Abstract
Previously tested n-hexane extracts of the Scorzonera latifolia showed promising bioactivity in vivo. Because triterpenes could account for this activity, n-hexane extracts were analyzed by HPLC to identify and quantify the triterpenes as the most abundant constituents. Other Scorzonera and Podospermum species, potentially containing triterpenic aglycones, were included in the study. An HPLC method for simultaneous determination of triterpene aglycones was therefore developed for analysis of Podospermum and Scorzonera species. n-Hexane extracts of root and aerial parts of S. latifolia, ten other Scorzonera species and two Podospermum species were studied to compare the content of triterpenes. HPLC was used for the qualitative and quantitative analysis of α-amyrin, lupeol, lupeol acetate, taraxasteryl acetate, 3-β-hydroxy-fern-7-en-6-one acetate, urs-12-en-11-one-3-acetyl, 3-β-hydroxy-fern-8-en-7-one acetate, and olean-12-en-11-one-3-acetyl. Limits of detection and quantification were determined for each compound. HPLC fingerprinting of n-hexane extracts of Podospermum and Scorzonera species revealed relatively large amounts of triterpenes in a majority of investigated taxa. Lupeol, lupeol acetate, and taraxasteryl acetate were found in a majority of the species, except S. acuminata. The presence of α-amyrin, 3β-hydroxy-fern-7-en-6-one-acetate, urs-12-en-11-one-3-acetyl, 3β-hydroxy-fern-8-en-7-one-acetate, and olean-12-en-11-one-3-acetyl was detected in varying amounts. The triterpene content could correlate with the analgesic and anti-inflammatory activity of Scorzonera, which was previously observed and Scorzonera species that have been determined to contain triterpenes in large amounts and have not yet been tested for their analgesic activity should be tested for their potential analgesic and anti-inflammatory potential. The presented HPLC method can be used for analysis of triterpene aglycones, for example dedicated to chemosystematic studies of the Scorzonerinae.
Keywords: HPLC, Podospermum, Scorzonera, triterpenes
1. Introduction
Scorzonera genus belonging to Asteraceae family is widely distributed in Eurasia and northern Africa with about 160 species. In Turkey, this genus is represented by 59 taxa, and 52 species, of which 31 are endemic [1]. Podospermum genus (Asteraceae), represented by several tens of species, is closely related to Scorzonera, and also grows mainly in Mediterranean and Western Asia. Members of the Scorzonera genus are used as vegetables and medicinal plants. Phenolic compounds such as dihydroisocoumarins [2,3], bibenzyl derivatives [4,5,6], flavonoids [7,8,9], lignans [6,10], stilbene derivatives [11], quinic and caffeic acid derivatives [8,12], sesquiterpenes [4,8,13] and triterpenes [12,13,14,15,16,17,18] have been isolated from Scorzonera species. Triterpenes are one of the largest groups of terpenes [19,20]. It has been estimated that more than 4000 triterpenoids are known to occur in nature [19]. Interest in the natural triterpenoids is growing because they display a wide spectrum of biological activities [19,20,21].
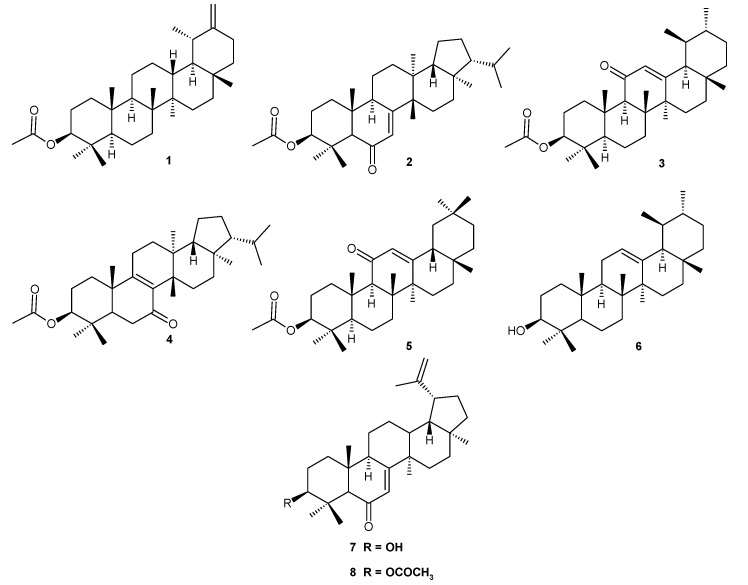
The current study is aimed at developing a fingerprint profile of n-hexane extracts of S. latifolia. In addition, the triterpenes taraxasteryl acetate (1), 3β-hydroxy-fern-7-en-6-one-acetate (2), urs-12-en-11-one-3-acetyl (3), 3β-hydroxy-fern-8-en-7-one-acetate (4), and olean-12-en-11-one-3-acetyl (5), which have been previously isolated from the n-hexane extracts of S. latifolia and the commercially available triterpenes α-amyrin (6), lupeol (7), and lupeol acetate (8) have been qualitatively and quantitatively determined first in S. latifolia, and later in other Scorzonera species. Because the fingerprint profiling of the plant extracts may be useful in chemotaxonomic classification of corresponding plants and also in predicting the potential bioactivity, several aerial as well as root extracts of Scorzonera species collected in Turkey have been analyzed by the same method to determine their triterpene profiles and to compare their triterpene contents.
2. Results
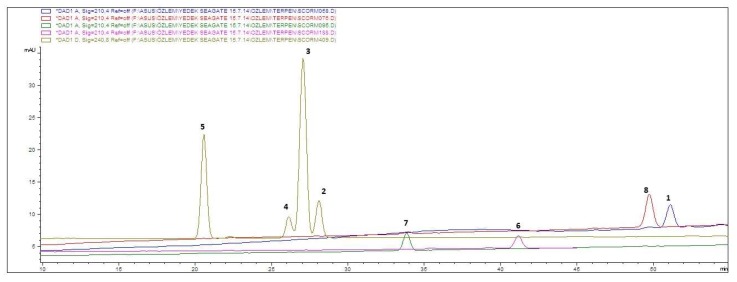
This paper describes the development and validation of an HPLC method for the identification of S. latifolia and other Scorzonera species in their n-hexane extracts as well as the quantification of the triterpenic compounds 1–8 in all of the Scorzonera and Podospermum species tested. The best separation of compounds 1–8 was obtained using a C8 stationary phase and linear gradient elution of acetonitrile in water. The absorbance at λ 200, 210 or 240 nm (Figure 1) was used to characterize the chromatogram for each compound. Table 1 shows the wavelength, calculated calibration curve, and LOD and LOQ results for each respective compound.
Figure 1.
Superimposed representative HPLC chromatograms of compounds 1 and 6–8 at 210 nm and 2–5 at 240 nm: taraxasteryl acetate (1) 33 µg·mL−1; 3β-hydroxy-fern-7-en-6-one acetate (2) 20 µg·mL−1; urs-12-en-11-one-3-acetyl (3) 65 µg·mL−1; 3β-hydroxy-fern-8-en-7-one acetate (4) 31 µg·mL−1; olean-12-en-11-one-3-acetyl (5) 65.5 µg·mL−1; α-amyrin (6) 23 µg·mL−1; lupeol (7) 26 µg·mL−1; lupeol acetate (8) 44 µg·mL−1.
Table 1.
Calibration curves, linearity, LOD, LOQ and precision of HPLC analysis for triterpenes 1–8.
| Compound | Calibration Curve | r 2 | LOD (µg/mL) |
LOQ (µg/mL) |
Precision % | |
|---|---|---|---|---|---|---|
| Intra-Day (n = 6) | Inter-Day (n = 3) | |||||
| 1 | Y = 5.1753X − 1.86223 | 0.9977 | 4.69 | 15.63 | 0.098 | 1.690 |
| 2 | Y = 8.5973X + 24.3984 | 0.9999 | 1.03 | 3.43 | 0.021 | 0.144 |
| 3 | Y = 3.1778X + 2.6354 | 0.9998 | 2.13 | 7.10 | 0.140 | 0.276 |
| 4 | Y = 12.8099X + 61.2355 | 0.9969 | 1.04 | 3.47 | 0.037 | 0.176 |
| 5 | Y = 7.2502X − 33.5294 | 0.9942 | 1.80 | 6.00 | 0.039 | 0.099 |
| 6 | Y = 6.0380X + 6.2415 | 0.9996 | 0.84 | 2.68 | 0.176 | 0.088 |
| 7 | Y = 6.1333X − 10.0885 | 0.9988 | 1.69 | 5.63 | 0.082 | 2.747 |
| 8 | Y = 7.3958X − 24.749 | 0.9993 | 2.84 | 9.46 | 0.060 | 2.525 |
X: Concentration of compound 1–8 (μg/mL), Y: area under the curve.
The results of precision tests (Table 1) indicate that the developed method is reproducible. All results demonstrated that this HPLC method is precise, reproducible and sensitive for analyzed compounds 1–8.
Afterwards, the roots and aerial parts of eleven different species of Scorzonera and two different species of Podospermum were subjected to extraction using n-hexane. The extracts were analyzed using the validated HPLC method to determine the triterpene profile and the amount of each of these triterpene aglycones (Figure 2). As shown in corresponding chromatograms presented in the Supplementary Materials, the compounds of interest were well separated in most cases (Figures S13–S38). Relatively high concentrations of taraxasteryl acetate (1), lupeol (7), and lupeol acetate (8) were found in the extracts of all species (with the exception of 1 in S. acuminata) and these compounds can therefore be referred to as major triterpenoid components of the Scorzonera species analyzed (Table 2). This HPLC method also enabled the qualitative and quantitative determination of 2, which had previously been isolated from S. latifolia only. The minor Scorzonera triterpenes, 3β-hydroxy-fern-7-en-6-one acetate (2), urs-12-en-11-one-3-acetyl (3), 3β-hydroxy-fern-8-en-7-one acetate (4), and olean-12-en-11-one-3-acetyl (5), were detected, mostly in small amounts, as shown in Table 2. Although urs-12-en-11-one-3-acetyl (3) and 3β-hydroxy-fern-8-en-7-one acetate (4) were detected in the majority of the extracts, it was not possible to quantify them, even under optimal conditions.
Figure 2.
Structures of the triterpenoids 1–8.
Table 2.
Quantification of triterpenoids 1–8.
| Species | Root or Aerial Part | Compound Content (µg·g−1; Calculated for Dry Weight of Plant Material) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total Content | ||
| P. canum (syn. S. cana var. jacquiniana) | R | 719 ± 3 | n.d. | tr. | n.d. | tr. | 920 ± 11 | 932 ± 2 | 4273 ± 12 | 6844 |
| AE | 81 ± 3 | n.d. | tr. | n.d. | tr. | 442 ± 5 | 932 ± 2 | 535 ± 4 | 1991 | |
| P. laciniatum (syn. S. laciniata subsp. laciniata) | R | 276 ± 3 | n.d. | tr. | n.d. | tr. | 146 ± 4 | 447 ± 2 | 3212 ± 13 | 4081 |
| AE | 69 ± 5 | n.d. | tr. | n.d. | tr. | 209 ± 3 | 1025 ± 6 | 892 ± 2 | 2195 | |
| S. acuminata | R | n.d. | n.d. | n.d. | n.d. | n.d. | 1646 ± 10 | 512 ± 1 | 297 ± 1 | 2456 |
| AE | n.d. | n.d. | n.d. | n.d. | n.d. | 1102 ± 6 | 327 ± 5 | 67 ± 1 | 1496 | |
| S. cinerea | R | 2171 ± 6 | 65 ± 1 | tr. | tr. | 115 ± 1 | 3221 ± 13 | 1073 ± 6 | 3645 ± 8 | 10,290 |
| AE | 417 ± 11 | tr. | tr. | tr. | tr. | 309 ± 2 | 1174 ± 16 | 839 ± 6 | 2738 | |
| S. eriophora | R | 3212 ± 17 | 20 ± 1 | tr. | tr. | tr. | n.d. | 244 ± 7 | 2195 ± 7 | 5672 |
| AE | 545 ± 5 | tr. | tr. | tr. | tr. | n.d. | 228 ± 6 | 368 ± 1 | 1142 | |
| S. incisa | R | 1191 ± 5 | n.d. | tr. | tr. | 151 ± 1 | n.d. | 283 ± 2 | 736 ± 10 | 2362 |
| AE | 280 ± 10 | n.d. | tr. | n.d. | n.d. | 644 ± 2 | 1090 ± 2 | 236 ± 9 | 2250 | |
| S. latifolia | R | 4201 ± 16 | 50 ± 1 | tr. | tr. | 135 ± 1 | n.d. | 213 ± 2 | 2261 ± 94 | 6861 |
| AE | 1062 ± 2 | 18 ± 1 | tr. | tr. | tr. | 827 ± 2 | 1538 ± 1 | 607 ± 1 | 4051 | |
| S. mirabilis | R | 2099 ± 4 | tr. | n.d. | tr. | n.d. | n.d. | 224 ± 1 | 1356 ± 2 | 3678 |
| AE | 1262 ± 728 | tr. | tr. | tr. | tr. | n.d. | 954 ± 14 | 998 ± 13 | 3214 | |
| S. mollis subsp. szowitsii | R | 3791 ± 14 | n.d. | tr. | tr. | tr. | 609 ± 6 | 282 ± 11 | 1244 ± 1 | 5926 |
| AE | 263 ± 4 | n.d. | tr. | n.d. | n.d. | 246 ± 8 | 321 ± 1 | 149 ± 7 | 979 | |
| S. parviflora | R | 811.96 ± 4 | n.d. | tr. | tr. | tr. | n.d. | 132 ± 4 | 711 ± 3 | 1656 |
| AE | 433 ± 2 | n.d. | tr. | tr. | tr. | n.d. | 649 ± 6 | 594 ± 5 | 1676 | |
| S. suberosa subsp. suberosa | R | 2340 ± 6 | n.d. | tr. | tr. | tr. | n.d. | 342 ± 4 | 1261 ± 5 | 3943 |
| AE | 535 ± 4 | n.d. | tr. | n.d. | tr. | n.d. | 1005 ± 17 | 312 ± 4 | 1853 | |
| S. sublanata | R | 4981 ± 2 | 35 ± 1 | tr. | tr. | tr. | n.d. | 415 ± 1 | 3920 ± 8 | 9351 |
| AE | 338 ± 6 | tr. | tr. | tr. | n.d. | n.d. | 169 ± 1 | 302 ± 1 | 809 | |
| S. tomentosa | R | 3168 ± 12 | 47 ± 1 | tr. | tr. | 187 ± 1 | 969 ± 11 | 564 ± 2 | 2502 ± 7 | 7435 |
| AE | 376 ± 13 | tr. | tr. | tr. | tr. | tr. | 509 ± 2 | 411 ± 1 | 1296 | |
Taraxasteryl acetate (1), α-amyrin (6), lupeol (7), lupeol acetate (8) (at 200 nm); and of 3β-hydroxy-fern-7-en-6-one-acetate (2), urs-12-en-11-one-3-acetyl (3), 3β-hydroxy-fern-8-en-7-one-acetate (4), and olean-12-en-11-one-3-acetyl (5) (at 240 nm) measured in μg·g−1. R, root; AE, aerial part; tr., traces (<LOQ level); n.d., not detected. The value was calculated as average of three independent measurements, with SD. Total content of triterpenes was calculated as a sum of amounts for compound 1–8.
Compounds 1, 7, and 8 were found in almost all of the extracts of both the aerial parts and the roots tested. The highest content of 1 was detected in the extract of the root of S. sublanata (4981 ± 2 µg·g−1), of 7 (1538± 1 µg·g−1) in the extract of the aerial parts of S. latifolia, and of 8 (4273 ± 12 µg·g−1) in the extract of the root of P. canum. Relatively high, but varying amounts of α-amyrin (6) were determined in Scorzonera species, as can be seen in Table 2. The highest content of 6 was determined to be the 3221 ± 13 µg·g−1 in S. cinerea root extract, and this, together with the high content of 8 (3645 ± 8 µg·g−1), 7 (1073 ± 6 µg·g−1), and 1 (2171 ± 6 µg·g−1), showed that the root of this Scorzonera species has the richest content of the triterpene aglycones monitored. The lowest triterpenoid content was determined in S. sublanata aerial parts with 338 ± 6 µg·g−1, 169 ± 1 µg·g−1, and 302 ± 1 µg·g−1 for compound taraxasteryl acetate (1), lupeol (7) and lupeol acetate (8), respectively. Total triterpenoid contents of the roots of investigated species were found to be higher than aerial parts. S. acuminata roots and aerial parts did not contain taraxasteryl acetate (1) and lupeol acetate (8) was detected in low amount (297 ± 1 µg·g−1 and 67 ± 1 µg·g−1). On the other hand, content of α-amyrin (6) was determined in relatively high amount as 1646 ± 10 µg·g−1 and 1102 ± 6 µg·g−1 for roots and aerial parts of S. acuminata, respectively.
3. Discussion
To our best knowledge, this is the first report of triterpenes in P. canum, P. laciniatum, S. acuminata, S. eriophora, S. incisa, S. mirabilis, S. mollis, S. parviflora, S. suberosa, and S. sublanata. Different triterpenes were previously isolated from other Scorzonera species: oleanane and ursane type from S. austriaca [18], and S. mongolica [17]; dammarane and tirucallane triterpenes from S. divaricata [13]; and β-amyrin, methyl oleate and methyl ursolate from S. undulata subsp. deliciosa [16]. Jehle et al. [12] described 3α-hydroxyolean-5-ene, lupeol (7), and magnificol in S. aristata. S. mongolica is a source of erythrodiol and moradiol (oleane derivatives) [17]. We here revealed that all tested Podospermum and Scorzonera species contain taraxasteryl acetate (1) except of S. acuminata. All Podospermum and Scorzonera species investigated here were also found to contain 7 and 8 in varying amounts. α-Amyrin (1), olean-12-en-11-one-3-acetyl (5), urs-12-en-11-one-3-acetyl (3), and two fernane derivatives 3β-hydroxy-fern-7-en-6-one-acetate (2), and 3β-hydroxy-fern-8-en-7-one-acetate (4) were detected here in varying amounts depending on the Podospermum and Scorzonera species as minor components. The method used allowed us to simply identify and quantify main triterpenic compounds in all Podospermum and Scorzonera species tested, and accordingly would be useful for work on other species.
Triterpenes are compounds distributed broadly in plant kingdom, with approximately 200 different skeletons showing great variability and diversity of triterpene metabolism [22]. Hill and Connoly reviewed the progress in triterpene isolation from 2012 [23]. More than 700 different triterpenes were described in this review, showing number of plant species as sources of various triterpenes and enormous progress in area of triterpene phytochemistry. Some triterpenoids can possess chemotaxonomic importance, as shown for example for triterpenes isolated from Conifers [24]. Interestingly, comparison of triterpenes which are present in soil and sediments with literature survey of triterpenes in Asteraceae showed the possibility of chemosystematic usage of some acetylated triterpenes [25]. Our analysis identified five types of triterpenes: taraxastane (1), ursanes (3 and 6), oleananes (5), lupanes (7 and 8), and fernanes (2 and 4), with six of the eight isolated compounds acetylated at the position 3 of the skeleton. However, the number of profiled species and identified compounds is still low.
Some attempts to give an overview of phytochemicals and chemosystematic analysis of Asteraceae were performed in the past, with a focus on presence of sesquiterpenic lactones, pyrrolizidine alkaloids, and polyacetylenes, and on the occurrence of in general highly oxidized compounds, which could be a structural feature shared by the majority of the Asteraceae chemicals [26,27]. Further studies showed phenolics as possible chemosystematic markers for the Asteraceae family (Cichoriae tribe) and for the Scorzonerinae subtribe in particular [28,29]. The work of Calabria [26] also tried to evaluate the presence of triterpenes in Asteraceae, showing their presence in 28 of 35 tribes of this family that time analyzed, 19 occurrences in Cichorieae. However, the information about the presence of triterpenes, and even the specific compounds, is still limited. Therefore, a reliable method for routine extraction and chromatographic analysis of triterpenic profile would be valuable not only for taxonomic evaluation of Podospermum and Scozonera, but also for other Cichorieae taxa and Asteraceae in general.
In Turkey and in some European countries, Scorzonera species are used mainly as a vegetable food [30,31]. However, the ethno-medicinal importance of the genus in Turkish, European, Chinese, Mongolian, and Libyan folk medicines has been reported [6,11,32,33,34]. Turkish folk medicine uses Scorzonera preparations to treat a variety of illnesses, including inflammation [35]. Triterpenic compounds are, besides inflammation, often connected with cytotoxicity and anticancer potential, as reviewed, for example, for lupeol derivatives [36,37,38,39,40,41]. For other triterpenes, cytotoxic triterpenes have previously been isolated from S. divaricata and S. hispanica. Furthermore, analgesic, anti-inflammatory, and wound healing activities of Scorzonera species have been reported by in vivo tests [15,42,43,44,45,46]. Some compounds responsible for the analgesic activity have been isolated by bioassay-guided fractionation from n-hexane extract of S. latifolia and identified as taraxasteryl acetate (1), taraxasteryl myristate [15], motiol and β-sitosterol [43]. n-hexane extract displayed higher activity than these isolated compounds, therefore analgesic activity of the Scorzonera extracts is suggested from possible synergistic interaction of the other triterpenes [15,45,46,47,48]. The same could be valid for anti-inflammatory activity of Scorzonera, and taraxasteryl acetate (1) [47,48], α-amyrin (6) [49,50], lupeol (7) [51], and lupeol acetate (8) [52], as visible from previously published studies anti-inflammatory active, could contribute to the antiphlogistic effect of Scorzonera species. Lupeol (7), which is relatively commonly found in several plant species, is reported to exhibit many kinds of biological activities, including anticancer, antiprotozoal, chemopreventive, and anti-inflammatory activities [53]. The anti-inflammatory activity of 7 is reportedly accompanied by immune modulatory and antitumor properties [39,41,51]. Compound 8 has demonstrated anti-inflammatory activity by regulating TNF-α and IL-2 specific mRNA and up-regulating the synthesis of IL-10 mRNA [40]. Taraxasteryl acetate (1), which is identified in all Scorzonera species investigated in concentrations ranging between 66.52 ± 1.0 and 4272.63 ± 11.61 µg·g−1, has been reported to have anti-inflammatory and analgesic activities [43,48]. Analgesic, anti-inflammatory, and wound healing activities of Scorzonera species have been reported by in vivo tests [15,42,44,45,46,47]. Some compounds responsible for the analgesic activity have been isolated by bioassay-guided fractionation from n-hexane extract of S. latifolia and identified as taraxasteryl acetate (1), taraxasteryl myristate [15], motiol and β-sitosterol [44] n-hexane extract displayed higher activity than these isolated compounds, therefore analgesic activity of the Scorzonera extracts is suggested from possible synergistic interaction of the other triterpenes [15,44]. According to the current study results, triterpene content of the S. tomentosa is found to be higher than S. latifolia which is followed by S. mollis subsp. mollis and S. suberosa ssp. suberosa roots. Analgesic activities of these mentioned species seem to be correlated with their triterpene contents, as we reported in our previous studies. S. tomentosa, S. latifolia, S. mollis subsp. mollis and S. suberosa ssp. suberosa roots displayed antinociceptive activities in acetic acid induced writhing test [47]. Therefore, these results encourage us to conduct further investigation, testing analgesic and anti-inflammatory activities of the species characterized by higher triterpene content, such as S. cinerea, S. sublanata, and S. cana var. jacquiniana.
4. Materials and Methods
4.1. Plant. Material
Podospermum and Scorzonera species were collected in different parts of Turkey. The plants were collected during flowering period, in eleven specimens. The taxonomic identification of the plants was confirmed by Prof. Hayri Duman, a plant taxonomist at the Department of Biological Sciences, Faculty of Sciences, Gazi University, Ankara, Turkey. Flora of Turkey and The East Aegean Islands was used for identification [54]. Voucher specimens were placed in the herbarium at the Faculty of Pharmacy of Ankara University (Table 3). The pictures of collected plant materials are available in the Supplementary Materials (Figures S1–S12). Basic rules for consistent characterization and documentation of plant source materials were followed [55].
Table 3.
Location of plant sample collection and the corresponding voucher specimen number.
| Plant Species of the Genus Scorzonera | Collection Locality and Coordinates | Herbarium No. |
|---|---|---|
| P. canum C. A. Meyer, (syn. S. cana (C.A. Meyer) Hoffm. var. jacquiniana (W. Koch) Chamberlain) | Ankara, Çamlıdere N 40°29′15.695″ E 32°28′9.862″ |
AEF 23834 |
| P. laciniatum (L.) DC. (syn. S. laciniata L. subsp. laciniata) | Ankara, Çamlıdere N 40°29′15.695″ E 32°28′9.862″ |
AEF 23835 |
| S. acuminata Boiss. | Çankırı, Yumaklı Village N 40°26′7.103″ E 32°45′41.665″ |
AEF 25938 |
| S. cinerea Boiss. | Sivas, Çetinkaya N 39°15′26.566″ E 37°38′7.844″ |
AEF 23829 |
| S. eriophora DC. | Ankara, Çubuk N 40°14′13.492″ E 33°01′52.513″ |
AEF 23832 |
| S. incisa DC. | Konya, Ermenek N 36°38′3.351″ E 32°53′32.567″ |
AEF 23833 |
| S. latifolia (Fisch. & Mey.) DC. | Kars, Arpaçay N 40°54′57.305″ E 43°21′2.969″ |
AEF 23830 |
| S. mirabilis Lipschitz | Van N 38°29′59.3412″ E 43°22′41.3148″ |
F 18386 |
| S. mollis Bieb. subsp. szowitsii (DC.) Chamberlain | Ankara, Kızılcahamam N 40°26′49.009″ E 32°37′6.269″ |
AEF 23844 |
| S. parviflora Jacq. | Ankara, Gölbaşı N 39°48′19.8″ E 32°48′10.799″ |
AEF 25894 |
| S. suberosa C. Koch subsp. suberosa | Kayseri, Pınarbaşı N 38°42′55.868″ E 36°24′26.345″ |
AEF 23843 |
| S. sublanata Lipschitz | Ankara, Kızılcahamam N 39°39′43.223″ E 35°51′40.547″ |
AEF 25937 |
| S. tomentosa L. | Yozgat, Akdağmadeni N 40°28′13.253″ E 32°39′0.73″ |
AEF 23841 |
4.2. HPLC Analysis
4.2.1. Optimization of Sample Extraction Procedure and Preparation of Samples
Air-dried and powdered aerial parts and roots (1 g for each) (homogenized mixtures of ten specimens) of the selected Podospermum and Scorzonera species (Table 3) were used for the extraction procedures. n-Hexane (50 mL per 1 g of plant material), petroleum ether, chloroform, and diethylether were tested for extraction of plant material. All prepared extracts were analyzed by HPLC and n-hexane was found to be a more suitable solvent than other solvents for extracting the triterpene aglycones found in Scorzonera species because the areas of peaks were greater than petroleum ether, and the selectivity was better than that of chloroform and diethylether, which allowed us to extract some flavonoids and isocoumarins complicating the chromatogram evaluation. Furthermore, triterpenes in chloroform and diethylether extracts were observed only as minor components. After selection of solvent, temperature was used as variable factor affecting extraction. Tests at 24 °C (room temperature), 50 °C, and 69 °C (the boiling temperature of n-hexane) were used to determine the most suitable conditions for the extraction procedure. n-Hexane at room temperature (24 °C) allowed us to extract the triterpenes more selectively than at higher temperatures. The extraction time was set to 8 h, using continuous stirring. Finally, 50 mL of n-hexane were used to prepare extract (1 g of plant material). Each prepared extract was later evaporated to dryness and the residual solids were dissolved in isopropanol (Merck, Darmstadt, Germany) and adjusted into 10 mL volumetric flasks. Each solution was filtered through a 0.45 μm membrane filter before injection.
4.2.2. Optimization of Conditions for HPLC Analysis
An HPLC method was developed to analyze the triterpenoids in Scorzonera extracts. An Agilent model LC 1100 chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a DAD (diode array detector) was used. The DAD was set to a wavelength of 200 or 240 nm. The chromatograms were analyzed and the peak areas integrated automatically using Agilent ChemStation Software.
Waters Spherisorb S5W normal-phase (25 cm × 4 mm, 5 µm), Supelcosil C18 reversed-phase (25 cm × 4 mm, 5 µm), and ACE 5 C8 reversed-phase (25 cm × 4.6 mm, 5 μm) columns were tested to obtain optimal separation. Methanol (HiPerSolv Chromanorm 20,864.320, VWR, Leuven, Belgium), acetonitrile (Merck 1.00030.2500, Darmstadt, Germany), and water (Extra pure water obtained from Millipore Milli Q Gradient A10, Milford, MA, USA) were used in different proportions as components of the mobile phase. Series of experiments showed the optimal separation to be achieved by using the ACE 5 C8 column with acetonitrile water gradient elution, with water (A) and acetonitrile (B) in a linear gradient elution: the initial composition at time 0 A:B 20:80 (v/v), after 70 min of following a linear gradient, was changed to A:B 6:94 (v/v), and after 70 min 100% B to wash column. The flow rate was 0.8 mL·min−1. The column temperature was maintained at 40 °C, and the sample injection volume was 10 μL.
4.2.3. Preparation and Calibration of Standard Solutions
Stock solutions of compounds 1–5 obtained from Scorzonera species [15,56] and 6–8 (from Sigma-Aldrich, St. Louis, MI, USA) were weighed and dissolved in isopropanol (Merck, Darmstadt, Germany). The purity of isolated compounds was determined from HPLC analysis (>98%) and 1H NMR analysis. Six different concentrations of each compound were prepared in the following ranges: 16.5-330 µg·mL−1 for taraxasteryl acetate (1), 8–160 µg·mL−1 for 3β-hydroxy-fern-7-en-6-one acetate (2), 26–520 µg·mL−1 for urs-12-en-11-one-3-acetyl (3), 12.5–250 µg·mL−1 for 3β-hydroxy-fern-8-en-7-one acetate (4), 25–500 µg·mL−1 for olean-12-en-11-one-3-acetyl (5), 11.5–230 µg·mL−1 for α-amyrin (6), 13–260 µg·mL−1 for lupeol (7), and 22–440 µg·mL−1 for lupeol acetate (8). Injections of 10 µL were performed in triplicate for each concentration of each standard solution. The area of the peak resulting from each injection was plotted against the known concentration of the substance to obtain the calibration curve.
4.2.4. Validation Procedure
Limits of Detection and Quantification
Standard HPLC validation procedures [57] were used to determine the limits of detection and quantification (LOD and LOQ), respectively. The LOD and LOQ were established at signal to noise ratios (S/N) of 3 and 10, respectively (Table 1). The LOD and LOQ concentrations were verified experimentally by repeating each analysis six times.
Precision
Intra-day precision tests were performed by analyzing the same standard solutions of all compounds at the LOQ level six times in a single day. Inter-day precision tests were performed by analyzing standard solutions at three different concentrations on three different days, respectively. The results of precision tests (Table 1) indicate that the developed method is reproducible. All results demonstrated that this HPLC method is precise, reproducible and sensitive.
5. Conclusions
An HPLC method for the identification and quantification of the triterpenes found in species of the genus Scorzonera was developed in the current study. This method allows establishing fingerprint chromatograms of n-hexane extracts of Scorzonera and Podospermum and quantifying taraxasteryl acetate (1), α-amyrin (6), lupeol (7), and lupeol acetate (8) as major triterpenes, and 3β-hydroxy-fern-7-en-6-one acetate (2), urs-12-en-11-one-3-acetyl (3), 3β-hydroxy-fern-8-en-7-one acetate (4), and olean-12-en-11-one-3-acetyl (5) as minor triterpenes. The amounts of the triterpenes, especially α-amyrin (6), lupeol (7), lupeol acetate (8), and taraxasteryl acetate (1), found in the different species could correlate with the analgesic and anti-inflammatory activity previously observed for preparations made from these plants. Further studies confirming this correlation are necessary.
Acknowledgments
Special thanks to Frank Thomas Campbell for language editing of the manuscript.
Supplementary Materials
The following are available online, Figures S1–S12: Pictures of voucher specimens, Figures S13–S38: Chromatograms of analyzed extracts.
Author Contributions
Conceptualization, Ö.B.-A. and K.Š.; Investigation, S.D.A. and F.Ö.; Methodology, Ö.B.-A.; Project administration, V.R. and K.Š.; Resources, Ö.B.-A.; Supervision, V.S.; Validation, S.Ö., G.S.-İ. and V.R.; Writing—original draft, Ö.B.-A. and K.Š.; and Writing—review and editing, Ö.B.-A., G.S.-İ. and V.S.
Funding
Authors are thankful for the support of IGA UVPS Brno 320/2016/FaF.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–8 are available from the authors.
References
- 1.Coskuncelebi K., Makbul S., Gultepe M., Okur S., Guzel M.E. A conspectus of Scorzonera s.l. in Turkey. Turk. J. Bot. 2015;39:76–87. doi: 10.3906/bot-1401-10. [DOI] [Google Scholar]
- 2.Paraschos S., Magiatis P., Kalpoutzakis E., Harvala C., Skaltsounis A.L. Three new dihydroisocoumarins from the Greek endemic species Scorzonera cretica. J. Nat. Prod. 2001;64:1585–1587. doi: 10.1021/np0103665. [DOI] [PubMed] [Google Scholar]
- 3.Çitoğlu G.S., Bahadir Ö., Dall’Acqua S. Dihydroisocoumarin derivatives isolated from the roots of Scorzonera latifolia. Turk. J. Pharm. Sci. 2010;7:205–212. [Google Scholar]
- 4.Zidorn C., Ellmerer-Müller E.P., Stuppner H. Tyrolobibenzyls-novel secondary metabolites from Scorzonera humilis L. Helv. Chim. Acta. 2000;83:2920–2925. doi: 10.1002/1522-2675(20001108)83:11<2920::AID-HLCA2920>3.0.CO;2-5. [DOI] [Google Scholar]
- 5.Zidorn C., Spitaler R., Ellmerer-Müller E.P., Perry N.B., Gerhauser C., Stuppner H. Structure of tyrolobibenzyl D and biological activity of tyrolobibenzyls from Scorzonera humilis L. Z. Naturforsch. 2002;57:614–619. doi: 10.1515/znc-2002-7-811. [DOI] [PubMed] [Google Scholar]
- 6.Zidorn C., Ellmerer E.P., Sturm S., Stuppner H. Tyrolobibenzyls E and F from Scorzonera humilis and distribution of caffeic acid derivatives, lignans and tyrolobibenzyls in European taxa of the subtribe Scorzonerinae (Lactuceae, Asteraceae) Phytochemistry. 2003;63:61–67. doi: 10.1016/S0031-9422(02)00714-8. [DOI] [PubMed] [Google Scholar]
- 7.Menichini F., Statti G. Flavonoid glycosides from Scorzonera columnae. Fitoterapia. 1994;65:555–556. [Google Scholar]
- 8.Tsevegsuren N., Edrada R.A., Lin W., Ebel R., Torre C., Ortlepp S., Wray V., Proksch P. Four new natural products from Mongolian medicinal plants Scorzonera divaritaca and S. Pseudodivaricata (Asteraceae) Planta Med. 2007;72:962–967. doi: 10.1021/np070013r. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y., Guo Q.S., Wang G.S. Flavonoid glycosides and their derivatives from the Herbs of Scorzonera austriaca Wild. Molecules. 2016;21:803. doi: 10.3390/molecules21060803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khobrakova V.B., Nikolaev S.M., Tolstikhina V.V., Semenov A.A. Immunomodulating properties of lignin glucoside from cultivated cells of Scorzonera hispanica. Pharm. Chem. J. 2003;37:10–11. doi: 10.1023/A:1026359206059. [DOI] [Google Scholar]
- 11.Wang Y., Edrada-Ebel R., Tseveqsuren N., Sendker J., Braun M., Wray V., Lin W., Proksch P. Dihydrostilbene Derivatives from the Mongolian Medicinal Plant Scorzonera radiata. J. Nat. Prod. 2009;72:671–675. doi: 10.1021/np800782f. [DOI] [PubMed] [Google Scholar]
- 12.Jehle M., Bano J., Ellmerer E.P., Zidorn C. Natural products from Scorzonera aristata (Asteraceae) Nat. Prod. Commun. 2010;5:725–727. [PubMed] [Google Scholar]
- 13.Yang Y.J., Yao J., Jin X.J., Shi Z.N., Shen T.F., Fang J.G., Yao X.J., Zhu Y. Sesquiterpenoids and tirucallane triterpenoids from the roots of Scorzonera divaricata. Phytochemistry. 2016;124:86–98. doi: 10.1016/j.phytochem.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Öksüz S., Gören N., Ulubelen A. Terpenoids from Scorzonera tomentosa. Fitoterapia. 1990;61:92–93. [Google Scholar]
- 15.Bahadir Ö., Citoglu G.S., Smejkal K., Dall’Acqua S., Ozbek H., Cvacka J., Zemlicka M. Analgesic compounds from Scorzonera latifolia (Fisch. and Mey.) DC. J. Ethnopharmacol. 2010;131:83–87. doi: 10.1016/j.jep.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Brahim H., Salah A., Bayet C., Laouer H., Dijoux-Franca M.-G. Evaluation of antioxidant activity, free radical scavenging and cuprac of two compounds isolated from Scorzonera undulata ssp. deliciosa. Adv. Environ. Biol. 2013;7:591–594. [Google Scholar]
- 17.Wang B., Li G.Q., Guan H.S., Yang L.Y., Tong G.Z. A new erythrodiol triterpene fatty ester from Scorzonera mongolica. Acta Pharm. Sin. 2009;44:1258–1261. [PubMed] [Google Scholar]
- 18.Wu Q.X., Su Y.B., Zhu Y. Triterpenes and steroids from the roots of Scorzonera austriaca. Fitoterapia. 2011;82:493–496. doi: 10.1016/j.fitote.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Patočka J. Biologically active pentacyclic triterpenes and their current medicine signification. J. Appl. Biomed. 2003;1:7–12. [Google Scholar]
- 20.Zwenger S., Basu C. Plant terpenoids: Applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008;3:1–7. [Google Scholar]
- 21.Thoppil R.S., Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011;3:228–249. doi: 10.4254/wjh.v3.i9.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu R., Fazio G.C., Matsuda S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry. 2004;65:261–291. doi: 10.1016/j.phytochem.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Hill R.A., Connolly J.D. Triterpenoids. Nat. Prod. Rep. 2015;32:237–327. doi: 10.1039/C4NP00101J. [DOI] [PubMed] [Google Scholar]
- 24.Otto A., Wilde V. Sesqui-, Di-, and Triterpenoids as Chemosystematic Markers in Extant Conifers—A Review. Bot. Rev. 2001;67:141–238. doi: 10.1007/BF02858076. [DOI] [Google Scholar]
- 25.Lavrieux M., Jacob J., LeMilbeau C., Zocatelli R., Masuda K., Breheret J.G., Disnar J.R. Occurrence of triterpenyl acetates in soil and their potential as chemotaxonomical markers of Asteraceae. Org. Geochem. 2011;42:1315–1323. doi: 10.1016/j.orggeochem.2011.09.005. [DOI] [Google Scholar]
- 26.Calabria L.M. Ph.D. Thesis. The University of Texas at Austin; Austin, TX, USA: 2008. The Isolation and Characterization of Triterpene Saponins from Silphium and the Chemosystematic and Biological Significance of Saponins in the Asteraceae. [Google Scholar]
- 27.Gemeinholzer B., Granica S., Moura M., Teufel L., Zidorn C. Leontodon x grassiorum (Asteraceae, Cichorieae), a newly discovered hybrid between an Azorean and a mainland European taxon: Morphology, molecular characteristics, and phytochemistry. Biochem. Syst. Ecol. 2017;72:32–39. doi: 10.1016/j.bse.2017.04.001. [DOI] [Google Scholar]
- 28.Sareedenchai V., Zidorn C. Flavonoids as chemosystematic markers in the tribe Cichorieae of the Asteraceae. Biochem. Syst. Ecol. 2010;38:935–957. doi: 10.1016/j.bse.2009.09.006. [DOI] [Google Scholar]
- 29.Granica S., Zidorn C. Phenolic compounds from aerial parts as chemosystematic markers in the Scorzonerinae (Asteraceae) Biochem. Syst. Ecol. 2014;58:102–113. doi: 10.1016/j.bse.2014.11.005. [DOI] [Google Scholar]
- 30.Bohm B.A., Stuessy T.F. Flavonoids of the Sunflower Family (Asteraceae) Springer; Wien, Austria: 2007. [Google Scholar]
- 31.Hamzaoğlu E., Aksoy A., Martin E., Pinar N.M., Colgecen H. A new record for the flora of Turkey: Scorzonera ketzkhovelii Grossh. (Asteraceae) Turk. J. Bot. 2010;34:57–61. [Google Scholar]
- 32.Jiang T.F., Wang Y.H., Lv Z.H., Yue M.E. Determination of kava lactones and flavonoid glycoside in Scorzonera austriaca by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 2007;43:854–858. doi: 10.1016/j.jpba.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y., Wu Q.-X., Hu P.-Z., Wu W.-S. Biguaiascorzolides A and B: Two novel dimeric guaianolides with a rare skeleton, from Scorzonera austriaca. Food Chem. 2009;114:1316–1320. doi: 10.1016/j.foodchem.2008.11.009. [DOI] [Google Scholar]
- 34.Granica S., Lohwasser U., Joehrer K., Zidorn C. Qualitative and quantitative analyses of secondary metabolites in aerial and subaerial of Scorzonera hispanica L. (black salsify) Food Chem. 2015;173:321–331. doi: 10.1016/j.foodchem.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Yıldırım B., Terz oglu O., Ozgokce F., Turkozu D. Ethnobotanical and pharmacological uses of some plants in the districts of Karpuzalan and Adıgüzel (Van-Turkey) J. Anim. Vet. Adv. 2008;7:873–878. [Google Scholar]
- 36.Setzer W.N., Setzer M.C. Plant-derived triterpenoids as potential antineoplastic agents. Mini-Rev. Med. Chem. 2003;3:540–556. doi: 10.2174/1389557033487854. [DOI] [PubMed] [Google Scholar]
- 37.Paduch R., Kandefer-Szerszen M. Antitumor and Antiviral Activity of Pentacyclic Triterpenes. Mini-Rev. Org. Chem. 2014;11:262–268. doi: 10.2174/1570193X1103140915105240. [DOI] [Google Scholar]
- 38.Zielinska S., Matkowski A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae) Phytochem. Rev. 2014;13:391–416. doi: 10.1007/s11101-014-9349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallo M.B.C., Sarachine M.J. Biological activities of lupeol. Int. J. Biomed. Pharm. Sci. 2009;3:46–66. [Google Scholar]
- 40.Lucetti D.L., Lucetti E.C.P., Bandeira A.M., Veras H.N.H., Silva A.H., Leal L.K.A.M., Lopes A.A., Alves V.C.C., Silva G.S., Brito G.A., et al. Anti-inflammatory effects and possible mechanism of action of lupeol acetate isolated from Himatanthus drasticus (Mart.) Plumel. J Inflamm. 2010;7:1–11. doi: 10.1186/1476-9255-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wal P., Wal A., Sharma G., Rai A.K. Biological activities of lupeol. Syst. Rev. Pharm. 2011;2:96–103. doi: 10.4103/0975-8453.86298. [DOI] [Google Scholar]
- 42.Bahadir Ö., Citoglu G.S., Smejkal K., Dall´Acqua S., Ozbek H., Cvacka J., Zemlicka M. Antinociceptive activity of some Scorzonera L. Species. Turk. J. Med. Sci. 2012;42:861–866. [Google Scholar]
- 43.Bahadir Ö., Citoglu G.S., Smejkal K., Dall’Acqua S., Ozbek H., Cvacka J., Zemlicka M. Bioassay-guided isolation of the antinociceptive compounds motiol and beta-sitosterol from Scorzonera latifolia root extract. Pharmazie. 2014;69:711–714. [PubMed] [Google Scholar]
- 44.Akkol E.K., Acikara O.B., Suntar I., Citoglu G.S., Keles H., Ergene B. Enhancement of wound healing by topical application of Scorzonera species: Determination of the constituents by HPLC with new validated reverse phase method. J. Ethnopharmacol. 2011;137:1018–1027. doi: 10.1016/j.jep.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 45.Süntar İ., Acikara O.B., Citoglu G.S., Keles H., Ergene B., Akkol E.K. In vivo and in vitro evaluation of the therapeutic potential of some Turkish Scorzonera species as wound healing agent. Curr. Pharm. Des. 2012;18:1421–1433. doi: 10.2174/138161212799504867. [DOI] [PubMed] [Google Scholar]
- 46.Küpeli Akkol E., Bahadir Acikara O., Suntar I., Ergene B., Saltan Citoglu G. Ethnopharmacological evaluation of some Scorzonera species: In vivo anti-inflammatory and antinociceptive effects. J. Ethnopharmacol. 2012;140:261–270. doi: 10.1016/j.jep.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Sing B., Ram S.N., Pandey B., Joshi V.K., Gambhir S.S. Studies on antiinflammatory activity of taraxasterol acetate from Echinops echinatus in rats and mice. Phytother. Res. 1991;5:103–106. doi: 10.1002/ptr.2650050303. [DOI] [Google Scholar]
- 48.Perez-Garcia F., Marin E., Parella T., Adzet T., Canigueral S. Activity of taraxasteryl acetate on inflammation and heat shock protein synthesis. Phytomedicine. 2005;12:278–284. doi: 10.1016/j.phymed.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho K.M., de Melo T.S., de Melo K.M., Quindere A.L., de Oliveira F.T., Viana A.F., Nunes P.I., Quetz J.S., Viana D.A., da Silva A.A., et al. Amyrins from Protium heptaphyllum Reduce High-Fat Diet-Induced Obesity in Mice via Modulation of Enzymatic, Hormonal And Inflammatory Responses. Planta Med. 2017;83:285–291. doi: 10.1055/s-0042-114222. [DOI] [PubMed] [Google Scholar]
- 50.Vitor C.E., Figueiredo C.P., Hara D.B., Bento A.F., Mazzuco T.L., Calixto J.B. Therapeutic action and underlying mechanisms of a combination of two pentacyclic triterpenes, α- and β-amyrin, in a mouse model of colitis. Br. J. Pharmacol. 2009;157:1034–1044. doi: 10.1111/j.1476-5381.2009.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai F.-S., Lin L.-W., Wu C.-R. Lupeol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016;929:145–175. doi: 10.1007/978-3-319-41342-6_7. [DOI] [PubMed] [Google Scholar]
- 52.Ashalatha K., Venkateswarlu Y., Priya A.M., Lalitha P., Krishnaveni M., Jayachandran S. Anti inflammatory potential of Decalepis hamiltonii (Wight and Arn) as evidenced by down regulation of pro inflammatory cytokines-TNF-α and IL-2. J. Ethnopharmacol. 2010;130:167–170. doi: 10.1016/j.jep.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 53.Salvador J.A.R. Pentacyclic Triterpenes as Promising Agents in Cancer. Nova Science Publishers; New York, NY, USA: 2010. [Google Scholar]
- 54.Davis P.H., Mill R.R., Tan K. Scorzonera L. Flora of Turkey and East Aegean Islands. Edinburgh. Edinburgh University Press; Edinburgh, UK: 1988. [Google Scholar]
- 55.Zidorn C. Guidelines for consistent characterisation and documentation of plant source materials for studies in phytochemistry and phytopharmacology. Phytochemistry. 2017;139:56–59. doi: 10.1016/j.phytochem.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Acıkara Ö.B., Çitoğlu G.S., Dall’Acqua S., Smejkal K., Cvačka J., Zemlička M. A new triterpene from Scorzonera latifolia (Fisch. and Mey.) DC. Nat. Prod. Res. 2012;26:1892–1897. doi: 10.1080/14786419.2011.625644. [DOI] [PubMed] [Google Scholar]
- 57.Kazakevych Y., Lobrutto R. HPLC for Pharmaceutical Scientists. John Wiley & Sons, Inc.; Hoboken, NY, USA: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.