Abstract
The incidence of infectious diseases caused by several bacterial pathogens such as Haemophilus influenzae type b, Streptococcus pneumoniae, and Neisseria meningitidis, has been dramatically reduced over the last 25 years through the use of glycoconjugate vaccines. The structures of the bacterial capsular polysaccharide (CPS) antigens, extracted and purified from microbial cultures and obtained with very high purity, show that many of them are decorated by O-acetyl groups. While these groups are often considered important for the structural identity of the polysaccharides, they play a major role in the functional immune response to some vaccines such as meningococcal serogroup A and Salmonella typhi Vi, but do not seem to be important for many others, such as meningococcal serogroups C, W, Y, and type III Group B Streptococcus. This review discusses the O-acetylation status of CPSs and its role in the immunological responses of these antigens.
Keywords: bacterial vaccines, conjugate vaccines, carbohydrate antigens, O-acetylation
1. Introduction
Glycoconjugation is a very well established ‘technology platform’ for bacterial vaccine development. Vaccines have been licensed and have strongly contributed to preventing Haemophilus influenzae type b, Streptococcus pneumoniae, and Neisseria meningitidis infections. Additional vaccines against other bacteria (e.g., Group B Streptococcus, Salmonella typhi Vi) or further Streptococcus pneumoniae and Neisseria meningitidis serogroups not included in the registered formulations are currently in clinical development [1].
Bacterial capsular polysaccharide (CPS) antigen structures, extracted and purified from microbial cultures or synthetically prepared (i.e., Quimi-Hib) [2], have been deeply investigated in the last decades. Nuclear magnetic resonance (NMR) spectroscopy, which provides fingerprint-type characteristics of the structure and is sensitive to small structural differences, has played a key role in the carbohydrate structure determination [3].
Bacterial polysaccharides often contain an array of substituents, such as O-acetyl and phosphate groups, which may constitute an important part of the immunodominant epitopes. CPSs from many pathogenic bacteria are O-acetylated, and a common feature is that the O-acetyl groups are easily removed at an alkaline pH. The O-acetyl content is considered an aspect of ‘identity’, as some immunologically distinguishable CPSs differ in the presence of O-acetyl groups only (e.g., pneumococcal types 9A–9V [4], and 11A–11E [5,6]); the immune responses evoked by these CPSs are not necessarily cross-protective.
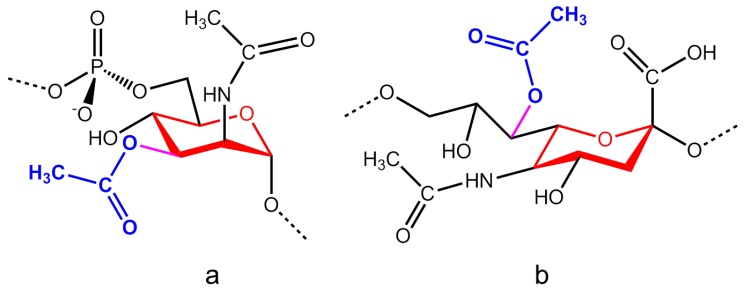
O-acetyl groups have been determined to be inside or outside the saccharide ring of monosaccharides constituting the repeating units of capsular polysaccharides. In Figure 1, the repeating unit structures of meningococcal serogroup A and C with O-acetyl groups inside or outside the saccharide ring are respectively reported.
Figure 1.
Repeating unit structures of meningococcal serogroup (a) A and (b) C, with O-acetyl groups inside or outside the saccharide ring, respectively.
Here we review the presence of the O-acetyl groups in capsular antigens and we try to address their role in the functional immune response.
2. O-acetylation in Conjugate Vaccines
O-acetylation patterns have been determined for several polysaccharide antigens (Table 1).
Table 1.
Structures of the repeating units containing O-acetyl groups of bacterial polysaccharides antigens of vaccines licensed or tested in clinical trials.
| Polysaccharide | Repeat Unit |
|---|---|
| Neisseria meningitidis | |
| Group A | →6)-α-d-ManpNAc(3/4OAc)-(1→OPO3→ |
| Group C | →9)-α-d-Neu5Ac(7/8OAc)-(2→ |
| Group W | →6)-α-d-Galp-(1→4)-α-d-Neu5Ac(7/9OAc)-(2→ |
| Group Y | →6)-α-d-Glcp-(1→4)-α-d-Neu5Ac(7/9OAc)-(2→ |
| Group B Streptococcus | |
| Type Ia | →4)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→4)-β-d-GlcpNAc-(1→3)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type Ib | →4)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→3)-β-d-GlcpNAc-(1→3)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type II | →3)-β-d-Glcp-(1→2)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)]-β-d-Galp-(1→4)-β-D-GlcpNAc-(1→3)-[β-d-Galp-(1→6)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type III | →6)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→4)]-β-d-GlcpNAc-(1→3)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type V | →4)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→4)-β-d-GlcpNAc-(1→6)]-α-d-Glcp-(1→4)-[β-d-Glcp-(1→3)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type VI | →6)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→3)]-β-d-Glcp-(1→3)-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Streptococcus pneumoniae | |
| Type 1 | →3)-d-AAT-α-Galp-(1→4)-α-d-GalpA(2/3OAc)-(1→3)-α-d-GalpA-(1→ |
| Type 7F | →6)-[β-d-Galp-(1→2)]-α-d-Galp-(1→3)-β-L-Rhap(2OAc)-(1→4)-β-d-Glcp-(1→3)-[α-d-GlcpNAc-(1→2)-α-L-Rhap-(1→4)]-β-d-GalpNAc-(1→ |
| Type 9V | →4)-α-d-Glcp(2/3OAc)-(1→4)-α-d-GlcpA-(1→3)-α-d-Galp-(1→3)-β-d-ManpNAc(4/6OAc)-(1→4)-β-d-Glcp-(1→ |
| Type 15B | →6)-[α-d-Galp(2/3/4/6OAc)-(1→2)-[Gro-(2→P→3)]-β-d-Galp-(1→2)]-β-d-GlcpNAc-(1→3)-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type 17F | →3)-β-L-Rhap-(1→4)-β-d-Glcp-(1→3)-α-d-Galp-(1→3)-β-L-Rhap(2OAc)-(1→4)-α-L-Rhap-(1→2)-d-Ara-ol-(1→P→ |
| Type 18C | →4)-β-d-Glcp-(1→4)-[α-d-Glcp(6OAc)-(1→2)][Gro-(1→P→3)]-β-d-Galp-(1→4)-α-d-Glcp-(1→3)-β-L-Rhap-(1→ |
| Type 22F | →4)-β-d-GlcpA-(1→4)-[α-d-Glcp-(1→3)]-β-L-Rhap(2OAc)-(1→4)-α-d-Glcp-(1→3)-α-d-Galf-(1→2)-α-L-Rhap-(1→ |
| Type 33F | →3)-β-d-Galp-(1→3)-[α-d-Galp-(1→2)]-α-d-Galp-(1→3)-β-d-Galf-(1→3)-β-d-Glcp-(1→5)-β-d-Galf(2OAc)-(1→ |
| Salmonella enterica | |
| typhi Vi | →)-α-d-GalpNAcA(3OAc)-(1→ |
| Staphylococcus aureus | |
| Type 5 | →4)-β-d-ManpNAcA-(1→4)-α-L-FucpNAc(3OAc)-(1→3)-β-d-FucpNAc-(1→ |
| Type 8 | →3)-β-d-ManpNAcA(4OAc)-(1→3)-α-L-FucpNAc-(1→3)-α-d-FucpNAc-(1→ |
Abbreviations: Glc, Glucose; Gal, Galactose; Neu5Ac, N-acetyl neuraminic acid (sialic acid); Rha, Rhamnose; GlcNAc, N-acetyl Glucosamine; GalNAc, N-acetyl Galactosamine; FucNAc, N-acetyl Fucosamine; ManNAcA, N-acetyl Mannuronic Acid; AAT, 2-acetamido-4-amino-2,4,6-trideoxygalactose; Gro, glycerol, Pne, 2-acetamido-2,6-dideoxytalose; Sug, 2-acetamido-2,6-deoxyhexose-4-ulose; P, phosphate in a phosphodiester linkage.
2.1. Neisseria Meningitidis
The α-1,6-linked N-acetylmannosamine phosphate constituting the repeating unit of meningococcal serogroup A CPS is partly O-acetylated at position C3 or C4 (Table 1). The meningococcal serogroups C, W, and Y, which all contain Neu5Ac moiety in the relative repeating units, are partially O-acetylated at the glycerol chain of Neu5Ac (at positions C7 and C8 for serogroup C and at positions C7 and C9 for serogroup W and Y) [7] (Table 1). The meningococcal genes coding O-acetylation of various serogroups have been analyzed [8]. On the contrary, the meningococcal serogroup X CPS [→4)-α-d-GlcpNAc-(1→OPO3→] is not O-acetylated [9,10].
The meningococcal serogroup A plain CPS is largely O-acetylated at position C3 (>90%) and slightly at position C4 [11,12] (Figure 1a). MynC was identified as new subclass of O-acetyltransferases that utilize acetyl-CoA to decorate the serogroup A CPS. Although a single or dual specificity of MynC (at positions C3 and/or C4) has not been demonstrated, the single specificity and a spontaneous migration of the O-acetyl group from the site of attachment around the ring is, perhaps, a more likely explanation. While a study demonstrated that O-acetylation is not required for capsule expression or to protect the meningococci from killing by normal human sera, investigation with O-acetylation-deficient mutant confirmed the importance of O-acetylation in serogroup A polysaccharide immunogenicity [11,13,14]. Berry et al. [15] confirmed that serogroup A-specific antibodies elicited in post-immunization human sera mostly identified O-acetylated CPS residues, impacting the degree of antibody inhibition. Comparative immunogenicity studies in mice revealed that de-O-acetylated antigens resulted in a marked loss of immunogenicity (total IgG), and, most relevantly, a loss in their ability to induce functional bactericidal antibodies. However, epitopes not involving O-acetyl groups may also contribute to the development of protective response since mice vaccinated with de-O-acetylated antigens elicited some functional antibodies.
In a phase III partially blinded, controlled study (where 1170 healthy subjects aged 18 to 25 years were randomized (1:1:1)), two lots of meningococcal ACWY-tetanus toxoid conjugate vaccines (MenACWY-TT) that differed in serogroup A CPS O-acetylation levels respectively (68% vs. 92% O-acetylation), were evaluated in terms of immunogenicity (i.e., rabbit serum bactericidal activity (rSBA)) and safety in comparison to a licensed MenACWY CPS vaccine (82% serogroup A O-acetylation). The MenA-TT conjugate with O-acetylation levels of 68% and 92% resulted in comparable vaccine immunogenicity [16]. As a matter of fact, all the meningococcal serogroup A conjugate contained in licensed vaccines (MenAfriVac–Serum Indian Institute; Menveo–GSK Vaccines; Menactra–Sanofi Pasteur; Nimenrix–GSK Vaccines (later taken over by Pfizer)) are largely O-acetylated. However, no evidence has been collected to define the minimal O-acetylation level affecting the immunogenicity of meningococcal serogroup A vaccines for the moment.
The high relevance of the O-acetyl moieties for serogroup A N. meningitidis could also be related to the presence of this decorative group within the saccharide ring, probably influencing and specifying the epitope in a unique arrangement that could not be easily mimicked in its absence.
Alternatively, the meningococcal serogroup C CPS is highly O-acetylated (>90%) at positions C8 and C7 of the sialic acid glycerol chain (out of the ring) (Figure 1b). The oatC gene required for O-acetylation of sialic acid residue was identified [8]. In meningococcal C CPSs freshly extracted or not exposed to chemical conditions (i.e., basic pH), which facilitates the migration from/to vicinal hydroxyl groups (i.e., at C8 and C7), most of the O-acetylation exists at position C8 with fewer CPSs repeating O-acetylation at C7, or results de-O-acetylated. After migration, most of the O-acetyl groups relocate from C8 to C7, leading to an epitope that is conformationally related, but not identical, to one contained in the fully de-O-acetylated serogroup C CPS. As demonstrated by Michon et al. [17] the immunogenicity in mice vaccinated with partially or completely de-O-acetylated serogroup C CPS antigens, obtained by mild basic treatment of the antigens, results in higher SBA titers toward the O-acetylated serogroup C strain C11. This strain was the dominant one for the de-O-acetylated antigen during the late 1990s in the UK. However, clinical data confirmed that both O-acetylated (Menjugate, Menveo, Menitorix, Menhibrix–GSK Vaccines; Menactra–Sanofi Pasteur; Nimenrix–Pfizer) and de-O-acetylated (NeisVac–North American Vaccine Inc., later taken over by Baxter Bioscience) conjugates were very immunogenic and efficacious in large vaccination campaigns [18,19,20,21] independent of the used carrier protein (TT, DT, CRM197). The high immunogenicity did not directly correlate to the C7 and C8 O-acetylation in meningococcal serogroup C. This differs from the serogroup A in which the O-acetylation seems to be determinant in the immunoresponse. This lack of correlation might be related to the positions of the O-acetyl groups which are located in the glycerol chain outside the saccharide ring where the influence on the epitope could be lower.
For meningococcal W and Y CPSs, minimal O-acetylation levels have been also revealed at positions C7 and C9 of sialic acid moieties of several strains. As suggested by Fusco et al. [22], for the meningococcal Y CPSs the O-acetyl is likely present on the surface of the organism and it seems to display significant conformational differences around its sialic acid linkages. This is not the same for the glycosidic bond between C6 glucose and C2 sialic acid, which does not seem to be affected by the O-acetylation status of the sialic acid. Furthermore, the authors proposed that conformational differences around the sialic acid bond between the C7 and C9 O-acetylated forms could be explained by the fact that the O-acetyl located at C7, in addition to the linkage at C4, influences the rotation of the sialic acid glycosidic bonds. One the other hand, the O-acetyl at C9, being away from these two centers, has no influence on the rotation (torsional angle), which therefore allows the conformational identity between C9 O-acetylated and de-O-acetylated forms of Y CPSs. The preferred position of the O-acetyl group on C9 Neu5Ac, which has been detected as most abundant form after purification of both meningococcal W and Y CPSs [12], might arise from its higher thermodynamic stability and as a consequence of the migration of O-acetyl group from the C7 to C9 positions.
Although the natural occurrence of both O-acetylated and non-O-acetylated W and Y isolates has been demonstrated (i.e., in the UK with a predominance of non-O-acetylated W and O-acetylated Y strains [23], and during the Hajj outbreak where all the W strains were non-O-acetylated [24]), the currently licensed serogroup W and Y conjugate vaccines contain the O-acetylated CPS. The general role of O-acetylation of these CPSs is still debated, however several studies conducted in the preclinical stage and using human sera seem to confirm that there is no difference in functional activity of O-acetylated or non-O-acetylated CPSs [22,25,26,27]. By investigating the meningococcal Y antigen, Fusco et al. [22] suggested that the O-acetyl groups may mask an important epitope to the immune system and thereby mislead the antibody response, resulting in an escape mechanism. Similar to meningococcal serogroup C, the O-acetylation in serogroup W and Y is outside the saccharide ring. It might confirm the poor immunological impact of this group when positioned outside the saccharide ring. However, the relatively low level of O-acetylation of several meningococcal serogroup W and Y strain might also explain the poor impact.
2.2. Group B Streptococcus
O-acetylation patterns at the ‘glycerol chain’ of the Neu5Ac residue, present at the terminal end of the branch, were also confirmed for several Group B Streptococcus (GBS) CPS serotypes (Ia, Ib, II, III, V, and VI) (Table 1), and were demonstrated to affect the inhibition of neutrophil suppression and virulence [28,29,30]. Data were consistent with an initial O-acetylation at position C7, and subsequent migration of the O-acetyl ester at positions C8 and C9. As for mammalian glycoproteins, O-acetylation of terminal sialic acid is a mechanism to prevent enzymatic de-sialylation; the exposure of terminal galactose and its excretion from the animal allow for speculation on a similar attitude of GBS.
Sera from healthy adults immunized with de-O-acetylated GBS CPS-TT conjugate vaccines were evaluated in opsonophagocytosis assays using 20 GBS clinical isolates (type Ia, Ib, II, III, or V CPSs with O-acetylation levels ranging from 2 to 40%). The data showed >90% opsonophagocytosis and killing of all strains, confirming that de-O-acetylated CPS-conjugate vaccines contain immunogenic epitopes that offer protection against GBS, independent of O-acetyl CPS modifications. Thus, presence of O-acetyl groups on the GBS CPSs is not essential for functional antibodies to be elicited by GBS glycoconjugate vaccines [31]. In the work of Carboni et al. the structure of a protective epitope of the group B Streptococcus type III capsular polysaccharide was investigated. The authors observed that the mAb interacts with the O7 of sialic acid through an H2O molecule, and dimensionally, this space could also host an acetyl group [32]. This could explain data in the literature reporting that O-acetylation does not interfere with protection.
2.3. Streptococcus Pneumoniae
The presence of O-acetyl groups has been observed in several S. pneumoniae CPSs (Table 1) but in only some has the potential role of O-acetylation been explored. A comparative study conducted both in the infant rhesus monkey model and in humans confirmed that antibodies against the de-O-acetylated backbone as well as against the O-acetylated serotype 9V CPS (O-acetyl groups revealed at positions C2 and C3 of the Glc residue and at positions C4 and C6 of the ManpNAc residue) [33,34] were detected. On the other hand, opsonophagocytic functional activity was observed in antisera in which the predominant antibody species recognize the de-O-acetylated serotype 9V CPS. The authors concluded that the O-acetylation side groups, while being recognized, were not essential to the ability of the serotype 9V CPS to elicit a functional antibody response [34].
For the serotype 18C CPS, which contains O-acetyl groups at position C6 of Glc residue in the branch [35], the role of O-acetylation was explored by using rabbit and human sera. No differences were shown in inhibition curves between any of the analyzed sera and CPSs with different degrees of O-acetylation [36].
2.4. Salmonella Typhi Vi
The α-1,4-linked N-acetylgalacturonic acid constituting the repeating unit of Salmonella typhi Vi CPS is partly O-acetylated (60–90%) at position C3 (Table 1). The O-acetylation level is one of the critical determinants of immunogenicity in the Vi polysaccharide antigen. Removal of O-acetyl groups resulted in a lower vaccine immunogenicity [37,38].
The molecular model of a pentamer (five repeating units) showed that the bulky nonpolar O-acetyl groups at position C3 make up most of the surface of Vi CPS by protruding on both sides. The O-acetyl groups dominate the surface of Vi CPS and it might explain their relevant influence on its immunogenicity [39].
2.5. Staphylococcus Aureus
Both S. aureus type 5 and 8 CPSs contain ManpNAcA and L- and D-FucpNAc residues (Table 1). The two CPSs differ in the stereochemical glycosidic linkages between the monosaccharides constituting the repeating units and the site of O-acetylation. The sites of O-acetylation are at the position C3 of L-FucpNAc for type 5 and at the position C4 of d-ManpNAcA type 8 [40].
As confirmed by Scully et al. [41] by collecting in vivo (mouse model) and in vitro experiments, the O-acetylation is necessary for CPS-CRM197 conjugates to induce effective opsonophagocytic killing responses. This data supports the high importance of a careful monitoring of the degree of O-acetylation during vaccine development and production.
The authors suggested that this finding may provide additional insight into the previous failure of a S. aureus CPS conjugate vaccine. Clashing with a previous clinical trial conducted in a similar population, which suggested short-term efficacy (up to 40 weeks) against S. aureus bacteremia after a single dose of a bivalent vaccine [42] (StaphVAX–Nabi; CPS types 5 and 8 conjugated with Pseudomonas aeruginosa exotoxin A) [43], a larger Phase III study failed despite robust antibody responses to primary vaccination. There was no demonstrative vaccine efficacy in preventing S. aureus bacteremia in end-stage renal disease patients receiving hemodialysis at any time interval studied or in any strategy considered [44]. There has been speculation on the reasons behind this failure [41,44]. Issues in the manufacturing consistency of the Phase III clinical trial material have been considered due to changes in contract facilities used, although the specific impacts of these changes were not further explained. Access to the material used in the phase 3 study, for instance to detect the potentially important critical quality attributes such as O-acetylation status, would be useful for a direct comparison with earlier results and to further correlate those data with recent evidence collected in the preclinical model [42].
3. Conclusions and Future Perspectives
The O-acetyl content of CPSs so far has not been considered an attribute to be monitored for quality control in the manufacturing of glycoconjugate vaccines. Therefore, content in vaccines may vary for several reasons, including the potential release of O-acetyl groups over time in solution because the group is labile in certain conditions (preferentially in a basic environment or as a consequence of repeated freeze and thawing). Also, variety in the initial content which may be attributed to different growth conditions and manufacturing processes (i.e., different manufacturers and lot-to-lot consistency).
By summarizing the available literature data around O-acetylation, a common driver could be speculated upon: for those antigens comprised of a repetition of single or few saccharide repeating units (e.g., meningococcal serogroup A, Staphylococcus types 5 and 8), the position and the role of the O-acetyl group weighs more on the overall structure due to the fact that the epitope might be involved in the repetition of the same saccharide. On the other hand, as confirmed by data available on meningococcal serogroup C, Y or Group B Streptococcus type III antigens, where the O-acetyl group is located on a ’glycerol’ chain out of the ring positions, its presence seems to be less relevant to the immunological response. This could also explain why a migration to vicinal sites of the O-acetyl group (e.g., in C7 from C8 of meningococcal serogroup C CPS) does not influence the immunoresponse.
Although the position of O-acetyl group is inside or outside the saccharide ring, its distribution and population in terms of percentage of O-acetylated portions should always be considered as a critical quality attribute for a candidate vaccine.
In several cases, including meningococcal serogroup A and pneunomococcal serotype 1, the presence of the O-acetyl groups prevents periodate oxidation and reductive amination coupling as a manufacturing strategy for the glycoconjugates. Controlled de-O-acetylation is required to produce vaccines by this route (e.g., MenAfriVac, Serum Institute India, PATH). For this reason, the O-acetylation pattern also may influence the choice of manufacturing strategy.
The O-acetyl content of CPSs has been historically determined by the Hestrin colorimetric assay [45]. However, during the last 15–20 years new advanced methods that are sufficiently sensitive and based on nuclear magnetic resonance (NMR) spectroscopy [12,46,47] or anion exchange chromatography coupled with conductivity detection (HPAEC-CD) [48] have been developed. While colorimetric assay and HPAEC-CD provides an overall estimation of O-acetyl content, NMR assay developed for meningococcal CPSs [12] also provides a precise distribution of the O-acetyl content at the different saccharide positions. For antigens eliciting an immune response dependent upon the O-acetyl status, it is considered as a key stability attribute which should be monitored carefully.
As reported by Jones et al. [46] and Lemercinier et al. [47], the kinetics of O-acetyl group release following a basic treatment with 0.2 M NaOD, which might be an indicator of its stability in aqueous solution, resulted more rapid (typically 20 min after NaOD addition) for meningococcal serogroups A, W, and Y, while it appeared slower for meningococcal serogroup C (the sample was typically incubated at 37°C for 1 h for complete de-O-acetylation) and for Salmonella typhi Vi CPSs, and also showed that with a 0.05 M final base concentration, the progress of de-O-acetylation could be followed in the spectrometer (narrowing of the CPS NMR peaks).
In conclusion, today we have tools to perform precise quantitative measurements of O-acetyl content in CPSs, and we should consider it a fundamental attribute to be monitored for quality control. However, each bacterial polysaccharide antigen should be considered and analyzed independently due to the intrinsic behavior that each one can exhibit. As shown for the selected bacterial polysaccharide antigens (Neisseria meningitidis, Group B Streptococcus, Streptococcus pneumoniae, Salmonella typhi Vi and Staphylococcus aureus) that have been used in licensed vaccines or vaccine clinical trials, data in the literature strongly demonstrate the fundamental role of the O-acetyl moieties influencing the immune response and, in some cases, imposing a different conformational epitope.
In the future, the ability to determine the crystal structure of protective epitopes [32] combined with the possibility of preparing synthetic conjugate vaccines and the precision of analytical tools such as NMR will allow for a better understanding of the role of O-acetyl groups in glycoconjugate vaccines.
Author Contributions
F.B., R.D.R., and R.R. wrote the paper.
Funding
This research received no external funding.
Conflicts of Interest
F.B, R.D.R. and R.R. are employees of the GSK companies. F.B. and R.R. are owners of patents on related topics.
Footnotes
Sample Availability: Not available.
References
- 1.Vella M., Pace D. Glycoconjugate vaccines: An update. Expert Opin. Biol. Ther. 2015;15:529–546. doi: 10.1517/14712598.2015.993375. [DOI] [PubMed] [Google Scholar]
- 2.Costantino P., Rappuoli R., Berti F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 2011;6:1045–1066. doi: 10.1517/17460441.2011.609554. [DOI] [PubMed] [Google Scholar]
- 3.Jones C. NMR assays for carbohydrate-based vaccines. J. Pharm. Biomed. Anal. 2005;38:840–850. doi: 10.1016/j.jpba.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Calix J.J., Saad J.S., Brady A.M., Nahm M.H. Structural characterization of Streptococcus pneumoniae serotype 9A capsule polysaccharide reveals role of glycosyl 6-O-acetyltransferase wcjE in serotype 9V capsule biosynthesis and immunogenicity. J. Biol. Chem. 2012;287:13996–14003. doi: 10.1074/jbc.M112.346924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calix J.J., Brady A.M., Du V.Y., Saad J.S., Nahm M.H. Spectrum of pneumococcal serotype 11A variants results from incomplete loss of capsule O-acetylation. J. Clin. Microbiol. 2014;52:758–765. doi: 10.1128/JCM.02695-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilli R., Spencer B.L., Moschioni M., Pinto V., Berti F., Nahm M.H., Pantosti A. Identification of Streptococcus pneumoniae serotype 11E, serovariant 11Av and mixed populations by high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and flow cytometric serotyping assay (FCSA) PLoS ONE. 2014;9:e100722. doi: 10.1371/journal.pone.0100722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An. Acad. Bras. Cienc. 2005;77:293–324. doi: 10.1590/S0001-37652005000200009. [DOI] [PubMed] [Google Scholar]
- 8.Claus H., Borrow R., Achtman M., Morelli G., Kantelberg C., Longworth E., Frosch M., Vogel U. Genetics of capsule O-acetylation in serogroup C, W-135 and Y meningococci. Mol. Microbiol. 2004;51:227–239. doi: 10.1046/j.1365-2958.2003.03819.x. [DOI] [PubMed] [Google Scholar]
- 9.Bundle D.R., Smith I.C., Jennings H.J. Determination of the structure and conformation of bacterial polysaccharides by carbon 13 nuclear magnetic resonance. Studies on the group-specific antigens of Neisseria meningitidis serogroups A and X. J. Biol. Chem. 1974;249:2275–2281. [PubMed] [Google Scholar]
- 10.Bröker M., Berti F., Costantino P. Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum. Vaccin. Immunother. 2016;12:1808–1824. doi: 10.1080/21645515.2016.1153206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gudlavalleti S.K., Datta A.K., Tzeng Y.L., Noble C., Carlson R.W., Stephens D.S. The Neisseria meningitidis serogroup A capsular polysaccharide O-3 and O-4 acetyltransferase. J. Biol. Chem. 2004;279:42765–42773. doi: 10.1074/jbc.M313552200. [DOI] [PubMed] [Google Scholar]
- 12.Bardotti A., Averani G., Berti F., Berti S., Carinci V., D’Ascenzi S., Fabbri B., Giannini S., Giannozzi A., Magagnoli C., et al. Physicochemical characterisation of glycoconjugate vaccines for prevention of meningococcal diseases. Vaccine. 2008;26:2284–2296. doi: 10.1016/j.vaccine.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Harrison O.B., Claus H., Jiang Y., Bennett J.S., Bratcher H.B., Jolley K.A., Corton C., Care R., Poolman J.T., Zollinger W.D., et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg. Infect. Dis. 2013;19:566–573. doi: 10.3201/eid1904.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiebig T., Freiberger F., Pinto V., Romano M.R., Black A., Litschko C., Bethe A., Yashunsky D., Adamo R., Nikolaev A., et al. Molecular cloning and functional characterization of components of the capsule biosynthesis complex of Neisseria meningitidis serogroup A: toward in vitro vaccine production. J. Biol. Chem. 2014;289:19395–19407. doi: 10.1074/jbc.M114.575142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry D.S., Lynn F., Lee C.H., Frasch C.E., Bash M.C. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 2002;70:3707–3713. doi: 10.1128/IAI.70.7.3707-3713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupisan S., Limkittikul K., Sosa N., Chanthavanich P., Bianco V., Baine Y., Van der Wielen M., Miller J.M. Meningococcal polysaccharide A O-acetylation levels do not impact the immunogenicity of the quadrivalent meningococcal tetanus toxoid conjugate vaccine: results from a randomized, controlled phase III study of healthy adults aged 18 to 25 years. Clin. Vaccine Immunol. 2013;20:1499–1507. doi: 10.1128/CVI.00162-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michon F., Huang C.H., Farley E.K., Hronowski L., Fusco P.C. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: Effect of O-acetylation on the nature of the protective epitope. Dev. Biol. (Basel) 2000;103:151–160. [PubMed] [Google Scholar]
- 18.Vodopija I., Baklaic Z., Hauser P., Roelants P., André F.E., Safary A. Reactivity and immunogenicity of bivalent (AC) and tetravalent (ACW135Y) meningococcal vaccines containing O-acetyl-negative or O-acetyl-positive group C polysaccharide. Infect. Immun. 1983;42:599–604. doi: 10.1128/iai.42.2.599-604.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peltola H., Safary A., Käyhty H., Karanko V., André F.E. Evaluation of two tetravalent (ACYW135) meningococcal vaccines in infants and small children: a clinical study comparing immunogenicity of O-acetyl-negative and O-acetyl-positive group C polysaccharides. Pediatrics. 1985;76:91–96. [PubMed] [Google Scholar]
- 20.Pichichero M., Anderson P., Gotschlich E., Kamm J., McMullen A., Nielsen S. Immunogenicity of O-acetyl-negative and -positive polysaccharide vaccines for infections with Neisseria meningitidis group C in infants. J. Infect. Dis. 1985;152:850–851. doi: 10.1093/infdis/152.4.850. [DOI] [PubMed] [Google Scholar]
- 21.Borrow R., Findlow J. Prevention of meningococcal serogroup C disease by NeisVac-C. Expert. Rev. Vaccines. 2009;8:265–279. doi: 10.1586/14760584.8.3.265. [DOI] [PubMed] [Google Scholar]
- 22.Fusco P.C., Farley E.K., Huang C.H., Moore S., Michon F. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin. Vaccine Immunol. 2007;14:577–584. doi: 10.1128/CVI.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longworth E., Fernsten P., Mininni T.L., Vogel U., Claus H., Gray S., Kaczmarski E., Borrow R. O-acetylation status of the capsular polysaccharides of serogroup Y and W135 meningococci isolated in the UK. FEMS Immunol. Med. Microbiol. 2002;32:119–123. doi: 10.1111/j.1574-695X.2002.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 24.Taha M.K., Achtman M., Alonso J.M., Greenwood B., Ramsey M., Fox A., Gray S., Kaczmarski E. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet. 2000;356:2159. doi: 10.1016/S0140-6736(00)03502-9. [DOI] [PubMed] [Google Scholar]
- 25.Giardina P.C., Longworth E., Evans-Johnson R.E., Bessette M.L., Zhang H., Borrow R., Madore D., Fernsten P. Analysis of human serum immunoglobulin G against O-acetyl-positive and O-acetyl-negative serogroup W135 meningococcal capsular polysaccharide. Clin. Diagn. Lab. Immunol. 2005;12:586–592. doi: 10.1128/CDLI.12.5.586-592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Z., Bohach G.A., Shiloach J., Norris S.E., Freedberg D.I., Deobald C., Coxon B., Robbins J.B., Schneerson R. Conjugates of group A and W135 capsular polysaccharides of Neisseria meningitidis bound to recombinant Staphylococcus aureus enterotoxin C1: preparation, physicochemical characterization, and immunological properties in mice. Infect. Immun. 2005;73:7887–7893. doi: 10.1128/IAI.73.12.7887-7893.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudlavalleti S.K., Lee C.H., Norris S.E., Paul-Satyaseela M., Vann W.F., Frasch C.E. Comparison of Neisseria meningitidis serogroup W135 polysaccharide-tetanus toxoid conjugate vaccines made by periodate activation of O-acetylated, non-O-acetylated and chemically de-O-acetylated polysaccharide. Vaccine. 2007;46:7972–7980. doi: 10.1016/j.vaccine.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Lewis A.L., Nizet V., Varki A. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc. Natl. Acad. Sci. USA. 2004;101:11123–11128. doi: 10.1073/pnas.0403010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiman S., Uchiyama S., Lin F.Y., Chaffin D., Varki A., Nizet V., Lewis A.L. O-acetylation of sialic acid on group B Streptococcus inhibits neutrophil suppression and virulence. Biochem. J. 2010;428:163–168. doi: 10.1042/BJ20100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto V., Berti F. Exploring the Group B Streptococcus capsular polysaccharides: the structural diversity provides the basis for development of NMR-based identity assays. J. Pharm. Biomed. Anal. 2014;98:9–15. doi: 10.1016/j.jpba.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Pannaraj P.S., Edwards M.S., Ewing K.T., Lewis A.L., Rench M.A., Baker C.J. Group B streptococcal conjugate vaccines elicit functional antibodies independent of strain O-acetylation. Vaccine. 2009;27:4452–4456. doi: 10.1016/j.vaccine.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carboni F., Adamo R., Fabbrini M., De Ricco R., Cattaneo V., Brogioni B., Veggi D., Pinto V., Passalacqua I., Oldrini D., et al. Structure of a protective epitope of group B Streptococcus type III capsular polysaccharide. Proc. Natl. Acad. Sci. USA. 2017;114:5017–5022. doi: 10.1073/pnas.1701885114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry M.B., Daoust V., Carlo D.J. The specific capsular polysaccharide of Streptococcus pneumoniae type 9V. Can. J. Biochem. 1981;59:524–533. doi: 10.1139/o81-073. [DOI] [PubMed] [Google Scholar]
- 34.McNeely T.B., Staub J.M., Rusk C.M., Blum M.J., Donnelly J.J. Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infect. Immun. 1998;66:3705–3710. doi: 10.1128/iai.66.8.3705-3710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lugowski C., Jennings H.J. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type 18C (56) Carbohydr. Res. 1984;131:119–129. doi: 10.1016/0008-6215(84)85409-9. [DOI] [PubMed] [Google Scholar]
- 36.Chang J., Serrano Y., Garrido R., Rodríguez L.M., Pedroso J., Cardoso F., Valdés Y., García D., Fernández-Santana V., Verez-Bencomo V. Relevance of O-acetyl and phosphoglycerol groups for the antigenicity of Streptococcus pneumoniae serotype 18C capsular polysaccharide. Vaccine. 2012;30:7090–7096. doi: 10.1016/j.vaccine.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 37.Jarvis F.G., Mesenko M.T., Martin D.G., Perrine T.D. Physiochemical properties of the Vi antigen before and after mild alkaline hydrolysis. J. Bacteriol. 1967;94:1406–1410. doi: 10.1128/jb.94.5.1406-1410.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szewczyk B., Taylor A. Immunochemical properties of Vi antigen from Salmonella typhi Ty2: Presence of two antigenic determinants. Infect. Immun. 1980;29:539–544. doi: 10.1128/iai.29.2.539-544.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szu S.C., Li X.R., Stone A.L., Robbins J.B. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect. Immun. 1991;59:4555–4561. doi: 10.1128/iai.59.12.4555-4561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones C. Revised structures for the capsular polysaccharides from Staphylococcus aureus Types 5 and 8 components of novel glycoconjugate vaccines. Carbohydr. Res. 2005;340:1097–1106. doi: 10.1016/j.carres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Scully I.L., Pavliak V., Timofeyeva Y., Liu Y., Singer C., Anderson A.S. O-acetylation is essential for functional antibody generation against Staphylococcus aureus capsular polysaccharide. Hum. Vaccin. Immunother. 2018;14:81–84. doi: 10.1080/21645515.2017.1386360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinefield H., Black S., Fattom A., Horwith G., Rasgon S., Ordonez J., Yeoh H., Law D., Robbins J.B., Schneerson R., et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 2002;346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 43.Fattom A.I., Horwith G., Fuller S., Propst M., Naso R. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine. 2004;22:880–887. doi: 10.1016/j.vaccine.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 44.Fattom A., Matalon A., Buerkert J., Taylor K., Damaso S., Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum. Vaccin. Immunother. 2015;11:632–641. doi: 10.4161/hv.34414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hestrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J. Biol. Chem. 1949;180:249–261. [PubMed] [Google Scholar]
- 46.Jones C., Lemercinier X. Use and validation of NMR assays for the identity and O-acetyl content of capsular polysaccharides from Neisseria meningitidis used in vaccine manufacture. J. Pharm. Biomed. Anal. 2002;30:1233–1247. doi: 10.1016/S0731-7085(02)00462-4. [DOI] [PubMed] [Google Scholar]
- 47.Lemercinier X., Martinez-Cabrera I., Jones C. Use and validation of an NMR test for the identity and O-acetyl content of the Salmonella typhi Vi capsular polysaccharide vaccine. Biologicals. 2000;28:17–24. doi: 10.1006/biol.1999.0238. [DOI] [PubMed] [Google Scholar]
- 48.Kao G., Tsai C.M. Quantification of O-acetyl, N-acetyl and phosphate groups and determination of the extent of O-acetylation in bacterial vaccine polysaccharides by high-performance anion-exchange chromatography with conductivity detection (HPAEC-CD) Vaccine. 2004;22:335–344. doi: 10.1016/j.vaccine.2003.08.008. [DOI] [PubMed] [Google Scholar]