive control. Only four compounds (11c, 18b, 18f and 18g) showed significant difference in their activities. Compounds 18c (IC50 = 75.9 ± 5.3 µM), 18d (IC50 = 77.1 ± 4.2 µM), 18h (IC50 = 73.2 ± 3.2 µM), 18j (IC50 = 77.4 ± 2.9 µM), 18k (IC50 = 74.0 ± 3.1 µM), 18l (IC50 = 78.7 ± 5.1 µM), 18o (IC50 = 72.2 ± 3.8 µM), 18q (IC50 = 72.8 ± 3.9 µM), 18r (IC50 = 73.0 ± 1.9 µM), 18s (IC50 = 78.2 ± 3.2 µM), 18t (IC50 = 78.7 ± 4.7 µM) and 25c (IC50 = 79.5 ± 4.8 µM) showed slightly higher activities than doxorubicin (IC50 = 80.9 ± 2.1 µM). In addition, compound 18m (IC50 = 80.3 ± 3.9 µM) was almost equipotent as doxorubicin (IC50 = 80.9 ± 2.1 µM), while, compounds 11a (IC50 = 81.3 ± 4.1 µM), 18e (IC50 = 81.2 ± 5.5 µM), 18n (IC50 = 82.5 ± 5.7 µM) and 25b (IC50 = 81.9 ± 5.9 µM) displayed slightly less activities compared to doxorubicin (IC50 = 80.9 ± 2.1 µM).
In case of MCF-7 cell lines, none of thecompounds showed any significant differences compared to the positive control. Compounds 18a (IC50 = 63.1 ± 3.1 µM), 18b (IC50 = 64.9 ± 3.1 µM), 18c (IC50 = 64.3 ± 4.2 µM), 18j (IC50 = 64.3 ± 3.1 µM), 11o (IC50 = 64.7 ± 1.9 µM) and 18u (IC50 = 64.5 ± 2.9 µM) showed slightly higher activities than doxorubicin (IC50 = 65.6 ± 4.2 µM). Whilst, compounds 11a (IC50 = 65.5 ± 4.3 µM), 18d (IC50 = 65.1 ± 2.8 µM), 18n (IC50 = 65.9 ± 3.1 µM), 18q (IC50 = 65.5 ± 2.1 µM) and 18r (IC50 = 65.9 ± 2.6 µM) displayed equipotent as doxorubicin (IC50 = 65.6 ± 4.2 µM). Whereas, compounds 18f (IC50 = 66.1 ± 2.9 µM), 18h (IC50 = 66.8 ± 2.6 µM), 18k (IC50 = 66.8 ± 3.9 µM), 18l (IC50 = 66.7 ± 3.2 µM), 18m (IC50 = 66.2 ± 3.8 µM), 18s (IC50 = 66.8 ± 5.0 µM), 25b (IC50 = 66.2 ± 2.9 µM) and 25c (IC50 = 66.5 ± 3.1 µM) displayed slightly less activities.
2.3. Structure Activity Relationship (SAR)
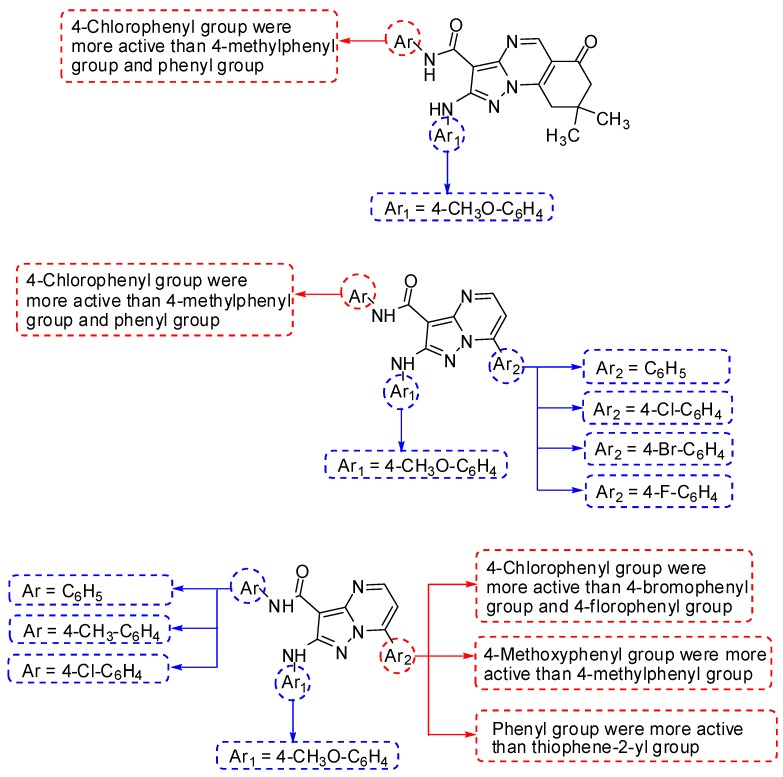
From the results of in vitrocytotoxic activity of the synthesized compounds against liver (HepG2) cell lines, we found that, 25c (IC50 = 79.5 ± 4.8 µM) >25b (IC50 = 81.9 ± 5.9 µM) >25a (IC50 = 87.9 ± 6.0 µM) in the series of pyrazolo[1,5-a]quinazolines 25a–c, in addition, 18o (IC50 = 72.2 ± 3.8 µM) >18h (IC50 = 73.2 ± 3.2 µM) >18a (IC50 = 85.4 ± 5.1 µM); 18r (IC50 = 73.0 ± 1.9 µM) >18k (IC50 = 74.0 ± 3.1 µM) >18d (IC50 = 77.1 ± 4.2 µM); 18s (IC50 = 78.2 ± 3.2 µM) >18l (IC50 = 78.7 ± 5.1 µM) >18c (IC50 = 75.9 ± 5.3 µM) and 18t (IC50 = 78.7 ± 4.7 µM) >18m (IC50 = 80.3 ± 3.92 µM) >18f (IC50 = 92.8 ± 6.7 µM) in the series of pyrazolo[1,5-a]pyrimidines 18a–u. This was concerning the effect of 4-Cl-C6H4 group (chloride atom as electron withdrawing group) and 4-CH3-C6H4 group (methyl as electron releasing group) in the two series. Whence, the derivatives bearing Ar = 4-Cl-C6H4 group (at position 3 in the two series) were slightly more active than those bearing Ar = 4-CH3-C6H4 group than those bearing Ar = C6H5 group.
In addition, we observed that, there was a ranking in the order of rings bearing halogen atoms (Cl, Br and F) in the series of 18a–u, where, 18d (IC50 = 77.1 ± 4.2 µM) >18e (IC50 = 81.2 ± 5.5 µM) >18f (IC50 = 92.8 ± 6.7 µM); 18k (IC50 = 74.0 ± 3.1 µM) >18l (IC50 = 78.7 ± 5.1 µM) >18m (IC50 = 80.3 ± 3.9 µM) and 18r (IC50 = 73.0 ± 1.9 µM) >18s (IC50 = 78.2 ± 3.2 µM) >18t (IC50 = 78.7 ± 4.7 µM). Therefore, the derivatives bearing Ar2 = 4-Cl-C6H4 group (at position 7) > Ar2 = 4-Br-C6H4 group > Ar2 = 4-F-C6H4 group.
Moreover, the derivatives bearing Ar2 = 4-CH3O-C6H4 group (at position 7) > Ar2 = 4-CH3-C6H4 group, where, 18c (IC50 = 75.9 ± 5.3µM) >18b (IC50 = 90.9 ± 6.5 µM); 18j (IC50 = 77.4 ± 2.9µM) >18i (IC50 = 83.3 ± 4.3 µM) and 18q (IC50 = 72.8 ± 3.9 µM) >18p (IC50 = 87.8 ± 5.4 µM). Therefore, the replacement of the 4-CH3-C6H4 group by 4-CH3O-C6H4 group was impacted and increased the activity against liver cancer.
Furthermore, we observed that, the derivatives bearing phenyl group (at position 7) more active than those bearing thiophen-2-yl group, where,18a (IC50 = 85.4 ± 5.1 µM) >18g (IC50 = 91.1 ± 6.4 µM); 18h (IC50 = 73.2 ± 3.2 µM) >18n (IC50 = 82.5 ± 5.7 µM) and 18o (IC50 = 72.2 ± 3.8 µM) >18u (IC50 = 83.1 ± 5.1 µM). Therefore, the introduction of thiophen-2-yl group in the series decreased the activity. A brief Structure-activity relationship (SAR) study has been presented in Figure 4.
Figure 4.
A brief Structure-activity relationship (SAR) study of 18a–u and 25a–c against liver (HepG2) cell lines.
2.4. Cell-Cycle Analysis and Apoptotic Changes
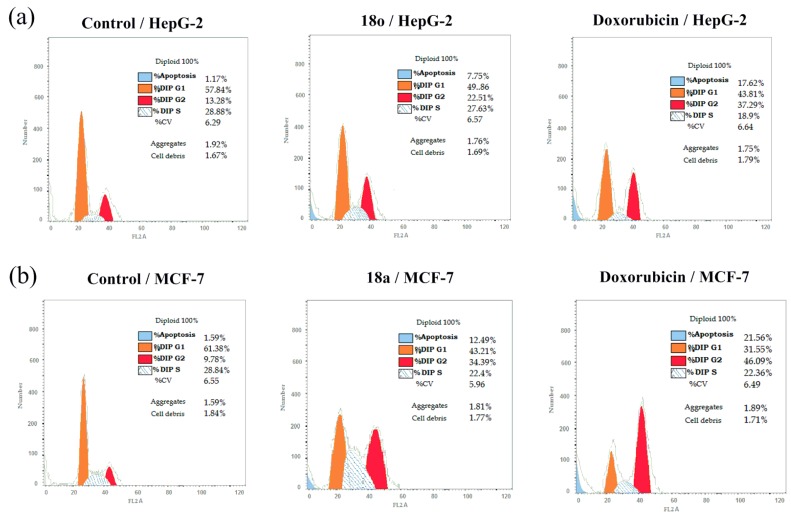
Cell cycle can be defined as cell reproduction via replication of the DNA followed by division of the nucleus and partitioning of the cytoplasm to yield two daughter cells. This cell cycle comprises four different phases. G1 phase occurs between nuclear division (M phase) and DNA synthesis (S phase); G2 phase occurs between S phase and M phase. These gaps allow for the repair of DNA damage and replication errors [34]. According to the cytotoxicity screening in Table 1, and because most of the compounds did not show statistical significant differences compared to the positive control, two compounds (18o and 18a) have been selected for further experiments. The effect of compounds 18o and 18a after 24 h of treatment by propidium iodide on cell cycle progression, using the flow cytometry (Figure 5a), was investigated against HepG-2 and MCF-7, respectively.
Figure 5.
(a) Effect of compound 18o on DNA-ploidy flow cytometric analysis of HepG-2 cancer cells, the cells were treated with DMSO as control and with doxorubicin as a positive control, for 24 h. (b) Effect of compound 18a on DNA-ploidy flow cytometric analysis of MCF-7 cancer cells, the cells were treated with DMSO as control and with doxorubicin as a positive control, for 24 h.
Compound 18o induced significant alterations in the cell-cycle phases of HepG2 cells when compared to control. Interestingly, exposure of HepG2 cells to 18o induced a significant increase in the percentage of cells at pre-G1 and G2/M phases by 6.6 folds and 1.7 folds, with a concurrent significant reduction in the percentage of cells at G0/G1 by 1.2 folds without any significant changes in S phase compared to control, respectively.
Moreover, treatment of MCF-7 cancer cells with compound 18a caused a significant increase in pre-G1 and G2/M phases percent by 7.9 folds and 3.5 folds with a significant reduction in the percentage of cells at G0/G1 by 1.4 folds and slightly increase in S phases by 0.9 folds compared to control, respectively (Figure 5b). However, the positive control showed better results. In case of HepG-2 cancer cells, Doxorubicin-induced a significant increase in the percentage of cells at pre-G1 and G2/M phases by 2.2 folds and 1.6 folds, with a significant reduction in the percentage of cells at G0/G1 and S phases by 1.14 and 1.46 folds compared to compound 18o. In addition, in case of MCF-7 cancer cells, Doxorubicin-induced a significant increase in the percentage of cells at pre-G1 and G2/M phases by 1.7-folds and 1.4 folds, with a significant reduction in the percentage of cells at G0/G1 and did not show any significant increase in S phases compared to compound 18o. From these results, it can be concluded that compounds 18o and 18a inhibit the cell growth through cell cycle arrest at G2/M phase, which in turn induces cell death by apoptosis. These results are in agreement with the cytotoxicity screening results.
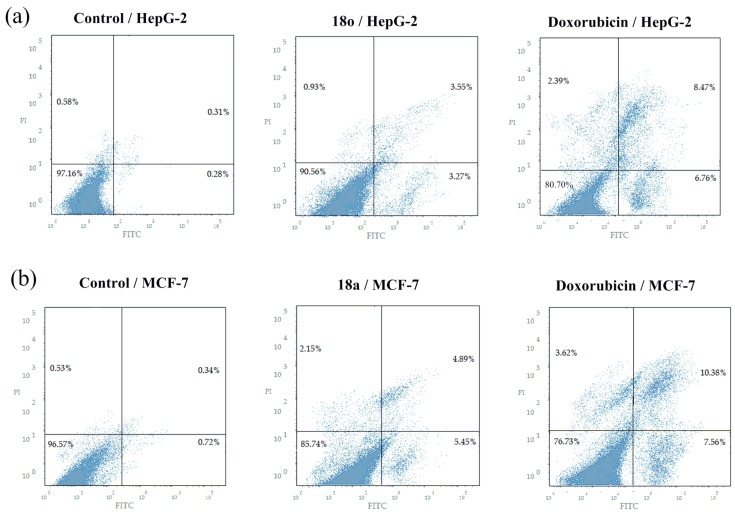
2.5. Annexin V-FITC Apoptosis Assay
The apoptotic effect of compounds 18o and 18a was carried out using Annexin V-FITC/PI (AV/PI) dual staining assay (Figure 6). The results revealed that HepG2 and MCF-7cells, treated with compounds 18o and 18a, respectively, showed a significant increase in the percent of annexin V-FITC positive apoptotic cells (UR & LR) by 11.6 folds and 9.8 folds compared to control, respectively. However, doxorubicin showed 2.2 folds and 1.7 folds increases in apoptotic cells % compared to compounds 18o and 18a, respectively. These results reveal that the cytotoxicity activities of compounds 18o and 18a are due to their potent pro-apoptotic activity.
Figure 6.
(a) Effect of compound 18o on the percentage of annexin V-FITC positive staining in HepG-2 cancer cells, the cells were treated with DMSO as control and with doxorubicin as a positive control, for 24 h. (b) Effect of compound 18a on the percentage of annexin V-FITC positive staining in MCF-7 cancer cells, the cells were treated with DMSO as control and with doxorubicin as a positive control, for 24 h.
3. Materials and Methods
3.1. Chemistry
All melting points were measured on a Gallenkamp melting point apparatus and are uncorrected. The IR spectra were recorded (KBr disk) on a 1650 FT-IR instrument (Perkin Elmer, Waltham, MA, USA). 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were recorded on a Varian spectrometer (Varian, Inc., Palo Alto, CA, USA) using DMSO-d6 or CDCl3 as solvent and TMS as an internal standard. Chemical shifts are reported in ppm. Coupling constants (J) are expressed in Hz. Mass spectra were recorded on a Varian MAT 112 spectrometer at 70 eV. Elemental analyses were performed at the Microanalytical Center, Cairo University, Egypt. The progress of the reactions was monitored by thin-layer chromatography (TLC) using aluminum sheets coated with silica gel F254 (Merck, Darmstadt, Germany), viewing under a short-wavelength UV lamp effected detection. All evaporations were carried out under reduced pressure at 40 °C.
Synthesis of 5-amino-3-(arylamino)-1H-pyrazole-4-carboxamides11a–c. Compounds of this series were prepared according to the literature procedure.
5-Amino-3-(4-methoxyphenylamino)-N-phenyl-1H-pyrazole-4-carboxamide (11a). White crystals; m.p. 175–177 °C [29].
5-Amino-3-(4-methoxyphenylamino)-N-(4-methylphenyl)-1H-pyrazole-4-carboxamide (11b). White crystals; m.p. 198–200 °C [29].
5-Amino-3-(4-methoxyphenylamino)-N-(4-chlorophenyl)-1H-pyrazole-4-carboxamide (11c). White crystals; m.p. 190–192 °C [29].
General Procedure for Synthesis of7-aryl-2-(arylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamides18a–u. A mixture of compounds 11a–c (0.01 mol) with enaminones 12a–g {e.g., 3-(dimethylamino)-1-phenylprop-2-en-1-one (12a), 3-(dimethylamino)-1-(4-methylphenyl)prop-2-en-1-one (12b), 3-(dimethylamino)-1-(4-methoxyphenyl)prop-2-en-1-one (12c), 1-(4-chlorophenyl)-3-(dimethyl-amino)prop-2-en-1-one (12d), 1-(4-bromophenyl)-3-(dimethylamino)prop-2-en-1-one (12e), 3-(dimethylamino)-1-(4-fluorophenyl)prop-2-en-1-one (12f) or 3-(dimethylamino)-1-(thiophen-2-yl)prop-2-en-1-one (12g)} (0.01 mol) in glacial acetic acid (25 mL), the reaction mixture was refluxed for 1 h and then left to cool. The solid product was filtered off, washed with ethanol, dried and finally recrystallized from DMF/H2O to afford the corresponding pyrazolo[1,5-a]pyrimidine derivatives 18a–u.
2-(4-Methoxyphenylamino)-N,7-diphenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18a). Yellow crystals, m.p. 218–220 °C, yield (72%). IR (KBr) νmax/cm−1 3346 (NH), 1658 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 3.80 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz,ArH), 6.96 (d, 1H, J = 4.8 Hz, pyrimidine), 7.12 (t, 1H, ArH), 7.36–7.42 (m, 5H, ArH), 7.62 (d, 2H, J = 9.0 Hz,ArH), 7.74 (d, 2H, J = 8.4 Hz,ArH), 8.11 (d, 2H, J = 8.3 Hz,ArH), 8.49 (d, 1H, J = 4.8 Hz, pyrimidine), 9.40 (s, 1H, NH), 10.05 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.8 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.2, 120.2, 123.7, 127.7, 129.1, 129.5, 129.6 (14C, Ar), 134.1 (C, C3a-pyrazolopyrimidine), 138.8, 142.4, 146.7 (3C, Ar), 147.9 (C, C7-pyrazolopyrimidine), 149.6 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 157.8 (C, C5-pyrazolopyrimidine), 163.3 (C=O). MS (m/z, %): 435 (M+, 73.86). Anal. Calcd. (%) for C26H21N5O2 (435.48): C, 71.71; H, 4.86; N, 16.08. Found: C, 71.80; H, 4.81; N, 16.00%.
2-(4-Methoxyphenylamino)-N-phenyl-7-(4-methylphenyl)-pyrazolo[1,5-a]pyrimidine-3-carboxamide (18b). Yellow crystals, m.p. 219–221 °C, yield (77%). IR (KBr) νmax/cm−1 3337 (NH), 1658 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.49 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.87 (d, 2H, J = 8.9 Hz, ArH), 6.91 (d, 1H, J = 4.7 Hz, pyrimidine), 7.12 (t, 1H, ArH), 7.36 (d, 2H, J = 8.3 Hz, ArH), 7.38 (t, 2H, ArH), 7.60 (d, 2H, J = 8.9 Hz, ArH), 7.73 (d, 2H, J = 7.6 Hz, ArH), 8.08 (d, 2H, J = 8.1 Hz,ArH), 8.43 (d, 1H, J = 4.7 Hz, pyrimidine), 9.38 (s, 1H, NH), 10.01 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.8 (C, CH3), 55.7 (C, OCH3), 87.7 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.1, 120.1, 123.7, 127.6, 129.1, 129.4, 129.6 (14C, Ar), 134.1 (C, C3a-pyrazolopyrimidine), 138.8, 142.3, 146.6 (3C, Ar), 147.8 (C, C7-pyrazolopyrimidine), 149.6 (C, Ar), 154.4 (C, C2-pyrazolopyrimidine), 157.7 (C, C5-pyrazolopyrimidine), 163.3 (C=O). MS (m/z, %): 449 (M+, 67.43). Anal. Calcd. (%) for C27H23N5O2 (449.50): C, 72.14; H, 5.16; N, 15.58. Found: C, 72.10; H, 5.20; N, 15.60%.
7-(4-Methoxyphenyl)-2-(4-methoxyphenylamino)-N-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18c). Yellow crystals, m.p. 206–208 °C, yield (76%). IR (KBr) νmax/cm−1 3340 (NH), 1646 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 3.80 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 6.87 (d, 2H, J = 8.9 Hz, ArH), 6.89 (d, 1H, J = 4.8 Hz, pyrimidine), 7.05 (d, 2H, J = 8.8 Hz, ArH), 7.11 (t, 1H, ArH), 7.37 (t, 2H, ArH), 7.60 (d, 2H, J = 8.9 Hz, ArH), 7.72 (d, 2H, J = 7.6 Hz, ArH), 8.18 (d, 2H, J = 8.8 Hz, ArH), 8.40 (d, 1H, J = 4.8 Hz, pyrimidine), 9.36 (s, 1H, NH), 10.02 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 55.5 (C, OCH3), 55.6 (C, OCH3), 87.4 (C, C3-pyrazolopyrimidine), 106.4 (C, C6-pyrazolopyrimidine), 113.9, 114.2, 119.0, 120.0, 122.4, 123.5, 128.9, 131.3 (14C, Ar), 134.0 (C, C3a-pyrazolopyrimidine), 138.6, 146.0 (2C, Ar), 147.7 (C, C7-pyrazolopyrimidine), 149.3 (C, Ar), 154.3 (C, C2-pyrazolopyrimidine), 157.5 (C, C5-pyrazolopyrimidine), 162.2 (C, Ar), 163.2 (C=O). MS (m/z, %): 465 (M+, 69.48). Anal. Calcd. (%) for C27H23N5O3 (465.50): C, 69.66; H, 4.98; N, 15.04. Found: C, 69.70; H, 4.95; N, 15.00%.
7-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-N-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18d). Yellow crystals, m.p. 252–253 °C, yield (72%). IR (KBr) νmax/cm−1 3343 (NH), 1648 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 3.81 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz, ArH), 6.94 (d, 1H, J = 4.7 Hz, pyrimidine), 7.13 (t, 1H, ArH), 7.39 (t, 2H, ArH), 7.58 (d, 4H, J = 8.8 Hz, ArH), 7.74 (d, 2H, J = 8.6 Hz, ArH), 8.15 (d, 2H, J = 8.7 Hz, ArH), 8.52 (d, 1H, J = 4.7 Hz, pyrimidine), 9.42 (s, 1H, NH), 9.99 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 55.7 (C, OCH3), 88.0 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.2, 120.2, 123.8, 129.1, 129.1, 130.9, 131.8 (14C, Ar), 133.9 (C, C3a-pyrazolopyrimidine), 134.6, 138.0, 138.7 (3C, Ar), 145.3 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.6 (C, C2-pyrazolopyrimidine), 157.9 (C, C5-pyrazolopyrimidine), 163.2 (C=O). MS (m/z, %): 469 (M+, 78.23). Anal. Calcd. (%) for C26H20ClN5O2 (469.92): C, 66.45; H, 4.29; N, 14.90. Found: C, 66.40; H, 4.30; N, 14.95%.
7-(4-Bromophenyl)-2-(4-methoxyphenylamino)-N-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18e). Yellow crystals, m.p. 278–280 °C, yield (69%). IR (KBr) νmax/cm−1 3365 (NH), 1650 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.73 (s, 3H, OCH3), 6.93 (d, 2H, J = 9.0 Hz, ArH), 7.12 (t, 1H, ArH), 7.39 (t, 2H, ArH), 7.41 (d, 1H, J = 4.8 Hz, pyrimidine), 7.59 (d, 2H, J = 9.0 Hz, ArH), 7.73 (d, 2H, J = 7.6 Hz, ArH), 7.90 (d, 2H, J = 8.7 Hz, ArH), 8.20 (d, 2H, J = 8.7 Hz, ArH), 8.74 (d, 1H, J = 4.8 Hz, pyrimidine), 9.26 (s, 1H, NH), 10.03 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.6 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 114.4, 119.1, 120.5, 123.3, 129.4, 129.8, 131.0, 131.6 (14C, Ar), 133.7 (C, C3a-pyrazolopyrimidine), 133.4, 136.1, 138.7 (3C, Ar), 145.2 (C, C7-pyrazolopyrimidine), 149.5 (C, Ar), 154.8 (C, C2-pyrazolopyrimidine), 157.1 (C, C5-pyrazolopyrimidine), 163.7 (C=O). MS (m/z, %): 514 (M+, 81.26). Anal. Calcd. (%) for C26H20BrN5O2 (514.37): C, 60.71; H, 3.92; N, 13.62. Found: C, 60.65; H, 3.97; N, 13.65%.
7-(4-Fluorophenyl)-2-(4-methoxyphenylamino)-N-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18f). Yellow crystals, m.p. 237–239 °C, yield (70%). IR (KBr) νmax/cm−1 3343 (NH), 1647 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.73 (s, 3H, OCH3), 6.91 (d, 2H, J = 9.0 Hz, ArH), 7.11 (t, 1H, ArH), 7.37 (d, 1H, J = 4.9 Hz, pyrimidine), 7.39 (d, 2H, J = 7.6 Hz, ArH), 7.52 (t, 2H, ArH), 7.58 (d, 2H, J = 9.0 Hz, ArH), 7.71 (d, 2H, J = 8.6 Hz, ArH), 8.31 (d, 2H, J = 8.9 Hz, ArH), 8.71 (d, 1H, J = 4.8 Hz, pyrimidine), 9.23 (s, 1H, NH), 10.01 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.2 (C, OCH3), 86.7 (C, C3-pyrazolopyrimidine), 108.3 (C, C6-pyrazolopyrimidine), 114.3, 115.7, 115.9, 118.8, 119.4, 123.5, 126.4, 129.1 (14C, Ar), 132.4 (C, C3a-pyrazolopyrimidine), 133.3, 138.4 (2C, Ar), 145.0 (C, C7-pyrazolopyrimidine), 147.1 (C, Ar), 151.1 (C, C2-pyrazolopyrimidine), 154.1 (C, C5-pyrazolopyrimidine), 156.6 (C, Ar), 162.2 (C=O). MS (m/z, %): 453 (M+, 87.33). Anal. Calcd. (%) for C26H20FN5O2 (453.47): C, 68.86; H, 4.45; N, 15.44. Found: C, 68.95; H, 4.40; N, 15.50%.
2-(4-Methoxyphenylamino)-N-phenyl-7-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18g). Yellow crystals, m.p. 233–235 °C, yield (71%). IR (KBr) νmax/cm−1 3356 (NH), 1652 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.78 (s, 3H, OCH3), 7.03 (d, 2H, J = 8.4 Hz, ArH), 7.12 (t, 1H, ArH), 7.40 (t, 2H, ArH), 7.47 (t, 1H, J = 4.9 Hz, thiophene), 7.74 (d, 2H, J = 7.8 Hz, ArH), 7.84 (d, 2H, J = 8.5 Hz, ArH), 7.90 (d, 1H, J = 4.6 Hz, pyrimidine), 8.28 (d, 1H, J = 4.4 Hz, thiophene), 8.58 (d, 1H, J = 2.8 Hz, thiophene), 8.71 (d, 1H, J = 4.4 Hz, pyrimidine), 9.44 (s, 1H, NH), 10.07 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 86.8 (C, C3-pyrazolopyrimidine), 107.3 (C, C6-pyrazolopyrimidine), 114.8, 119.2, 120.8, 126.3 (7C, Ar), 128.1, 129.8 (2C, thiophene), 130.1 (2C, Ar), 133.2 (C, thiophene), 133.9 (C, C3a-pyrazolopyrimidine), 134.5, 137.1 (2C, Ar), 139.4 (C, thiophene), 147.3 (C, Ar), 150.8 (C, C2-pyrazolopyrimidine), 154.4 (C, C5-pyrazolopyrimidine), 156.3 (C, C7-pyrazolopyrimidine), 162.9 (C=O). MS (m/z, %): 441 (M+, 100). Anal. Calcd. (%) for C24H19N5O2S (441.50): C, 65.29; H, 4.34; N, 15.86. Found: C, 65.35; H, 4.30; N, 15.90%.
2-(4-Methoxyphenylamino)-7-phenyl-N-(4-methylphenyl)-pyrazolo[1,5-a]pyrimidine-3-carboxamide (18h). Yellow crystals, m.p. 251–253 °C, yield (76%). IR (KBr) νmax/cm−1 3374 (NH), 1660 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.35 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz, ArH), 6.95 (d, 1H, J = 4.8 Hz, pyrimidine), 7.18 (d, 2H, J = 8.2 Hz, ArH), 7.40 (d, 2H, J = 8.2 Hz, ArH), 7.60–7.64 (m, 5H, ArH), 8.11 (d, 2H, J = 8.2 Hz, ArH), 8.48 (d, 1H, J = 4.8 Hz, pyrimidine), 9.42 (s, 1H, NH), 9.97 (s, 1H, NH).13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 87.8 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 114.4, 119.1, 120.2, 127.7, 129.4, 129.6, 133.3 (14C, Ar), 134.2 (C, C3a-pyrazolopyrimidine), 136.2, 142.4, 146.6 (3C, Ar), 147.8 (C, C7-pyrazolopyrimidine), 149.6 (C, Ar), 154.4 (C, C2-pyrazolopyrimidine), 157.8 (C, C5-pyrazolo-pyrimidine), 163.2 (C=O). MS (m/z, %): 449 (M+, 92.11). Anal. Calcd. (%) for C27H23N5O2 (449.50): C, 72.14; H, 5.16; N, 15.58. Found: C, 72.20; H, 5.11; N, 15.50%.
2-(4-Methoxyphenylamino)-N,7-di-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18i). Yellow crystals, m.p. 261 °C, yield (74%). IR (KBr) νmax/cm−1 3293 (NH), 1642 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.35 (s, 3H, CH3), 2.49 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.87 (d, 2H, J = 9.0 Hz, ArH), 6.91 (d, 1H, J = 4.8 Hz, pyrimidine), 7.18 (d, 2H, J = 8.2 Hz, ArH), 7.37 (d, 2H, J = 8.0 Hz, ArH), 7.60 (d, 2H, J = 9.0 Hz, ArH), 7.61 (d, 2H, J = 8.5 Hz, ArH), 8.08 (d, 2H, J = 8.3 Hz, ArH), 8.43 (d, 1H, J = 4.8 Hz, pyrimidine), 9.40 (s, 1H, NH), 9.94 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 21.8 (C, CH3), 55.7 (C, OCH3), 87.7 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 114.4, 119.1, 120.1, 127.6, 129.4, 129.5, 129.6, 133.2 (14C, Ar), 134.1 (C, C3a-pyrazolopyrimidine), 136.2, 142.3, 146.5 (3C, Ar), 147.7 (C, C7-pyrazolopyrimidine), 149.5 (C, Ar), 154.4 (C, C2-pyrazolopyrimidine), 157.7 (C, C5-pyrazolopyrimidine), 163.2 (C=O). MS (m/z, %): 463 (M+, 100). Anal. Calcd. (%) for C28H25N5O2 (463.53): C, 72.55; H, 5.44; N, 15.11. Found: C, 72.55; H, 5.44; N, 15.11%.
7-(4-Methoxyphenyl)-2-(4-methoxyphenylamino)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18j). Yellow crystals, m.p. 244–245 °C, yield (75%). IR (KBr) νmax/cm−1 3368 (NH), 1649 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.35 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz, ArH), 6.93 (d, 1H, J = 4.8 Hz, pyrimidine), 7.10 (d, 2H, J = 9.0 Hz, ArH), 7.18 (d, 2H, J = 8.2 Hz, ArH), 7.61–7.64 (m, 4H, ArH), 8.22 (d, 2H, J = 8.9 Hz, ArH), 8.45 (d, 1H, J = 4.8 Hz, pyrimidine), 9.42 (s, 1H, NH), 9.98 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 55.7 (C, OCH3), 87.7 (C, C3-pyrazolopyrimidine), 106.5 (C, C6-pyrazolopyrimidine), 114.1, 114.4, 119.1, 120.2, 122.7, 129.6, 131.5, 133.2 (14C, Ar), 134.2 (C, C3a-pyrazolopyrimidine), 136.2, 146.2 (2C, Ar), 147.9 (C, C7-pyrazolopyrimidine), 149.5 (C, Ar), 154.4 (C, C2-pyrazolopyrimidine), 157.8 (C, C5-pyrazolopyrimidine), 162.3 (C, Ar), 163.3 (C=O). MS (m/z, %): 479 (M+, 92.77). Anal. Calcd. (%) for C28H25N5O3 (479.53): C, 70.13; H, 5.25; N, 14.60. Found: C, 70.05; H, 5.30; N, 14.55%.
7-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18k). Yellow crystals, m.p. 267–269 °C, yield (71%). IR (KBr) νmax/cm−1 3315 (NH), 1662 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.35 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz, ArH), 6.93 (d, 1H, J = 4.7 Hz, pyrimidine), 7.19 (d, 2H, J = 8.2 Hz, ArH), 7.57–7.62 (m, 6H, ArH), 8.14 (d, 2H, J = 8.7 Hz, ArH), 8.50 (d, 1H, J = 4.7 Hz, pyrimidine), 9.43 (s, 1H, NH), 9.91 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 88.0 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.2, 120.2, 129.1, 129.6, 130.9, 131.7, 133.4 (14C, Ar), 134.1 (C, C3a-pyrazolopyrimidine), 134.4, 136.0, 137.9 (3C, Ar), 146.1 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.6 (C, C2-pyrazolopyrimidine), 159.4 (C, C5-pyrazolopyrimidine), 163.1 (C=O). MS (m/z, %): 483 (M+, 87.08). Anal. Calcd. (%) for C27H22ClN5O2 (483.95): C, 67.01; H, 4.58; N, 14.47. Found: C, 67.10; H, 4.50; N, 14.50%.
7-(4-Bromophenyl)-2-(4-methoxyphenylamino)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18l). Yellow crystals, m.p. 278–279 °C, yield (68%). IR (KBr) νmax/cm−1 3325 (NH), 1649 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.30 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 6.95 (d, 2H, J = 9.3 Hz, ArH), 7.20 (d, 2H, J = 8.6 Hz, ArH), 7.44 (d, 1H, J = 4.1 Hz, pyrimidine), 7.62 (d, 2H, J = 8.4 Hz, ArH), 7.63 (d, 2H, J = 7.8 Hz, ArH), 7.93 (d, 2H, J = 8.3 Hz, ArH), 8.22 (d, 2H, J = 8.3 Hz, ArH), 8.77 (d, 1H, J = 4.3 Hz, pyrimidine), 9.31 (s, 1H, NH), 9.98 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 88.0 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.9, 119.2, 120.5, 125.2, 129.7, 129.4, 131.0, 133.5 (14C, Ar), 134.2 (C, C3a-pyrazolopyrimidine), 134.4, 135.9, 137.9 (3C, Ar), 146.0 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 159.3 (C, C5-pyrazolopyrimidine), 163.1 (C=O). MS (m/z, %): 528 (M+, 26.25). Anal. Calcd. (%) for C27H22BrN5O2 (528.40): C, 61.37; H, 4.20; N, 13.25. Found: C, 61.45; H, 4.16; N, 13.30%.
7-(4-Fluorophenyl)-2-(4-methoxyphenylamino)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18m). Yellow crystals, m.p. 255–257 °C, yield (69%). IR (KBr) νmax/cm−1 3329 (NH), 1652 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.30 (s, 3H, CH3), 3.73 (s, 3H, OCH3), 6.93 (d, 2H, J = 9.0 Hz, ArH), 7.19 (d, 2H, J = 8.3 Hz, ArH), 7.39 (d, 1H, J = 4.8 Hz, pyrimidine), 7.54 (d, 2H, J = 8.9 Hz, ArH), 7.60 (d, 2H, J = 9.0 Hz, ArH), 7.62 (d, 2H, J = 8.4 Hz, ArH), 8.32 (d, 2H, J = 9.0 Hz, ArH), 8.74 (d, 1H, J = 4.8 Hz, pyrimidine), 9.27 (s, 1H, NH), 9.97 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 21.0 (C, CH3), 55.8 (C, OCH3), 87.9 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.6, 116.1, 119.5, 120.5, 129.5, 130.9, 131.7 (13C, Ar), 134.2 (C, C3a-pyrazolopyrimidine), 134.2, 136.1, 138.0 (3C, Ar), 146.2 (C, C7-pyrazolopyrimidine), 149.6 (C, Ar), 154.3 (C, C2-pyrazolopyrimidine), 159.0 (C, C5-pyrazolopyrimidine), 160.1 (C, Ar), 162.9 (C=O). MS (m/z, %): 467 (M+, 45.13). Anal. Calcd. (%) for C27H22FN5O2 (467.49): C, 69.37; H, 4.74; N, 14.98. Found: C, 69.30; H, 4.80; N, 15.05%.
2-(4-Methoxyphenylamino)-7-(thiophen-2-yl)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18n). Yellow crystals, m.p. 278–279 °C, yield (70%). IR (KBr) νmax/cm−1 3345 (NH), 1652 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.30 (s, 3H, CH3), 3.78 (s, 3H, OCH3), 7.03 (d, 2H, J = 8.9 Hz, ArH), 7.20 (d, 2H, J = 8.1 Hz, ArH), 7.47 (t, 1H, thiophene), 7.62 (d, 2H, J = 8.0 Hz, ArH), 7.83 (d, 2H, J = 8.6 Hz, ArH), 7.90 (d, 1H, J = 3.6 Hz, pyrimidine), 8.28 (d, 1H, J = 4.7 Hz, thiophene), 8.59 (d, 1H, J = 2.3 Hz, thiophene), 8.70 (d, 1H, J = 4.8 Hz, pyrimidine), 9.46 (s, 1H, NH), 10.00 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 87.5 (C, C3-pyrazolopyrimidine), 107.1 (C, C6-pyrazolopyrimidine), 114.1, 119.5, 120.3 (6C, Ar), 127.6, 128.1, 129.8 (3C, thiophene), 130.0 (2C, Ar), 133.6 (C, C3a-pyrazolopyrimidine), 133.5, 134.5, 137.1 (3C, Ar), 139.8 (C, thiophene), 147.8 (C, Ar), 151.5 (C, C2-pyrazolopyrimidine), 154.1 (C, C5-pyrazolopyrimidine), 157.2 (C, C7-pyrazolopyrimidine), 163.0 (C=O). MS (m/z, %): 455 (M+, 65.71). Anal. Calcd. (%) for C25H21N5O2S (455.53): C, 65.92; H, 4.65; N, 15.37. Found: C, 66.00; H, 4.60; N, 15.31%.
N-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-7-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18o). Yellow crystals, m.p. 252–254 °C, yield (73%). IR (KBr) νmax/cm−1 3336 (NH), 1650 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.72 (s, 3H, OCH3), 6.90 (d, 2H, J = 9.0 Hz, ArH), 7.40 (d, 1H, J = 4.8 Hz, pyrimidine), 7.44 (d, 2H, J = 8.8 Hz, ArH), 7.61 (d, 2H, J = 9.0 Hz, ArH), 7.68–7.70 (m, 3H, ArH), 7.78 (d, 2H, J = 8.9 Hz, ArH), 8.23 (d, 2H, J = 7.2 Hz, ArH), 8.75 (d, 1H, J = 4.8 Hz, pyrimidine), 9.20 (s, 1H, NH), 10.11 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.9 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.5, 119.1, 120.2, 124.0, 128.4, 129.1, 130.9, 131.8 (13C, Ar), 134.0 (C, C3a-pyrazolopyrimidine), 134.6, 135.9, 138.0, 138.7 (4C, Ar), 145.6 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.8 (C, C2-pyrazolopyrimidine), 158.0 (C, C5-pyrazolopyrimidine), 163.8 (C=O). MS (m/z, %): 469 (M+, 29.83). Anal. Calcd. (%) for C26H20ClN5O2 (469.92): C, 66.45; H, 4.29; N, 14.90. Found: C, 66.40; H, 4.35; N, 14.85%.
N-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-7-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18p). Yellow crystals, m.p. 261 °C, yield (75%). IR (KBr) νmax/cm−1 3322 (NH), 1658 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.32 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 6.93 (d, 2H, J = 7.6 Hz, ArH), 7.42 (d, 1H, J = 4.8 Hz, pyrimidine), 7.45 (d, 2H, J = 7.7 Hz, ArH), 7.51 (d, 2H, J = 8.6 Hz, ArH), 7.64 (d, 2H, J = 7.8 Hz, ArH), 7.79 (d, 2H, J = 8.4 Hz, ArH), 8.19 (d, 2H, J = 7.5 Hz, ArH), 8.74 (d, 1H, J = 4.8 Hz, pyrimidine), 9.23 (s, 1H, NH), 10.14 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 87.5 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.3, 120.9, 129.0, 129.6, 131.0, 131.7, 133.4 (14C, Ar), 134.0 (C, C3a-pyrazolopyrimidine), 134.3, 136.0, 137.9 (3C, Ar), 146.1 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 159.4 (C, C5-pyrazolopyrimidine), 163.1 (C=O). MS (m/z, %): 483 (M+, 22.71). Anal. Calcd. (%) for C27H22ClN5O2 (483.95): C, 67.01; H, 4.58; N, 14.47. Found: C, 67.10; H, 4.50; N, 14.55%.
N-(4-Chlorophenyl)-7-(4-methoxyphenyl)-2-(4-methoxyphenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18q). Yellow crystals, m.p. 266–267 °C, yield (74%). IR (KBr) νmax/cm−1 3365 (NH), 1661 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.74 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 6.95 (d, 2H, J = 8.8 Hz, ArH), 7.24 (d, 2H, J = 8.7 Hz, ArH), 7.40 (d, 1H, J = 4.7 Hz, pyrimidine), 7.44 (d, 2H, J = 8.7 Hz, ArH), 7.65 (d, 2H, J = 8.8 Hz, ArH), 7.78 (d, 2H, J = 8.6 Hz, ArH), 8.31 (d, 2H, J = 8.6 Hz, ArH), 8.70 (d, 1H, J = 4.7 Hz, pyrimidine), 9.22 (s, 1H, NH), 10.15 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.6 (C, OCH3), 55.7 (C, OCH3), 87.8 (C, C3-pyrazolopyrimidine), 106.4 (C, C6-pyrazolopyrimidine), 114.2, 114.6, 119.0, 120.5, 122.8, 129.5, 131.6 (13C, Ar), 133.1 (C, C3a-pyrazolopyrimidine), 134.8, 135.0, 136.1 (3C, Ar), 147.9 (C, C7-pyrazolopyrimidine), 149.4 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 157.8 (C, C5-pyrazolopyrimidine), 161.5 (C, Ar), 163.2 (C=O). MS (m/z, %): 499 (M+, 18.46). Anal. Calcd. (%) for C27H22ClN5O3 (499.95): C, 64.86; H, 4.44; N, 14.01. Found: C, 64.95; H, 4.40; N, 14.05%.
N,7-bis(4-Chlorophenyl)-2-(4-methoxyphenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18r). Yellow crystals, m.p. 282–284 °C, yield (70%). IR (KBr) νmax/cm−1 3317 (NH), 1653 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.74 (s, 3H, OCH3), 6.94 (d, 2H, J = 8.8 Hz, ArH), 7.44 (d, 2H, J = 8.6 Hz, ArH), 7.45 (d, 1H, J = 3.8 Hz, pyrimidine), 7.61 (d, 2H, J = 8.8 Hz, ArH), 7.78 (d, 4H, J = 8.4 Hz, ArH), 8.29 (d, 2H, J = 8.6 Hz, ArH), 8.76 (d, 1H, J = 4.7 Hz, pyrimidine), 9.23 (s, 1H, NH), 10.10 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.8 (C, OCH3), 87.4 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 115.0, 119.3, 120.4, 129.1, 129.7, 129.9, 131.6 (13C, Ar), 133.1 (C, C3a-pyrazolopyrimidine), 133.8, 134.3, 136.0, 137.9 (4C, Ar), 146.0 (C, C7-pyrazolopyrimidine), 149.8 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 159.4 (C, C5-pyrazolopyrimidine), 162.9 (C=O). MS (m/z, %): 504 (M+, 22.87). Anal. Calcd. (%) for C26H19Cl2N5O2 (504.37): C, 61.91; H, 3.80; N, 13.89. Found: C, 62.00; H, 3.75; N, 13.80%.
7-(4-Bromophenyl)-N-(4-chlorophenyl)-2-(4-methoxyphenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18s). Yellow crystals, m.p. 275–277 °C, yield (67%). IR (KBr) νmax/cm−1 3327 (NH), 1648 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.74 (s, 3H, OCH3), 6.94 (d, 2H, J = 7.3 Hz, ArH), 7.44 (d, 2H, J = 7.2 Hz, ArH), 7.45 (d, 1H, J = 4.4 Hz, pyrimidine), 7.61 (d, 2H, J = 7.1 Hz, ArH), 7.79 (d, 2H, J = 7.9 Hz, ArH), 7.92 (d, 2H, J = 7.3 Hz, ArH), 8.21 (d, 2H, J = 7.8 Hz, ArH), 8.76 (d, 1H, J = 4.3 Hz, pyrimidine), 9.23 (s, 1H, NH), 10.11 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.6 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 114.4, 119.2, 120.2, 123.2, 129.1, 129.6, 130.7, 131.4 (14C, Ar), 132.7 (C, C3a-pyrazolopyrimidine), 134.2, 136.0, 137.5 (3C, Ar), 146.0 (C, C7-pyrazolopyrimidine), 149.8 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 159.5 (C, C5-pyrazolopyrimidine), 163.2 (C=O). MS (m/z, %): 548 (M+, 20.55). Anal. Calcd. (%) for C26H19BrClN5O2 (548.82): C, 56.90; H, 3.49; N, 12.76. Found: C, 57.00; H, 3.40; N, 12.80%.
N-(4-Chlorophenyl)-7-(4-fluorophenyl)-2-(4-methoxyphenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18t). Yellow crystals, m.p. 251–252 °C, yield (67%). IR (KBr) νmax/cm−1 3339 (NH), 1651 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.74 (s, 3H, OCH3), 6.93 (d, 2H, J = 7.8 Hz, ArH), 7.44 (d, 2H, J = 8.7 Hz, ArH), 7.45 (d, 1H, J = 4.2 Hz, pyrimidine), 7.56 (d, 2H, J = 8.0 Hz, ArH), 7.62 (d, 2H, J = 8.0 Hz, ArH), 7.78 (d, 2H, J = 7.4 Hz, ArH), 8.33 (d, 2H, J = 8.1 Hz, ArH), 8.75 (d, 1H, J = 4.5 Hz, pyrimidine), 9.21 (s, 1H, NH), 10.10 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.8 (C, OCH3), 86.7 (C, C3-pyrazolopyrimidine), 108.3 (C, C6-pyrazolopyrimidine), 114.3, 115.9, 119.0, 119.4, 124.6, 128.1, 129.1 (13C, Ar), 132.3 (C, C3a-pyrazolopyrimidine), 133.2, 134.3, 138.4 (3C, Ar), 145.0 (C, C7-pyrazolopyrimidine), 147.3 (C, Ar), 151.1 (C, C2-pyrazolopyrimidine), 154.0 (C, C5-pyrazolopyrimidine), 156.6 (C, Ar), 162.3 (C=O). MS (m/z, %): 487 (M+, 21.30). Anal. Calcd. (%) for C26H19ClFN5O2 (487.91): C, 64.00; H, 3.93; N, 14.35. Found: C, 64.10; H, 4.00; N, 14.30%.
N-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-7-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18u). Yellow crystals, m.p. 289–291 °C, yield (71%). IR (KBr) νmax/cm−1 3293 (NH), 1644 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.78 (s, 3H, OCH3), 7.04 (d, 2H, J = 9.0 Hz, ArH), 7.33 (d, 2H, J = 9.2 Hz, ArH), 7.47 (t, 1H, thiophene), 7.79 (d, 2H, J = 9.1 Hz, ArH), 7.84 (d, 2H, J = 8.9 Hz, ArH), 7.91 (d, 1H, J = 4.8 Hz, pyrimidine), 8.29 (d, 1H, J = 5.1 Hz, thiophene), 8.60 (d, 1H, J = 2.9 Hz, thiophene), 8.71 (d, 1H, J = 5.7 Hz, pyrimidine), 9.39 (s, 1H, NH), 10.14 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.8 (C, C3-pyrazolopyrimidine), 107.1 (C, C6-pyrazolopyrimidine), 114.0, 119.5, 120.4, (6C, Ar), 127.6, 128.2, 129.7 (3C, thiophene), 130.0 (2C, Ar), 133.1 (C, C3a-pyrazolopyrimidine), 133.6, 134.5, 136.9 (3C, Ar), 139.7 (C, thiophene), 148.0 (C, Ar), 151.4 (C, C2-pyrazolopyrimidine), 154.2 (C, C5-pyrazolopyrimidine), 157.2 (C, C7-pyrazolopyrimidine), 162.9 (C=O). MS (m/z, %): 475 (M+, 74.59). Anal. Calcd. (%) for C24H18ClN5O2S (475.95): C, 60.56; H, 3.81; N, 14.71. Found: C, 60.50; H, 3.90; N, 14.80%.
General Procedure for Synthesis of N-aryl-2-(arylamino)-8,8-dimethyl-6-oxo-6,7,8,9-tetrahydropyrazolo[1,5-a]quinazoline-3-carboxamides25a–c. A mixture of compounds 11a–c (0.01 mol) with 2-((dimethyl-amino)methylene)-5,5-dimethylcyclohexane-1,3-dione (19, 0.01 mol, 1.95 g) in glacial acetic acid (25 mL), the reaction mixture was refluxed for 1 h and then left to cool. The solid product was filtered off, washed with ethanol, dried and finally recrystallized from DMF/H2O to afford the corresponding pyrazolo[1,5-a]quinazolines 25a–c.
2-(4-Methoxyphenylamino)-8,8-dimethyl-6-oxo-N-phenyl-6,7,8,9-tetrahydropyrazolo[1,5-a]quinazoline-3-carboxamide (25a). Yellow crystals, m.p. 270–272 °C, yield (73%). IR (KBr) νmax/cm−1 3302 (NH), 1655 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 1.24 (s, 6H, 2CH3), 2.58 (s, 2H, CH2), 3.31 (s, 2H, CH2), 3.82 (s, 3H, OCH3), 6.95 (d, 2H, J = 9.0 Hz, ArH), 7.14 (t, 1H, ArH), 7.39 (t, 2H, ArH), 7.64 (d, 2H, J = 9.0 Hz, ArH), 7.71 (d, 2H, J = 7.5 Hz, ArH), 8.99 (s, 1H, quinazoline), 9.48 (s, 1H, NH), 9.88 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 28.7 (2C, 2CH3), 32.7 (C, C8-quinazoline), 37.7 (C, CH2), 51.1 (C, CH2), 55.7 (C, OCH3), 90.9 (C, C3-quinazoline), 113.8 (C, C5a-quinazoline), 114.5, 119.6, 120.3, 124.2, 129.2 (9C, Ar), 133.4 (C, C3a-quinazoline), 138.2, 147.1, 148.6 (3C, Ar), 151.5 (C, C2-quinazoline), 155.0 (C, C5-quinazoline), 159.1 (C=O), 162.6 (C, C9a-quinazoline), 193.9 (C=O). MS (m/z, %): 455 (M+, 71.06). Anal. Calcd. (%) for C26H25N5O3 (455.51): C, 68.56; H, 5.53; N, 15.37. Found: C, 68.50; H, 5.55; N, 15.40%.
2-(4-Methoxyphenylamino)-8,8-dimethyl-6-oxo-N-(4-methylphenyl)-6,7,8,9-tetrahydropyrazolo[1,5-a]quinazoline-3-carboxamide (25b). Yellow crystals, m.p. 266–268 °C, yield (77%). IR (KBr) νmax/cm−1 3316 (NH), 1659 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 1.19 (s, 6H, 2CH3), 2.34 (s, 3H, CH3), 2.52 (s, 2H, CH2), 3.22 (s, 2H, CH2), 3.81 (s, 3H, OCH3), 6.91 (d, 2H, J = 9.0 Hz, ArH), 7.16 (d, 2H, J = 8.2 Hz, ArH), 7.54 (d, 2H, J = 8.3 Hz, ArH), 7.58 (d, 2H, J = 9.0 Hz, ArH), 8.90 (s, 1H, quinazoline), 9.41 (s, 1H, NH), 9.72 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 28.7 (2C, 2CH3), 32.6 (C, C8-quinazoline), 37.5 (C, CH2), 50.9 (C, CH2), 55.6 (C, OCH3), 90.7 (C, C3-quinazoline), 113.7 (C, C5a-quinazoline), 114.4, 119.4, 120.1, 129.6 (8C, Ar), 133.4 (C, C3a-quinazoline), 133.7, 135.7, 146.8, 148.5 (4C, Ar), 151.5 (C, C2-quinazoline), 154.8 (C, C5-quinazoline), 158.8 (C=O), 162.3 (C, C9a-quinazoline), 194.0 (C=O). MS (m/z, %): 469 (M+, 93.88). Anal. Calcd. (%) for C27H27N5O3 (469.53): C, 69.07; H, 5.80; N, 14.92. Found: C, 69.15; H, 5.75; N, 15.00%.
N-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-8,8-dimethyl-6-oxo-6,7,8,9-tetrahydropyrazolo[1,5-a]quinazoline-3-carboxamide (25c). Yellow crystals, m.p. 291–293 °C, yield (72%). IR (KBr) νmax/cm−1 3299 (NH), 1662 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 1.16 (s, 6H, 2CH3), 2.59 (s, 2H, CH2), 3.36 (s, 2H, CH2), 3.76 (s, 3H, OCH3), 6.98 (d, 2H, J = 8.8 Hz, ArH), 7.45 (d, 2H, J = 8.6 Hz, ArH), 7.72 (d, 2H, J = 8.6 Hz, ArH), 7.76 (d, 2H, J = 8.8 Hz, ArH), 8.95 (s, 1H, quinazoline), 9.30 (s, 1H, NH), 10.01 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 28.7 (2C, 2CH3), 32.6 (C, C8-quinazoline), 37.6 (C, CH2), 50.0 (C, CH2), 55.7 (C, OCH3), 90.8 (C, C3-quinazoline), 113.7 (C, C5a-quinazoline), 114.4, 119.3, 120.1, 129.3 (8C, Ar), 133.4 (C, C3a-quinazoline), 133.8, 136.3, 146.9, 148.1 (4C, Ar), 151.5 (C, C2-quinazoline), 154.8 (C, C5-quinazoline), 158.9 (C=O), 162.4 (C, C9a-quinazoline), 193.9 (C=O). MS (m/z, %): 489 (M+, 63.07). Anal. Calcd. (%) for C26H24ClN5O3 (489.95): C, 63.74; H, 4.94; N, 14.29. Found: C, 63.80; H, 5.00; N, 14.20%.
3.2. Biological Evaluation
3.2.1. In-Vitro Anticancer Activity
Cell culture of HepG-2 (human liver carcinoma) and MCF-7 (human breast adenocarcinoma) cell lines were purchased from the American Type Culture Collection (Rockville, MD, USA) and maintained in DMEM medium which was supplemented with 10% heat-inactivated FBS (fetal bovine serum), 100 U/mL penicillin and 100 U/mL streptomycin. The cells were grown at 37 °C in a humidified atmosphere of 5% CO2.
3.2.2. MTT Cytotoxicity Assay
The antitumor activity against HepG-2 and MCF-7 human cancer cell lines was estimated using the 3-[4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, which is based on the cleavage of the tetrazolium salt by mitochondrial dehydrogenases in viable cells [31,32,33]. Cells were dispensed in a 96 well sterile microplate (5 × 104 cells/well), and incubated at 37 °C with series of different concentrations, in DMSO, of each tested compound or Doxorubicin® (positive control) for 48 h in a serum free medium prior to the MTT assay. After incubation, media were carefully removed, 40 µL of MTT (2.5 mg/mL) were added to each well and then incubated for an additional 4 h. The purple formazan dye crystals were solubilized by the addition of 200 µL of DMSO. The absorbance was measured at 590 nm using a SpectraMax®, Paradigm® Multi-Mode microplate reader. The relative cell viability was expressed as the mean percentage of viable cells compared to the untreated control cells.
3.2.3. Statistical Analysis
All experiments were conducted in triplicate and repeated on three different days. All the values were represented as mean ± SD. IC50s were determined by probit analysis using the SPSS software program (SPSS Inc., Chicago, IL, USA).
3.2.4. Cell Cycle Analysis and Apoptosis Detection
Cell cycle analysis and apoptosis detection were carried out by flow cytometry [35]. Both HepG-2 and MCF-7 cells were seeded at 8 × 104 and incubated at 37 °C, 5% CO2 overnight, after treatment with the tested compounds, for 24 h. Cell pellets were collected and centrifuged (300 g, 5 min). For cell cycle analysis, cell pellets were fixed with 70% ethanol on ice for 15 min and collected again. The collected pellets were incubated with propidium iodide (PI) staining solution (50 µg/mL PI, 0.1 mg/mL RNaseA, 0.05% Triton X-100) at room temperature for 1 h and analyzed by Gallios flow cytometer (Beckman Coulter, Brea, CA, USA). Apoptosis detection was performed by FITC Annexin-V/PI commercial kit (Becton Dickenson, Franklin Lakes, NJ, USA) following the manufacture protocol. The samples were analyzed by fluorescence-activated cell sorting (FACS) with a Gallios flow cytometer (Beckman Coulter) within 1 h after staining. Data were analyzed using Kaluza v. 1.2 (Beckman Coulter).
4. Conclusions
A series of N-aryl-7-aryl-pyrazolo[1,5-a]pyrimidines 18a–u and N-aryl-pyrazolo[1,5-a]quinazolines 25a–c have been synthesized and investigated for their in vitroantitumor activity. All the investigated compounds showed dose-dependent cytotoxic activities against two cancer types (liver and breast cancer). The IC50 values of these compounds did not reveal statistical significant differences compared to the positive control (doxorubicin). Therefore, two compounds (18o and 18a) have been selected to study their cell cycle and apoptotic effect against HepG2 and MCF-7 cancer cell lines. Compounds 18o and 18ashowed slightly higher cytotoxicity compared to doxorubicin against HepG-2 cells (IC50 = 72.2 ± 3.8 vs. 80.9 ± 2.1 µM) and against MCF-7 cells (IC50 = 63.1 ± 3.1 vs. 65.6 ± 4.2µM), respectively. Cell cycle analysis of HepG-2 cells treated with 18o and MCF-7 cells treated with 18a revealed a significant G2/M phase arrest coupled with an increase in the percentage of cells in pre-G phase, which is indicative of apoptosis. The pro-apoptotic activity of 18a and 18o was inferred by the significant increase in the percentage of annexin V-FITC-positive apoptotic cells.
Supplementary Materials
Spectra of compounds are available online.
Author Contributions
A.S.H. formulated the research idea; M.E.-N., A.S.H. and M.F.M. carried out the experimental, interpreted the data and prepared the manuscript; H.M.A. performed the biological screening. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds are all available from the authors.
References
- 1.Abd El Razik H.A., Abdel Wahab A.E. Synthesis and Biological Evaluation of Some Novel Fused Pyrazolopyrimidines as Potential Anticancer and Antimicrobial Agents. Arch. Pharm. Chem. Life Sci. 2011;11:184–196. doi: 10.1002/ardp.201000188. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal R., Masan E., Kaushik P., Kaushik D., Sharma C., Aneja K.R. Synthesis and biological evaluation of 7-trifluoromethylpyrazolo[1,5-a]pyrimidines as anti-inflammatory and antimicrobial agents. J. Fluor. Chem. 2014;168:16–24. doi: 10.1016/j.jfluchem.2014.08.017. [DOI] [Google Scholar]
- 3.Kaping S., Kalita U., Sunn M., Singha L.I., Vishwakarma J.N. A facile, regioselective synthesis of pyrazolo[1,5-a]pyrimidine analogs in the presence of KHSO4 in aqueous media assisted by ultrasound and their anti-inflammatory and anti-cancer activities. Monatsh. Chem. 2016;147:1257–1276. doi: 10.1007/s00706-015-1638-x. [DOI] [PubMed] [Google Scholar]
- 4.Deshmukh S., Dingore K., Gaikwad V., Jachak M. An efficient synthesis of pyrazolo[1,5-a]pyrimidines and evaluation of their antimicrobial activity. J. Chem. Sci. 2016;128:1459–1468. doi: 10.1007/s12039-016-1141-x. [DOI] [Google Scholar]
- 5.El-Mekabaty A., Habib O.M.O., Moawad E.B., Hasel A.M. Synthesis and Antioxidant Activity of New Pyrazolo[1,5-a]Pyrimidine Derivatives Incorporating a Thiazol-2-yldiazenyl Moiety. J. Heterocycl. Chem. 2016;53:1820–1826. doi: 10.1002/jhet.2492. [DOI] [Google Scholar]
- 6.Hassan A.S., Masoud D.M., Sroor F.M., Askar A.A. Synthesis and biological evaluation of pyrazolo[1,5-a]pyrimidine-3-carboxamide as antimicrobial agents. Med. Chem. Res. 2017;26:2909–2919. doi: 10.1007/s00044-017-1990-y. [DOI] [Google Scholar]
- 7.Kumar A.K.A., Bodke Y.D., Lakra P.S., Sambasivam G., Bhat K.G. Design, synthesis and anti-cancer evaluation of a novel series of pyrazolo[1,5-a]pyrimidine substituted diamide derivatives. Med. Chem. Res. 2017;26:714–744. doi: 10.1007/s00044-016-1770-0. [DOI] [Google Scholar]
- 8.Rahmouni A., Souiei S., Belkacem M.A., Romdhane A., Bouajila J., Ben Jannet H. Synthesis and biological evaluation of novel pyrazolopyrimidines derivatives as anticancer and anti-5-lipoxygenase agents. Bioorg. Chem. 2016;66:160–168. doi: 10.1016/j.bioorg.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhao M., Ren H., Chang J., Zhang D., Yang Y., He Y., Qi C., Zhang H. Design andSynthesis of pyrazolo[1,5-a]pyrimidine derivativesbearing nitrogen mustard moiety and evaluation of their antitumor activity in vitro and in vivo. Eur. J. Med. Chem. 2016;119:183–196. doi: 10.1016/j.ejmech.2016.04.068. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Zhao Y.F., Zhao X.L., Yuan X.Y., Gong P. Synthesis and Anti-tumor Activities of Novel Pyrazolo[1,5-a]pyrimidines. Arch. Pharm. Chem. Life Sci. 2006;339:593–597. doi: 10.1002/ardp.200600098. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed O.M., Mohamed M.A., Ahmed R.R., Ahmed S.A. Synthesis and anti-tumor activities of some new pyridines and pyrazolo[1,5-a]pyrimidines. Eur. J. Med. Chem. 2009;44:3519–3523. doi: 10.1016/j.ejmech.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Aziz H.A., Saleh T.S., El-Zahabi H.S.A. Facile Synthesis and In-Vitro Antitumor Activity of Some Pyrazolo[3,4-b]pyridines and Pyrazolo[1,5-a]pyrimidines Linked to a Thiazolo[3,2-a]benzimidazoleMoiety. Arch. Pharm. Chem. Life Sci. 2010;343:24–30. doi: 10.1002/chin.201019155. [DOI] [PubMed] [Google Scholar]
- 13.Hassan A.S., Mady M.F., Awad H.M., Hafez T.S. Synthesis and antitumor activity of some new pyrazolo[1,5-a]pyrimidines. Chin. Chem. Lett. 2017;28:388–393. doi: 10.1016/j.cclet.2016.10.022. [DOI] [Google Scholar]
- 14.Hassan A.S., Hafez T.S., Osman S.A.M., Ali M.M. Synthesis and In Vitro cytotoxic activity of novel pyrazolo[1,5-a]pyrimidines and related Schiff bases. Turk. J. Chem. 2015;39:1102–1113. doi: 10.3906/kim-1504-12. [DOI] [Google Scholar]
- 15.Gopalsamy A., Ciszewski G., Hu Y., Lee F., Feldberg L., Frommer E., Kimb S., Collins K., Wojciechowicz D., Mallon R. Identification of pyrazolo[1,5-a]pyrimidine-3-carboxylates as B-Raf kinase inhibitors. Bioorg. Med. Chem. Lett. 2009;19:2735–2738. doi: 10.1016/j.bmcl.2009.03.129. [DOI] [PubMed] [Google Scholar]
- 16.Mukaiyama H., Nishimura T., Kobayashi S., Komatsu Y., Kikuchi S., Ozawa T., Kamada N., Ohnota H. Novel pyrazolo[1,5-a]pyrimidines as c-Src kinase inhibitors that reduce IKr channel blockade. Bioorg. Med. Chem. 2008;16:909–921. doi: 10.1016/j.bmc.2007.10.068. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Laufer R., Patel N.K., Ng G., Sampson P.B., Li S.-W., Lang Y., Feher M., Brokx R., Beletskaya I., et al. Discovery of Pyrazolo[1,5-a]pyrimidine TTK Inhibitors: CFI-402257 is a Potent, Selective, Bioavailable Anticancer Agent. Med. Chem. Lett. 2016;7:671–675. doi: 10.1021/acsmedchemlett.5b00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukaiyama H., Nishimura T., Shiohara H., Kobayashi S., Komatsu Y., Kikuchi S., Tsuji E., Kamada N., Ohnota H., Kusama H. Discovery of Novel 2-Anilinopyrazolo[1,5-a]pyrimidine Derivatives a c-Src Kinase Inhibitors for the Treatment of Acute Ischemic Stroke. Chem. Pharm. Bull. 2007;55:881–889. doi: 10.1248/cpb.55.881. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A.K.A., Bodke Y.D., Sambasivam G., Lakra P.S. Design, synthesis, and evaluation of novel hydrazide hydrochlorides of 6-aminopyrazolo[1,5-a]pyrimidine-3-carboxamides as potent Aurora kinase inhibitors. Monatsh. Chem. 2017;148:1767–1780. doi: 10.1007/s00706-017-1943-7. [DOI] [Google Scholar]
- 20.Hassan A.S., Awad H.M., Magd-El-Din A.A., Hafez T.S. Synthesis and in vitro antitumor evaluation of novel Schiff bases. Med. Chem. Res. 2018;27:915–927. doi: 10.1007/s00044-017-2113-5. [DOI] [Google Scholar]
- 21.Hassan A.S., Hafez T.S., Ali M.M., Khatab T.K. Design, synthesis and cytotoxic activity of some new pyrazolines bearing benzofuran and pyrazole moieties. Res. J. Pharm. Biol. Chem. Sci. 2016;7:417–429. [Google Scholar]
- 22.Abd El-All A.S., Hassan A.S., Osman S.A., Yosef H.A.A., Abdel-Hady W.H., El-Hashash M.A., Atta-Allah S.R., Ali M.M., El Rashedy A.A. Synthesis, characterization and biological evaluation of new fused triazine derivatives based on 6-methyl-3-thioxo-1,2,4-triazin-5-one. Acta Poloniae Pharm. Drug Res. 2016;73:79–92. [PubMed] [Google Scholar]
- 23.Osman S.A., Mousa H.A., Yosef H.A.A., Hafez T.S., El-Sawy A.A., Abdallah M.M., Hassan A.S. Synthesis, characterization and cytotoxicity of mixed ligand Mn(II), Co(II) and Ni(II) complexes. J. Serb. Chem. Soc. 2014;79:953–964. doi: 10.2298/JSC130813134O. [DOI] [Google Scholar]
- 24.Hafez T.S., Osman S.A., Yosef H.A.A., Abd El-All A.S., Hassan A.S., El-Sawy A.A., Abdallah M.M., Youns M. Synthesis, structural elucidation and in vitro antitumor activities of some pyrazolopyrimidines and Schiff bases derived from 5-amino-3-(arylamino)-1H-pyrazole-4-carboxamides. Sci. Pharm. 2013;81:339–357. doi: 10.3797/scipharm.1211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osman S.A., Yosef H.A.A., Hafez T.S., El-Sawy A.A., Mousa H.A., Hassan A.S. Synthesis and antibacterial activity of some novel chalcones, pyrazoline and 3-cyanopyridine derivatives based on khellinone as well as Ni(II), Co(II) and Zn(II) complexes. Aust. J. Basic Appl. Sci. 2012;6:852–863. [Google Scholar]
- 26.Elgemeie G.H., Elsayed S.H., Hassan A.S. Design and synthesis of the first thiophene thioglycosides. Synth. Commun. 2009;39:1781–1792. doi: 10.1080/00397910802590928. [DOI] [Google Scholar]
- 27.Elgemeie G.H., Elsayed S.H., Hassan A.S. Direct route to a new class of acrylamide thioglycosides and their conversions to pyrazole derivatives. Synth. Commun. 2008;38:2700–2706. doi: 10.1080/00397910802222605. [DOI] [Google Scholar]
- 28.Hassan A.S., Moustafa G.O., Awad H.M. Synthesis and in vitro anticancer activity of pyrazolo[1,5-a]pyrimidines and pyrazolo[3,4-d][1,2,3]triazines. Synth. Commun. 2017;47:1963–1972. doi: 10.1080/00397911.2017.1358368. [DOI] [Google Scholar]
- 29.Hassan A.S., Hafez T.S., Osman S.A. Synthesis, characterization, and cytotoxicity of some new 5-aminopyrazole and pyrazolo[1,5-a]pyrimidine derivatives. Sci. Pharm. 2015;83:27–39. doi: 10.3797/scipharm.1409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadek K.U., Mekheimer R.A., Mohamed T.M., Moustafa M.S., Elnagdi M.H. Regioselectivity in the multicomponent reaction of 5-aminopyrazoles, cyclic 1,3-diketones and dimethyl formamide dimethylacetal under controlled microwave heating. Beilstein J. Org. Chem. 2012;8:18–24. doi: 10.3762/bjoc.8.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamdy N.A., Anwar M.M., Abu-Zied K.M., Awad H.M. Synthesis, tumor inhibitory and antioxidant activity of new polyfunctionally 2-substituted 5,6,7,8-tetrahydronaphthalene derivatives containing pyridine, thioxopyridine and pyrazolopyridine moieties. Acta Poloniae Pharm. Drug Res. 2013;70:987–1001. [PubMed] [Google Scholar]
- 32.Awad H.M., Abd-Alla H.I., Mahmoud K.H., El-Toumy S.A. In vitro anti-nitrosative, antioxidant, and cytotoxicity activities of plant flavonoids: A comparative study. Med. Chem. Res. 2014;23:3298–3307. doi: 10.1007/s00044-014-0915-2. [DOI] [Google Scholar]
- 33.Soliman H.A., Yousif M.N.M., Said M.M., Hassan N.A., Ali M.M., Awad H.M., Abdel-Megeid F.M.E. Synthesis of novel 1,6-naphthyridines, pyrano[3,2-c]pyridines and pyrido[4,3-d]pyrimidines derived from 2,2,6,6-tetramethylpiperidin-4-one for in vitro anticancer and antioxidant evaluation. Der Pharma Chem. 2014;6:394–410. [Google Scholar]
- 34.Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 35.Thornton T.M., Rincon M. Non-classical P38 map kinase functions: Cell cycle checkpoints and survival. Int. J. Biol. Sci. 2009;5:44–52. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.