Abstract
Freezing of barley (Hordeum vulgare), Hordeum murinum, and Holcus lanatus was studied using infrared video thermography. In the field, ice could enter H. lanatus leaves through hydathodes. In laboratory tests with barley, initially 0.4% of the leaf water froze, spreading in alternate strips of high and low freezing intensity longitudinally at 1 to 4 cm s−1, and simultaneously spreading laterally at 0.3 cm s−1. Similar results were obtained in the field with H. lanatus. A distinct second, more intense, freezing event spread slowly from the margins of the leaves toward the midrib. Organs of uprooted barley tested in the laboratory froze in this order: nucleated leaf, roots, older leaves, younger leaves, and secondary tillers. When ice spread from one leaf to the rest of the plant the crown delayed spread to the roots and other leaves. There was a longer delay above than below −2°C, helping to protect the crown from freezing during mild frosts. Initial spread of freezing was not damaging. However, the initial spread is a prerequisite for the second freezing event, which can cause damage. The route of the initial spread of ice may be extracellular, drawing water from more gel-like parts of the cell wall.
The survival and performance of temperate wild and crop species depends on their ability to tolerate some degree of freezing. Understanding freezing tolerance creates means to improve crop performance and helps explain the distribution of wild species. Current strategies for achieving this understanding focus on molecular analysis of cold acclimation (Pearce, 1999; Thomashow, 1999). However, it is equally important to understand the freezing process itself. This can identify similarities or differences between species in how they are affected by freezing, and is essential for eventually differentiating aspects of freezing that the plant does and does not control.
During freezing, substantial amounts of ice accumulate between the cells, growing at the expense of water drawn from inside the cells and thus dehydrating them (Pearce, 1988; Pearce and Ashworth, 1992). The cells are killed when their dehydration tolerance is exceeded. It is clear that this will only happen to a cell or tissue if freezing reaches that part of the plant. Understanding the spread of freezing within the plant is one factor helping to explain susceptibility of different plant parts to freezing damage and may indicate ways of controlling or avoiding damage.
Different plant organs or tissues may freeze at different temperatures. This is particularly well known in woody plants and is explained by different ice nucleation events in different parts of the plant and by barriers to spread of ice (Burke et al., 1976; Ashworth, 1996; Wisniewski et al., 1997; Wisniewski and Fuller, 1999). It is argued that ice forms in xylem vessels and then spreads to other parts of the plant through the vessels (Sakai and Larcher, 1987). The idea of rapid spread through vessels is a reasonable suggestion. There is direct evidence for ice propagation through xylem tissue (Kitaura, 1967). Although the rate of spread of freezing through stems can be fast, of the order of 1 to 2 cm s−1 (Single and Marcellos, 1981; Sakai and Larcher, 1987), direct evidence for nucleation in vessels and spread through vessels is lacking.
In cereals, stem nodes and the base of the roots pose a barrier to spread of ice, possibly related to xylem structure (Single and Marcellos, 1981; Zámećník et al., 1994). Proteins and polysaccharides with antifreeze properties are present in cereal leaves and the crown, respectively (Olien and Smith, 1981; Griffith and Antikainen, 1996). On the other hand, a comprehensive account of spread of freezing in cereals is lacking, yet is essential for understanding the relative roles of any specific features identified. Our purpose was to use infrared video thermography (IRVT) to obtain a more comprehensive account of spread of freezing in cereals and to seek new and informative details.
When supercooled water freezes it causes a release of heat (an exotherm). Freezing is detected by the consequent rise in temperature. Freezing after even slight supercooling is detectable. The usual method to study freezing has been by attaching thermocouples to parts of a plant and recording the time and temperature at which exotherms occur. Though this has provided important information (Burke et al., 1976; Sakai and Larcher, 1987), the approach has limitations: it cannot identify the site of nucleation of freezing, is not ideal for identifying the pathway of spread of ice, and gives only partial information on rates of spread. In addition, thermocouples themselves may induce freezing (Fuller and Wisniewski, 1999).
IRVT has recently been used to study freezing (Wisniewski et al., 1997). Data obtained using IRVT comprises video images in which false color indicates the temperature. IRVT provides detailed real-time images of the surface temperature of plant organs and allows the site of nucleation and initial spread of freezing to be observed. So far it has mainly been applied to tender species such as bean, tomato, and potato, and to woody species such as rhododendron and fruit crops (Wisniewski et al., 1997; Carter et al., 1999; Fuller and Wisniewski, 1999; Wisniewski and Fuller, 1999; Workmaster et al., 1999). These studies have shown that freezing may occur first on the leaf surface and that ice enters leaves through stomata, but also that in some woody species freezing may first occur inside the plant (Wisniewski et al., 1997; Wisniewski and Fuller, 1999). They have also verified the importance of internal barriers to the spread of freezing (Carter et al., 1999; Workmaster et al., 1999).
IRVT studies have not included hardy herbaceous plants, nor any grasses or cereals, and have been entirely based on laboratory studies. In a radiation frost moisture condenses on the plant and freezes, and this may nucleate freezing in the plant. This is difficult to achieve in the laboratory, and therefore nucleation is ensured by artificial methods. For this reason it is important to include field experiments to help verify the ideas about plant freezing developed on the basis of laboratory experiments.
Thus we used IRVT to characterize freezing in whole plants of barley (Hordeum vulgare) and in barley and grass leaves (Hordeum murinum and Holcus lanatus), mostly in the laboratory, but also in nature, including determining rates of spread, spatial distribution, directions of spread, and sequences of organ freezing, and we drew conclusions about the possible routes of spread and role of barriers to spread.
RESULTS
Shoots of cereal plants usually require moisture on their surface to freeze in laboratory tests at temperatures similar to those at which they freeze in the field. To verify this in the present experiments eight well-watered plants in pots, but having dry shoot surfaces, were cooled to a shoot temperature of −4.0°C. No parts of the plants froze (data not shown). In addition, when ice or single droplets of water or of a suspension of ice-nucleation active (INA) bacteria were placed on leaves, freezing was initiated at the point of application (below), and not at any other point. Hence for shoot freezing to be studied in the laboratory, water, ice, or a suspension of INA bacteria had to be brought into contact with the shoot surface.
Interpretation of the Images
A freezing event is exothermic and therefore its initiation can be detected as a rise in temperature. When the freezing event has finished, no more heat is released and the temperature falls. In each experiment the temperature range covered by the false-color scale (e.g. Fig. 1) was reset as cooling progressed, to keep the temperature of unfrozen parts of the plant at the bottom-end of the temperature range, shown by cold-pink or blue. Thus freezing would be detected by warming, giving a blue (if the specimen was initially cold-pink) or white color. With further warming the color would change again, to green, yellow, or red. Black indicates a temperature below, and pale pink indicates a temperature above, the set range. It was important to identify the termination of the freezing event, when all the ice that could form at that time in the experiment had formed, and when the specimen would recool. This was seen as a change in color toward the cold-end (blue or cold-pink) of the temperature scale.
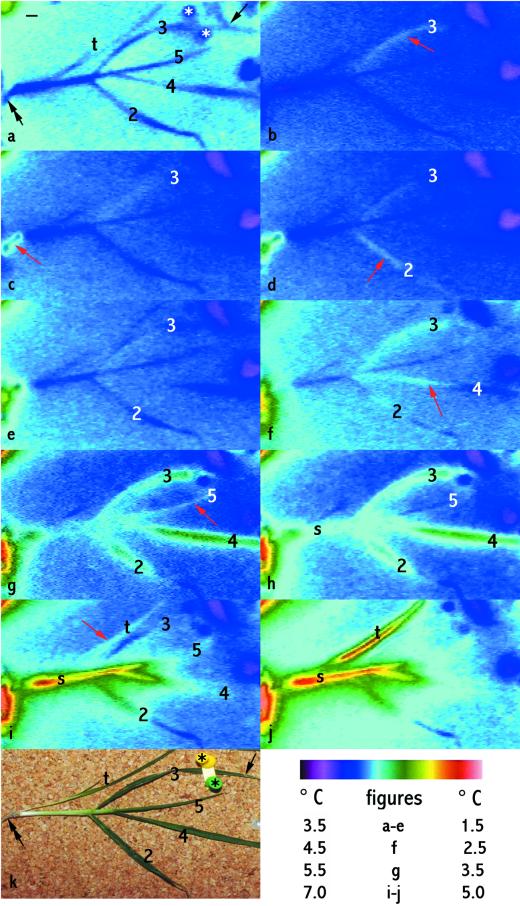
Figure 1.
Sequential freezing of parts of a barley plant during cooling in a controlled environment chamber showing spread of ice through the plant, including the first (red arrows) and second freezing events in a number of leaves (leaves 2–5) and freezing of roots (black, double-headed arrow), a leaf sheath (s), and secondary tiller (t). IRVT images are shown in time order from before the plant began to freeze (a) to when extensive freezing had occurred (j). k is a visible light image taken when freezing was complete. Leaves are numbered from the second to the fifth leaf to emerge (the first leaf was removed before the test). The plant was removed from soil and placed flat on a cork surface and exposed to subzero cooling. The stars in a and k mark two pins holding down a strip of plastic that gently held leaf 3 in contact with ice (indicated by a single-headed black arrow) to initiate freezing. Black bar (in a) = 1 cm. The color scale table (bottom right) indicates the temperature scale for each figure. Leaf 3 froze first and showed two freezing events. The warming of leaf 3 in b (red arrow) compared with in a indicates freezing. The leaf then cooled (c–e). It warmed again (f), indicating a second freezing event, and reached a higher temperature than in the first freezing event (compare g and h with b), and then cooled again (i). Roots froze second (red arrow in c). Leaf 2 froze next (red arrow indicates warming of the leaf in d) and also showed two freezing events: it cooled (e) and then warmed again (starting in f and continuing in g and h), finally cooling again. The sheath of leaf 2 (s) also froze (second freezing event shown in h–j). Leaf 4 froze next, ice spreading up the leaf (red arrow in f; the second freeze is shown in g–h and cooling in i) and the last leaf to freeze was leaf 5 (red arrow in g, cooled in h, second freezing event shown in i). The secondary tiller froze (red arrow in i; the second freezing event is shown in j).
More than one warming and recooling could occur sequentially at one location. This would indicate that only part of the water that could freeze at the location had frozen during the first warming and recooling event, with more water freezing later.
Freezing of Whole Plants in the Laboratory
The effect on the spread of ice of simultaneous exposure of all parts of the plant to the same temperature was tested. To do this, young barley plants were placed on cardboard or polystyrene and ice was placed in contact with the youngest fully expanded leaf of each plant, and then freezing was followed during cooling.
Table I shows the time interval between ice reaching the region where the sheath base joins the stem at the base of the plant, and the first occurrence of freezing in other organs. It therefore indicates the time to spread across the stem at the base of the crown and reach each of the organs. These time intervals differed significantly between organs, varying from seconds to reach the roots, to minutes or about 1 h to reach the youngest leaves and secondary tillers. The time-interval data for the acclimated (n = 5) and nonacclimated plants (n = 4) tested was not significantly different (P > 0.05; details not shown) and the data were therefore pooled in Table I. The order of time interval for spread to these organs was: roots < leaf older than the nucleated leaf < leaves younger than the nucleated leaves and secondary tillers.
Table I.
Time between the freezing front reaching the bottom of the sheath of the first leaf to freeze, and reaching other organs
| Organ to Which the Freezing Front Spread | Temperature of the Shoot Basea When the Freezing Front Reached It | Time ± se | n |
|---|---|---|---|
| °C | s | ||
| Roots | −1.5 to −2.0 | 28.9 ± 5.4 | 5 |
| Roots | −2.9 to −3.0 | 2.61 ± 0.57 | 4 |
| Next older leaf | −1.5 to −1.9 | 125 ± 48b | 4 |
| Next older leaf | −2.9 to −3.0 | 2.34, 6.32 | 2 |
| Next younger leaf | −1.5 to −2.0 | 3.50 × 103 | 5 |
| ±0.69 × 103 | |||
| Next younger leaf | −2.9 to −3.5 | 0.32 × 103 | 6 |
| ±0.30 × 103 | |||
| Secondary tiller | −1.5 to −3.0 | 3.70 × 103 | 5 |
| ±1.12 × 103 |
Plants of barley cv Gleam were grown in either a greenhouse (nonacclimated: four plants) or in the field (acclimated: five plants) and were analyzed in February 1999. Freezing was initiated by placing a piece of ice in contact with the youngest fully expanded leaf. Nine plants analyzed from three separate cooling runs, which each included acclimated and nonacclimated plants.
The shoot base comprised the stem and the basal 1 mm of surrounding leaf sheaths.
Data range from 38 to 268 s.
The time intervals varied between plants and a more precise account of the order in which freezing occurred was obtained by documenting it separately for each plant. Figure 1 is typical of the initiation and spread of freezing in the nine plants used in Table I: The leaf in contact with ice froze first, the ice spread rapidly and nucleated the roots, ice then spread to the next older leaf and subsequently to the younger leaves, and finally, it spread to secondary tiller. The same order occurred in the acclimated and nonacclimated plants tested (P > 0.05 in all comparisons; details not shown). In two of the plants used in Table I there were two leaves younger than the nucleated leaf. Further tests were made of two more young barley plants and of two reproductive wheat plants each with two leaves younger than the one nucleated. In all six specimens the leaf next youngest after the nucleated leaf froze before the most recently emerged leaf.
Figure 1 also shows typical freezing of the leaves in two stages. The first freezing event involved rapid spread of an area of slight warming. This recooled and subsequently, a second freezing event occurred that was more prolonged than the first freezing event and involved a larger rise in temperature. Thus, in Figure 1, leaf 3 showed the first freezing event (arrow in Fig. 1b), recooled (Fig. 1, c–e), and then a second freezing event occurred (Fig. 1, f–h). The same sequence occurred in the other leaves, older and younger than the first leaf to freeze (Fig. 1), and all leaves of the nine plants analyzed in Tables I through III, acclimated and nonacclimated, froze like this. Thus the same pattern of freezing occurred in each leaf whether it was the first leaf to freeze, initiated by external ice or froze later, nucleated by ice spreading within the plant.
Table III.
Rate of longitudinal spread of the first freezing event in leaves of whole barley cv Gleam plants
| Organs and Treatments | Rate ± se | 95% Confidence Interval | n |
|---|---|---|---|
| cm s−1 | |||
| Leaf blades | |||
| All samples | 2.36 ± 0.22 | 30 | |
| Effect of nucleation temperature | |||
| −1.5°C to −2.0°C | 1.64 | 0.78 | 9 |
| −2.5°C to −4.0°C | 2.67 | 0.52 | 21 |
| Effect of environment | |||
| Greenhouse (nonacclimated) | 2.16 | 0.74 | 11 |
| Field (acclimated) | 2.47 | 0.61 | 19 |
| Effect of leaf position relative to nucleated leaf | |||
| Nucleated leaf | 1.90 | 0.43 | 10 |
| Next older leaf | 1.57 | 1.21 | 6 |
| Younger leaf | 3.02 | 0.69 | 14 |
| Sheatha | |||
| All samples | 1.2 ± 0.42 | 6 | |
| Tillersb | |||
| All samples | 2.89 ± 0.88 | 4 |
Leaves and secondary tillers were analyzed from nine plants from three separate cooling runs. Experimental details were the same as in Table I. The data for leaf blades was analyzed by ANOVA to test for effects of temperature, growth environment, and the position of the leaf on the plant. ANOVA indicated significant differences (P < 0.01) between factors, and means were compared using confidence intervals.
The warming due to the spread of ice within the leaf sheath was only clearly detectable for some sheaths.
Only some plants had sufficiently large secondary tillers for accurate measurement.
Table I also shows that spread of ice across the stem at the base of the crown was much slower, between −1.5°C and −2.0°C, than below −2.9°C. Temperature also affected the time between the first and second freezing event in the leaves: at and below −2.2°C it was a few minutes, but at and above −1.9°C the average was 48 min (Table II).
Table II.
Time in each leaf of whole barley cv Gleam plants between the start of the first and second freezing events
| Temperature of the Leaf When Freezing Began | Time ± se | n |
|---|---|---|
| °C | min | |
| −1.4 to −1.9 | 47.8 ± 11.1 | 8 |
| −2.2 to −3.5 | 2.74 ± 0.35 | 25 |
Experimental details were the same as in Table I. Leaves were analyzed from 15 plants from three separate cooling runs, including four nonacclimated plants and 11 acclimated plants.
The spread of the ice front in leaves was very rapid within each leaf: 1 to 3 cm s−1 in leaf blades and leaf sheaths of the main and secondary tillers (Table III). This did not differ significantly between field-grown (acclimated) and greenhouse-grown (nonacclimated) plants. However, spread was about twice as fast in younger leaves than in older leaves. Again, spread was slower at −1.5°C and −2.0°C than at and below −2.5°C, though the difference was smaller than the effect of temperature on spread between organs.
Visible damage symptoms were assessed 7 d after testing. The lowest temperature that the plants in Tables I through III were exposed to was −7.0°C. The nonacclimated plants showed damage symptoms, whereas the acclimated plants did not.
Plants in pots or dug from the field and used with an intact root ball were also tested. In these laboratory tests the soil was expected to cool more slowly than the leaves and it was expected that the base of the crown embedded in the soil surface would therefore also cool more slowly than the leaves. Therefore, it was predicted that ice nucleated in one leaf in each plant would not initially spread to other leaves or other tillers. The first test was with four greenhouse-grown (nonacclimated) plants in which one drop of a suspension of INA bacteria was placed on one leaf of each plant. Figure 2A was typical; as expected, freezing in the nucleated leaf did not immediately spread to other parts of the plants.
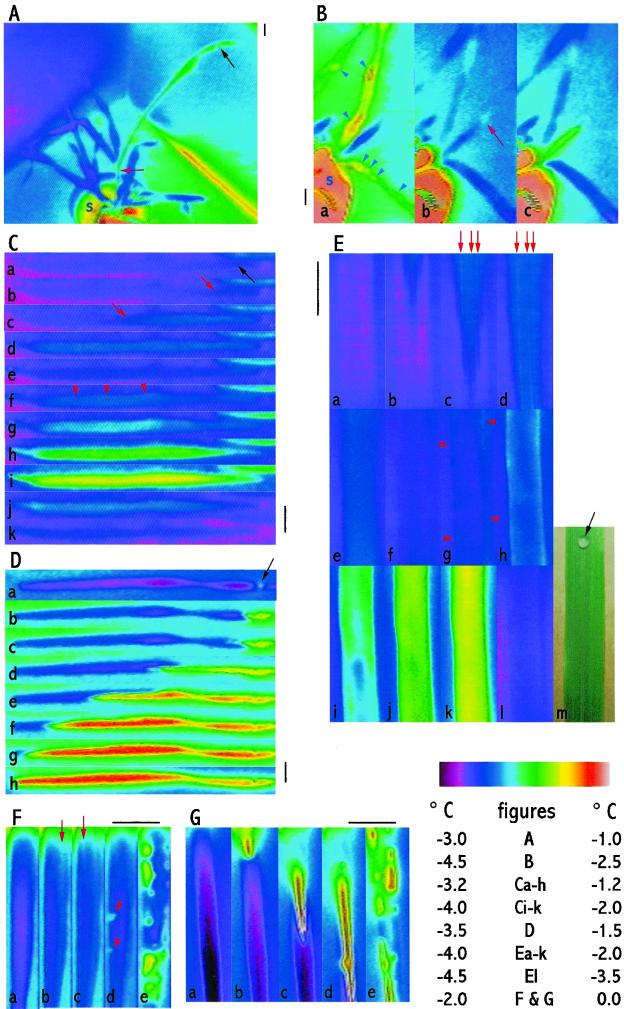
Figure 2.
Freezing of barley plants in pots and of excised leaves of barley during cooling in a controlled environment chamber. IRVT images in each of B through G are shown in time order. In A and C through G, freezing was initiated by placing a droplet of a suspension of INA bacteria on or touching the leaf surface. In B the plants were sprayed with water till run-off. Black bars = 1 cm. The color scale table (bottom right) indicates the temperature scale for each figure. A, Freezing of a greenhouse-grown (nonacclimated) plant of barley cv Gleam nucleated by a droplet of INA bacteria (black arrow) placed on the surface of a leaf blade. In the image this leaf is warmer than the other leaves. It had already begun to freeze, whereas the other leaves (which remained cooler) had not. Note that freezing had progressed into the sheath (red arrow) of the freezing leaf, but not to the rest of the plant. At this stage in the experiment the soil surface (s) was −1.00°C (in the pot showing at the base of plant), whereas the unfrozen leaves were −3.0°C. Freezing did not spread to the rest of the plant during the subsequent 75 min of observation, by when the soil surface had cooled to −2.6°C. B, Freezing of a field-grown (acclimated) plant of barley cv Gleam sprayed with water. The images show three leaves from one tiller. (The semicircle of light pink at the bottom left of the image is due to the soil [s] in the plant pot.) In a, several leaves were freezing, indicated by their warm color, including freezing of surface droplets (arrow heads). Later, these leaves had completed their freezing (b), but had not nucleated freezing of the youngest leaf (center of each figure). In this youngest leaf a droplet at its tip froze first (red arrow in b) and this initiated freezing in the whole leaf (warming shown in c). C and D, Successive exposure to freezing of whole leaf blades excised from plants of barley cv Igri grown in a 20°C/15°C (nonacclimating) environment. In C the leaves were cooled to −7.0°C. They were then thawed and kept for 3 h in a dark humid environment, during which they remained water-soaked. In D they were then exposed again to subzero cooling. In C there were two freezing events. Freezing began from the droplet of INA bacteria (black arrow in a) and was first indicated by a localized warming (change in color from pink/blue to blue then to blue/white), which spread down the blade (red arrows in b and c). The area of warming spread to the base of the blade (d) and then cooled (e), indicating the end of the first freezing event. Shortly after, the second freezing event began throughout the blade (warming indicated by blue/white area in f, red arrowheads). The leaf progressively warmed further (white to yellow in g–i), indicating continuation of freezing, and then recooled (j and k), indicating completion of the second freezing event. In D there was one freezing event. The spread of warming was indicated by the spread of the area of yellow and red down the leaf (b–h), beginning from the droplet of INA bacteria (black arrow in a). The warming of the leaf was greater than in the first freezing event in C, as indicated by the yellow and red false color. E, Typical details of a time-sequence of freezing in part of a leaf blade of barley cv Gleam. Freezing was initiated by a drop of a suspension of INA bacteria placed on the middle of the lower surface of the leaf, outside the upper edge of the field of view of the IRVT images (m is a visible-light photograph of the leaf piece showing the droplet: black arrow). a shows the leaf piece just before freezing. Freezing first appeared as a wedge of warmer tissue (blue) in the upper part of the image (b). This spread down the leaf (c and d) and cooled (becoming pink in f). The second freezing event was indicated by progressive warming in g through k (white to green to yellow) followed by recooling (to pink/blue in l). Note that the warming that initially spread down the leaf was most intense in narrow strips (red arrows in c and d). The second freezing event began at the edge of the leaf (red arrowheads in g) and spread inwards from there (h–j). F and G, Details of freezing of excised pieces of partially water-soaked leaf blades from plants of H. murinum (induced by 24 h in a 22°C, dark, humid environment). In F (typical of five out of eight of the leaves tested) initial warming spread in strips down the leaf (red arrows in b and c). Immediately after, localized freezing events occurred (red arrowheads in d) and rapidly recooled. This was followed by a period in which many localized freezing events, which lasted for varied times, occurred throughout the leaf (warmer patches in e). In G (typical of three out of eight of the leaves tested) there was initial spread of substantial warming down the middle of the leaf (b–e), and again, this was followed by many localized freezing events throughout the leaf (e).
The plant surface in many climates acquires condensed moisture early during a freezing night. To simulate this, three greenhouse-grown (nonacclimated) and three field-grown (acclimated) plants were sprayed with deionized water, to run-off. In these tests the soil surface remained at or above −1.0°C and did not freeze during the course of the experiment. Below a leaf and droplet temperature of −3°C, the droplets of water on the shoots froze sporadically, some before the leaves on which they were froze and some after. Most leaves froze during the course of the experiment. Figure 2B shows typical results. The leaves indicated froze at different times, and each froze by an initial spread of ice from at or near the tip.
In these experiments using potted plants or plants dug from the field and tested with root ball intact, freezing of a total of seven greenhouse-grown (nonacclimated) and 10 field-grown (acclimated) plants was examined. Damage to the leaves was visually assessed 7 d after freezing. The lowest temperature of the leaves was −2.5°C to −5.0°C in different tests. In no tests did leaves of the acclimated plants show damage symptoms. In nonacclimated plants no damage was seen when the lowest temperature reached was −2.5°C, whereas, in shoots that cooled to −4.5°C, the leaves that froze showed damage symptoms and those that did not freeze showed no symptoms.
Freezing of Excised Leaf Blades in the Laboratory
When leaf blades were cut from the plants and immediately cooled there was again a very rapid spread through the leaf of an area of slight warming followed by recooling, and then a second and larger rise in temperature, which persisted for longer (Fig. 2C). Whereas the first freezing event spread down the leaf, the second freezing event did not start at one point and spread from there. Instead it started simultaneously in many parts of the leaf.
Leaves from nonacclimated plants (grown in a 20°C/15°C environment) were tested during two successive freezing tests in which they were cooled to −7.0°C. Because they were not acclimated, the leaves were killed by the first exposure to −7.0°C (confirmed by visible symptoms). During the first test they froze typically, with an initial, very rapid spread of an exotherm that quickly disappeared, followed shortly by a second, more intense exotherm (Fig. 2C). When thawed they had a water-soaked appearance. The leaves were stored for 3 h in a dark, humid environment before retesting. They remained water-soaked, indicating that the previous freeze killed the leaves and redistributed water, filling the extracellular spaces. During the second test, freezing occurred differently from the first test. The first freezing event was much more intense (the leaf warmed much more and for much longer) than in the preceding freezing test, and freezing spread much more slowly down the leaf (4.13 ± 0.42 cm s−1 in the first freezing test and 0.40 ± 0.08 cm s−1 in the second; n = 6). In addition, although in the first test the second freezing event occurred 11.5 ± 0.8 s (n = 6) after the first freezing event, in the second test there was only a single extended freezing event (Fig. 2D).
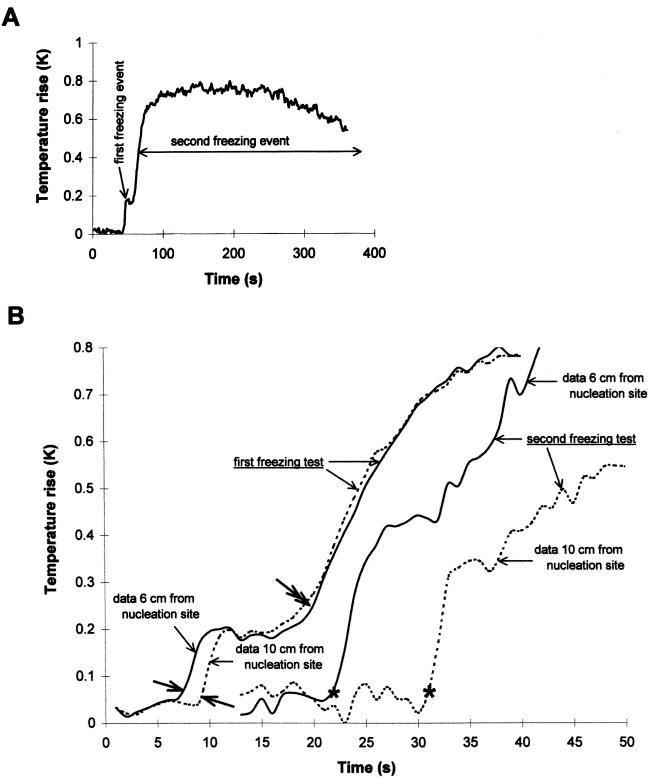
Digital analysis was used to obtain more precise temperature data than could be obtained directly from the color video images. This confirmed that the first freezing event involved a much smaller rise in temperature and the rise lasted for a much shorter time than in the second freezing event. Less than 0.37% ± 0.12% of leaf water froze during the initial spread of freezing (first freezing event, Fig. 3A). The digital analysis showed a clear separation in time between the first occurrence of freezing at different places in the leaf, and this was observed during the first spread of ice in the first and second freezing tests (Fig. 3B). However, in the first test the second freezing event was clearly shown as occurring at the same time in different parts of the leaf (Fig. 3B).
Figure 3.
Rise in temperature with time at one (A) or two (B) sites on leaf blades of barley cv Igri during typical freezing. Temperature was averaged at one (A) or two (B) lines crossing the image of the leaf, and averaged background temperature was subtracted. In B the two lines were spaced 4 cm apart, 6 and 10 cm, respectively, from the droplet of INA bacteria that nucleated freezing in the leaf. A, First freezing event compared with the second freezing event. B, Initial stages in freezing at the two positions in the leaf. Results are shown for the same leaf during exposure to freezing for the first time (first freezing test) or after cooling to −7.0°C and thawing, during exposure for the second time (second freezing test). In both tests ice spread down the leaf from the site of nucleation. Thus, the position on the leaf that the ice reached first warmed first, and warming occurred at the second site shortly after. Note that there was a shorter time gap between the start of freezing at the two sites in the first test (start of warming indicated by the single-headed bold arrows) than between the start of freezing at the two sites in the second test (start of warming indicated by stars). This indicates the different rates of spread of ice in the two tests, 2.0 and 0.4 cm s−1, respectively. Note also that in the first test the start of the second freezing event (indicated by the double-headed bold arrow) began at the same time at both sites, and the subsequent curves of warming at the two sites remained closely similar. On the other hand, in the second test the curves of warming at the two sites remained separate.
Details of freezing were studied by close-up examination of leaf pieces of only a few centimeters length (Fig. 2E). These confirmed the rapid initial spread of ice and the higher more prolonged second freezing event. In addition, they showed (n = 10) that the warming of the specimen during the initial freezing event was not evenly distributed across the leaf surface. Instead, more warming occurred in narrow strips down the leaf (equal numbers of leaves nucleated on the upper or lower surface with a drop of INA bacterial solution gave the same appearance). This indicated some lateral localization of the initial freezing event. The leaf pieces also showed that the second freezing event began at the edge of the leaf and progressed toward the mid-rib (Fig. 2E, g–j).
In these experiments with short pieces of leaf, freezing was initiated by placing a drop of a suspension of INA bacteria in the center of the leaf surface or touching one edge. This nucleated freezing throughout the leaf, and not just along the central region or edge with which the drop was in contact. This made it possible to measure the rate of the initial spread of ice laterally across the leaf, as well as longitudinally. The lateral spread was an order of magnitude slower than the longitudinal spread (Table IV).
Table IV.
Rate of longitudinal and lateral spread of first freezing event in excised youngest fully expanded leaves from plants of barley cv Gleam (grown in the greenhouse, nonacclimated, in the field, acclimateda) and analyzed in February 1999
| Leaf Part and Direction of Spread of Ice | Rate ± se | n |
|---|---|---|
| cm s−1 | ||
| Whole leaves | ||
| Longitudinal spread | 2.85 ± 0.18 | 7 |
| Four-cm lengths of leaf | ||
| Longitudinal spread | 2.18 ± 0.18 | 10 |
| Lateral spread | 0.31 ± 0.08 | 10 |
Freezing was initiated by freezing of a droplet of a suspension of INA bacteria placed near the tip of each leaf. First spread of ice occurred when leaves were at −2.5°C to −4.0°C.
There was no significant difference in the data for the acclimated and nonacclimated plants.
Short pieces of leaf were also used to examine spread of ice in partially water-soaked samples. Partial water soaking was obtained by placing potted plants of H. murinum in a growth chamber at 22°C with no lights and 100% relative humidity for 24 h before excising leaves and exposing them to subzero cooling. This resulted in one of two patterns of freezing. In five out of eight leaves tested, the initial spread of ice occurred in the usual way, by a rapid spread of ice, especially in strips. However, this was immediately followed by localized, more intense, but short-lasting (1 to several s) freezing events (Fig. 2F), which continued to occur in new locations until the leaf had finished freezing. The time gap between the initial spread of ice and the first localized higher-intensity freeze was 1.96 ± 0.82 s (mean ± se; n = 5) and the two shortest intervals were 0.40 and 0.56 s. In three out of eight leaves tested the initial spread of ice was slow and localized to the mid-rib region of the blade, and the warming in this initial spread of ice was much higher than usual. However, this was again immediately followed by localized spread laterally of the area of warming accompanied by, as in the first type, localized high-intensity short-lasting freezing (Fig. 2G).
Freezing of Lawn Grass Leaves in Situ
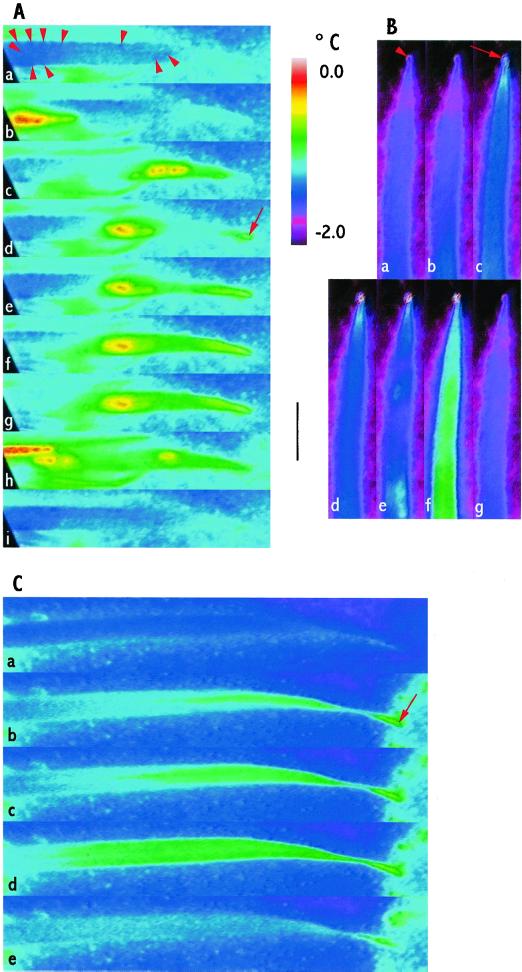
To verify the relevance of parts of the laboratory observations to Poaceae in the field, freezing of leaves of H. lanatus in a lawn was studied during a mild winter frost. Two leaves froze at −1.5°C and two at −2.5°C. All leaves showed an initial rapid spread of ice, seen as rapid spread of slight warming lasting for a short time (Fig. 4; Table V). At the higher temperature a second freezing event did not occur within the period of observation, whereas at the lower temperature a second freezing event occurred less than 1 min after the initial spread of ice. The initial spread of ice in two leaves was basipetal. In one of these, droplets of water on the leaf surface froze sequentially, starting near the base of the leaf and freezing droplets further up the leaf. When freezing of these surface droplets reached the tip, it nucleated freezing within the leaf. In the other leaf the tip was nucleated, causing spread of ice through the leaf, when ice spread on an adjacent frozen surface touching a droplet at the leaf tip (Fig. 4, A and C). In the other two leaves the initial spread of ice was acropetal. In one leaf, when the ice spreading internally reached the tip of the leaf, it froze a guttated drop located there (Fig. 4B). In the three leaves where freezing of the droplet at the tip occurred, no difference in time could be detected between the moment when the guttated droplet froze and the moment when freezing spread from or to the tip, indicating a direct connection between water freezing in the leaf and the guttated drops.
Figure 4.
Freezing of leaves of H. lanatus in situ in a lawn during a mild natural frost. Images in each of A through C are shown in time order. No artificial aid to nucleation of freezing was applied. Black bar = 1 cm. The color scale (top middle) indicates the temperature for the figures. A, Spread of freezing on the leaf surface in which droplets initiated freezing in adjacent droplets. This progressed toward the tip and froze a droplet there that then initiated the first freezing event in the leaf. Numerous water droplets had condensed on the leaf surface (some indicated by the red arrowheads in a). Droplets froze outside the field of view and nucleated droplets toward the base of the leaf (indicated by a warmer area at the left in b). Nucleation of surface droplets progressed sporadically, mainly acropetally (c). When a droplet at the tip was nucleated (red arrow in d) slight warming spread basipetally through the leaf (spread of the green, warmed, area from the tip toward the base in e–g). Other surface droplets subsequently froze (warmer areas in h), but eventually further surface freezing events ceased (i). A second freezing event within the leaf was not seen during the period of observation. B, The first freezing event spread up the leaf and nucleated a guttated droplet at the leaf tip. Warming spread up the leaf starting from outside the field of view (slight warming indicated by white/blue at the bottom of b). This spread to the tip where it nucleated freezing in a guttated droplet. The red arrowhead in a indicates the droplet before freezing; the red arrow in c indicates the droplet at the moment of freezing; the guttated droplet continued to emit heat in d through f, but had cooled again in g. The leaf cooled slightly (d) following the spread of freezing to the tip of the leaf. The second freezing event, indicated by warming, began at several points in the leaf (white and white/blue patches in e). This then spread and increased in warmth (green and white areas in f) and then cooled (g), completing the second freezing event. C, Contact between frozen droplets on the background surface and a droplet at the leaf tip initiated the first freezing event in the leaf. The leaf tip was touching a nearby surface on which moisture had condensed and was freezing. When freezing of this moisture spread to droplets that touched the tip of the leaf it initiated freezing in the leaf (red arrow in b) and warming spread basipetally through the leaf (white and green areas in b–d) and then cooled (e).
Table V.
Rate of longitudinal spread of first freezing event in fully expanded leaves of H. lanatus tested in situ during a natural frost
| Temperature of the Leaf When Freezing Began | Rate ± se | Time Gap to Second Freezing Event | n |
|---|---|---|---|
| °C | cm s−1 | ||
| −1.5 (n = 2); −2.5 (n = 2) | 2.51 ± 0.66 | Second event not seen during 3 h in the two leaves which first froze at −1.5°C | 4 |
| 22 and 38 s in the two leaves which first froze at −2.5°C |
No artificial aids to nucleate freezing were used.
DISCUSSION
IRVT showed freezing throughout the barley plant, allowing a comprehensive account of the spread of ice to be obtained: (a) The first and second freezing events in leaves are distinct. The first involves a rapid longitudinal spread of freezing, mainly in narrow strips, involving only a small fraction of leaf water. In contrast, the second freezing event spreads slowly and locally, from the margin of the leaf toward the mid-rib, and occurs simultaneously throughout the length of the leaf. It involves freezing of most of the leaf's water, causing the intracellular dehydration that stresses the plant. (b) Above −2°C the initial spread of freezing through the crown is delayed much more than at lower temperatures. This sets an important limitation on the ability of freezing initiated in one organ to spread further. Consistent with this, our results also indicate that nucleation of freezing occurs independently in different leaves. Thus any function the barriers have in adaptation to freezing could be in protecting the crown rather than preventing spread to other leaves. (c) Without the initial spread of ice from a point of nucleation, the plant will not experience freezing stress. Thus any strategy to modify freezing behavior in plants must address the route by which freezing spreads. The water that freezes during the initial spread of ice is probably drawn from cell walls. If so, it is only a fraction of the total water present in cell walls, and may be drawn only from the most gel-like parts. (d) The species used acclimated only moderately in the environments used, so the results need not be typical of all cereals or the most strongly acclimatory environments. Nevertheless, our experiments showed no differences in freezing behavior between the cold-acclimated and nonacclimated plants.
Natural Freezing
Freezing in the leaves during a natural frost was similar to that in leaves tested in the laboratory. There was an initial freezing event that comprised rapid spread of ice, but freezing of only a fraction of the leaf water. When the temperature fell further, there was a second freezing event during which much more water froze. The rate of spread of ice in the first freezing event was also similar to in the laboratory. Thus the more detailed analysis in the laboratory tests was likely to be indicative of behavior and mechanisms in leaves in the field.
Laboratory experiments have shown that ice may enter leaves through stomata (Wisniewski and Fuller, 1999). However, at night, stomata will be closed. In our field experiments done at night, the hydathodes at the tip of the leaves, through which water guttated, was an important channel for ice to spread into or out of the plant (Fig. 4). Laboratory tests indicated that guttated water itself does not have a high nucleation temperature (not shown), and in the limited observations in the field it was frozen by contact with ice nucleated from elsewhere. Nevertheless, in our field experiments droplets that froze on the leaf surface did not nucleate freezing in the leaf except when the droplet at the leaf tip was nucleated.
Intercellular spaces in hydathodes are connected to the termination of vascular strands. However, there is not a free path of water between the guttating drop and the lumen of the xylem elements since the strands terminate in tracheids (Esau, 1965). It is generally thought that there would be a delay in ice crossing into tracheids or crossing the end-walls of vessels, due to the presence of pit membranes (Single and Marcellos, 1981; Zámećník et al., 1994). However, we could not detect any delay, which may indicate that the initial rapid spread of ice through the leaf was not through the xylem vessels or that pore size in these pit membranes was not restrictive.
Rates of Spread of Freezing in Leaves
The rate of longitudinal spread of freezing in the leaf blades was rapid, between 1 and 4 cm s−1, depending on age and freezing temperature, with an overall average of 2.6 cm s−1. These are among the fastest rates of spread recorded in plants, though earlier estimates are close; up to 2 cm s−1 in nonacclimated wheat (Single and Marcellos, 1981), 1 cm s−1 in mulberry (Kitaura, 1967), and 0.4 cm s−1 in barley seminal internodes and bean internodes (Zámećník et al., 1994; Wisniewski et al., 1997).
The fastest rates of spread of freezing in plants are somewhat faster than the linear growth rate of ice in water, which at atmospheric pressure and when supercooled by about 1° K, is 0.5 cm s−1 (Hobbs, 1974). To obtain a rate comparable with the fastest found in plants, above 2 cm s−1, supercooling of about 5° K is needed (Hobbs, 1974). Allowing for a depression of freezing point of about 1° K (Levitt, 1980), this is several degrees more supercooling than occurred in our experiments. Growth of ice from the vapor phase is much slower than in water, of the order of 10−4 cm s−1 (Hobbs, 1974). Thus plants show unusual freezing behavior compared with physical experiments, speeding the spread of ice.
Rapid dissipation of the heat released from the very small amount of water frozen during the initial spread of ice could explain this. Dissipation of the heat of fusion is a major factor determining the rate of growth of ice (Hobbs, 1974). The amount of water that froze during the initial spread of ice accounted for less than 0.4% of the total leaf water. Consistent with this explanation, the warming at the spreading front was much higher and the associated rate of spread was slower in the water-soaked leaves, in which more water froze initially.
The Route of the Initial Spread of Ice in Leaves
The IRVT method used does not resolve ice formation in or around individual cells. However, it did show differences in freezing between adjacent areas within leaves at distances of less than 1 mm. In addition, it provided circumstantial evidence relating to the precise locations of ice.
The initial longitudinal spread of freezing in leaves occurred mainly in strips that could have corresponded to main ribs, and if so, this spread could have been associated closely with the vascular bundles. However, this need not indicate freezing in the vessels themselves. In Solanum and Brassica, freezing in petioles first occurred in vascular tissue and adjacent intercellular spaces (Hudson and Idle, 1962). Several of our observations indicate that the initial spread of freezing was not through the xylem elements themselves. Freezing withdraws water from leaf cells, forming ice in the intercellular spaces (Pearce, 1988; Pearce and Ashworth, 1992). If the leaf is damaged, then on thawing, this water remains in the intercellular spaces causing a water-soaked appearance. However, water would have remained in the xylem vessels. Thus, one would expect to see the same rapid initial spread of freezing in ribs in non-damaged, non-water-soaked leaves and in damaged, fully water-soaked leaves. However, the patterns of spread (Figs. 2, C and D and 3B) and initial rates of spread, which differed by a factor of 10, were quite different. This may indicate that the initial longitudinal spread of ice was in both cases extracellular.
In five of the partially water-soaked leaves there was a typical first freezing event involving rapid spread of freezing in strips down the leaf (Fig. 2F). However, these then initiated localized short-lived freezing events in between under 1 s and a few seconds, again suggesting initial spread was extracellular.
The rate of lateral spread in leaves was an order of magnitude less than the rate of longitudinal spread. Minor vein connections run laterally between the longitudinal veins. In festucoid grasses they occur with a frequency connecting each pair of longitudinal vascular bundles of about 1 per mm (illustration in Pate and Gunning, 1969) or more (illustration in Esau, 1965). The lateral veins do not form a linear path across the leaf, instead, any ice spreading laterally through xylem vessels would have to take a tortuous route, which might then partially explain the slower rate of spread laterally than longitudinally. However, it is equally plausible that spread occurred outside the vascular bundles since paths of travel at right angles to each other might lead to different rates of travel due the different structure of the leaf in the lateral and longitudinal directions.
The proportion of the leaf water that froze during the initial spread of ice was under 0.4%. Assuming the initial spread of ice was extracellular, then there are two sources of water for this initial freeze: the vapor phase in the gas spaces and the cell walls. The former would contribute a negligible amount: a fall in temperature from 0°C to −5°C would contribute through condensation about 4 × 10−5% of the leaves water content (based on a change in water content of the vapor phase of 62.5 × 10−6 mol L−1 between 0°C and −5°C). The proportion of the leaf water volume accounted for by water in the cell walls is about 6% (assuming the cell wall accounts for about 10% of the leaf fresh weight and that it has a water content of 60%–70%). Thus much less than the full amount of water in the cell walls froze during this initial spread of ice. The small pore sizes in plant cell walls, averaging 4 nm (Preston, 1974), would reduce the average freezing point of water in the wall to between −15°C and −25°C (Ashworth and Ables, 1984). The converse will follow, that the vapor pressure of ice outside the cell walls will not be low enough at temperatures above these to draw water out from such pores. On the other hand, water might be drawn from more gel-like parts of cell walls such as the middle lamella or from water-rich wall layers found in some fibers. If so, the distribution of these sources of wall water at the tissue and organ level could influence the route of the initial spread of ice.
The Second Freezing Event
In leaves of freeze-stressed temperate cereals ice is extracellular (Pearce, 1988; Pearce and Ashworth, 1992). The second freezing event accounts for most of the freezing in the leaf and therefore must comprise formation of this extracellular ice.
The ice formed during the first freezing event probably initiates the second freezing event. In other respects, the two freezing events are separable. When the first freezing event began above −2°C the occurrence of the second freezing event could be delayed (using a slow cooling rate) for a considerable time. However, if the first freezing event occurred below −2°C, the second freezing event followed within a few minutes or seconds. This indicates that the two freezing events involved separate fractions of plant water with different freezing points, one above −2°C and one just below. This is consistent with the first freezing event being apoplasmic, whereas the second involved drawing water from the symplast.
Other details confirm the distinctness of the two freezing events. The first freezing event spread longitudinally through the leaf and thus reached different parts of the leaf at different times, but the second freezing event began simultaneously at different points along the length of the leaf (Figs. 2C and 3B). Also, the second freezing event began first at the edge and spread toward the center of the leaf from there, whereas the first freezing event involved longitudinal spread across the leaf's width (Fig. 2E).
Spread of Freezing through the Plant
In festucoid grasses the largest vascular bundles originating in a leaf have major connections in the stem with the vascular bundles of the leaves inserted two nodes above and below, and are connected only indirectly via these main connections and via the nodal plexus with the leaves inserted one node to either side (Hitch and Sharman, 1971). When the joined vasculatures reach the base of the crown they connect with the root vasculature via the complex network of the vascular transition zone. Thus if the spread of freezing were confined to the xylem vessels, at least parts of the rest of the shoot should freeze before any of the roots, and leaves two-above and two-below the nucleated leaf should freeze before the leaves adjacent to it. However, when the freezing front reached the crown it always first initiated freezing in the roots, and the next leaf above the nucleated one froze before the one next above that.
Embolisms may form in vessels as a result of freezing, though the vessels can refill when thawed (Canny, 1997). The vascular system of angiosperms is segmented; vessels are terminated by tracheids at nodes, and this prevents the spread of embolisms between organs (Aloni, 1987). These termini are more frequent in the vascular transition zone between the root and shoot in hardy than in tender cereals (Aloni and Griffith, 1991), and spread of ice is delayed in this zone (Zámećník et al., 1995). The node is also a barrier to the spread of freezing in the reproductive stem of wheat. Single and Marcellos (1981) suggested that the barrier is the tracheal termini in the node, and that passage of ice across the node is in or around living cells. The same pattern of spread occurs in the first barley leaf to freeze as in the barley leaf that freezing spreads to last. This would be unlikely if spread were first in the vessels and then outside them.
Our results show that below −2.0°C the delay to spread of ice caused by these barriers is only a few seconds, whereas above −2.0°C the delay may be prolonged or indefinite. Thus, in young barley plants the barriers are unlikely to be effective in reducing spread of ice throughout the plant except in the mildest frosts. In many frost situations the base of the plant will tend to be warmer than the exposed parts of the leaves. This difference in temperature could, in vegetative plants, also be an effective barrier delaying spread from one leaf to the rest of the plant (Fig. 2A). However, in the laboratory and in nature, individual leaves of barley are capable of freezing separately (Fig. 2B). Fuller and Wisniewski (1998) made similar observations in potato. Ice did not travel from one shoot growing up from the tuber to another, because of, it was suggested, insulation by the soil. Shoot morphology is quite different in potato and barley. Nevertheless, as in barley, leaves of potato sprayed with water froze independently, thus structural or biochemical barriers were important in both species.
Thus the role of these barriers in cereals, if they have one, would be to control freezing of the crown, rather than to protect leaves from freezing. This is interesting because the crown is often as frost-sensitive or more sensitive than other parts of cereal and grass shoots (Tanino and McKersie, 1985; Shibata and Shimada, 1986) and the survival of the crown is essential for plant survival (Olien, 1967).
MATERIALS AND METHODS
Plant Material and Growth Environments
Barley (Hordeum vulgare cv Gleam) was grown in nonacclimating and cold-acclimating environments. The nonacclimating environment comprised a greenhouse at the Seale-Hayne campus of the University of Plymouth (Devon, UK) maintained with a minimum temperature of 10°C and supplementary lighting during an 8-h day. The acclimating environment comprised a field site also at the Seale Hayne campus of the University of Plymouth (50° 32′ N, 3° 34′ W; January mean temperature of 5.7°C). At this site the daily minimum and maximum air temperatures in the 7 d preceding use of the plants in February 1999 varied from −1.5°C to −8.8°C and from 6.4°C to 10.6°C, respectively. Barely cv Igri was grown in a nonacclimating environment of 20°C/15°C (day/night temperature, 10-h photoperiod, 250 μmol m−2 s−1 photon flux density). Hordeum murinum was grown in a similar controlled environment using a cold-acclimating temperature of 6°C/2°C. Holcus lanatus was growing wild in a lawn in Newcastle upon Tyne, UK (55° 0′ N, 1° 40′ W; January mean temperature 3.7°C) and was tested in February 2000. In the 7 d immediately preceding the test the daily grass minimum temperature varied between −1.2°C and 3.1°C and daily grass maximum temperature varied between 6.4°C and 12.0°C, as recorded at the nearby University of Newcastle Close House Field Station.
Freezing-Test Environments and IRVT
Freezing of barley cv Gleam was tested at Seale Hayne in a controlled environment radiation freezing chamber giving a steady cooling profile and using a cooling rate of between 1°C h−1 and 3°C h−1 (Fuller and LeGrice, 1998). Freezing of H. lanatus was observed in situ during a mild natural frost in February 2000. Freezing of barley cv Igri and of H. murinum was done at Newcastle University in a controlled environment chamber giving a steady cooling rate of 2°C h−1 and a minimum temperature that could be maintained for prolonged periods with less than 0.5°C variation.
An infrared imaging camera using a HgCdTe long-wave detector (model 760, Inframetrics, North Billerica, MA) was used to monitor exothermic events in the freezing plants (Wisniewski et al., 1997). Frame averaging of 16 frames s−1 was used to smooth the image. The results were recorded on video tape as color and black-and-white images using two recorders. Thermocouples placed in the field of view in contact with the background, but not touching the plant were used as an independent check of the temperatures recorded. The temperature recorded by the camera was also standardized against an ice-water mixture at 0°C.
The image delivered by the camera used false color to show the temperature. The temperature scale used covered a 2°C range from “cold” colors (pink and blue) to “warm” colors (yellow and red). The plant material was placed against a background chosen to be of a different temperature to the plant, warmer or cooler (depending on the background material). This provided sufficient contrast for the specimen to be visualized by the camera before it froze, and thus to position the plant in the camera's field of view and to focus the image. It was important to avoid confounding warming of the plant due to any warming in the environment with warming due to heat released during freezing. Therefore we discounted any warming of the plant that was accompanied by warming in the background. The color image facilitated visual detection of exotherms with a temperature resolution of between 0.1°C and 0.2°C.
The black-and-white recording was analyzed using the program Thermagram Plot (Inframetrics). This allowed quantification of freezing. Warming (in Kelvin) due to freezing was detected by subtracting the average temperature of pixels in a 0.7-cm line in the background from the average temperature of pixels in a 0.7-cm line in the image of the leaf. Freezing occurred during a period of slow cooling and raised the temperature of the sample by less than 1°K, and thus gave only small changes in the driving force (gradient of temperature between leaf and the condenser of the cooling unit) for removal of heat throughout the test period. Thus the amount of water freezing was proportional to the area under the graph of temperature against time during freezing. The proportion of water freezing in the first freezing event was calculated as the area under the graph of temperature against time during the first freezing event relative to the area under the whole graph of first and second freezing event, from initiation of freezing to completion. A remaining small amount of unfrozen water would freeze subsequently as temperature fell further, and therefore this calculation gave a maximum estimate of the proportion of leaf water frozen in the first freezing event.
The video images were studied frame-by-frame to obtain the time and location of start and finish of freezing events. The dimensions of excised leaves and of the organs of plants used in the experiments was measured. From this data the rate of spread within organs and the time for spread between organs was calculated.
Statistically significant effects were detected by t tests or in Table III, by ANOVA and estimates of 95% confidence intervals.
After plants had been used in freezing tests they were placed in the greenhouse environment described above and assessed after 1 week for visual symptoms of damage: loss of turgor and dryness indicated killed regions and premature yellowing of leaves indicated a sublethal effect.
INA Bacteria
The ice-nucleating bacterium, Pseudomonas syringae strain Cit7 (Lindow, 1985), was grown for 72 h on plates of King's medium B (King et al., 1954) at 20°C. The plates were then stored at 4°C to induce expression of the ice-nucleation protein. Cells were harvested with a loop and suspended in sterile deionized water and stored on ice before use.
Footnotes
This work was supported by the Biotechnology and Biological Science Research Council (grant no. 321/JEI09421).
LITERATURE CITED
- Aloni R. Differentiation of vascular tissue. Annu Rev Plant Physiol. 1987;38:179–204. [Google Scholar]
- Aloni R, Griffith M. Functional xylem anatomy in root-shoot junctions of six cereal species. Planta. 1991;184:123–129. doi: 10.1007/BF00208245. [DOI] [PubMed] [Google Scholar]
- Ashworth EN. Responses of bark and wood cells to freezing. Adv Low Temp Biol. 1996;3:65–106. [Google Scholar]
- Ashworth EN, Ables FB. Freezing behavior of water in small pores and the possible role in the freezing of plant tissues. Plant Physiol. 1984;76:201–204. doi: 10.1104/pp.76.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MJ, Gusta LV, Quamme HA, Weiser CJ, Li PH. Freezing and injury in plants. Annu Rev Plant Physiol. 1976;27:507–528. [Google Scholar]
- Canny MJ. Vessel contents during transpiration: embolisms and refilling. Am J Bot. 1997;84:1223–1230. [PubMed] [Google Scholar]
- Carter J, Brennan R, Wisniewski M. Low-temperature tolerance of blackcurrant flowers. Hortsci. 1999;34:855–859. [Google Scholar]
- Esau K. Plant Anatomy. Ed 2. New York: John Wiley & Sons; 1965. [Google Scholar]
- Fuller MP, LeGrice P. A chamber for the simulation of radiation freezing of plants. Ann Appl Biol. 1998;133:111–121. [Google Scholar]
- Fuller MP, Wisniewski M. The use of infrared thermal imaging in the study of ice nucleation and freezing of plants. J Therm Biol. 1998;23:81–89. [Google Scholar]
- Griffith M, Antikainen M. Extracellular ice formation in freezing-tolerant plants. Adv Low-Temp Biol. 1996;3:107–139. [Google Scholar]
- Hitch PA, Sharman BC. The vascular patterns of festucoid grass axes, with particular reference to nodal plexi. Bot Gaz. 1971;132:38–56. [Google Scholar]
- Hobbs PV. Ice Physics. Oxford: Clarendon Press; 1974. [Google Scholar]
- Hudson MA, Idle DB. The formation of ice in plant tissues. Planta. 1962;57:718–730. [Google Scholar]
- King EO, Ward MK, Raney DE. Two simple media for demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- Kitaura K. Freezing and injury of mulberry trees by late spring frost. Bull Sericult Exp Station (Tokyo) 1967;22:202–323. [Google Scholar]
- Levitt J. Responses of Plants to Environmental Stresses. Vol. 1. New York: Academic Press; 1980. [Google Scholar]
- Lindow SE. Integrated control and role of antibiosis in biological control of fireblight and frost injury. In: Windels C, Lindow SE, editors. Biological Control on the Phylloplane. Minneapolis: American Phytopathological Society; 1985. pp. 83–115. [Google Scholar]
- Olien CR. Freezing stresses and survival. Annu Rev Plant Physiol. 1967;18:387–408. [Google Scholar]
- Olien CR, Smith MN. Protective systems that have evolved in plants. In: Olien CR, Smith MN, editors. Analysis and Improvement of Plant Cold Hardiness. Boca Raton, FL: CRC Press; 1981. pp. 61–87. [Google Scholar]
- Pate JS, Gunning BES. Vascular transfer cells in angiosperm leaves: a taxonomic and morphological survey. Protoplasma. 1969;68:135–156. [Google Scholar]
- Pearce RS. Extracellular ice and cell shape in frost-stressed cereal leaves: a low temperature scanning electron microscopy study. Planta. 1988;175:313–324. doi: 10.1007/BF00396336. [DOI] [PubMed] [Google Scholar]
- Pearce RS. Molecular analysis of acclimation to cold. Plant Growth Reg. 1999;29:47–76. [Google Scholar]
- Pearce RS, Ashworth EN. Cell shape and localization of ice in leaves of overwintering wheat during frost stress in the field. Planta. 1992;188:324–331. doi: 10.1007/BF00192798. [DOI] [PubMed] [Google Scholar]
- Preston RD. The Physical Biology of Plant Cell Walls. London: Chapman & Hall; 1974. [Google Scholar]
- Sakai A, Larcher W. Frost Survival of Plants. Berlin: Springer-Verlag; 1987. [Google Scholar]
- Shibata S, Shimada T. Anatomical observation of the development of freezing injury in orchardgrass crown. J Jap Grass Sci. 1986;32:197–204. [Google Scholar]
- Single WV, Marcellos H. Ice formation and freezing injury in actively growing cereals. In: Olien CR, Smith MN, editors. Analysis and Improvement of Plant Cold Hardiness. Boca Raton, FL: CRC Press; 1981. pp. 17–33. [Google Scholar]
- Tanino KK, McKersie BD. Injury within the crown of winter wheat seedlings after freezing and icing stress. Can J Bot. 1985;63:432–436. [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Wisniewski M, Fuller MP. Ice nucleation and deep supercooling in plants: new insights using infrared thermography. In: Margesin R, Schinner F, editors. Cold-Adapted Organisms: Ecology, Physiology, Enzymology and Molecular Biology. Berlin: Springer-Verlag; 1999. pp. 105–118. [Google Scholar]
- Wisniewski M, Lindow SE, Ashworth EN. Observation of ice nucleation and propagation in plants using infrared video thermography. Plant Physiol. 1997;113:327–334. doi: 10.1104/pp.113.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workmaster BAA, Palta JP, Wisniewski M. Ice nucleation and propagation in cranberry uprights and fruit using infrared video thermography. J Am Soc Hort Sci. 1999;124:619–625. [Google Scholar]
- Zámećník J, Bieblovaá J, Grospietch M. Safety zone as a barrier to root-shoot ice propagation. Plant Soil. 1994;167:149–155. [Google Scholar]