Abstract
Background:
Mechanic’s hands is a well characterized manifestation of select idiopathic inflammatory myopathy (IIM) syndromes. Less well characterized is the hyperkeratosis of the toes and plantar surface of the feet that can also accompany these disorders. We aim to describe common pedal signs in the context of IIM, and suggest that it may be another key feature in the presentation of these syndromes.
Methods & Findings:
A cohort of 2145 myositis patient charts gathered since 2003 were retrospectively reviewed using the key search terms “mechanic’s feet” and/or “mechanic’s foot”. Charts that included either phrase were further reviewed for clinical characteristics. Nine patients were identified with documentation describing “mechanic’s feet” or “mechanic’s foot”. All nine affected individuals carried a diagnosis of DM, seven of whom also met criteria for antisynthetase syndrome. In eight patients (89%), it presented in conjunction with mechanic’s hands. Six (67%) presented with anti-Jo-1 antibodies, and three (33%) were seronegative.
Conclusions:
Although the term “mechanic’s feet” has been used to describe this clinical finding in patients in our myositis cohort, we propose the term “hiker’s feet”, given that the presentation resembles a callousing pattern more typical of avid hikers or long-distance walkers. Prevalence data are not yet known but should be considered for further study. If the presenting signs of IIM are expanded to include hiker’s feet, it could aid in not only diagnosis and management, but also provide insights into the pathophysiology of these diseases.
Introduction
Idiopathic inflammatory myopathies (IIM) are a subset of autoimmune diseases that include dermatomyositis, polymyositis, and immune mediated necrotizing myopathy. The prevalence of these syndromes is estimated between 0.1 and 6 per 100,000 in the US, with the majority of cases presenting in women and the average age at presentation varying depending upon the subset of IIM. 1–8 IIM syndromes are usually characterized by chronic muscle inflammation and muscle weakness and may have associated extramuscular signs and symptoms as part of a syndrome complex.
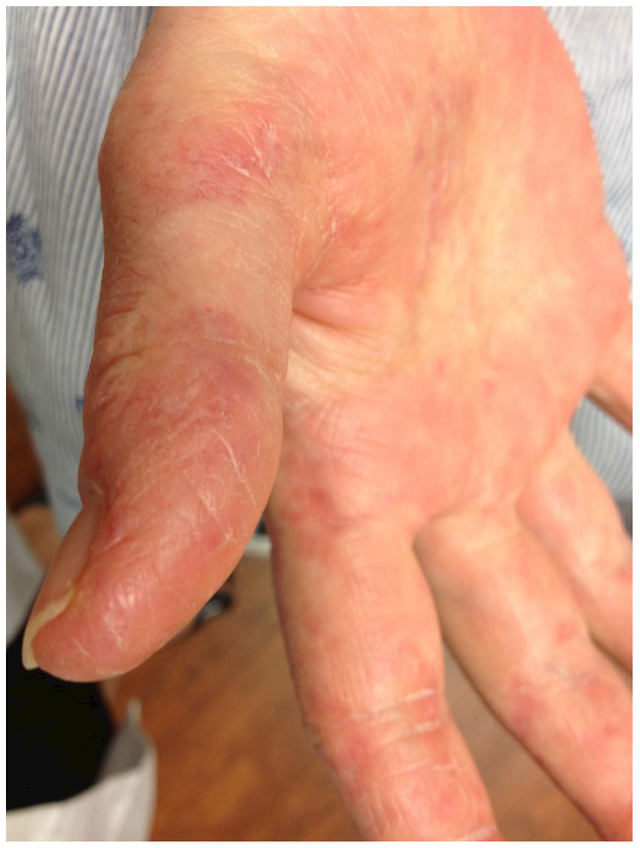
The clinical finding of “mechanic’s hands” is a cutaneous condition associated with patients with inflammatory myopathy, most often in dermatomyositis and the anti-synthetase syndrome9–12 First described in 1979, this sign is characterized by cracking and hyperkeratosis of the finger pads, palms, and lateral surfaces of the digits, and commonly involving the first, second, and third fingers9 [Figure 1]. Aside from being painful and aesthetically unpleasant for myositis patients, mechanic’s hands are also frequently associated with antisynthetase autoantibodies, anti-MDA5 autoantibodies, and anti-PM-Scl autoantibodies with interstitial lung disease (ILD).10,13,14 Thus, this sign may be clinically useful in helping to delineate unique clinical phenotypes.15,16
Figure 1.
Mechanic’s hands
Despite widespread familiarity with mechanic’s hands amongst specialists who diagnose and treat myositis patients, there is little mention of a similar sign found in the feet of IIM patients. A literature review revealed only one study, a case series of three patients, describing cutaneous involvement on the feet of patients with inflammatory myopathies.17 We aim to describe our experience with this physical exam finding and increase awareness with the term “hiker’s feet”.
Methods
This study was approved by our Institutional Review Board, and written consent was obtained for all patient participation and photography. Throughout a 13-year period (2003–2016) 2,145 patients have been evaluated. Patient charts were retrospectively reviewed using the key search terms “mechanic’s feet” and/or “mechanic’s foot”. Charts that included either phrase were further reviewed for phenotype information including demographics and clinical characteristics. Interstitial lung disease was considered present if the chart indicated a history of ILD based on high-resolution chest CT and/or pulmonary function testing.
Results
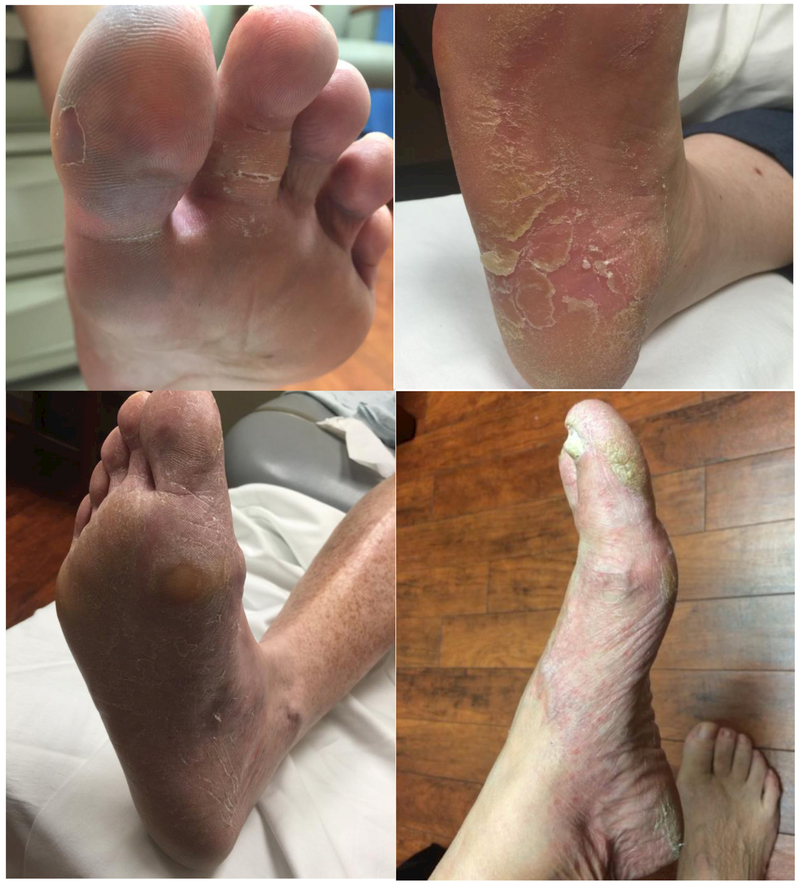
Nine patients were identified with documentation describing “mechanic’s feet” or “mechanic’s foot” [Figure 2]. Eight of the nine (89%) individuals were female, and seven (78%) were Caucasian. The median age at diagnosis was 43 (range 37– 63). All nine affected individuals carried a diagnosis of DM, seven of which who also met criteria for antisynthetase syndrome. In eight patients (89%), it presented in conjunction with mechanic’s hands. Six (67%) presented with anti-Jo-1 antibodies, and three (33%) were seronegative.
Figure 2.
Examples of “Hiker’s feet” in four of our patients.
Discussion
During the course of 13 years in our clinic, our team has identified hiker’s feet in nine patients. Eight of the nine patients presented with concomitant mechanic’s hands. The exact incidence and prevalence of this sign is unknown but is likely under-recognized, given that we did not record its presence systematically due to not all patients receiving a thorough pedal exam and the search terms used relying on the physician noting “mechanic’s feet/foot” to describe the physical examination finding.
The finding of “hiker’s feet” is similar in gross appearance to mechanic’s hands. It presents as bilateral dryness, cracking, and hyperkeratosis predominantly on the plantar aspect of feet and toes. Patients generally report pain and are self-conscious of the appearance of their feet. Given the many similarities between hiker’s feet and mechanic’s hands and the fact that 89% of our patients presented with concomitant mechanic’s hands, we suspect that they may share common pathophysiologic characteristics. However, further research into the epidemiology, histology, and clinical associations of hiker’s feet is warranted for further characterization.
Should hiker’s feet prove to share pathophysiologic characteristics with mechanic’s hands, this may serve to further elucidate the etiology of these symptoms and perhaps other characteristics of IIM syndromes as well.
It is worth noting that we could not definitively exclude other possible causes of such hyperkeratosis and epithelial cracking of the plantar foot. Other potential causes include: calluses secondary to extensive standing, walking, or running, repeated trauma secondary to peripheral neuropathy, pedal dermatitis, dyshidrotic eczema, hyperkeratotic eczema, and acral peeling skin syndrome. However, each patients’ chart was reviewed extensively and there were no other recorded findings either in the history or physical exam that would suggest any of these patients had any of these conditions. Furthermore, its coexistence with mechanic’s hands in ~90% of patients supports its link to the underlying myopathy. Lastly, Shibuya et al. has also described this exam finding in three patients with inflammatory myopathy, further strengthening the association with hiker’s feet and IIM.17
It is important to consider the clinical implications of this sign: (i) It may aid in the diagnosis of antisynthetase patients, especially in a subset of patients who may have developed hiker’s feet but lack mechanic’s hands. (ii) If mechanic’s hands and hiker’s feet are essentially a manifestation of the same phenomenon distinguished only by their anatomical locations, then hiker’s feet would also presumably be associated with antisynthetase autoantibodies and ILD.13,14 This is consistent with the presence of ILD in 89% of our patients and anti-Jo-1 antibodies in 67%. Thus, this sign could serve useful in earlier diagnosis of antisynthetase syndrome and potentially warrants increased screening for interstitial lung disease.
Table 1.
Clinical Characteristics of 9 patients with Hiker’s Feet.
| Patient No. |
Sex | Race | Age at diagnosis |
Antibody | Bohan & Peter |
Mechanic’s Hands |
ILD |
|---|---|---|---|---|---|---|---|
| 1 | F | W | 44 | Jo-1, U1RNP |
DM | + | + |
| 2 | F | W | 55 | Jo-1 | DM | + | + |
| 3 | F | W | 39 | Negative | DM | − | − |
| 4 | F | B | 51 | Negative | DM | + | + |
| 5 | F | W | 39 | Negative | DM | + | + |
| 6 | F | W | 50 | Jo-1 | DM | + | + |
| 7 | F | W | 38 | Jo-1 | DM | + | + |
| 8 | F | W | 63 | Jo-1 | DM | + | + |
| 9 | M | B | 37 | Jo-1 | DM | + | + |
“DM” = dermatomyositis; “PM” = polymyositis; “ILD” = interstitial lung disease; “Jo-1” = anti-Jo-1 autoantibodies; “U1RNP” = anti-U1-ribonucleoprotein autoantibodies.
Acknowledgements:
None.
Funding: None.
Funding disclosure for this work: none
Abbreviations:
- IIM
Idiopathic inflammatory myopathies
- ILD
Interstitial lung disease
Footnotes
Competing and Conflicting Interests: We, the authors, have no competing or conflicting interests to declare, financial or otherwise, as relates to this manuscript. We state this having reviewed the NIH guidelines on conflicts of interest available at http://grants.nih.gov/grants/policy/coi/.
Co-first-authors;
References
- 1.Furst DE, Amato AA, Iorga SR, Gajria K, Fernandes AW. Epidemiology of adult idiopathic inflammatory myopathies in a U.S. managed care plan. Muscle Nerve. 2012;45(5):676–683. [DOI] [PubMed] [Google Scholar]
- 2.Smoyer-Tomic KE, Amato AA, Fernandes AW. Incidence and prevalence of idiopathic inflammatory myopathies among commercially insured, medicare supplemental insured, and medicaid enrolled populations: An administrative claims analysis. BMC Musculoskelet Disord. 2012;13:103–2474–13–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prieto S, Grau JM. The geoepidemiology of autoimmune muscle disease. Autoimmun Rev 2010;9(5):A330–4. [DOI] [PubMed] [Google Scholar]
- 4.Wilson FC, Ytterberg SR, St Sauver JL, Reed AM. Epidemiology of sporadic inclusion body myositis and polymyositis in olmsted county, minnesota. J Rheumatol 2008;35(3):445–447. [PubMed] [Google Scholar]
- 5.Briani C, Doria A, Sarzi-Puttini P, Dalakas MC. Update on idiopathic inflammatory myopathies. Autoimmunity. 2006;39(3):161–170. [DOI] [PubMed] [Google Scholar]
- 6.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971–982. [DOI] [PubMed] [Google Scholar]
- 7.Oddis CV, Conte CG, Steen VD, Medsger TA Jr. Incidence of polymyositis-dermatomyositis: A 20-year study of hospital diagnosed cases in Allegheny County, PA 1963–1982. J Rheumatol 1990;17(10):1329–1334. [PubMed] [Google Scholar]
- 8.Benbassat J, Geffel D, Zlotnick A. Epidemiology of polymyositis-dermatomyositis in israel, 1960–76. Isr J Med Sci 1980;16(3):197–200. [PubMed] [Google Scholar]
- 9.Stahl NI, Klippel JH, Decker JL. A cutaneous lesion associated with myositis. Ann Intern Med 1979;91(4):577–579. [DOI] [PubMed] [Google Scholar]
- 10.Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep 2011;13(3):175–181. [DOI] [PubMed] [Google Scholar]
- 11.Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med 1991;325(21):1487–1498. [DOI] [PubMed] [Google Scholar]
- 12.Indart F, Espana A, Idoate MA, Lucas I, Quintanilla E. A cutaneous lesion associated with primary polymyositis. Arch Dermatol 1993;129(9):1207–1208. [PubMed] [Google Scholar]
- 13.Ang CC, Anyawu CO, Robinson E, Okawa J, Feng R, et al. Clinical signs associated with an increased risk of interstitial lung disease: a retrospective study of 101 patients with dermatomyositis. Br J Dermatol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Török L, Dankó K, Cserni G, Szûcs G. PM-SCL autoantibody positive scleroderma with polymyositis (mechanic’s hand: clinical aid in the diagnosis). J Eur Acad Dermatol Venerol 2004;18(3)356–359. [DOI] [PubMed] [Google Scholar]
- 15.Taggart AJ, Finch MB, Courtney PA, Gormley GJ. Anti jo-1 myositis. ‘mechanic’s hands’ and interstitial lung disease. Ulster Med J 2002;71(1):68–71. [PMC free article] [PubMed] [Google Scholar]
- 16.Marie I, Josse S, Hatron PY, et al. Interstitial lung disease in anti-jo-1 patients with antisynthetase syndrome. Arthritis Care Res (Hoboken). 2013;65(5):800–808. [DOI] [PubMed] [Google Scholar]
- 17.Shibuya H, Arakawa S, Kai Y, Hatano Y, Okamoto O, Takayasu S, et al. Three cases of ‘mechanic’s hands’ associated with interstitial pneumonia: possible involvement with foot lesions. J Dermatol 2003. December;30(12):892–7. [DOI] [PubMed] [Google Scholar]