Abstract
The endoplasmic reticulum (ER) of columella root cap cells has been postulated to play a role in gravity sensing. We have re-examined the ultrastructure of columella cells in tobacco (Nicotiana tabacum) root tips preserved by high-pressure freezing/freeze-substitution techniques to gain more precise information about the organization of the ER in such cells. The most notable findings are: the identification of a specialized form of ER, termed “nodal ER,” which is found exclusively in columella cells; the demonstration that the bulk of the ER is organized in the form of a tubular network that is confined to a peripheral layer under the plasma membrane; and the discovery that this ER-rich peripheral region excludes Golgi stacks, vacuoles, and amyloplasts but not mitochondria. Nodal ER domains consist of an approximately 100-nm-diameter central rod composed of oblong subunits to which usually seven sheets of rough ER are attached along their margins. These domains form patches at the interface between the peripheral ER network and the ER-free central region of the cells, and they occupy defined positions within central and flanking columella cells. Over one-half of the nodal ER domains are located along the outer tangential walls of the flanking cells. Cytochalasin D and latrunculin A cause an increase in size and a decrease in numbers of nodal ER domains. We postulate that the nodal ER membranes locally modulate the gravisensing signals produced by the sedimenting amyloplasts, and that the confinement of all ER membranes to the cell periphery serves to enhance the sedimentability of the amyloplasts in the central region of columella cells.
Root cap columella cells exhibit a distinct structural polarity, which has been postulated to be related to their gravity-sensing function (Sack, 1991; Sievers et al., 1991; Konings, 1995; Chen et al., 1999). This polarity develops during the differentiation of the meristematic calyptrogen cells into columella cells and coincides with the initial sedimentation of the amyloplast-type statoliths (Barlow, 1975; Moore and McClelen, 1983a, 1983b, 1985). Amyloplast sedimentation appears to be involved in the perception of gravity by columella cells (Konings, 1995), but how this sedimentation is transduced into a differential growth signal has yet to be determined.
Sievers and coworkers (Volkmann and Sievers, 1979; Hensel and Sievers, 1981) have postulated that the sedimentation of statoliths onto sheets of endoplasmic reticulum (ER) cisternae lying parallel to the distal plasma membrane of columella cells could mediate the gravitropic response. This hypothesis has stimulated numerous studies of the ER of columella cells, including investigations of changes in the organization of ER membranes during columella cell development (Barlow et al., 1984; Moore and McClelen, 1985; Sack and Kiss, 1989; Baluska et al., 1997), in the distribution of ER cisternae in response to microtubule- and microfilament-disrupting drugs (Hensel, 1984a, 1984b, 1985, 1986, 1987; Went et al., 1987), and in ER architecture and amyloplast-ER relationships in response to different types of gravitropic treatments (Hensel and Sievers, 1980; Went et al., 1987). These investigations have shown that the normal polar organization of ER membranes in columella cells is dependent on microtubules and microfilaments, that this organization is susceptible to gravitational forces, and that changes in this organization can affect gravisensing. However, none of these studies has provided unambiguous support for the idea that direct statolith-ER interactions mediate the gravisensing response. For this reason, the hypothesis that amyloplast sedimentation onto ER membranes produces a growth signal by triggering a local Ca2+ release (Sievers and Busch, 1992) has yet to be substantiated.
Despite the lack of direct experimental support for this hypothesis, the notion of an involvement of ER in the gravitropic response remains attractive for two reasons. First, ER cisternae serve as reservoirs from which Ca2+ involved in signaling and the control of other cellular activities is released (Sinclair and Trewavas, 1997). Second, the non-random organization of ER cisternae in columella cells appears to be important for the gravitropic response (Sievers et al., 1991). Furthermore, due to the limited ability of chemical fixatives to preserve the structural organization of cells in a natural state for viewing in the electron microscope (Mersey and McCully, 1978; Gilkey and Staehelin, 1986), one cannot rule out the possibility that the earlier studies of ER membranes in columella cells failed to detect critical statolith-ER relationships due to technical limitations. Differences in appearance of ER cisternae in permanganate versus glutaraldehyde-osmium tetroxide fixed cells have been noted (Hensel, 1984a).
In this investigation we have overcome many of the limitations of classical chemical fixation methods by preserving the ultrastructure of columella cells in tobacco (Nicotiana tabacum) root tips by means of high-pressure freezing/freeze-substitution techniques (Kiss and Staehelin, 1995). High-pressure freezing immobilizes all cellular components within milliseconds versus the selected cross-linking of classes of molecules by chemical fixatives over seconds and minutes, and freeze-substitution at low temperatures greatly reduces the amount of post-fixation changes in cellular morphology compared with dehydration at room temperature. Our micrographs provide not only a much clearer picture of the general architecture of columella cells but also demonstrate a unique form of ER, termed “nodal ER.” This specialized form of ER, which is observed exclusively in columella cells, exhibits a non-random distribution that differs in systematic ways between central and flanking columella cells. Based on these findings, we postulate that in conjunction with the sedimenting statoliths, the nodal ER domains could provide directional cues to the gravitropic response system to enable it to determine which side of the root is up and which side is down.
RESULTS
Organization of the Tobacco Root Tip
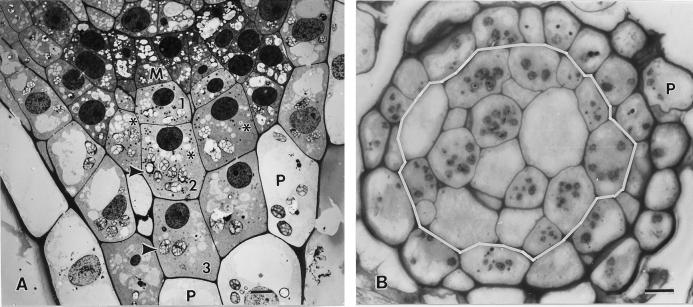
The root cap of 5-d-old tobacco seedlings (root length 5–10 mm) possesses three types of cells: meristematic columella initials (calyptrogen cells), columella cells, and peripheral cells (Fig. 1A). The gravisensing columella cells are organized into approximately 18 files (Fig. 1B), each of which displays three horizontal tiers (tiers 1–3; Fig. 1A). The tier-1 columella cells that derive from the initials have the smallest, and the tier-3 cells that differentiate into the peripheral cells have the largest dimensions. However, because the different files of cells are offset from one another, not all cells in a given tier are at exactly the same stage of growth and development. The one to two cell thick layer of mucilage-secreting peripheral cells forms a protective sheath around the columella cell region.
Figure 1.
Longitudinal (A) and cross (B) sections through 5-d-old tobacco root tips grown vertically but placed horizontally for approximately 2 min during the mounting of the samples for high pressure freezing. A, Electron micrographs depicting a meristematic (M) columella cell initial, and three stories of derived columella cells (nos. 1, 2, and 3). Most of the columella cell amyloplasts (arrowheads) appear sedimented toward the lower, distal cell wall. B, Light micrograph of a root tip cross section at the level of the second tier columella cells (cells demarcated by the white line). Due to the offset organization of the columella cells (see asterisks), the section includes the amyloplast-containing layer (arrowheads) of some cells but not others. The columella cells are surrounded by one or two layers of vacuolated peripheral (P) cells. Bar = 5 μm.
Changes in Columella Cell Architecture during Differentiation and Conversion into Peripheral Cells
The meristematic cells that give rise to the columella cells are typically small and possess a centrally located nucleus that occupies nearly one-half of the cell volume. The plastids, which contain sizable amounts of starch, as well as the mitochondria and the Golgi stacks, are positioned around the nucleus in a random distribution. Both sheet-like and tubular ER domains are seen throughout the cytoplasm, and both types of domains carry polysomes. Only few vacuoles are present.
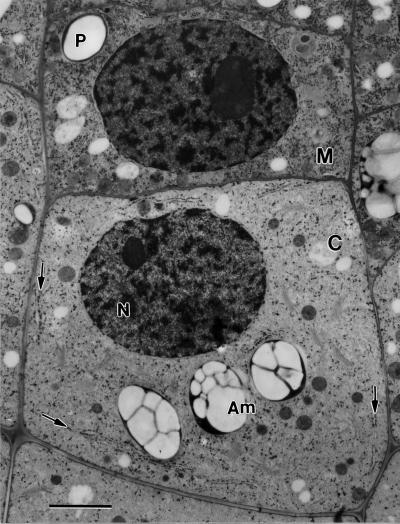
The onset of differentiation of the tier-1 columella cells is marked by an increase in cell size, a reduction in the general staining of the cytoplasm, and a polarization of organelles (Figs. 1A and 2). Thus, while the nucleus remains close to the proximal end of the cell, the starch-rich amyloplasts (statoliths) sediment to the distal end. Most notable, however, is the appearance of a specialized form of ER membranes, termed nodal ER (Figs. 2 and 3; arrows). As detailed below, the nodal ER membranes exhibit a unique morphology and are only observed at specific sites in the cortex of columella cells. Both the mitochondria and the Golgi stacks remain dispersed throughout the central cytoplasm, and the enlarged vacuoles also do not display any polar organization.
Figure 2.
Meristematic (M) columella cell initial and a derived first story columella (C) cell. The arrows point to nodal ER domains in a columella cell. N, Nucleus; Am, amyloplast. Bar = 2 μm.
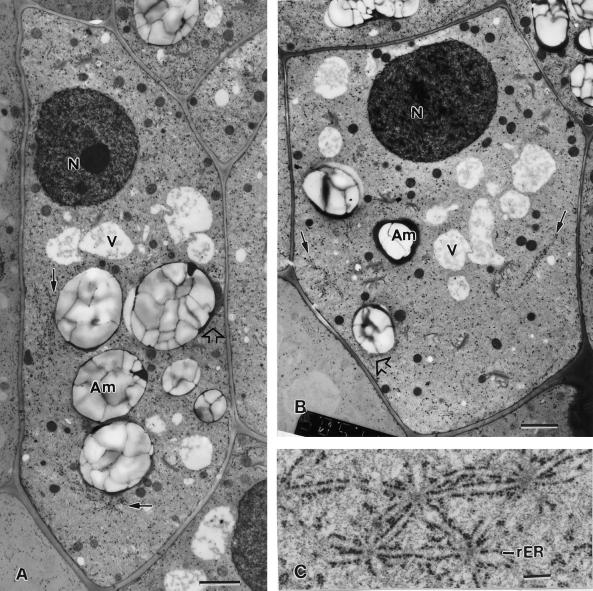
Figure 3.
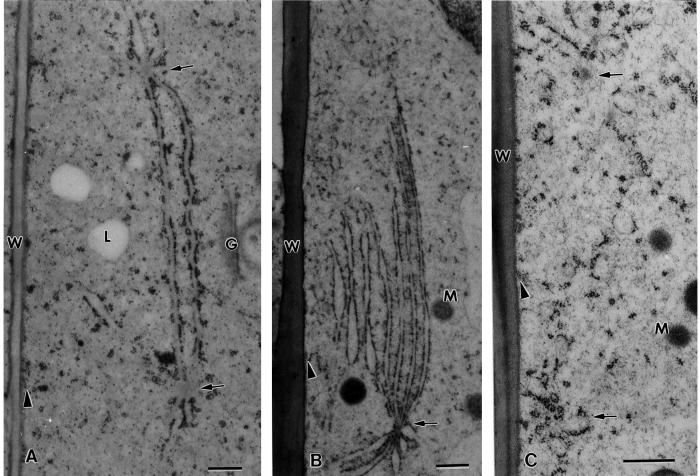
Electron micrographs of a flanking file (A) and a central file (B) columella cell of a tobacco root tip. In the flanking file cell, nodal ER membranes (small arrows) are seen along the external lateral wall and in the basal region, whereas in the central file cell the nodal ER membranes are seen only adjacent to the lateral walls. All of the nodal ER domains are positioned at the interface between the ER-rich cell cortex and the amyloplast-containing central region of the cells. Note how in the flanking cell the nodal ER regions form barriers that prevent the adjacent amyloplasts (Am) from approaching the plasma membrane. In contrast, where the tubular ER in the cell cortex is not shielded by nodal ER membranes the amyloplasts can get much closer to the plasma membrane (white arrows). C, Set of closely spaced and interconnected nodal ER domains from the basal region of a flanking file cell. rER, Rough ER; N, nucleus. A and B, bar = 2 μm; C, bar = 0.2 μm.
The increase in volume of developing columella cells (Figs. 1A, 2, and 3, A and B) is brought about primarily by an increase in the volume of the cytosol and an increase in size of the vacuolar compartment. The nuclei typically lie close to the proximal cell wall, whereas the amyloplasts occupy the distal regions of the cells. The lack of apparent sedimentation of amyloplasts in several of our tier-2 and -3 cell micrographs relates to the fact that during mounting of the samples in the freezing holders the root tips had to be reoriented, and we usually did not allow time for the statoliths to resediment after the mounting. Two types of changes in ER membranes are observed during columella cell differentiation. The first relates to the clearing of most ER membranes from the central region of the cells and the organization of the bulk of the ER into a three-dimensional tubular network in the cell periphery (Fig. 4). The second change in ER membranes relates to the formation of nodal ER domains (Figs. 2, 3, and 5). These specialized ER membrane domains begin to appear at the same time that amyloplast sedimentation is observed in the first tier columella cells (Fig. 2). The number of nodal domains per cell increases until the first tier cells become second tier cells and decreases again in the third tier cells. Also, as will be discussed in greater detail below, distinct differences in the distribution of nodal ER domains between central and flanking types of columella cells become evident in the tier-2 cells (Figs. 3, 5–7) but then begin to disappear in the tier-3 cells. The loss of cell polarity during the conversion of tier-3 cells into peripheral cells coincides with the loss of nodal ER domains, which become regular rough ER cisternae.
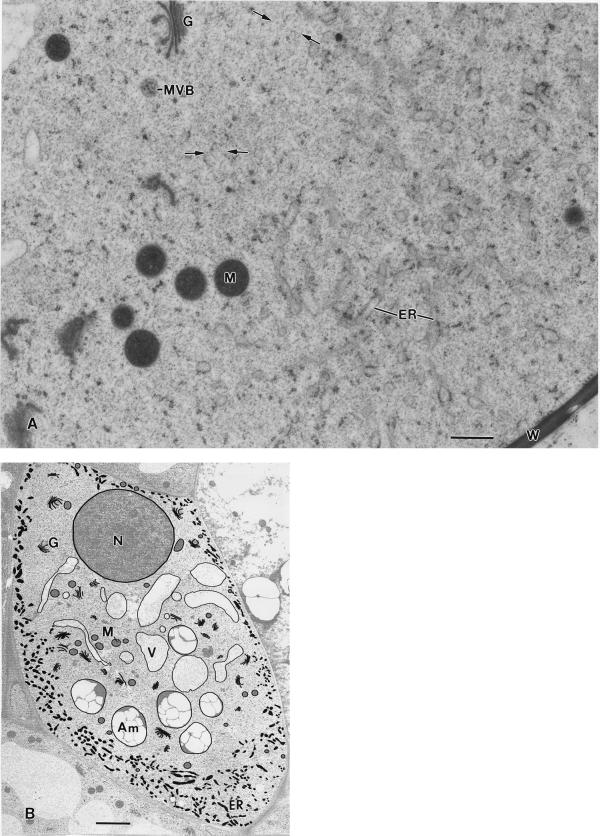
Figure 4.
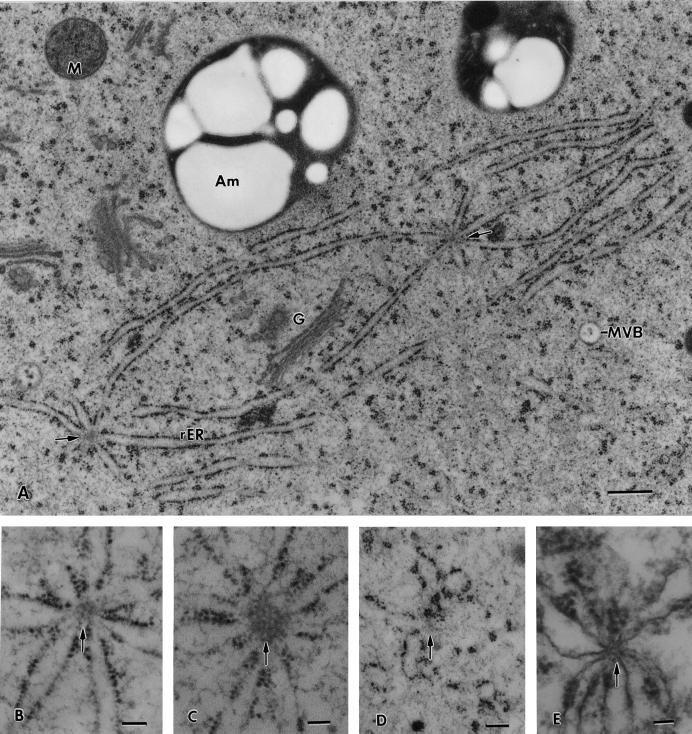
A, Electron micrograph of the interface region between the ER-rich cortical and the ER-devoid central region of a columella cell. The ER is organized in the form of interconnected 100- to 150-nm diameter tubules that carry small patches of polysomes. The spaces between the ER tubules are filled by a cytosolic matrix that seamlessly blends with the cytosolic matrix in the central region. The scarcity of organelles in the central region is striking, and in some places (small arrows) thin, actin-like filaments can be detected (see also Fig. 3 B). MVB, Multivesicular body. B, Micrograph of a longitudinal/slightly tangential section through a tobacco flanking file columella cell in which the major membranous organelles have been traced to highlight their distribution and particularly the distribution of the ER in the cell cortex. Note that the Golgi stacks (G), vacuoles (V), and amyloplasts (Am) are all confined to the central ER-devoid region of the cells and that only some mitochondria (M) have penetrated the tubular ER network region in the cell cortex. A, Bar = 0.5 μm; B, bar = 2 μm.
Figure 5.
Higher magnification micrographs of nodal ER membrane domains. A, Two nodal ER domains from the basal region of a flanking file cell that are interconnected by two shared ER cisternae. All of the ER cisternae that appear attached to the nodal rods (arrows) are typical sheet-like, rough ER (rER) membranes. B through E, Effects of drugs and fixatives on the structural organization of the nodal rods. B, Control cell nodal ER domain in which the nodal rod appears composed of a small number of oblong subunits. C, Nodal ER from a root tip exposed to 40 μm cytochalasin D for 1 h to disrupt actin filaments. Note the increased diameter of the nodal rod and the oblong shape of the subunits that form the rod. D, Likely former nodal ER domain from a root tip sample treated with 1 μm propyzamide, a microtubule disrupting drug, for 1 h. The central rod of the nodal ER domain appears completely disrupted. E, Nodal ER domain as seen in a root tip preserved by chemical fixation (2% [v/v] glutaraldehyde, 1% [v/v] OsO4 with 0.8% [v/v] potassium ferricyanide). The central rod structure is greatly reduced in diameter and resembles a cross-sectioned microtubule. G, Golgi; M, mitochondrion; MVB, multivesicular body. A, Bar = 0.5 μm; B through E, bar = 0.1 μm.
Figure 7.
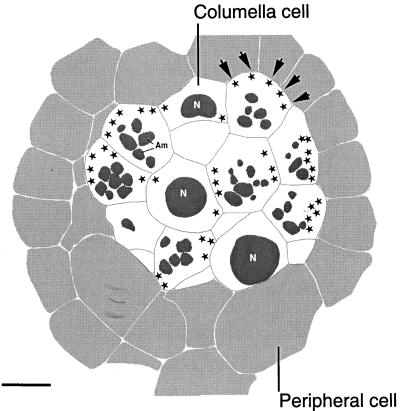
Reconstruction of a 0.2-μm-thick cross-section at the level of the second tier columella cells of a tobacco root cap. The columella cells are shown in white and the surrounding peripheral cells in light gray. Due to the offset type of arrangement of the columella cells, some of the cells appear cross-sectioned at the level of their nuclei (N), whereas others are cross-sectioned at the level of their amyloplasts (Am; see also Fig. 1B). The sites of nodal ER domains are indicated with stars. Note that the majority of the nodal ER domains are located along the external periclinal walls of the outer flanking file cells (arrows). Bar = 5 μm.
We have also examined lateral roots for the presence of nodal ER domains. As in the primary roots, nodal ER domains appeared in columella cells as soon as they began to exhibit a typical polar organization (data not shown). This finding is consistent with earlier studies in which the columella cells of primary and secondary roots were shown to exhibit the same ultrastructural features (Ransom and Moore, 1983; Moore and Pasieniuk, 1984).
The Tubular ER Network of the Cell Cortex Forms a Sharp Interface with the ER-Depleted Central Cell Region and Excludes Amyloplasts, Vacuoles, and Golgi
As mentioned above, columella cells of tobacco roots contain two types of ER membrane domains, tubular network and nodal ER domains. Both of these ER membrane domains are located in the cell periphery and, as their name suggests, they represent subdomains of the larger ER membrane system. The tubular ER network forms a continuum in the cell cortex and abuts the inner surface of the plasma membrane. In contrast, the nodal ER domains are organized into patches that occupy the interface between the cortical tubular network and the ER-poor cytoplasm in the interior of the cells. Of the larger organelles, only the mitochondria seem to penetrate the ER-rich cortical regions of columella cells on a regular basis; Golgi stacks, vacuoles, and amyloplasts are confined to the interior region.
The distinct interface between the ER-rich cell periphery and the ER-poor central region of a tier-2 columella cell is shown in Figure 4A. In this semitangential section through the cell cortex, the 100- to 150-nm-diameter ER tubules are seen to be organized into a three-dimensional network and to possess a limited number of bound polysomes (Fig. 4A). Free polysomes are also seen in the spaces between the membrane tubules. The transition to the ER-depleted central cytoplasm appears quite abrupt due the relatively sharp boundary of the cortical ER network (Fig. 4B). The cytosolic material that makes up the bulk of the central cytoplasm is comprised of a network of fine filamentous molecules, some of which are straight and resemble single actin molecules (Fig. 4A). Dispersed within this cytoskeletal network are fairly evenly distributed free polysomes, some Golgi-derived vesicles, as well as individual Golgi stacks, vacuoles, amyloplasts, and mitochondria. The fine filamentous network material of the central region also extends seamlessly into the restricted spaces between the tubules of the cortical ER network.
Structural Organization of Nodal ER Domains
Nodal ER domains appear to arise from the attachment of between three and eight, but usually seven, rough ER cisternae to a central “nodal rod” element (Figs. 3C and 5). The three-dimensional organization of such structures can be seen in the model shown in Figure 6A, which is based on a reconstruction from six 70-nm-thick serial section electron micrographs. In this model, the nodal rod is shown in yellow, and the seven attached cisternae (blue) are seen to be connected to the rod along their edges. This association appears to induce a sheet-like organization of the ER membranes and to stimulate the binding of polysomes as evidenced by the higher density of polysomes on the flat nodal ER membranes than on the tubular ER membranes in the cell cortex (compare Figs. 4A and 5A). Adjacent nodal domains frequently appear to be linked together by one or more shared ER cisternae. In most instances these membrane sheets tend to run parallel to the cell walls and extend over considerable distances (microns; Figs. 5A and 8A), but in some cases the links are shorter and connect groups of nodal domains into lattice-like arrays (Fig. 3C).
Figure 6.
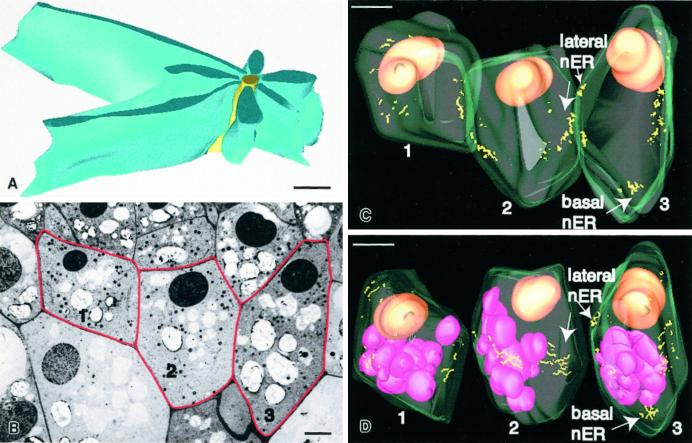
A, Model of a nodal ER domain based on six 70-nm-thick serial sections. The yellow structure corresponds to the approximately 100-nm-diameter central rod element and the blue structures to the rough ER cisternae that are attached along their margins to the central rod. B, One of 198 serial 100-nm-thick serial sections used for the three-dimensional reconstruction of the three columella cells depicted in Figure 6, C and D. The red lines highlight the three reconstructed cells. Cell 1 corresponds to a very young second tier flanking file cell and contains only a limited number of nodal ER domains; cell 2 corresponds to a partially expanded, central file second tier cell, and cell 3 to a nearly fully expanded, flanking file second tier cell. C, Model of the three cells outlined in B. The cell walls are displayed in light green, the nuclei are colored orange, and the nodal rods correspond to the yellow lines. Note that most of the lateral nodal ER domains are organized into patches, most of which are found near the equatorial regions of the cells. Only in the more mature flanking cells are basal nodal ER domains seen. D, Model in which the three cells of C are shown as separate units. The cells have also been rotated to optimize the viewing of the nodal ER domain patches. The purple structures correspond to amyloplasts, which are not sedimented due to the root tip manipulations prior to freezing. A, Bar = 0.5 μm; B, bar = 3 μm; C and D, bar = 5 μm.
Figure 8.
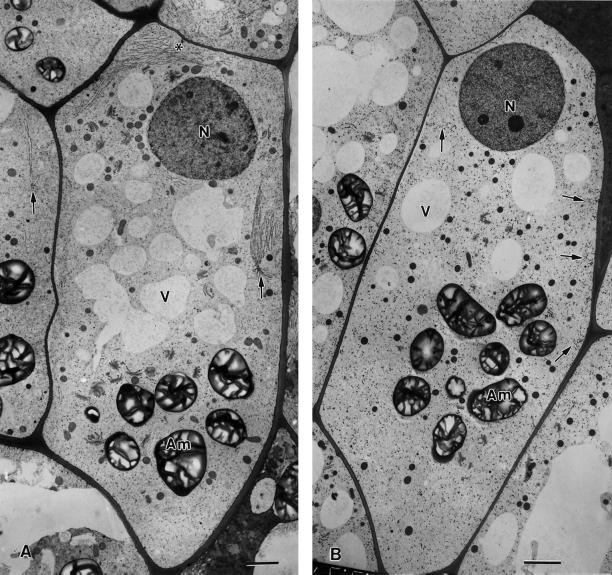
Effects of drugs that disrupt microfilaments (A) and microtubules (B) on the distribution and organization of nodal ER domains (arrows) in flanking file columella cells. In A, the 1-h treatment with 1 μm latrunculin A is seen to lead to much larger nodal ER domains (see also Figs. 5C and 9B) along the lateral walls, but the number of these domains decreases (data not shown). Note also the proliferation of rough ER membrane sheets (asterisk) between the nucleus and the adjacent upper cell wall. The cell in B was treated with 10 μm propyzamide for 1 h. Only small fragments of what might have been former nodal ER domains (arrows) are seen. N, Nucleus; Am, amyloplast; V, vacuole. Higher magnification views of the nodal ER domains of these samples are shown in Figure 9. Bars = 2 μm.
Our micrographs suggest that the nodal rods are composed of subunits (Fig. 5, B and C), but the exact organization of the subunits in the rods has yet to be determined. In control cells, the rods have a diameter of approximately 100 nm and variable lengths, and in cross-sectional views they often display more lightly staining, oblong “subunits” with a diameter of approximately 10 nm and a length of approximately 20 nm. These subunits become more evident in cells incubated with the actin depolymerizing drugs cytochalasin D (Fig. 5C) and latrunculin A (not shown), treatments that also lead to an increase in diameter of the rod elements. Propyzamide, a drug that disrupts microtubules, leads to the breakdown of the nodal ER domains and to the disruption of the nodal rods (Fig. 5D). In samples where nodal ER domains can still be recognized in chemically fixed cells, the diameter of their central rods is reduced by approximately 50% compared with those seen in freeze-substituted cells, and the rods stain more like microtubules (Fig. 5E).
In the context of columella cell function, we have also analyzed the spatial relationship of the amyloplast statoliths to the different types of ER domains. This analysis has shown that the nodal ER domains are sufficiently stable platforms to resist deformation by sedimented amyloplasts and that they physically prevent amyloplasts from approaching the plasma membrane and from perturbing the underlying tubular ER domains (Figs. 2, 3A [filled arrows], and 5A). In cortical cytoplasmic regions that are not covered by nodal ER domains the amyloplast can sediment closer to the plasma membrane (Fig. 3, A and B, white arrows) but seem to be prevented from contacting the plasma membrane by the tubular ER network. We have not observed any significant redistribution of ER membranes in response to root tip reorientation.
Spatial Distribution of Nodal ER Regions in Columella Cells and Tissues
To learn more about the three-dimensional organization of nodal ER regions in central and flanking columella cells, we have reconstructed three adjacent tier-2 cells from 198 serial 100-nm sections using 556 electron micrographs (Fig. 6, C and D). The large number of micrographs was needed because nodal ER domains could not be reliably identified on negatives taken at magnifications below 3,000×. This required a separate set of negatives for each cell and the joining of the three independent models by means of the IMOD software program (Kremer et al., 1996). In Figure 6C the three joined cells, one central (no. 2) and two flanking (nos. 1 and 3), are shown in their natural spatial relationship, whereas in Figure 6D the cells are illustrated as individual entities and have been rotated for optimal visualization of the nodal ER regions (yellow lines). Each yellow line corresponds to the axis of a nodal rod. The associated nodal ER cisternae are not displayed. The orange spheres correspond to nuclei that are positioned close to the top of the cells and the pink spheres to amyloplasts. The most notable feature of the models is that most of the nodal ER domains are clustered into discrete cortical patches. Furthermore, most of these variably sized patches are located along the lateral walls near the equatorial regions of the cells. The flanking cell (no. 3) contains a nodal ER patch near its basal (distal) wall, whereas the less developed other flanking cell (no. 1) like the central number 2 does not display such a distal nodal ER system. The absence of a distal nodal ER system in cell number 2 is typical of central columella cells. Differences in distribution of nodal ER domains between central and flanking file columella cells are also evident in the reconstruction presented in Figure 7.
Figure 7 displays on a more global scale the distribution of nodal ER domains within a 200-nm-thick slice of tier-2 columella cells. In this model, the cells depicted in light gray correspond to peripheral cells and the cells shown in white to columella cells (compare with Fig. 1B). In the latter cells, the sectioned nuclei and the starch granules are shaded dark gray; the nodal ER domains are depicted as black stars. Clearly the most striking aspect of this model is the high concentration of nodal ER domains along the outer periclinal wall of the flanking columella cells (Fig. 7). Of the 42 nodal domains shown in this reconstruction, 66% are located along the outer periclinal walls of the flanking cells and 34% along all of the other walls of the flanking and central cells.
Effects of Cytoskeleton-Disrupting Drugs on Nodal ER Domains
Cytoskeleton-disrupting drugs were applied to 5-d-old tobacco root tips to evaluate the relationship between the cytoskeleton and the nodal ER domains. As already reported by Hensel (1985), microfilament-disrupting drugs such as cytochalasin B have a profound effect on the distribution of ER membranes in columella cells. For the structural studies reported here we treated the root tips with either cytochalasin D (40 μm) or latrunculin A (1 μm) for 1 h. In addition, we investigated the effects of latrunculin A on root growth and the gravitropic response for periods of up to 24 h. These latter studies demonstrated a slowdown in root growth and a reduced gravitropic response, but no necrotic changes in the roots. At the ultrastructural level of analysis, both of the microfilament-disrupting drugs were found to cause the sheet-like ER membranes to consolidate into large, nodal ER-associated parallel membrane arrays along the lateral walls and between the nucleus and the proximal cell wall (Figs. 8A and 9B). These massive nodal ER structures have at their center nodal rod elements with significantly increased diameters (compare Fig. 5, B and C). The increase in size of the individual nodal ER domains is paralleled by a significant decrease in their numbers. The latrunculin A and cytochalasin D treatments also led to a more pronounced sedimentation of amyloplasts, sometimes to positions very close to the plasma membrane, the redistribution of Golgi stacks and mitochondria to locations around the nucleus and the amyloplasts, and the concentration of vacuoles between the nucleus and the sedimented amyloplasts (Fig. 8A).
Figure 9.
Higher magnification views of nodal ER domains (arrows) of a control cell (A), a cell treated with 1 μm latrunculin A for 1 h (B), and a cell treated with 10 μm propyzamide for 1 h (C). Note the greatly expanded rough ER membrane sheets associated with the nodal ER domain in the latrunculin A sample and the loss of nodal ER domains in the propyzamide-treated specimen. W, Cell wall; G, Golgi; M, mitochondrion; L, lipid body; arrowheads, cortical microtubules. Compare with Figure 8. Bar = 0.5 μm.
Exposure of root tips to the microtubule-disrupting drug propyzamide (10 μm, 1 h) produced a significant amount of ER fragmentation and a complete loss of nodal ER domains (Figs. 8B and 9C). In the columella cells treated for 1 h it was hard to even discern ER cisternae that might have been associated previously with nodal ER domains (Fig. 9C) or to identify structures that might correspond to mechanically disrupted nodal rods (Fig. 5D). Treatment of root tips with 0.1% (v/v) dimethyl sulfoxide (DMSO) without drugs produced no change in ER ultrastructure.
DISCUSSION
In this study we have re-investigated the ultrastructure of tobacco columella cells preserved by high-pressure freezing and freeze-substitution techniques, methods that allow for a much improved preservation of cellular architecture for electron microscope analysis (Gilkey and Staehelin, 1986). The four most notable new findings are: (a) the identification of a specialized form of ER, termed nodal ER; (b) the demonstration that the bulk of the columella cell ER is tubular and not sheet-like; (c) the finding that virtually all ER cisternae are confined to a clearly delineated layer underlying the plasma membrane; and (d) the discovery that this ER-rich peripheral layer excludes Golgi stacks, vacuoles, and amyloplasts but not mitochondria.
Images of Chemically Fixed Roots Do Not Provide an Accurate View of the ER of Columella Cells
Numerous papers describing the deleterious effects of chemical fixatives on the morphology of ER and other membrane systems have been published. For example, in 1973 Buckley demonstrated that chemical fixation of cultured chick embryo cells caused the ER to vesiculate, and similar changes of vacuoles were subsequently reported for tomato petiolar hair cells (Mersey and McCully, 1978). The problems with chemical fixatives have been traced to their slow mode of action compared with the rate of cellular activities, the selective nature of the cross-linking reactions, and the inability of chemical fixatives to preserve the osmotic conditions of cellular compartments (for review, see Gilkey and Staehelin, 1986; McCauly and Hepler, 1992). Although all of these problems can be overcome by means of ultrarapid freezing/freeze-substitution (or freeze-fracture/-etch) techniques (for example, see Linder and Staehelin, 1979; Gilkey and Staehelin, 1986), electron microscopists have been slow in embracing these techniques because they are technically more demanding than conventional fixation methods. Nevertheless, the feasibility of cryofixing entire root caps for ultrastructural analysis has been demonstrated previously (Craig and Staehelin, 1988; Kiss and Sack, 1990), and the results reported here confirm the superiority of cyrofixation over chemical fixation methods.
Virtually all of the ER cisternae of columella cells are located in a clearly defined layer underlying the plasma membrane, which completely encompasses the central cytoplasm (Fig. 4B). Within this layer, the bulk of the ER cisternae have a tubular architecture and form a three-dimensional membrane network with the rest being organized in the sheet-like cisternae of the nodal ER domains (Figs. 4A and 5A). The 100- to 150-nm-diameter tubules carry some polysomes, but most exhibit a smooth morphology (Fig. 4A). The tubular network is slightly thicker along the distal (lower) end of the cells, but the width seen in Figure 4A is somewhat misleading due to the tangential nature of the section in that region of the cell. Nevertheless, the tubular appearance of the bulk of the ER membranes in our cryofixed columella cells is in stark contrast to the much publicized sheet-like appearance of the majority of the ER cisternae in cells fixed with potassium permanganate (Sievers and Volkmann, 1972; Juniper and French, 1973; Hensel, 1987). For comparative reasons we have also fixed tobacco root tips with 2% potassium permanganate and observed the same meandering, sheet-like architecture as reported in the literature (data not shown). Thus, the extensive sheet-like morphology of columella cell ER cisternae seen in permanganate fixed cells is an artifact caused by the fixative. Although other chemical fixatives and en bloc staining protocols (e.g. zinc-iodide-osmium and osmium ferricyanide) can yield more faithful images of ER membranes in columella cells (Sack and Kiss, 1989), they are far from perfect as evidenced by the failure of such studies to visualize the unique morphological features of nodal ER domains and their ability to induce a reticulation of cis- and trans-Golgi cisternae (for review, see Ladinsky et al., 1999). Due to these uncertainties, it is unclear whether the local ER aggregates reported by Hepler (1981) in osmium ferricyanide-stained cells correspond to the nodal ER domains described in this paper.
Confinement of the ER to the Cortex of Columella Cells Enhances the Sedimentability of the Statoliths
One of the intriguing aspects of the cortical tubular ER network of columella cells is its ability to exclude Golgi stacks, vacuoles, and amyloplasts, but not mitochondria (Fig. 4B). This selective type of exclusion does not seem to be based on organelle size alone, since the mitochondria that do permeate the network are only marginally smaller than the Golgi stacks. However, this exclusionary ability may help maintain the compactness of the ER system in the cell periphery and thereby maximize the volume of the central region of the cell where amyloplasts can sediment without impedance by ER cisternae.
Due to their small size, neither the dispersed Golgi stacks nor the mitochondria in the ER-free central region should significantly affect amyloplast movement, and due to the deformability of the highly branched vacuolar compartment it too should not significantly reduce statolith sedimentation. In contrast, the protein-containing ER cisternae with bound polysomes could potentially present a barrier to sedimenting amyloplasts, and even if the cisternae could be easily stretched, displaced, or disrupted by the sedimenting amyloplasts, the associated physical changes in the ER membranes could lead to uncontrolled calcium fluxes or to the destruction of ER-bound polysomes. Based on these considerations, the confinement of the bulk of the ER to a tight peripheral network both protects the ER system from being damaged by the sedimenting amyloplasts as well as enhances their ability to sediment through the central region of the cell.
The Spatial Distribution of Nodal ER Membranes within the Root Cap Suggests a Role in Gravisensing Modulation
The discovery of yet another specialized ER domain, the nodal ER, adds to an already long list of structurally defined ER domains each of which performs a specific cellular function (Staehelin, 1997). Nodal ER domains are defined by a central rod-like element to which sheets of rough ER membranes (most often seven) are attached along their edges (Fig. 5). These domains lie at the interface between the cortical ER tubular network and the central, ER-devoid region of columella cells. Furthermore, our three-dimensional reconstructions demonstrate that they are organized into patches whose spatial distribution differs between central and flanking cells. Although we have no direct evidence that would point to a specific function of these unique ER structures, the following observations suggest that they may be part of the gravisensing system.
Nodal ER membranes are observed exclusively in columella cells of tobacco seedlings. During columella cell development they appear at about the time that statolith sedimentation becomes evident, and they disappear when the columella cells are converted to peripheral cells. Since the principle function of columella cells is gravisensing, the expression of nodal ER membranes only in columella cells makes it likely that they participate in the gravisensing process.
Which other observations might support this hypothesis? Since gravisensing involves detection of the spatial orientation of the root tip with respect to the orientation of the gravity vector, the sensing system is likely to depend on elements whose spatial distribution within the cells and within the tissue is non-uniform. The nodal ER domains clearly meet this criterion, since their spatial organization differs between central and flanking cells (Fig. 6C), and in the flanking cells they are positioned primarily along the outer tangential walls (Fig. 7). As evidenced by cell ablation studies carried out on Arabidopsis root cap cells, not all columella cells contribute equally to the gravisensing response (Blancaflor et al., 1998). In particular, ablation of the central columella cells affected root curvature to a much greater extent than ablation of the flanking cells. Thus, the differences in the spatial organization of the nodal ER domains in central and flanking cells is consistent with their differential responses in the cell ablation experiments.
The centro-symmetric organization of the root cap around the longitudinal root axis raises another question related to root tip gravitropism: how do columella cells know if they are located in the upper or lower one-half of a tilted root? One solution to this problem would be to have a sensing system that differs in its properties between the outer and inner tangential walls. Again, as illustrated in Figure 7, the distribution of the nodal ER domains is consistent with the requirements for such an asymmetric gravisensing signal-producing system.
Hypothetical Function of Nodal ER Membrane Domains
The two already discussed features of nodal ER domains that could be functionally important are (a) their positioning at the interface between the central cytoplasm and the cortical tubular ER membrane network, and (b) their non-random distribution in central and flanking columella cells. A third potentially important property is their apparent mechanical rigidity as evidenced by the regular organization of the membranes around the central core structures (Figs. 3C and 5A) and their lack of deformation by sedimented amyloplasts. Such “stiff” ER domains positioned in specific locations of columella cells could participate in gravisensing in two ways. They could be directly responsible for producing the physiological signals, e.g. via local Ca2+ fluxes as envisaged by the ER signaling hypothesis (Sack, 1991; Sievers and Busch, 1992), or they could serve as local “shields” to prevent the amyloplasts from perturbing specific regions of the cortical cytoplasm and the plasma membrane. We favor the latter hypothesis for the following reasons.
During the past decade numerous researchers have obtained indirect evidence for a role of Ca2+ in gravisensing (for review, see Chen et al., 1999), but more direct investigations involving Ca2+-reporter systems have failed to demonstrate any gravity-induced transient changes in cytosolic Ca2+ levels (Legue et al., 1997). These findings do not rule out a role of Ca2+ in the gravisensing response, but make Ca2+ release by ER membranes a less likely primary signal in the gravisensing pathway. Thus, the production of gravisensing signals by direct amyloplast-nodal ER interactions is not supported by the experimental evidence currently available.
The shielding hypothesis of the nodal ER domains suggests that these domains serve as directional modulators of the gravisensing system by producing “protected” cortical ER/plasma membrane domains, which could locally alter the signaling patterns produced by the sedimented amyloplasts. This hypothesis is consistent with the recently formulated tensegrity model of gravisensing (Staehelin et al., 2000; Yoder et al., 2001), which postulates that statolith signaling in columella cells is brought about by the sedimenting amyloplasts disrupting postulated links between the actin-based cytoskeletal network and stretch receptors in the plasma membrane. By shielding cortical cytoplasmic domains from approaching amyloplasts, the nodal ER domains could locally prevent the disruption of the links to the receptors in the plasma membrane and thereby modulate the signaling pattern produced by the sedimented statolith amyloplasts. There is no information currently available as to the nature of the receptors or to the presence of the postulated links to the receptors. However, the recent discovery that cytosolic pH plays a key role in the early events of the gravisensing signaling pathway of roots (Scott and Allen, 1999) suggests that the postulated stretch receptors could be functionally coupled to H+-pumps in the plasma membrane, since gravistimulation leads to alkalinization of the cytoplasm.
Drug Experiments Indicate That Nodal ER Membranes Are Linked to the Actin-Based Cytoskeletal Network
As documented in Figures 8 and 9, both actin filament and microtubule-disrupting drugs affect the organization of nodal ER membranes. Thus, whereas actin filament-disrupting drugs (latrunculin A and cytochalasin D) cause a decrease in number but an increase in size of nodal ER domains (Figs. 8A and 9B), the microtubule-disrupting drug propyzamide causes a complete disruption and loss of such domains (Figs. 8B and 9C). These perturbations can be explained if one assumes that the nodal ER domains are linked to the actin-based cytoskeletal network that pervades the central cytoplasm and is postulated to also connect to the plasma membrane receptors discussed in the preceding section. It is well known that the organization of ER membranes in plant cells is dependent on the actinomyosin system (Knebel et al., 1990; Staehelin, 1997), and direct links between ER membranes and actin filament bundles have been documented in Drosera tentacles preserved by cryofixation/freeze-substitution techniques (Lichtsheidl et al., 1990).
The consolidation of the nodal ER regions into fewer but larger structures in the presence of latrunculin A suggests that the size of nodal ER domains reflects an equilibrium between actin filament-based dispersive (pulling) forces and nodal ER assembly forces (e.g. nodal ER rod assembly and rod-membrane binding forces). Upon disruption of the actin filaments, the dispersive forces are weakened and a new equilibrium develops that enables the nodal rod elements to assemble into larger diameter rods (Fig. 5C) that can bind more and larger sheets of ER membranes (Fig. 9B). Since the number of rod subunits is most likely constant, any increase in size of some rods will be at the expense of others, thereby leading to a reduction in the number of nodal ER domains. A re-organization of dispersed tubular and cisternal ER elements into ER stacks has also been observed in cytochalasin-treated germinating pollen tubes preserved by cryfixation/freeze-substitution techniques (Lancelle and Hepler, 1988).
The disruption of nodal ER membranes in the presence of propyzamide, in turn, suggests that microtubules of columella cells regulate the forces exerted by actin filaments on nodal ER domains. In the absence of these actin “brakes,” the randomly organized actinomyosin elements of columella cells (Fig. 4A; Driss-Ecole et al., 2000; Yoder et al., 2001) may disrupt the nodal ER domains by simply tearing these specialized membrane domains apart.
MATERIALS AND METHODS
Seeds of tobacco (Nicotiana tabacum) were surface sterilized, imbibed, and germinated on sterile filter paper moistened by medium containing macronutrients as described by Haughn and Sommerville (1986) in vertically positioned Petri dishes. Seeds were geminated in darkness for 2 d, then grown in continuous light from 60-W fluorescent lamps for 3 d at room temperature. Five days after sowing, 1-mm-long root tip segments from 5- to 10-mm roots were excised while submerged under a 3.5% (v/v) Suc solution and mounted in high-pressure freezing specimen cups coated with lecithin (Craig and Staehelin, 1988).
For the cytochalasin D, latrunculin A, and propyzamide treatments, seedlings were inserted with their roots into glass capillaries glued onto a microscope slide (Hensel, 1984a) and then placed for 1 h into the cytochalasin D (40 μm), latrunculin A (1 μm), and propyzamide (10 μm) solutions containing 0.1% (v/v) DMSO. Control experiments involved treatment with 0.1% (v/v) DMSO but no drugs.
After freezing, the samples were substituted in either 4% (v/v) OsO4 in acetone at −80°C for 3 d or in 1% (v/v) glutaraldehyde with 0.1% (v/v) tannic acid in acetone at −80°C for 2.5 d, 2% (v/v) OsO4 with 0.01% (v/v) uranyl acetate in acetone at −50°C for 6 h, then −20°C for 1 d, 4°C overnight (Ladinsky et al., 1999), and warmed to room temperature. After a dry acetone wash at room temperature, samples were infiltrated in Spurr's resin and polymerized at 70°C for 8 h.
Several series of 200 to 300 serial thin sections (100 nm thick) were produced to reconstruct the nucleus, the amyloplasts, the nodal ER domains, and the plasma membranes of sets of adjacent columella cells. Shorter series of 70-nm sections were used to reconstruct the nodal ER regions. The sections were stained with uranyl acetate in 70% (v/v) methanol 15 min, lead citrate for 4 min, and examined at 80 kV in a Philips CM10 electron microscope.
The negatives used for the modeling experiments were digitized using a Dage-18 video camera (Dage-MTI, Wabash, MI) into a Silicon Graphics INDY computer. Serial images were aligned using the MIDAS program. Features in the aligned image stack were modeled using the IMOD software program (Kremer et al., 1996). Each cell was modeled individually, and each type of organelle was recorded as an individual “object” and assigned a different color. The IMOD software was also used to join the individual cell models into columella tissue models.
ACKNOWLEDGMENT
We would like to thank Dr. Marisa Otegui for her helpful comments on the manuscript.
Footnotes
This work was supported by the National Aeronautics and Space Administration (grant no. NAG5–3967).
LITERATURE CITED
- Baluska F, Kreibaum A, Vitha S, Parker JS, Barlow PW, Sievers A. Central root cap cells are depleted of endoplasmic microtubules and actin microfilament bundles: implications for their role as gravity-sensing statocytes. Protoplasma. 1997;196:212–223. doi: 10.1007/BF01279569. [DOI] [PubMed] [Google Scholar]
- Barlow PW. The root cap. In: Torrey J, Clarkson DT, editors. The Development and Function of Roots. London: Academic Press; 1975. pp. 21–54. [Google Scholar]
- Barlow PW, Hawes CR, Horne JC. Structure of amyloplasts and endoplasmic reticulum in the root caps of Lepidium sativum and Zea mays observed after selective membrane staining and by high-voltage electron microscopy. Planta. 1984;160:363–371. doi: 10.1007/BF00393418. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley IK. Studies in fixation for electron microscopy using cultures cells. Lab Invest. 1973;29:398–410. [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson P. Gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S, Staehelin LA. High pressure freezing of intact plant tissue: evaluation and characterization of novel features of the endoplasmic reticulum and associated membrane systems. Eur J Cell Biol. 1988;46:80–93. [PubMed] [Google Scholar]
- Driss-Ecole D, Vassy J, Rembur J, Guivarc'h A, Prouteau M, Dewitte W, Perbal G. Immunolocalization of actin in root statocytes of Lens culunaris L. Exp Bot. 2000;51:521–528. doi: 10.1093/jexbot/51.344.521. [DOI] [PubMed] [Google Scholar]
- Gilkey JC, Staehelin LA. Advances in ultrarapid freezing for the preservation of cellular ultrastructure. J Electron Microsc Tech. 1986;3:177–210. [Google Scholar]
- Guikema JA, Gallegos GL. Plastids: dynamic components of plant cell development. Trans Kans Acad Sci. 1992;95:50–54. [PubMed] [Google Scholar]
- Haughn GW, Sommerville C. Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet. 1986;204:430–434. [Google Scholar]
- Hensel W. Microtubules in statocytes from roots of cress (Lepidium sativum L.) Protoplasma. 1984a;119:121–134. doi: 10.1007/BF01287824. [DOI] [PubMed] [Google Scholar]
- Hensel W. A role of microtubules in the polarity of statocytes from roots of Lepidium sativum L. Planta. 1984b;162:404–414. doi: 10.1007/BF00393452. [DOI] [PubMed] [Google Scholar]
- Hensel W. Cytochalasin B affects the structural polarity of statocytes from cress roots (Lepidium sativum L.) Protoplasma. 1985;129:178–187. doi: 10.1007/BF01279915. [DOI] [PubMed] [Google Scholar]
- Hensel W. Demonstration of microfilaments in statocytes of cress roots. Naturwissenschaften. 1986;73:510–511. doi: 10.1007/BF00367204. [DOI] [PubMed] [Google Scholar]
- Hensel W. Cytodifferentiation of polar plant cells: formation and turnover of endoplasmic reticulum in root statocytes. Exp Cell Res. 1987;172:377–384. doi: 10.1016/0014-4827(87)90395-8. [DOI] [PubMed] [Google Scholar]
- Hensel W, Sievers A. Effects of prolonged omnilateral gravistimulation on the ultrastructure of statocytes and on the graviresponse of roots. Planta. 1980;150:338–346. doi: 10.1007/BF00384664. [DOI] [PubMed] [Google Scholar]
- Hensel W, Sievers A. Induction of gravity-dependent plasmatic responses in root statocytes by short time contact between amyloplasts and the distal endoplasmic reticulum complex. Planta. 1981;153:303–307. doi: 10.1007/BF00384246. [DOI] [PubMed] [Google Scholar]
- Hepler PK. The structure of the endoplasmic reticulum revealed by osmium tetroxide-potassium ferricyanide staining. Eur J Cell Biol. 1981;26:102–110. [PubMed] [Google Scholar]
- Juniper BE, French A. The distribution and redistribution of endoplasmic reticulum (ER) in geoperceptive cells. Planta. 1973;109:211–224. doi: 10.1007/BF00387085. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Sack FD. Severely reduced gravitropism in dark-grown hypocotyls of a starch-deficient mutant of Nicotiana sylvestris. Plant Physiol. 1990;94:1867–1873. doi: 10.1104/pp.94.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Staehelin LA. High pressure freezing. In: Severs NJ, Shotton DM, editors. Rapid Freezing, Freeze Fracture and Deep Etching. New York: Wiley-Liss; 1995. pp. 89–104. [Google Scholar]
- Knebel W, Quader H, Schnepf E. Mobile and immobile endoplasmic reticulum in onion bulb epidermis cells: short- and long-term observations with a confocal laser scanning microscope. Eur J Cell Biol. 1990;52:328–340. [PubMed] [Google Scholar]
- Konings Gravitropism of roots: an evaluation of progress during the last three decades. Acta Bot Neerl. 1995;44:195–223. doi: 10.1111/j.1438-8677.1995.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelle SA, Hepler PK. Cytochalasin-induced ultrastructural alterations in Nicotiana pollen tubes. Protolasm Suppl. 1988;2:65–75. [Google Scholar]
- Legue V, Blancaflor E, Wymer C, Perbal G, Fanttin D, Dilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtsheidl IK, Lancelle SA, Hepler PK. Actin-endoplasmic reticulum complexes in Drosera: their structural relationship with the plasmalemma, nucleus, and organelles in cells prepared by high pressure freezing. Protoplasma. 1990;155:116–126. [Google Scholar]
- Linder JC, Staehelin LA. A novel model for fluid secretion by the trypanosomeatid contractile vacuole apparatus. J Cell Biol. 1979;83:371–382. doi: 10.1083/jcb.83.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauly MM, Hepler PK. Cortical ultrastructure of freeze-substituted protonemata of the moss Funaria hygrometrica. Protoplasm. 1992;169:168–178. [Google Scholar]
- Mersey B, McCully ME. Monitoring the course of fixation in plant cells. J Microsc. 1978;114:49–76. [Google Scholar]
- Moore R, McClelen CE. Amorphometric analysis of cellular differentiation in the root cap of Zea mays. Am J Bot. 1983a;70:611–617. [Google Scholar]
- Moore R, McClelen CE. Ultrastructural aspects of cellular differentiation in the root cap of Zea mays. Can J Bot. 1983b;61:1566–1572. [Google Scholar]
- Moore R, McClelen CE. Changes in the distribution of plastids and endoplasmic reticulum during cellular differentiation in root caps of Zea mays. Ann Bot. 1985;56:73–81. doi: 10.1093/oxfordjournals.aob.a086996. [DOI] [PubMed] [Google Scholar]
- Moore R, Pasieniuk J. Structure of columella cells in primary and lateral roots of Ricinus communis (Euphorbiaceae) Am J Bot. 1984;53:517–726. [Google Scholar]
- Ransom JS, Moore R. Geoperception in primary and lateral roots of Phaseolus vulgaris (Fabaceae): I. Structure of columella cells. Am J Bot. 1983;70:1048–1056. [PubMed] [Google Scholar]
- Sack FD. Plant gravity sensing. Int Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Sack FD, Kiss JZ. Rootcap structure in wild type and in a starchless mutant of Arabidopsis. Am J Bot. 1989;76:454–464. [PubMed] [Google Scholar]
- Scott AC, Allen NS. Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol. 1999;121:1291–1298. doi: 10.1104/pp.121.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers A, Buchen B, Volkmann D. Role of the cytoskeleton in gravity perception. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. London: Academic Press; 1991. pp. 169–182. [Google Scholar]
- Sievers A, Busch MB. An inhibitor of the Ca2+-ATPases in the sarcoplasmic and endoplasmic reticula inhibits transduction of the gravity stimulus in cress roots. Planta. 1992;188:619–622. doi: 10.1007/BF00197057. [DOI] [PubMed] [Google Scholar]
- Sievers A, Volkmann D. Verursacht differentieller Druck der Amyloplasten auf ein komplexes Endomembransystem die Geoperzeption in Wurzeln? Planta. 1972;102:160–172. doi: 10.1007/BF00384870. [DOI] [PubMed] [Google Scholar]
- Sinclair W, Trewavas AJ. Calcium in gravitropism: a re-examination. Planta. 1997;203:S85–S90. doi: 10.1007/pl00008120. [DOI] [PubMed] [Google Scholar]
- Staehelin LA. The plant ER: adynamic organelle composed of a large number of discrete functional domains. Plant J. 1997;11:1151–1165. doi: 10.1046/j.1365-313x.1997.11061151.x. [DOI] [PubMed] [Google Scholar]
- Staehelin LA, Zheng HQ, Yoder TL, Smith JD, Todd P. Columella cells revisited: novel structures, novel properties and a novel gravisensing model. Grav Space Biol Bull. 2000;13:95–100. [PubMed] [Google Scholar]
- Volkmann D, Sievers A. Graviperception in multicellular organs. In: Haupt W, Feinleib M, editors. Encyclopedia of Plant Physiology, New Series, 7. Berlin: Springer-Verlag; 1979. pp. 573–600. [Google Scholar]
- Went M, Kuo-Huang L, Sievers A. Gravitropic bending of cress roots without contact between amyloplasts and complexes of endoplamsic reticulum. Planta. 1987;172:321–329. doi: 10.1007/BF00398660. [DOI] [PubMed] [Google Scholar]
- Yoder TL, Zheng H-Q, Todd P, Staehelin LA (2001) Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]