Key Points
Question
Is DNA methylation in peripheral blood cells associated with circulating tumor necrosis factor α levels and risk of coronary heart disease?
Findings
In this epigenome-wide study, top methylation loci associated with circulating tumor necrosis factor α concentration in whole blood or CD4+ T cells were located in or near the DTX3L-PARP9 gene complex, NLRC5, and ABO. The findings in NLRC5 and DTX3L-PARP9 were successfully replicated and linked to gene expression, and methylation at these loci was robustly inversely associated with the risk of incident coronary heart disease.
Meaning
After further validation, these epigenetic associations might be useful in the pursuit of new or improved therapeutic applications.
This epigenome-wide meta-analysis of 8 cohort studies analyzes blood-derived DNA methylation and tumor necrosis factor α levels across the epigenome and assesses the association of findings with incident coronary heart disease.
Abstract
Importance
Tumor necrosis factor α (TNF-α) is a proinflammatory cytokine with manifold consequences for mammalian pathophysiology, including cardiovascular disease. A deeper understanding of TNF-α biology may enhance treatment precision.
Objective
To conduct an epigenome-wide analysis of blood-derived DNA methylation and TNF-α levels and to assess the clinical relevance of findings.
Design, Setting, and Participants
This meta-analysis assessed epigenome-wide associations in circulating TNF-α concentrations from 5 cohort studies and 1 interventional trial, with replication in 3 additional cohort studies. Follow-up analyses investigated associations of identified methylation loci with gene expression and incident coronary heart disease; this meta-analysis included 11 461 participants who experienced 1895 coronary events.
Exposures
Circulating TNF-α concentration.
Main Outcomes and Measures
DNA methylation at approximately 450 000 loci, neighboring DNA sequence variation, gene expression, and incident coronary heart disease.
Results
The discovery cohort included 4794 participants, and the replication study included 816 participants (overall mean [SD] age, 60.7 [8.5] years). In the discovery stage, circulating TNF-α levels were associated with methylation of 7 cytosine-phosphate-guanine (CpG) sites, 3 of which were located in or near DTX3L-PARP9 at cg00959259 (β [SE] = −0.01 [0.003]; P = 7.36 × 10−8), cg08122652 (β [SE] = −0.008 [0.002]; P = 2.24 × 10−7), and cg22930808(β [SE] = −0.01 [0.002]; P = 6.92 × 10−8); NLRC5 at cg16411857 (β [SE] = −0.01 [0.002]; P = 2.14 × 10−13) and cg07839457 (β [SE] = −0.02 [0.003]; P = 6.31 × 10−10); or ABO, at cg13683939 (β [SE] = 0.04 [0.008]; P = 1.42 × 10−7) and cg24267699 (β [SE] = −0.009 [0.002]; P = 1.67 × 10−7), after accounting for multiple testing. Of these, negative associations between TNF-α concentration and methylation of 2 loci in NLRC5 and 1 in DTX3L-14 PARP9 were replicated. Replicated TNF-α–linked CpG sites were associated with 9% to 19% decreased risk of incident coronary heart disease per 10% higher methylation per CpG site (cg16411857: hazard ratio [HR], 0.86; 95% CI, 0.78-1.95; P = .003; cg07839457: HR, 0.89; 95% CI, 0.80-0.94; P = 3.1 × 10−5; cg00959259: HR, 0.91; 95% CI, 0.84-0.97; P = .002; cg08122652: HR, 0.81; 95% CI, 0.74-0.89; P = 2.0 × 10−5).
Conclusions and Relevance
We identified and replicated novel epigenetic correlates of circulating TNF-α concentration in blood samples and linked these loci to coronary heart disease risk, opening opportunities for validation and therapeutic applications.
Introduction
Tumor necrosis factor α (TNF-α) is a proinflammatory cytokine with pleiotropic effects in human health and disease. In addition to its well-characterized pathogenic contributions to inflammatory and autoimmune diseases, atherosclerosis, type 2 diabetes, and cancer, TNF-α also plays a key homeostatic role in pathogen defense, tissue repair and regeneration, and organ development.1 Therapeutic inhibition of TNF-α has been used in clinical settings, both successfully (eg, in various forms of autoimmune diseases) and unsuccessfully (eg, in multiple sclerosis).2 Furthermore, treatment with TNF inhibitors has long been known to lower the risk of cardiovascular disease among patients with autoimmune disease,3 and currently several trials4 are assessing cardioprotective effects of inhibiting inflammatory cytokines. Recently, the randomized placebo-controlled CANTOS trial5,6 reported significant reductions in recurrent cardiovascular risk associated with interleukin 1β inhibition, which was achieved independently of changes in lipid levels. While such findings highlight the clinical promise of targeting systemic inflammation in the context of cardiovascular disease, the underlying mechanisms of action remain elusive.
Circulating concentrations of TNF-α have a moderate genetic determinant, with heritability estimates ranging from 17%7 to 39%8 in large-scale European twin studies to 68% in a Ugandan community with a high prevalence of tuberculosis.9 Known common mutations account for a small fraction of that heritable component, explaining less than 4% of TNF-α variance in a recent meta-analysis of genome-wide association studies (written communication, B. Z. Alizadeh, MD, MSc, PhD, June 30, 2017). Emerging evidence suggests that epigenetic processes, such as DNA methylation, that reflect changes in gene expression that occur without sequence mutations, may offer promising clues in the search for missing TNF-α heritability. For example, methylation of cytosine-phosphate-guanine (CpG) loci in the TNF promoter was associated with TNF-α expression and plasma TNF-α levels in several population-based studies.10,11 In vitro, experimental manipulation of DNA methylation has been shown to alter the ability of cells to produce TNF-α,12 offering causal support for the association observed in population studies. To our knowledge, however, no study to date has comprehensively examined DNA methylation across the entire genome in association with circulating levels of TNF-α in large human populations, or has assessed TNF-α epigenetics with regards to cardiovascular risk.
Therefore we conducted what is, to our knowledge, the first epigenome-wide meta-analysis of associations between circulating TNF-α levels and DNA methylation in whole-blood samples or isolated lymphocytes in 4794 individuals in the Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) consortium. We subsequently achieved replication of the top CpG loci in an independent population of 816 individuals, evaluated the associations between DNA methylation and cis gene expression, and assessed genotype contributions to the observed CpG methylation variation in the regions of interest.
Finally, we investigated the association of the top epigenetic correlates of circulating TNF-α concentrations with incident coronary heart disease (CHD) in a meta-analysis that included 11 461 participants who experienced 1895 CHD events (coronary insufficiency, coronary revascularization, recognized myocardial infarction, and coronary death). For this purpose, we added the data of 4 additional study cohorts.
Methods
Discovery and Replication Populations
In the discovery phase, the epigenome-wide study included individuals of European descent from 6 studies participating in the CHARGE consortium13: the Framingham Heart Study (FHS), the Genetics of Lipid-Lowering Drugs and Diet Network (GOLDN) study, the Invecchiare in Chianti Study (InCHIANTI), the Kooperative Gesundheitsforschung in der Region Augsburg cohort (KORA), the Lothian Birth Cohort 1921 (LBC1921), and the Normative Aging Study (NAS). Two additional Finnish cohorts, the Northern Finland Birth Cohort 1966 (NFBC66) and the Helsinki Birth Cohort Study (HBCS), were designated for replication. (The NAS and HBCS cohorts were excluded from the main analysis because of the extreme variability in TNF-α measurements [Table 1], which could be because of the poor performance of multiplexed assays compared with enzyme-linked immunosorbent assays [ELISA], as previously reported.14) Finally, a Dutch cohort, the Rotterdam Study (RS), was excluded from the main epigenetic analyses because it does not have TNF-α measurements, but was included in the subsequent analyses because the data set did include DNA methylation, DNA sequence, and gene expression data.
Table 1. Characteristics of Participating Cohortsa.
| Study | No. of Participants | Country | Mean Age, (SD), y | Women, No. (%) | Mean TNF-α (SD), pg/mL | TNF-α Assay | Coefficient of Variation of TNF-α Measurements |
|---|---|---|---|---|---|---|---|
| Discovery phase | |||||||
| Framingham Heart Study | 1730 | US | 67.0 (9.0) | 898 (51.9) | 1.4 (1.2) | ELISA (R&D Systems) | Intra 6%-8%, inter 5%-11% |
| Genetics of Lipid Lowering Drugs and Diet Network | 970 | US | 47.8 (16.3) | 504 (52.0) | 3.2 (1.7) | ELISA (R&D Systems) | Intra 5%, inter 10% |
| InCHIANTI | 498 | Italy | 62.8 (15.8) | 275 (55.2) | 4.3 (2.2) | ELISA (R&D Systems) | Intra 7%, inter <21% |
| KORA | 800 | Germany | 69.0 (4.3) | 392 (49.0) | 2.5 (2.2) | ELISA (R&D Systems) | Intra 6%, inter 14% |
| Lothian Birth Cohort 1921 | 165 | UK | 86.7 (0.4) | 89 (53.9) | 1.5 (1.6) | Immunonepheometry (Dade-Behring) | Not available |
| Normative Aging Studyb | 631 | US | 74.6 (6.8) | 0 | 55.0 (155.8) | Milliplex Human Cytokine/ Chemokine Panel (EMD Millipore) | Not available |
| Replication phase | |||||||
| Northern Finland Birth Cohort 1966 | 667 | Finland | 31.0 (0.3) | 374 (56.0) | 7.8 (9.0) | ELISA (Merck) | Intra 3%, inter 7% |
| Helsinki Birth Cohortb | 149 | Finland | 63.3 (2.7) | 0 | 16.7 (47.6) | Milliplex Map Human Metabolic Hormone Panel Kit (HMH-34K) | Not available |
Abbreviations: InCHIANTI, Invecchiare in Chianti Study; inter, inter-assay coefficients of variation; intra, intra-assay coefficients of variation; KORA, Kooperative Gesundheitsforschung in der Region Augsburg Study; TNF-α, tumor necrosis factor α; UK, United Kingdom; US, United States.
The Rotterdam Study (Netherlands) is excluded from this Table because it did not include TNF-α measurements.
Excluded from the analyses because of concerns over reliability of TNF-α measurements.
Individuals in any of the studies who had reported an autoimmune diagnosis or were taking immune-modulating agents (eg, TNF-α blockers) were not included in the analyses. Further details about each study are included in Table 1 and in eMethods 1 in the Supplement.
This study was approved by the institutional review board of the University of Alabama at Birmingham. All protocols of analyzed studies were approved by the institutional review boards of the participating study sites. All participants provided written informed consent.
Laboratory Measurements
Circulating TNF-α concentrations were measured in picograms per milliliter using the approaches listed in Table 1. In all but 2 cohort (FHS and RS), TNF-α concentrations were measured at the same time and center visit as blood samples were drawn for the quantification of DNA methylation. In the FHS cohort, TNF-α was measured in the same individuals approximately 7 years prior to the DNA methylation assay. In the RS cohort, no TNF-α measurements were ever made.
Circulating TNF-α concentration was natural log-transformed (lnTNF-α) to reduce the skewness of the distribution. Individuals whose lnTNF-α measurements were more than 4 SDs away from the cohort mean (n = 19) were excluded from subsequent analyses.
DNA Methylation Measurements, Normalization, and Quality Control
All studies used the Illumina Infinium Human Methylation 450 Beadchip (Illumina Inc) to quantify epigenome-wide DNA methylation. In all studies that conducted TNF-α measurements but 1, these measurements were performed on DNA extracted from whole-blood samples; the GOLDN study isolated and quantified DNA methylation on CD4+ T cells. Study-specific approaches to methylation data processing are summarized in eTable 1 in the Supplement.
Statistical Analyses
In the discovery phase, each cohort fit 3 linear mixed-effect regression models to assess associations between lnTNF-α (predictor) and normalized methylation β scores (outcomes). The base model adjusted for age and sex as fixed effects and for technical covariates (array, row, and/or column number) as a random effect. The second model additionally adjusted for white blood cells subtypes for studies reporting methylation in whole-blood samples. The third model adjusted for the same covariates as the second model, plus smoking (current, former, or never) and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared). All covariates were selected based on their known associations with DNA methylation. Cohorts additionally adjusted for relatedness or other study-specific covariates as necessary (eTable 2 in the Supplement). Results from the 5 cohorts participating in the main discovery analysis were meta-analyzed using a fixed-effects, inverse-variance–weighted approach in METASOFT, version 2.0 (University of California–Los Angeles).15 Because the GOLDN study was the only cohort that used CD4+ T cells and not whole-blood samples, we ran a sensitivity meta-analysis excluding GOLDN data. We performed additional sensitivity analyses including the NAS and the HBCS, which were cohorts with extremely variable Milliplex-measured TNF-α results.
To maximize statistical power of discovery, we carried CpG sites forward to the replication phase if the false discovery rate for the specific CpG site was less than 0.05. The models used in the replication analysis were identical to those implemented in the discovery phase. To minimize the chance of false positives, we implemented a more stringent Bonferroni correction in the replication phase: 0.05 per number of statistically significant hits from the discovery meta-analysis.
Gene Expression Measurements and Analysis
The CpG sites that significantly replicated in the independent replication sample were further tested for association with cis gene expression in whole-blood samples from 3738 participants with available gene expression measurements (from the FHS, KORA, and RS cohorts). Methods for the expression measurements and analysis are described in eMethods 2 in the Supplement. Messenger RNA (mRNA) transcripts that achieved statistical significance in at least 2 cohorts were further evaluated for association with circulating TNF-α concentration in the FHS cohort, using regression models adjusted for age, sex, imputed white blood cell counts, smoking, BMI, and technical covariates.
Integrating DNA Methylation and Sequence Data
To establish genetic contributions to the observed methylation of the top loci, we studied genetic associations with DNA methylation in cis genes (within 20 kb) using the GOLDN study as the discovery cohort and RS as the replication population. Genotyping, imputation procedures, and statistical analysis for both cohorts are described in eMethods 3 in the Supplement. The variants that achieved nominal significance in the replication phase were tested for association with circulating TNF-α concentration in the GOLDN cohort using linear mixed models adjusting for age, sex, study site (fixed effects), and relationships between participating family members (random effect). In addition to methylation quantitative trait loci analyses, we searched for overlap with the genomic regions containing the replicated sites in 2 genome-wide association study (GWAS) catalogs16,17 to assess previously reported associations of sequence variants in the regions of interest with disease traits.
Associations With Incident CHD
We tested associations between the replicated epigenetic correlates of circulating TNF-α concentrations and incident CHD in fixed-effects meta-analysis of the CHARGE consortium that included 470 346 CpG sites, 1895 disease events, and 11 641 participants from the FHS, InCHIANTI, KORA, and NAS cohorts, plus additional participants from 4 more cohorts: Atherosclerosis Risk in Communities, Cardiovascular Health Study, Long-term Follow-up of Antithrombotic Management Patterns in Acute Coronary Syndrome Patients, and Women’s Health Initiative (details in eMethods 1 in the Supplement). The definition of CHD events included coronary insufficiency, coronary revascularization, recognized myocardial infarction (defined as hospitalization with diagnostic electrocardiographic changes and/or biomarkers of myocardial infarction), and coronary death. Participants with CHD at enrollment were excluded.
In each cohort, associations were adjusted for age, sex, smoking status, education, BMI, differential white blood cell counts (either directly measured or imputed), and technical covariates. Data were meta-analyzed using an inverse-variance weighted fixed-effects method. This lookup was restricted to the top 4 CpG sites from the circulating TNF-α meta-analysis; findings were considered statistically significant if P was less than .05/4 (in other words, .0125). Further details about the incident CHD meta-analysis18 are available in eMethods 4 in the Supplement.
Functional Annotation
We used Hudson Alpha Institute for Biotechnology Encyclopedia of DNA Elements project custom methylation tracks, implemented in the University of California–Santa Cruz genome browser and the Illumina annotation file, to visualize and annotate the functional potential of the top CpG sites, including such indicators of regulatory activity as H3K27Ac marks, DNaseI hypersensitivity elements in relevant cell types, and genomic location of the CpG sites (eg, in the promoter vs the gene body, in an exon vs an intron).
Results
General Characteristics
Demographic and TNF-α characteristics of all participating cohorts are summarized in Table 1. The cohorts ranged in age from a mean (SD) of 31 (0.3) years in the NFBC66 cohort to a mean (SD) of 87 (0.4) years in the LBC1921 cohort. The cohorts were approximately half female, with 2532 women included overall (45.1%); the exceptions were the NAS and HBCS cohorts, which only recruited men (Table 1). The NAS and HBCS cohorts were also unique in reporting exceptionally high and variable concentrations of TNF-α (NAS: mean [SD] concentration, 55.0 [155.8] pg/mL; HBCS: mean [SD], 16.7 [47.6] pg/mL) compared with all other cohorts (FHS: 1.4 [1.2]; GOLDN, 3.2 [1.7]; InCHIANTI: 4.3 [2.2]; KORA: 2.5 [2.2]; LBC1921: 1.5 [1.6]; NFBC66: 7.8 [9.0]). The observed discrepancy may be because of technical differences between approaches to TNF-α quantification (Milliplex vs ELISA) (Table 1).
Meta-analysis, Replication, and Sensitivity Analyses
The 3 models fit to test associations between epigenome-wide methylation and TNF-α levels yielded similar results (Table 2; eTables 3 and 4 in the Supplement); namely, the cg16411857 site in the NLR family CARD domain containing 5 genes (NLRC5) emerged as the top hit with all approaches. Based on prior evidence that supports adjusting epigenetic models for smoking and BMI,19 all subsequent analyses focused on the third model.
Table 2. Associations of Methylation Sites and Circulating Tumor Necrosis Factor αa.
| CpG site | Chromosome | Positionb | Gene | Discovery | I2,c % | Replication | ||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P Value | β (SE) | P Value | |||||
| cg16411857 | 16 | 57023191 | NLRC5 | −0.01 (0.002) | 2.14 × 10−13 | 15 | −0.009 (0.003) | .003d |
| cg07839457 | 16 | 57023022 | NLRC5 | −0.02 (0.003) | 6.31 × 10−10 | 70 | −0.01 (0.004) | <.001d |
| cg00959259 | 3 | 122281975 | DTX3L;PARP9 | −0.01 (0.003) | 7.36 × 10−8 | 56 | NAe | NAe |
| cg22930808 | 3 | 122281881 | DTX3L;PARP9 | −0.01 (0.002) | 6.92 × 10−8 | 58 | −0.008 (0.004) | .04 |
| cg13683939 | 9 | 136152547 | Intergenic; proximal to ABO | 0.04 (0.0080) | 1.42 × 10−7 | 71 | −0.02 (0.01) | .18 |
| cg24267699 | 9 | 136137106 | ABO | −0.009 (0.002) | 1.67 × 10−7 | 89 | 0.002 (0.002) | .47d |
| cg08122652 | 3 | 122281939 | DTX3L;PARP9 | −0.008 (0.002) | 2.24 × 10−7 | 78 | −0.007 (0.002) | .003d |
Abbreviations: CpG, cytosine-phosphate-guanine; β, regression coefficient; NA, not available.
Model adjusted for age, sex, white blood cell proportions, technical covariates, smoking, and body mass index with false discovery rate of less than 0.05 in the discovery stage.
Human genome 19/Genome Reference Consortium Human Build 37 from University of California, Santa Cruz.
I2 is the heterogeneity statistic.
Results that met the Bonferroni threshold in the replication stage (P = .05/7 = .007).
Results are not available because these data did not pass quality control.
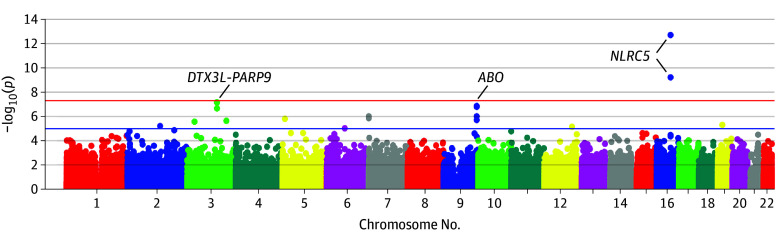
The results of the epigenome-wide analyses are summarized in Table 2 and visualized in Figure and eFigure 1 in the Supplement. Seven CpG sites located in 3 genomic regions emerged as the top hits in the discovery stage (each of which had a false-discovery rate <0.05). Of those, 3 CpG sites, 2 located in NLRC5 and 1 in the deltex E3 ubiquitin ligase 3L/poly(ADP-ribose) polymerase family member 9 gene complex (DTX3L/PARP9), were replicated in the 677 individuals from the NFBC66 cohort. All replicated associations were in the same direction and had comparable effect sizes. Another locus in DTX3L/PARP9 (cg00959259) was unable to undergo replication because of insufficient quality control procedures in the NFBC66 cohort; however, because of its proximity and similarity of associations to the replicated site cg08122652, we included it in subsequent analyses.
Figure. Epigenome-Wide Associations Between DNA Methylation and Circulating Tumor Necrosis Factor α in the Discovery Stage.
This graph includes the data of 4163 participants in the discovery analyses. The red horizontal line denotes the threshold false detection rate of .05 for statistical significance.
Inclusion of the 2 cohorts that measured TNF-α levels using the Milliplex method, namely the NAS cohort in the discovery phase (eTable 5 in the Supplement) and the HBCS cohort in the replication sample, increased genomic inflation by 19% (λ = 0.94 in the main analysis vs λ = 1.13 with the NAS cohort included). While the NLRC5 and DTX3L/PARP9 hits still emerged as significant in the discovery stage, none were replicated in the HBCS cohort. Excluding the GOLDN cohort from the discovery stage because of differences in methylation measurements (CD4+ T cells vs whole-blood samples) yielded 5 of the 7 significant hits observed in the main analysis, including the NLRC5 and DTX3L/PARP9 loci (eTable 6 in the Supplement).
Methylation vs Expression vs Circulating TNF-α
In an expression Quantitative Trait Methylation (eQTMs) assessment of the association between DNA expression and methylation, we observed 9 cis-eQTMs (methylation-expression pairs), pairs of 4 TNF-α–associated methylation loci and expression of cis genes in the FHS cohort. All were robust in the RS cohort, but none reached significance in the KORA cohort. The CpG-transcript pairs that satisfied the Bonferroni threshold in at least 2 cohorts are presented in Table 3. The direction of association was negative for all transcripts except the methylation with karyopherin subunit alpha 1 gene (KPNA1) pair, and the magnitude of associations were consistent between the FHS and RS cohorts. Of the 5 transcripts that were significantly associated with TNF-α–linked CpG sites in both the FHS cohort and the RS cohort (NLRC5, DTX3L, KPNA1, PARP9, and the poly [ADP-ribose] polymerase family member 14 gene, PARP14), 3 were positively associated with circulating TNF-α concentrations in the FHS cohort (NLRC5: β [SE] = 0.03 [0.007]; P = 5.47 × 10−5; DTX3L: β [SE] = 0.05 [0.02]; P = .003; PARP14: β [SE] = 0.04 [0.01]; P = .003 after meeting the Bonferroni threshold).
Table 3. Associations Between Methylation Status of Top Tumor Necrosis Factor α Cytosine-Phosphate-Guanine Sites and Neighboring Gene Expression.
| Transcript |
P Value in RS (n = 750) |
P Value in FHS (n = 2262) |
P Value in KORA (n = 726) |
Direction of Association |
|---|---|---|---|---|
| CpG site cg16411857a | ||||
| NLRC5 | .0002 | 2.56 × 10−8 | .16 | Negative |
| CpG site cg07839457a | ||||
| NLRC5 | 2.10 × 10−7 | 1.85 × 10−8 | .44 | Negative |
| CpG site cg00959259b | ||||
| DTX3L | 1.07 × 10−6 | 1.80 × 10−9 | .80 | Negative and positive |
| PARP9 | 2.86 × 10−22 | 2.58 × 10−13 | .81/.07c | Negative |
| PARP14 | 9.21 × 10−23 | 6.51 × 10−17 | NAd | Negative and uncertain |
| DTX3L | 2.91 × 10−7 | 2.13 × 10−6 | 0.61 | Negative |
| KPNA1 | .0003 | .00004 | NAd | Positive and uncertain |
| CpG site cg08122652b | ||||
| PARP9 | 1.04 × 10−25 | 1.09 × 10−9 | 0.12/0.26c | Negative |
| PARP14 | 1.25 × 10−24 | 8.48 × 10−15 | NA | Negative and uncertain |
Abbreviations: FHS, Framingham Heart Study; KORA, Kooperative Gesundheitsforschung in der Region Augsburg Study; NA, not available; RS, Rotterdam Study.
On chromosome 16.
On chromosome 3.
Two probes (ILMN_1731224 and ILMN_2053527) corresponded to PARP9 in KORA.
Reliable measurements of this gene's expression were not available in the KORA cohort.
Methylation vs Sequence Variation vs Circulating TNF-α Levels
Of all significant methylation correlates of TNF-α concentration, only cg07839457 showed nominally significant replication of associations with 2 neighboring NLRC5 sequence variants: rs17369768 and a deletion at the 57042641 position on chromosome 16. Of all significant methylation correlates of TNF-α concentration, only cg07839457 showed nominally significant replication of associations with 2 neighboring NLRC5 sequence variants: rs17369768 (β [SE] = −0.73 [0.33]; P = .03) and a deletion at the 57042641 position on chromosome 16 (β [SE] = −0.66 [0.33]; P = .05; eTable7 in the Supplement). Neither locus was significantly associated with circulating TNF-α in the GOLDN study. However, rs17369768 was nominally associated with visceral adipose tissue volume, waist circumference, weight, psoriasis, and rheumatoid arthritis in public databases.17
Associations With Incident CHD
Methylation at all 4 TNF-α–associated loci was robustly negatively associated with the risk of incident CHD in the meta-analysis of CHARGE consortium cohorts (Table 4; eFigure 2 in the Supplement). Adjusted for the appropriate covariates, each 10% increase in methylation of a given TNF-α–associated locus was associated with a 9% to 19% decrease in the risk of an adverse CHD event (cg16411857: hazard ratio [HR], 0.86; 95% CI, 0.78-1.95; P = .003; cg07839457: HR, 0.89; 95% CI, 0.80-0.94; P = 3.1 × 10−5; cg00959259: HR, 0.91; 95% CI, 0.84-0.97; P = .002; cg08122652: HR, 0.81; 95% CI, 0.74-0.89; P = 2.0 × 10−5).
Table 4. Associations Between Incident Coronary Heart Disease and Methylation Status of Top Tumor Necrosis Factor α Cytosine-Phosphate-Guanine Sites in a Meta-analysis of 8 Cohorts With 1895 Disease Events and 11 641 Participants.
| Site | Chromosome | Positiona | Gene | Hazard Ratiob (95% CI) | P Value |
|---|---|---|---|---|---|
| cg16411857 | 16 | 57023191 | NLRC5 | 0.86 (0.78-0.95) | .003 |
| cg07839457 | 16 | 57023022 | NLRC5 | 0.89 (0.80-0.94) | 3.1 × 10−5 |
| cg00959259 | 3 | 122281975 | DTX3L; PARP9 | 0.91 (0.84-0.97) | .002 |
| cg08122652 | 3 | 122281939 | DTX3L; PARP9 | 0.81 (0.74-0.89) | 2.0 × 10−5 |
Positions are per Human genome 19/Genome Reference Consortium Human Build 37 from University of California Santa Cruz.
Hazard ratios are per 10% increase in methylation.
GWAS Catalog Lookup and Functional Annotation
Of the 3 common single-nucleotide polymorphisms (SNPs) in or near NLRC5 that were reported in the GWAS catalog, 2 (rs821470 and rs17290922) were associated with schizophrenia-related phenotypes.20,21 The closest reported variant to the DTX3L/PARP9 locus (rs2173763) was associated with major depressive disorder.22 Conversely, a search for SNPs previously reported to be associated with circulating TNF-α levels in the regions harboring the replicated epigenetic hits yielded no results. However, an ABO polymorphism from a region that emerged as a top hit, yet did not replicate, was identified as a TNF-α protein quantitative trait locus in an earlier analysis of KORA data.23
Bioinformatic regulatory annotations for the DTX3L/PARP9 and NLRC5 regions are presented in eFigures 3 and 4, respectively, in the Supplement. Both sets of loci overlap or are adjacent to regulatory elements, supporting observed associations with gene expression.
Discussion
Using epigenome-wide data from adult participants of European descent, we have identified and replicated novel associations between leukocyte DNA methylation loci in 2 genomic regions mapping to NLRC5 and DTX3L/PARP9, the expression of corresponding genes, and circulating TNF-α levels. Most notably, DNA methylation at the same loci that were correlated with lower plasma TNF-α levels was also associated with a substantial reduction in the risk of incident CHD in this multiethnic, well-powered meta-analysis.
Both genomic regions that were discovered and validated in our analysis encode proteins that play a pivotal role in the immune response. The NLRC5 genomic region is a specific transactivator of major histocompatibility complex class I genes,24 which encode human leukocyte antigen proteins that set off the adaptive immune reaction.25 These processes are induced chiefly by interferon γ (IFN-γ) stimulation, although also by toll-like receptor ligands, other interferons, and viral infections.26 By activating CD8+ T cells via major histocompatibility complex class I proteins, NLRC5 has also been shown to upregulate IFN-γ, creating a positive feedback loop that ensures an effective response to intracellular pathogens.27
The role of NLRC5 as a master regulator of the immune response, combined with its remarkable specificity, has positioned it as a promising therapeutic target in multiple clinical settings. The specific methylation loci that emerged as our top findings, cg16411857 and cg07839457, have been shown to be significantly more likely to be hypomethylated in blood samples from immune-suppressed individuals living with HIV and to also correlate negatively with viral load.28 In another whole-blood DNA methylation study, both CpG sites were linked to circulating interleukin 18, which offered a possible mechanism for the association we observed with CHD incidence.28 A clinically interesting detail was that the NLRC5 promoter (and specifically the cg16411857 locus) was shown to be hypermethylated in 13 distinct cancer types, with a corresponding reduction in expression of not only NLRC5, but also other genes in the major histocompatibility complex class I family, which provides a mechanism for evasion of CD8+ T lymphocyte antitumor activity.29 Therefore, this study adds to the robust body of evidence in support of NLRC5 involvement in a wide range of pathophysiologic conditions.
Similarly to NLRC5, increased expression of DTX3L-PARP9 has been shown to enhance IFN-γ signaling and therefore host immune response.30 Recent evidence suggests that DTX3L-PARP9 may also play a key role in vascular inflammation and atherosclerosis. In macrophage-like cell lines stimulated with IFN-γ, experimental silencing of PARP9 has suppressed the induction of TNF-α (which was consistent with the directions of association observed in our analyses); silencing of PARP14 has had opposite effects (in contrast with our observations). Additionally, PARP14 deficiency was shown to promote atherogenesis in mice.31 Possible explanations for the discrepancy in the direction of association may include the tested cell type (macrophages vs T lymphocytes or whole blood); tightly controlled experimental conditions in cell culture or murine models vs observational data from free-living humans; chance; or other factors. Therapeutic inhibition of other PARP enzymes (specifically PARP1) has also been shown to confer cardioprotective effects,32 as well as to reduce circulating TNF-α levels in vivo.33 Although the inconsistency of the PARP14 finding across studies merits close attention in future investigations, our analysis contributes to growing evidence linking PARP enzymes with systemic inflammation and CHD.31
In follow-up analyses, we found only limited evidence of genotype contributions to the methylation of the CpG sites of interest, suggesting the importance of environmental determinants. A prior analysis of the GOLDN study reported moderate heritabilities for the top loci associated with TNF-α levels in our analysis, with some of them (eg, cg07839457) likely to be enriched in the genomic regions that evade erasure during embryogenesis.34 It is therefore possible that the methylation of loci such as cg07839457 in NLRC5 could be programmed by environmental exposures (notably pathogens) and transmitted across generations, although further targeted studies are needed to rigorously test this hypothesis.
To our knowledge, the presented analysis is the largest epigenetic study of circulating TNF-α levels to date, both in sample size and scope. Previously, a number of studies have assessed relationships between methylation in the tumor necrosis factor gene (TNF) gene promoter, corresponding gene expression (where available), and circulating TNF-α levels in various disease contexts (eg, rheumatoid arthritis,35 chronic periodontitis,35,36 type 1 diabetes,37 or obesity10). Interestingly, TNF was not among the top regions associated with circulating TNF-α levels in our meta-analysis or in published GWAS of TNF-α levels.23 Furthermore, there was little overlap between findings of our epigenome-wide meta-analysis and previous GWAS of TNF-α levels. The only exception concerns our observed but unreplicated association between TNF-α levels and methylation loci in ABO, α 1-3-N-acetylgalactosaminyltransferase, and α 1-3-galactosyltransferase gene (ABO), which were also observed in a previous protein quantitative trait loci GWAS,23 which presented evidence that the effect was assay-specific and may be driven by cross-reactivity with ABO antigens. Finally, to the best of our knowledge, the NLRC5 and DTX3L-PARP9 findings have not been reported in epigenetic studies of other proinflammatory cytokines, although a recent meta-analysis of C-reactive protein reported multiple associations with methylation loci in other interferon pathway genes,38 demonstrating distinct yet associated epigenetic determinants of the human immune response.
Given the inflammatory relevance of the TNF-α phenotype, the use of leukocyte-derived DNA for methylation measurements constitutes a clear strength of the study. Furthermore, the accessibility of blood facilitates future translational applications of the findings (eg, development of risk stratification tools or other personalized approaches). Another strength of our study stems from restricting the main analyses to cohorts that measured TNF-α levels using ELISA tests, considered the gold standard for clinical use,39 which reduced spurious variation. Third, we achieved independent replication of our top hits in the NFBC66 cohort, increasing confidence in the validity of the findings. Finally, DNA methylation measurements were available in multiple cohorts that also offered genotype and expression data, enabling an integrative approach to identify the mechanisms linking methylation and circulating TNF-α levels.
Limitations
However, several limitations of our integrative analyses must be noted. First, the expression findings replicated robustly between the FHS cohort and RS, but not in the KORA cohort. Possible reasons include discrepancies in population characteristics, gene expression measurements, or chance. Second, the FHS measurements of methylation and TNF-α levels were taken several years apart, while all other cohorts performed them contemporaneously. However, the FHS findings were similar to those derived from cross-sectional studies, and the difference in time between the measurements would bias the effect estimates toward the null, further reassuring our results. Third, the reported associations cannot be interpreted as causal because they were established in observational data that do not preclude bias (eg, because of residual confounding). Causal inference methods such as mendelian randomization, used widely to corroborate findings of epigenome-wide studies, were not optimal for this study because strong genetic instruments for either (1) TNF-α itself or (2) the methylation at the top loci, which we showed to be only weakly related to the genotype were not currently available. Future studies may consider directly assessing the relationship between DNA methylation in NLRC5 and PARP9-DTX3L and systemic inflammation in experimental models.
Conclusions
In summary, we report novel evidence linking DNA methylation in 2 immune response-related regions (NLRC5 and PARP9-DTX3L) with corresponding gene expression, circulating TNF-α levels, and incident CHD in a population-based meta-analysis, highlighting the potential of these regions as translational targets. These findings elucidate the usefulness of hypothesis-free methylome-wide studies in identifying physiologically meaningful phenomena. Together with evidence from in vitro and in vivo functional studies, these findings yield valuable insights into immunopathology of CHD.
eMethods 1 Descriptions of participating studies.
eMethods 2 Methods for gene expression measurement and analysis.
eMethods 3 Methods for genotyping and methylation quantitative trait loci analysis.
eMethods 4 Methods for the DNA methylation vs. incident coronary heart disease meta-analysis.
eFigure 1 Forest plots for the associations between top NLRC5 and DTX3L-PARP9 loci and circulating TNF-α.
eFigure 2 Forest plots for the associations between top NLRC5 and DTX3L-PARP9 loci and incident CHD.
eFigure 3 Bioinformatic annotation of the genomic region containing the top NLRC5 methylation loci.
eFigure 4 Bioinformatic annotation of the genomic region containing the top DTXL3/PARP9 methylation loci.
eTable 1 Quality control procedures for Illumina Infinium Human Methylation450K BeadChip data across cohorts.
eTable 2 Analyses of Illumina Infinium Human Methylation450K BeadChip data across cohorts.
eTable 3 Associations of methylation loci and circulating TNF-α in the model adjusted for age, sex, family and technical artifacts (where needed) with FDR<0.05 in the discovery stage.
eTable 4 Associations of methylation loci and circulating TNF-α in the model adjusted for age, sex, family, technical artifacts (where needed), and blood cell counts with FDR<0.05 in the discovery stage.
eTable 5 Associations of methylation loci and circulating TNF-α in the model adjusted for age, sex, family, technical artifacts (where needed), blood cell counts, body mass index, and smoking with FDR<0.05 in the discovery stage, including participants from the Normative Aging Study removed from the main analysis due to extreme TNF-α values.
eTable 6 Associations of methylation loci and circulating TNF-α in the model adjusted for age, sex, family, technical artifacts (where needed), blood cell counts, body mass index, and smoking with FDR<0.05 in the discovery stage, removing participants from the GOLDN study due to difference in sample (CD4+ T cells in GOLDN vs. whole blood elsewhere).
eTable 7 Associations between methylation status of top TNF-α CpG sites and neighboring DNA sequence variation (FDR < 0.05 in the discovery phase).
References
- 1.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12(1):49-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15(6):362-374. [DOI] [PubMed] [Google Scholar]
- 3.Pope JE. Rheumatoid arthritis: TNF inhibitors and cardiovascular risk management in RA. Nat Rev Rheumatol. 2016;12(6):317-318. [DOI] [PubMed] [Google Scholar]
- 4.Graf J. Study of adalimumab to lower cardiovascular risk in RA patients with well controlled joint disease. https://clinicaltrials.gov/ct2/show/NCT01893996. Published May 2, 2017. Accessed February 26, 2018.
- 5.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119-1131. [DOI] [PubMed] [Google Scholar]
- 6.Novartis Pharmaceuticals . Cardiovascular risk reduction study (reduction in recurrent major CV disease events) (CANTOS). https://clinicaltrials.gov/ct2/show/NCT01327846. Published February 26, 2018. Accessed February 26, 2018.
- 7.Sas AA, Jamshidi Y, Zheng D, et al. The age-dependency of genetic and environmental influences on serum cytokine levels: a twin study. Cytokine. 2012;60(1):108-113. [DOI] [PubMed] [Google Scholar]
- 8.Neijts M, van Dongen J, Kluft C, Boomsma DI, Willemsen G, de Geus EJ. Genetic architecture of the pro-inflammatory state in an extended twin-family design. Twin Res Hum Genet. 2013;16(5):931-940. [DOI] [PubMed] [Google Scholar]
- 9.Stein CM, Guwatudde D, Nakakeeto M, et al. Heritability analysis of cytokines as intermediate phenotypes of tuberculosis. J Infect Dis. 2003;187(11):1679-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermsdorff HH, Mansego ML, Campión J, Milagro FI, Zulet MA, Martínez JA. TNF-alpha promoter methylation in peripheral white blood cells: relationship with circulating TNF-α, truncal fat and n-6 PUFA intake in young women. Cytokine. 2013;64(1):265-271. [DOI] [PubMed] [Google Scholar]
- 11.Marques-Rocha JL, Milagro FI, Mansego ML, Mourão DM, Martínez JA, Bressan J. LINE-1 methylation is positively associated with healthier lifestyle but inversely related to body fat mass in healthy young individuals. Epigenetics. 2016;11(1):49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan KE, Reddy AB, Dietzmann K, et al. Epigenetic regulation of tumor necrosis factor alpha. Mol Cell Biol. 2007;27(14):5147-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Psaty BM, O’Donnell CJ, Gudnason V, et al. ; CHARGE Consortium . Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. 2009;2(1):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu MY, Xydakis AM, Hoogeveen RC, et al. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin Chem. 2005;51(7):1102-1109. [DOI] [PubMed] [Google Scholar]
- 15.Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet. 2011;88(5):586-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GWAS Catalog . GWAS Catalog: The NHGRI-EBI catalog of published genome-wide association studies. http://www.ebi.ac.uk/gwas/search. Published 2017. Accessed April 25, 2017. [DOI] [PMC free article] [PubMed]
- 17.University of Cambridge Cardiovascular Epidemiology Unit . Phenoscanner version 1.1. http://www.phenoscanner.medschl.cam.ac.uk/phenoscanner. Published 2017. Accessed August 7, 2017.
- 18.Agha G, Group IHDCEW. DNA methylation is associated with incident cardiovascular disease. Gerontologist. 2016;56(suppl 3):34-35. [Google Scholar]
- 19.Mendelson MM, Marioni RE, Joehanes R, et al. Association of body mass index with dna methylation and gene expression in blood cells and relations to cardiometabolic disease: a mendelian randomization approach. PLoS Med. 2017;14(1):e1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergen SE, O’Dushlaine CT, Ripke S, et al. Genome-wide association study in a Swedish population yields support for greater CNV and MHC involvement in schizophrenia compared with bipolar disorder. Mol Psychiatry. 2012;17(9):880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanous AH, Zhou B, Aggen SH, et al. ; Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium . Genome-wide association study of clinical dimensions of schizophrenia: polygenic effect on disorganized symptoms. Am J Psychiatry. 2012;169(12):1309-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ripke S, Wray NR, Lewis CM, et al. ; Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium . A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melzer D, Perry JR, Hernandez D, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet. 2008;4(5):e1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meissner TB, Li A, Biswas A, et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc Natl Acad Sci U S A. 2010;107(31):13794-13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr Opin Immunol. 1999;11(1):100-108. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Shao F. NLRC5: a NOD-like receptor protein with many faces in immune regulation. Cell Res. 2012;22(7):1099-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y, Wang Y, Chen F, et al. NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res. 2012;22(5):836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Justice AC, Hu Y, et al. Epigenome-wide differential DNA methylation between HIV-infected and uninfected individuals. Epigenetics. 2016;11(10):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshihama S, Roszik J, Downs I, et al. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc Natl Acad Sci U S A. 2016;113(21):5999-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Mao D, Roswit WT, et al. PARP9-DTX3L ubiquitin ligase targets host histone H2BJ and viral 3C protease to enhance interferon signaling and control viral infection. Nat Immunol. 2015;16(12):1215-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwata H, Goettsch C, Sharma A, et al. PARP9 and PARP14 cross-regulate macrophage activation via STAT1 ADP-ribosylation. Nat Commun. 2016;7:12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacher P, Szabó C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25(3):235-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zakaria EM, El-Bassossy HM, El-Maraghy NN, Ahmed AF, Ali AA. PARP-1 inhibition alleviates diabetic cardiac complications in experimental animals. Eur J Pharmacol. 2016;791:444-454. [DOI] [PubMed] [Google Scholar]
- 34.Day K, Waite LL, Alonso A, et al. Heritable DNA methylation in CD4+ cells among complex families displays genetic and non-genetic effects. PLoS One. 2016;11(10):e0165488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojima A, Kobayashi T, Ito S, Murasawa A, Nakazono K, Yoshie H. Tumor necrosis factor-alpha gene promoter methylation in Japanese adults with chronic periodontitis and rheumatoid arthritis. J Periodontal Res. 2016;51(3):350-358. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Barros SP, Moretti AJ, et al. Epigenetic regulation of TNFA expression in periodontal disease. J Periodontol. 2013;84(11):1606-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arroyo-Jousse V, Garcia-Diaz DF, Codner E, Pérez-Bravo F. Epigenetics in type 1 diabetes: TNFa gene promoter methylation status in Chilean patients with type 1 diabetes mellitus. Br J Nutr. 2016;116(11):1861-1868. [DOI] [PubMed] [Google Scholar]
- 38.Ligthart S, Marzi C, Aslibekyan S, et al. ; WHI-EMPC Investigators; CHARGE epigenetics of Coronary Heart Disease . DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol. 2016;17(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38(4):317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1 Descriptions of participating studies.
eMethods 2 Methods for gene expression measurement and analysis.
eMethods 3 Methods for genotyping and methylation quantitative trait loci analysis.
eMethods 4 Methods for the DNA methylation vs. incident coronary heart disease meta-analysis.
eFigure 1 Forest plots for the associations between top NLRC5 and DTX3L-PARP9 loci and circulating TNF-α.
eFigure 2 Forest plots for the associations between top NLRC5 and DTX3L-PARP9 loci and incident CHD.
eFigure 3 Bioinformatic annotation of the genomic region containing the top NLRC5 methylation loci.
eFigure 4 Bioinformatic annotation of the genomic region containing the top DTXL3/PARP9 methylation loci.
eTable 1 Quality control procedures for Illumina Infinium Human Methylation450K BeadChip data across cohorts.
eTable 2 Analyses of Illumina Infinium Human Methylation450K BeadChip data across cohorts.
eTable 3 Associations of methylation loci and circulating TNF-α in the model adjusted for age, sex, family and technical artifacts (where needed) with FDR<0.05 in the discovery stage.
eTable 4 Associations of methylation loci and circulating TNF-α in the model adjusted for age, sex, family, technical artifacts (where needed), and blood cell counts with FDR<0.05 in the discovery stage.
eTable 5 Associations of methylation loci and circulating TNF-α in the model adjusted for age, sex, family, technical artifacts (where needed), blood cell counts, body mass index, and smoking with FDR<0.05 in the discovery stage, including participants from the Normative Aging Study removed from the main analysis due to extreme TNF-α values.
eTable 6 Associations of methylation loci and circulating TNF-α in the model adjusted for age, sex, family, technical artifacts (where needed), blood cell counts, body mass index, and smoking with FDR<0.05 in the discovery stage, removing participants from the GOLDN study due to difference in sample (CD4+ T cells in GOLDN vs. whole blood elsewhere).
eTable 7 Associations between methylation status of top TNF-α CpG sites and neighboring DNA sequence variation (FDR < 0.05 in the discovery phase).