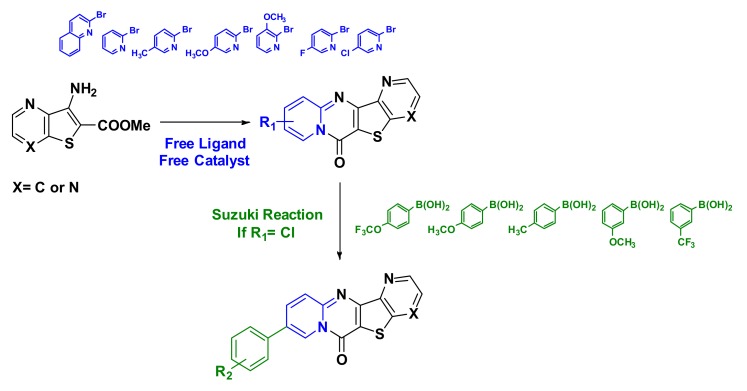
Abstract
We report herein a new metal free synthetic pathway to generate tetracyclic compounds from 3-aminothieno[3,2-b]pyridine-2-carboxylate. To enlarge the molecular diversity, we studied the Suzuki coupling of 9-chloro-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one and several boronic acids were easily introduced.
Keywords: metal-free, thienopyrimidinone, tetracyclic compounds
1. Introduction
Angiogenesis, one of the critical processes that affect the growth and development of cancerous cells, is the generation of new blood vessels from the existing vasculature. It is the key factor in the advancement of various human diseases, including cancer, where it is essential for the growth, spread, and survival of tumors. Angiogenesis is a complex process regulated by multiple growth factors and cytokines. Among these factors, vascular endothelial growth factor (VEGF) is one of the most potent angiogenic factors involved in tumor growth. It stimulates endothelial cell proliferation, migration, and tube formation by binding to its two main receptor tyrosine kinases (RTKs) expressed on endothelial cells, VEGF receptor 1 (VEGFR-1) and VEGF receptor 2 (VEGFR-2) [1]. Thienopyridine derivatives have applications in many areas and have shown interesting biological activities in medicinal chemistry or agricultural chemistry [2,3,4]. Various thieno[3,2-b]pyridine, thieno[2,3-d]pyrimidine and thieno[3,2-d]pyrimidine skeletons have frequently attracted the interest of medicinal chemistry researchers due to their promising anticancer properties. They were shown to be inhibitors of the vascular endothelial growth factor receptor (VEGFR-2) related to angiogenesis and metastasis. These compounds are also involved in cyclin dependent kinase (CDK4) and many other anti-cancer therapies [5]. In this work, we describe the simple and efficient metal free synthesis of several new tetracyclic compounds from 3-aminothieno[3,2-b]pyridine-2-carboxylate (Figure 1) followed by suzuki cross-coupling to enlarge the molecular diversity. The advantages of using transition metal catalysts in synthesis are now widely known, however it is also necessary to take into account certain essential factors for efficient and environmentally friendly syntheses: cost, toxicity, and durability. Our team has focused its research activities on the development of greener methodologies and approaches. In recent years, several studies have been reported in the literature with the demonstration of alternative synthetic routes to obtain sulfur and nitrogen containing heterocycles [6,7]. As far as we know, there are only three descriptions of the synthesis of these tetracyclic structures in the literature, all of which reported moderate yields and involved a Buchwald–Hartwig C-N coupling [8,9,10,11,12] of substituted 3-bromobenzo[b]thiophenes with anilines and amino-pyridines [13], 3-aminobenzo[b]thiophenes with bromopyridines [14].
Figure 1.
Metal free synthesis of several new tetracyclic compounds from 3-aminothieno[3,2-b]pyridine-2-carboxylate.
2. Results and Discussion
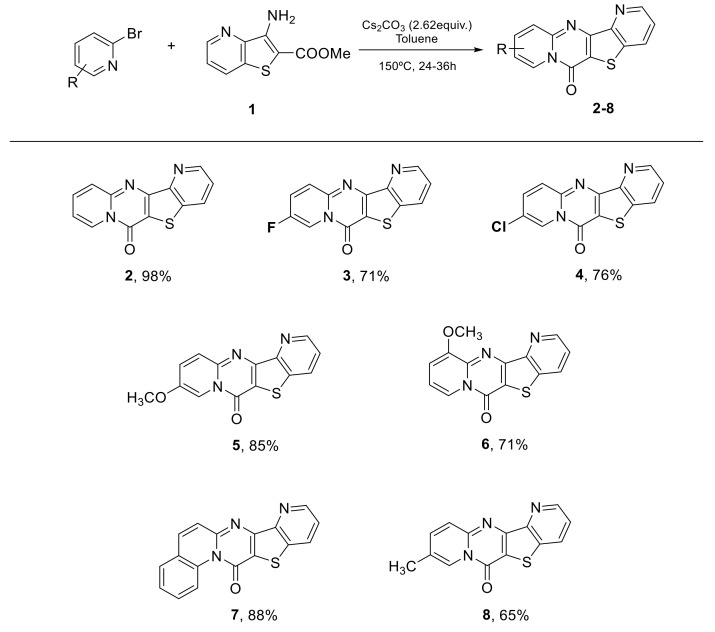
In 2010, this reaction was performed and described once from 3-aminothieno[3,2-b]pyridine-2-carboxylate and 2-bromopyridine [15]. The 3-aminothieno[3,2-b]pyridine-2-carboxylate 1 as starting material for palladium-catalyzed coupling by reaction on the 2-bromopyridine was prepared in excellent yield, reacting the 3-fluoropicolinonitrile with methyl thioglycolate in DMF using an excess of KOH (aq), following the known procedure [14]. The optimization of C-N Buchwald–Hartwig coupling of 3-amino-thieno[3,2-b]pyridine-2-carboxylate 1 with 2-bromopyridine was performed to obtain tetracyclic compounds in good yields (Table 1). These compounds are generated, after Palladium coupling, by intramolecular cyclization involving the nucleophilic attack of the pyridine nitrogen on the carbonyl of the ester group, described on the benzo[b]thiophene series [15].
Table 1.
Optimization of conditions to generate tetracyclic compound 2.
| Entry | [Cat] (mol %) | Solvent | Ligand (mol %) | T (°C) | Base 2.62 equiv | Yield (%) [a] |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 (3) | Dioxane | Xantphos (4) | 120 | Cs2CO3 | 67 |
| 2 | Pd(OAc)2 (3) | Dioxane | PPh3 (6) | 120 | Cs2CO3 | 33 |
| 3 | Pd(OAc)2 (5) | Dioxane | 10-phenanthroline (10) | 120 | Cs2CO3 | 18 |
| 4 | Pd(OAc)2 (3) | Dioxane | - | 120 | Cs2CO3 | 14 |
| 5 | Pd(OAc)2 (3) | Toluene | - | 120 | Cs2CO3 | [b] |
| 6 | Pd(OAc)2 (3) | Toluene | PPh3 (6) | 120 | Cs2CO3 | 8 |
| 7 | Pd(OAc)2 (3) | Toluene | PPh3 (6) | 150 | Cs2CO3 | 10 |
| 8 | Pd(OAc)2 (3) | Toluene/EtOH (2:1) | - | 120 | Cs2CO3 | - |
| 9 | Pd(OAc)2 (3) | Toluene | - | 150 | Cs2CO3 | 41 |
| 10 | Pd(OAc)2 (3) | Toluene | - | 150 | K3PO4 | - |
| 11 | Pd(OAc)2 (3) | DMA | - | 150 | Cs2CO3 | [c] |
| 12 | Pd(TFA)2 (5) | Toluene | - | 120 | Cs2CO3 | 61 |
| 13 | Pd(dppf)Cl2·DCM (3) | Toluene | - | 120 | Cs2CO3 | - |
| 14 | - | Toluene | - | 150 | Cs2CO3 | 98 |
[a] Isolated yield after column chromatography. [b] 120 °C, t = 10 min. in MW 100% of starting material was recovered. [c] 150 °C, t = 1 h, MW degradation in mixture.
Our goal was to perform the Buchwald–Hartwig Reaction under the conditions reported in the literature [8,9,10,11,12], in order to optimize the yield and achieve a more environmentally benign process by decreasing the quantity of palladium and reagents. To generate the tetracyclic compound 2 we first used 2-bromopyridine and methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate 1 in presence of Pd(OAc)2 and Xantphos as ligand in 1,4 dioxane at 120 °C for 24 h (entry 1, Table 1) to led to 2 in 67% yield using the conditions usually applied in our team to optimize cross-coupling methodologies [11,12]. We then used several less complex ligands and various solvents. Unfortunately, with PPh3 and 10-phenanthroline or without ligand a decrease in the yields was obtained (entries 2–4 and 6–7, Table 1). When the temperature was increased, the reaction could be performed without any ligand (entry 5 versus entry 9). When K3PO4 was used instead of Cs2CO3 no product was obtained (entry 10). The same result was found when we used a mixture of toluene/EtOH: 2/1 instead of toluene (entry 8). Degradation was observed under microwave irradiation (MW) at 150 °C in a sealed reaction vessel (entry 11). This reaction was carried out in a Biotage Initiator microwave synthesis instrument, the temperature was measured by IR-sensor and was maintained during the experiment. With Palladium(II) trifluoroacetate without ligand, a reasonable yield of 61% was obtained at 120 °C (entry 12) but was unsuccessful with Pd(dppf)Cl2·DCM (entry 13). We finally explored the reaction without Palladium catalyst and to our surprise, in view of the methods already published using exclusively palladium catalyst, the expected product was obtained in quasi quantitative yield using only Cs2CO3 and toluene as solvent (entry 14). With these optimized conditions, the scope and limitation of the reaction were examined (Scheme 1).
Scheme 1.
Synthesis of new tetracyclic compounds from 3-aminothieno[3,2-b]pyridine-2-carboxylate.
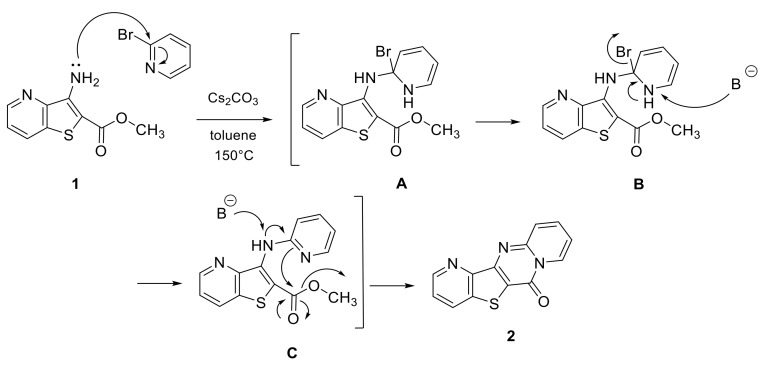
Different 2-bromo pyridines were then used for the reaction. Several 6H-pyrido[1,2-a]pyrido [2′,3′:4,5]thieno[3,2-d]pyrimidin-6-ones were obtained in good to excellent yield. Some bromopyridine derivatives required more time to achieve total consumption of the starting material. In contrast to already published results, this reaction did not proceed via C-N Buchwald–Hartwig Cross Coupling. The mechanism is represented in Scheme 2: an electrophilic attack of amine followed by an elimination of HBr in presence of Cs2CO3 gave the intermediate C (Scheme 2) which underwent an intramolecular cyclization process.
Scheme 2.
Plausible mechanism.
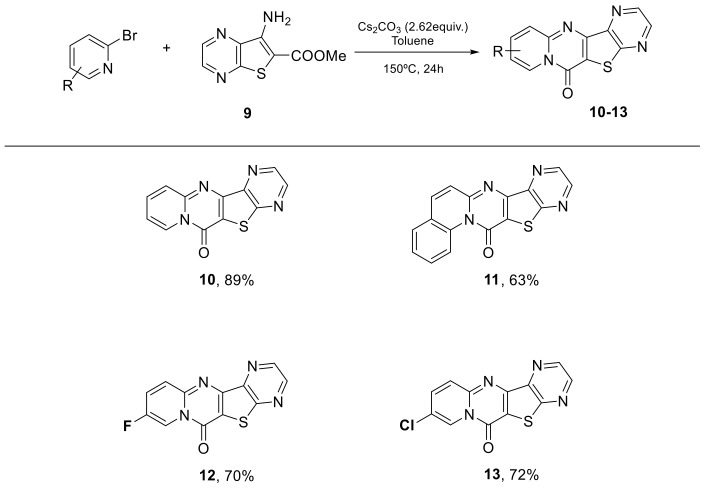
The conditions found were tested on pyrazine scaffold. From methyl 7-aminothieno[2,3-b]pyrazine-6-carboxylate (9), several 6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidinones were synthesized in moderate to good yields that demonstrated the generality of this method. Based on the latest results of the team, as we expected, the pyrazine showed lower yields than the pyridine (Scheme 3).
Scheme 3.
Synthesis of new tetracyclic compounds from methyl 7-aminothieno[2,3-b]pyrazine-6-carboxylate.
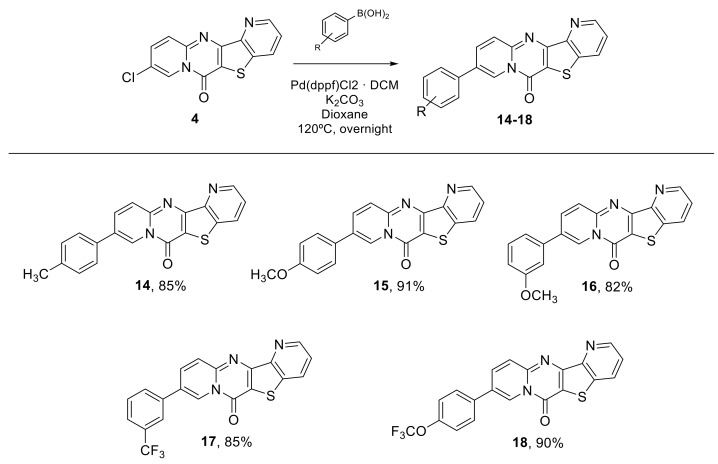
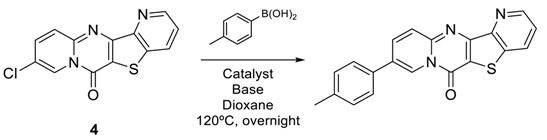
In the second phase of this work, we studied the Suzuki Reaction of 6H-pyrido[1,2-a]pyrido [2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one 4 with p-tolylboronic acid (Table 2).
Table 2.
Suzuki reaction optimization in dioxane.
| Entry | [Cat] | Base | % [a] |
|---|---|---|---|
| 1 | Pd(dppf)Cl2·DCM | K2CO3 | 85 |
| 2 | Pd(dppf)Cl2·DCM | Na2CO3 | 57 |
| 3 | Pd(PPh3)4 | Na2CO3 | [b] |
[a] Isolated yield after column chromatography; [b] mixture of products.
The experimental conditions used were those previously reported by our team [12,16,17,18,19,20]. The best result, 85% yield, was obtained with K2CO3 as base and Pd(dppf)Cl2·DCM complex as catalyst. The use of Na2CO3 decreased the yield (entry 2, Table 2) and changing the catalyst led to a mixture of various compounds. With our optimized conditions, the scope and limitation of the reaction were examined (Table 2 and Scheme 4). Several boronic acids were used for the reaction. Using Pd(dppf)Cl2·DCM (10 mol%), K2CO3 (2 equiv.) in dioxane at 120 °C for 24 h, the various 6H-pyrido[1,2-a]pyrido [2′,3′:4,5]thieno[3,2-d]pyrimidin-6-ones were obtained in excellent yield (Scheme 4).
Scheme 4.
Synthesis of 6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one from compound 4.
3. Materials and Methods
3.1. General Methods
All reagents were purchased from commercial suppliers and were used without further purification except for DMF, which was stored under argon and activated molecular sieves. The reactions were monitored by thin-layer chromatography (TLC) analysis using silica gel (60 F254) plates. Compounds were visualized by UV irradiation. Flash column chromatography was performed on silica gel 60 (230–400 mesh, 0.040–0.063 mm). Melting points (m.p. [°C]) were taken on samples in open capillary tubes and are uncorrected. 1H and 13C NMR spectra were recorded on a Bruker DPX 250 (13C, 62.9 MHz) (Bruker, Wissembourg, France), Bruker Avance II 250.13 (13C, 63 MHz), Bruker Avance 400.13 (13C, 101 MHz) (Bruker, Wissembourg, France), or on a Bruker Avance III HD nanobay 400.13 (13C, 101 MHz) (Bruker, Wissembourg, France). Chemical shifts are given in parts per million from tetramethylsilane (TMS) as internal standard. The following abbreviations are used for the proton spectra multiplicities: b: broad, s: singlet, d: doublet, t: triplet, q: quartet, p: pentuplet, m: multiplet. Coupling constants (J) are reported in hertz (Hz). Multiplicities were determined by the DEPT 135 sequence. Attributions of protons and carbons were made with the help of HSQC and HMBC 2D NMRs. Eudesmane numbering of carbons was used instead of the IUPAC numbering. Microwaves-assisted reactions were carried out in a Biotage Initiator microwave synthesis instrument and temperatures were measured by IR-sensor (Biotage, Uppsala, Sweden), High-resolution mass spectra (HRMS) were performed on a Maxis UHR-q-TOF mass spectrometer Bruker 4G (Bruker, Wissembourg, France), with an electrospray ionisation (ESI) mode.
3.2. General Methods
General Procedure for Synthesis of 6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-ones (2–8 and 10–13). A solution of compound 1 or 9 (100 mg; 0.480 mmol), 2-bromo pyridine (2 equiv., 0.960 mmol) and Cs2CO3 (2.62 equiv., 1,258 mmol) was heated at 150 °C in dry toluene (2 mL) for 24–36 h. The reaction was followed by TLC. After completion, the mixture was concentrated under vacuum. The solid obtained was submitted to a column chromatography. The increase of polarity in solvent gradient was made from neat petroleum ether to mixture of AcOEt/petroleum ether (9:1). 1H NMR and 13C NMR of compounds 2–8, 10–18 are available in Supplementary Materials.
6H-Pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (2) [15]: Yellow solid (121 mg, 98%), m.p. 280–282 °C. 1H NMR (400 MHz, CDCl3) δ 7.17 (td, J = 1.4, 7.2 Hz, 1H), 7.53 (dd, J = 4.5, 8.3 Hz, 1H), 7.74 (ddd, J = 1.6, 6.6, 8.3 Hz, 1H), 7.98 (d, J = 9.0 Hz, 1H), 8.30 (dd, J = 1.4, 8.3 Hz, 1H), 8.93 (dd, J = 1.4, 4.5 Hz, 1H), 9.11 (d, J = 7.2 Hz, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 115.1, 116.4, 123.4, 126.5, 127.3, 131.8, 135.0, 137.5, 148.5, 149.7, 150.0, 152.7, 155.1 ppm.
9-Fluoro-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (3): Yellow solid (92 mg, 71%), m.p. 334–336 °C. 1H NMR (400 MHz, CDCl3) δ 7.57 (dd, J = 8.3, 4.5 Hz, 1H), 7.68 (ddd, J = 2.8, 6.5, 9.6 Hz, 1H), 8.02 (dd, J = 5.3, 9.9 Hz, 1H), 8.33 (dd, J = 1.4, 8.3 Hz, 1H), 8.95 (dd, J = 1.4, 4.5 Hz, 1H), 9.03 (dd, J = 2.8, 4.8 Hz, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 112.7, 116.6, 123.6, 128.1, 129.2, 131.9, 137.5, 148.7, 149.6, 152.5, 152.8, 154.6, 155.2 ppm. HRMS: calcd for C13H7FN3OS [M + H]+ 272.0288, found 272.0289.
9-Chloro-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (4): Yellow solid (105 mg, 76%), m.p. 323–325 °C. 1H NMR (400 MHz, CDCl3) δ 7.57 (dd, J = 4.3, 8.3 Hz, 1H), 7.68 (d, J = 9.6 Hz, 1H), 7.95 (d, J = 9.6 Hz, 1H), 8.34 (d, J = 8.3 Hz, 1H), 8.96 (d, J = 3.6 Hz, 1H), 9.16 (s, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 123.6, 123.9, 124.2, 128.2, 131.9, 136.4, 137.6, 148.4, 148.8, 149.5, 152.5, 154.2, 162.6 ppm. HRMS: calcd for C13H7ClN3OS [M + H]+ 287.9993, found 287.9994.
9-Methoxy-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (5): Yellow solid (116 mg, 85%), m.p. 361–363 °C. 1H NMR (250 MHz, CDCl3) δ 3.99 (s, 3H), 7.50–7.58 (m, 2H), 7.90–7.99 (m, 1H), 8.31 (dd, J = 1.5, 8.3 Hz, 1H), 8.61 (d, J = 2.6 Hz, 1H), 8.94 (dd, J = 1.4, 4.5 Hz, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 56.5, 105.7, 115.9, 123.1, 127.8, 130.9, 131.7, 137.1, 147.2, 148.3, 149.8, 150.6, 151.8, 154.7 ppm. HRMS: calcd for C14H10N3O2S [M + H]+ 284.0488, found 284.0491.
11-Methoxy-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (6): Yellow solid (96 mg, 71%), m.p. 347–349 °C. 1H NMR (250 MHz, CDCl3) δ 4.07 (s, 3H), 6.98 (dd, J = 1.4, 7.7 Hz, 1H), 7.07 (t, J = 7.4 Hz, 1H), 7.51 (dd, J = 4.5, 8.3 Hz, 1H), 8.28 (dd, J = 1.4, 8.3 Hz, 1H), 8.73 (dd, J = 1.4, 7.2 Hz, 1H), 8.93 (dd, J = 1.4, 4.5 Hz, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 53.4, 122.3, 125.1, 125.3, 127.1, 128.2, 129.6, 131.7, 135.7, 148.6, 149.4, 149.8, 150.7, 159.8 ppm. HRMS: calcd for C14H10N3O2S [M + H]+ 284.0488, found 284.0488.
13H-Pyrido[2′’,3′’:4′,5′]thieno[3′,2′:4,5]pyrimido[1,2-a]quinolin-13-one (7): Yellow solid (128 mg, 88%), m.p. 282–284 °C. 1H NMR (250 MHz, CDCl3) δ 7.51–7.60 (m, 2H), 7.65–7.74 (m, 3H), 7.80 (d, J = 9.3 Hz, 1H), 8.32 (d, J = 7.3 Hz, 1H), 8.94 (d, J = 4.6 Hz, 1H), 9.94 (d, J = 8.7 Hz, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 122.2, 122.3, 122.9, 125.1, 125.3, 127.1, 128.2, 129.6, 131.7, 135.6, 135.7, 137.3, 148.6, 149.5, 149.8, 150.7, 159.8 ppm. HRMS: calcd for C17H10N3OS [M + H]+ 304.0539, found 304.0541.
9-Methyl-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (8): Yellow solid (83 mg, 65%), m.p. 277–279 °C. 1H NMR (400 MHz, CDCl3) δ 2.49 (s, 3H), 7.54 (dd, J = 4.5, 8.3 Hz, 1H), 7.62 (dd, J = 2.1, 9.2 Hz, 1H), 7.94 (d, J = 9.2 Hz, 1H), 8.31 (dd, J = 1.5, 8.2 Hz, 1H), 8.94–8.95 (m, 2H) ppm. 13C NMR (101 MHz, CDCl3) δ 18.7, 116.2, 123.3, 123.8, 125.4, 126.7, 131.8, 137.5, 138.2, 148.5, 149.1, 149.9, 152.6, 155.0 ppm. HRMS: calcd for C14H10N3OS [M + H]+ 268.0539, found 268.0541.
6H-Pyrazino[2′,3′:4,5]thieno[3,2-d]pyrido[1,2-a]pyrimidin-6-one (10): Yellow solid (108mg, 89%), m.p. 294–296 °C. 1H NMR (400 MHz, CDCl3) δ 7.26–7.30 (m, 1H), 7.83–7.86 (m, 1H), 8.03 (d, J = 9.1 Hz, 1H), 8.83 (s, 1H), 8.95 (s, 1H), 9.20 (d, J = 7.3 Hz, 1H) ppm. 13C NMR (100.6 MHz, CDCl3) δ 115.4, 116.1, 126.6, 127.1, 135.4, 142.8, 144.2, 144.9, 150.2, 150.3, 154.7, 159.0 ppm. HRMS: calcd for C12H7N4OS [M + H]+ 255.0335, found 255.0334.
13H-Pyrazino[2′’,3′’:4′,5′]thieno[3′,2′:4,5]pyrimido[1,2-a]quinolin-13-one (11): Yellow solid (92mg, 63%), m.p. 180–182 °C. 1H NMR (400 MHz, CDCl3) δ 7.60–64 (m, 1H). 7.70 (d, J = 9.4 Hz, 1H), 7.74–7.78 (m, 2H), 7.86 (d, J = 9.4 Hz, 1H), 8.78 (d, J = 2.3 Hz, 1H), 8.91 (d, J = 2.3 Hz, 1H), 9.96 (d, J = 8.9 Hz, 1H) ppm. 13C NMR (100.6 MHz, CDCl3) δ 115.6, 122.3, 125.0, 125.1, 127.4, 127.9, 128.3, 129.9, 130.7, 135.5, 136.3, 140.9, 142.9, 144.5, 159.5, 183.6 ppm. HRMS: calcd for C16H9N4OS [M + H]+ 305.0492, found 305.0492.
9-Fluoro-6H-pyrazino[2′,3′:4,5]thieno[3,2-d]pyrido[1,2-a]pyrimidin-6-one (12): Yellow solid (91 mg, 70%), m.p. 321–323 °C. 1H NMR (400 MHz, CDCl3) δ 7.71–7.76 (m, 1H), 8.00–8.04 (m, 1H), 8.80 (d, J = 2.3 Hz, 1H), 8.92 (d, J = 2.3 Hz, 1H), 9.06–9.08 (m, 1H) ppm. 13C NMR (100.6 MHz, CDCl3) δ 112.9, 128.2, 128.5, 128.9, 129.0, 142.9, 144.0, 145.0 ppm. HRMS: calcd for C12H6FN4OS [M + H]+ 273.0241, found 273.0242.
9-Chloro-6H-pyrazino[2′,3′:4,5]thieno[3,2-d]pyrido[1,2-a]pyrimidin-6-one (13): Yellow solid (99 mg, 72%), m.p. 342–344 °C. 1H NMR (400 MHz, CDCl3) δ 7.73 (dd, J = 9.6, 2.3 Hz, 1H), 7.94 (d, J = 9.6 Hz, 1H), 8.81 (d, J = 2.3 Hz, 1H), 8.92 (d, J = 2.3 Hz, 1H), 9.18 (d, J = 1.8 Hz, 1H) ppm. 13C NMR (100.6 MHz, CDCl3) δ 80.0, 124.2, 128.0, 136.8, 143.0, 145.1 ppm. HRMS: calcd for C12H6ClN4OS [M + H]+ 288.9945, found 288.9945.
General Procedure for Synthesis of 9-phenyl-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (14–18). A solution of compound 4 (100 mg; 0.348 mmol), boronic acid (2 equiv., 0.696 mmol), Pd(dppf)Cl2·DCM (10 mol%) and K2CO3 (2 equiv., 0.696 mmol) was heated at 120 °C in dioxane (2mL), overnight. After completion, the mixture was concentrated under vacuum. The solid obtained was submitted to a column chromatography. The increase of polarity in solvent gradient was made from neat petroleum ether to mixture of AcOEt/petroleum ether (6:4).
9-(p-Tolyl)-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (14): white solid (101 mg, 85%), m.p. 259 – 261 °C. 1H NMR (400 MHz, CDCl3) δ 2.44 (s, 3H), 7.34 (d, J = 7.7 Hz, 2H), 7.54–7.63 (m, 3H), 8.05 (d, J = 4.0 Hz, 2H), 8.33 (d, J = 8.1 Hz, 1H), 8.96 (d, J = 3.7 Hz, 1H), 9.32 (s, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 21.2, 115.8, 116.2, 122.8, 123.3, 126.8, 127.1, 128.8, 129.1, 130.1, 131.7, 132.6, 135.2, 137.4, 139.0, 148.4, 149.0, 149.7, 152.4, 155.0 ppm. HRMS: calcd for C20H14N3OS [M + H]+ 344.0852, found 344.0851.
9-(4-Methoxyphenyl)-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (15): white solid (113 mg, 91%), m.p. 274–276 °C. 1H NMR (400 MHz, CDCl3) δ 3.91 (s, 3H), 7.05–7.07 (m, 2H), 7.54–7.57 (m, 1H), 7.63–7.66 (m, 2H), 8.04 (dd, J = 2.0, 9.3 Hz, 2H), 8.33 (dd, J = 1.4, 8.2 Hz, 1H), 8.96 (dd, J = 1.5, 4.5 Hz, 1H), 9.29 (dd, J = 0.9, 2.0 Hz, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 55.5, 112.7, 114.2, 116.3, 119.3, 123.3, 123.3, 127.1, 128.9, 130.5, 131.7, 135.2, 136.9, 137.4, 148.4, 149.0, 149.6, 152.4, 155.0, 160.3 ppm. HRMS: calcd for C20H14N3O2S [M + H]+ 360.0801, found 360.0797.
9-(3-Methoxyphenyl)-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (16): white solid (102 mg, 82%), m.p. 278–280 °C. 1H NMR (400 MHz, CDCl3) δ 3.96 (s, 3H), 7.04–7.07 (m, 1H), 7.26–7.33 (m, 2H), 7.50 (t, J = 8.0 Hz, 1H), 7.61 (dd, J = 4.5, 8.2 Hz, 1H), 8.09–8.11 (m, 2H), 8.38 (dd, J = 1.5, 8.1 Hz, 1H), 9.01 (dd, J = 1.4, 4.4 Hz, 1H), 9.37–9.38 (m, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 55.5, 112.7, 114.2, 116.3, 119.3, 123.3, 123.3, 127.1, 128.9, 130.5, 131.7, 135.2, 136.9, 137.4, 148.4, 149.0, 149.6, 152.4, 155.0, 160.3 ppm. HRMS: calcd for C20H14N3O2S [M + H]+ 360.0801, found 360.0805.
9-(3-(Trifluoromethyl)phenyl)-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (17): white solid (118 mg, 85%), m.p. 265–267 °C. 1H NMR (400 MHz, CDCl3) δ 7.56 (dd, J = 4.5, 8.2 Hz, 1H), 7.67 (t, J = 7.7 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.89 (d, J = 7.7 Hz, 1H), 7.93 (s, 1H), 8.01 (dd, J = 2.1, 9.3 Hz, 1H), 8.09 (d, J = 9.3 Hz, 1H), 8.33 (dd, J = 1.5, 8.3 Hz, 1H), 8.95 (dd, J = 1.5, 4.5 Hz, 1H), 9.33 (d, J = 2.1 Hz, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 29.7, 116.6, 119.7, 122.4, 123.4, 123.8, 125.2, 125.6, 127.6, 130.0, 130.2, 131.7, 134.5, 136.5, 137.5, 148.6, 148.9, 149.5, 152.5, 154.9 ppm. HRMS: calcd for C20H11F3N3OS [M + H]+ 398.0569, found 398.0567.
9-(4-(Trifluoromethoxy)phenyl)-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one (18): white solid (129 mg, 90%), m.p. 272–274 °C. 1H NMR (250 MHz, CDCl3) δ 7.37 (d, J = 8.3 Hz, 2H), 7.54 (dd, J = 4.5, 8.3 Hz, 1H), 7.71 (d, J = 8.6 Hz, 2H), 7.97 (dd, J = 2.2, 9.3 Hz, 1H), 8.06 (d, J = 9.3 Hz, 1H), 8.31 (dd, J = 1.4, 8.1 Hz, 1H), 8.94 (dd, J = 1.4, 4.3 Hz, 1H), 9.28 (d, J = 2.1 Hz, 1H) ppm. 13C NMR (101 MHz, CDCl3) δ 116.5, 116.6, 119.1, 121.8, 123.3, 123.4, 127.4, 127.6, 128.4, 131.7, 134.2, 134.6, 137.4, 148.5, 148.9, 149.5, 149.7, 149.7, 152.4, 154.9 ppm. HRMS: calcd for C20H11F3N3O2S [M + H]+ 414.0519, found 414.0518.
4. Conclusions
In summary, we have developed the first efficient method to obtain various new tetracyclic compounds from 3-aminothieno[3,2-b]pyridine-2-carboxylate in toluene without using ligand and catalyst. This procedure could be applied to various similar heterocycles to generate various biologically active compounds in an environmentally sound manner. In our case, to show the possible diversity we introduced several boronic acids by Suzuki reaction of 9-chloro-6H-pyrido[1,2-a]pyrido[2′,3′:4,5]thieno[3,2-d]pyrimidin-6-one in excellent yields.
Acknowledgments
We acknowledge the Region Centre for financial support.
Supplementary Materials
The following are available online: 1H NMR and 13C NMR of compounds 2–8, 10–18.
Author Contributions
Joana F. Campos and Sabine Berteina-Raboin conceived and designed the experiments; Mohammed Aounzou and Joana F. Campos performed the experiments; Mohammed Aounzou, Joana F. Campos, Mohammed Loubidi and Sabine Berteina-Raboin analyzed the data. Joana F. Campos and Sabine Berteina-Raboin wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all compounds are available from the authors.
References
- 1.Davis P., Mousa S. Anti-Angiogenesis Strategies in Cancer Therapeutics. 1st ed. Academic Press; Amsterdam, The Netherlands: 2016. pp. 1–19. [Google Scholar]
- 2.Loidreau Y., Deau E., Marchand P., Nourrisson M.-R., Loge C., Coadou G., Loaec N., Besson T. Synthesis and molecular modelling studies of 8-arylpyrido[30,20:4,5] thieno[3,2-d]pyrimidin-4-amines as multitarget Ser/Thr kinases inhibitors. Eur. J. Med. Chem. 2015;92:124–134. doi: 10.1016/j.ejmech.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 3.Loidreau Y., Marchand P., Dubouilh-Benard C., Nourrisson M.-R., Duflos M., Loaec N., Meijer L., Besson T. Synthesis and biological evaluation of N-aryl-7-methoxybenzo[b]furo[3,2-d]pyrimidin-4-amines and their N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amine analogues as dual inhibitors of CLK1 and DYRK1A kinases. Eur. J. Med. Chem. 2013;59:283–295. doi: 10.1016/j.ejmech.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Loidreau Y., Marchand P., Dubouilh-Benard C., Nourrisson M.-R., Duflos M., Lozach O., Loaec N., Meijer L., Besson T. Synthesis and biological evaluation of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors. Eur. J. Med. Chem. 2012;58:171–173. doi: 10.1016/j.ejmech.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Bozorov K., Zhao J.-Y., Elmuradov B., Pataer A., Aisa H.A. Recent developments regarding the use of thieno[2,3-d]pyrimidin-4-one derivatives in medicinal chemistry, with a focus on their synthesis and anticancer properties. Eur. J. Med. Chem. 2015;102:552–573. doi: 10.1016/j.ejmech.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Kaur N., Jangid N.K., Sharma V. Metal- and nonmetal-catalyzed synthesis of five-membered S,N-heterocycles. J. Sulfur. Chem. 2018;39:193–236. doi: 10.1080/17415993.2017.1415338. [DOI] [Google Scholar]
- 7.Kumari S., Kishore D., Paliwal S., Chauhan R., Dwivedi J., Mishra A. Transition metal-free one-pot synthesis of nitrogen-containing heterocycles. Mol. Divers. 2016;20:185–232. doi: 10.1007/s11030-015-9596-0. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Castillo P., Buchwald S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016;116:12564–12649. doi: 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentabed-Ababsa G., Sid Ely S.C., Hesse S., Nassar E., Chevallier F., Nguyen T.T., Derdour A., Mon-gin F. Direct Metalation of Heteroaromatic Esters and Nitriles Using a Mixed Lithium−Cadmium Base. Subsequent Conversion to Dipyridopyrimidinones. J. Org. Chem. 2010;75:839–847. doi: 10.1021/jo902385h. [DOI] [PubMed] [Google Scholar]
- 10.Yuan Q., Ma D. A One-Pot Coupling/Hydrolysis/Condensation Process to Pyrrolo[1,2-a]quinoxaline. J. Org. Chem. 2008;73:5159–5162. doi: 10.1021/jo8008098. [DOI] [PubMed] [Google Scholar]
- 11.Loubidi M., Moutardier A., Campos J.F., Berteina-Raboin S. Pd-catalyzed Suzuki/Sonogashira cross-coupling reaction and the direct sp3 arylation of 7-chloro-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine. Tetrahedron Lett. 2018;59:1050–1054. doi: 10.1016/j.tetlet.2018.02.001. [DOI] [Google Scholar]
- 12.El Akkaoui A., Berteina-Raboin S., Mouaddib A., Guillaumet G. Direct Arylation of Imidazo[1,2-b]pyridazines: Microwave-Assisted One-Pot Suzuki Coupling/Pd-Catalysed Arylation. Eur. J. Org. Chem. 2010;5:862–871. doi: 10.1002/ejoc.200900849. [DOI] [Google Scholar]
- 13.Queiroz M.-J.R.P., Begouin A., Ferreira I.C.F.R., Kirsch G., Calhelha R.C., Barbosa S., Estevinho L.M. Palladium-Catalysed Amination of Electron-Deficient or Relatively Electron-Rich Benzo[b]thienyl Bromides-Preliminary Studies of Antimicrobial Activity and SARs. Eur. J. Org. Chem. 2004;17:3679–3685. doi: 10.1002/ejoc.200400218. [DOI] [Google Scholar]
- 14.Queiroz M.-J.R.P., Calhelha R.C., Kirsch G. Reactivity of several deactivated 3-aminobenzo[b]thiophenes in the Buchwald-Hartwig C-N coupling. Scope and limitations. Tetrahedron. 2007;63:13000–13005. doi: 10.1016/j.tet.2007.10.029. [DOI] [Google Scholar]
- 15.Calhelha R.C., Queiroz M.-J.R.P. Synthesis of new thieno[3,2-b]pyridine derivatives by palladium-catalyzed couplings and intramolecular cyclizations. Tetrahedron Lett. 2010;51:281–283. doi: 10.1016/j.tetlet.2009.10.138. [DOI] [Google Scholar]
- 16.Loubidi M., Pillard C., El Hakmaoui A., Bernard P., Akssira M., Guillaumet G. A new synthetic approach to the imidazo[1,5-a]imidazole-2-one scaffold and effective functionalization through Suzuki-Miyaura cross coupling reactions. RSC Adv. 2016;6:7229–7238. doi: 10.1039/C5RA25520A. [DOI] [Google Scholar]
- 17.Fresneau N., Hiebel M.-A., Agrofoglio L.A., Berteina-Raboin S. Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2′-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media. Molecules. 2012;17:14409–14417. doi: 10.3390/molecules171214409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Akkaoui A., Bassoude I., Koubachi J., Berteina-Raboin S., Mouaddib A., Guillaumet G. Pd-catalyzed regiocontrolled Sonogashira and Suzuki cross-coupling reaction of 3,6-dihalogenoimidazo[1,2-a]pyridines: One-pot double-coupling approach. Tetrahedron. 2011;67:7128–7138. doi: 10.1016/j.tet.2011.06.108. [DOI] [Google Scholar]
- 19.Koubachi J., El Kazzouli S., Berteina-Raboin S., Mouaddib A., Guillaumet G. Synthesis of Polysubstituted Imidazo[1,2-a]pyridines via Microwave-Assisted One-Pot Cyclization/Suzuki Coupling/Palladium-Catalyzed Heteroarylation. J. Org. Chem. 2007;72:7650–7655. doi: 10.1021/jo0712603. [DOI] [PubMed] [Google Scholar]
- 20.Wendeborn S., Berteina S., Brill W.K.-D., De Mesmaeker A.D. Pd-mediated C-C and C-S bond formation on solid support: A scope and limitations study. Synlett. 1998;6:671–675. doi: 10.1055/s-1998-22661. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.