Abstract
Background
Following vestibular loss, standard tests of vestibular function do not correlate with the patients’ symptoms and functional disability.
Objectives
1) To determine if head movements in patients with a vestibular deficiency differ from those in normal subjects during daily life activities. 2) To assess if these differences can be more tightly correlated with patients symptoms and perception of dizziness-induced handicap.
Methods
Head movements during ten standardized tasks from daily life were recorded using body-worn movement sensors in 31 vestibular schwannoma (VS) patients with documented postoperative unilateral vestibular loss. The maximal self-generated peak head velocities, the time to complete the task, the average head velocity during the task and the number of head turns performed were compared to those generated by 31 age-, gender- and physical activity level-matched controls who have symmetric vestibulo-ocular reflexes (VOR) and no symptoms of dizziness. These performance parameters of movement were then correlated with the self-reported Dizziness Handicap Inventory (DHI) scores of the patients.
Results
Patients with a unilateral vestibular deficit took significantly longer to perform most daily life activities compared to controls. Their head movements, however, were not always slower. They adopted a different movement strategy, in certain instances less efficient and more disorganised. There was, however, no correlation between the DHI and patients’ performance in tasks of daily living.
Conclusion
Vestibular loss, even when compensated, affects patients’ movements, which can be measured in an ambulatory setting of daily life activities. This has significant implications in vestibular rehabilitation. The differences in movements associated with vestibular loss do not correlate with the degree of patients’ perceived dizziness and self-reported handicap. A combination of both subjective and objective measures of dizziness and disequilibrium during the assessment of patients with vestibular dysfunction is therefore required.
Introduction
Following injury to the peripheral vestibular system, standard diagnostic tests (caloric testing, harmonic acceleration with vestibulo-ocular reflex (VOR) testing, computerized dynamic posturography, visual feedback posturography) are currently used to assess vestibular function. They inform us on the physiologic state of the vestibular system, the presence of dysfunction and document the status of the compensation process of the central nervous system. These tests, however, are not useful indicators of recovery from the patient’s point of view since their results do not correlate well with the severity of dizziness-related symptoms once the patient has reached a plateau of compensation.1 Several studies have documented large discrepancies between subjective and objective measurements of vestibular function.2–10 Overall, most of these vestibular tests do not provide an accurate picture of either the extent of dizziness-induced functional limitation (disability) or the impact of the vestibular disorder on the patient’s life (handicap). Following vestibular injury, the deranged function of the vestibular system leads to difficulty maintaining balance and disability performing tasks that require balance. Handicap on the other hand is the avoidance of tasks perceived as potentially disequilibrating.5 Therefore, vestibular handicap and what the patient feels are not solely explained with measurements of the severity of the impairment or injury.
Because objective measures of impairment or improvement are of little significance unless the patient perceives a benefit, there is a need for an objective measure of functional status that correlates with the presence of vestibular dysfunction and with patients’ subjective perception of handicap. With the vestibular impairment having an impact on patients’ movements, we believe that an assessment of movements in daily life is the closest approach that can be used to objectify the resulting disability and handicap.
From short tests performed in an artificial setting, we know that following unilateral vestibular loss, patients tend to walk and move their arms slower,11,12 have more sway of their center of gravity,13–15 and take longer to perform ambulatory test such as the “Timed Up and Go” and the “Five times sit to stand” test.16 Overall, these studies suggest that patients with vestibular loss move slower. It is however not known how these tests translate to daily life realities and how movements are affected in an unrestricted setting. Are patients also slower during tasks of daily life? Are all their movements slower or are they simply using different movement strategies? Are all planes of movement equally affected?
Routine daily activities are complex but well-learned motor tasks, practiced thousands of times over the course of a lifetime. It has been suggested that vestibular disorders may introduce some inefficiency in performance with these tasks requiring more energy than they did before. This idea is supported by the fact that many patients complain of fatigue, difficulty concentrating, and decreased attention span after a vestibular injury.17 Identifying the nature of these inefficiencies and adaptation strategies through careful movement analysis has implications in retraining motor skills during rehabilitation by directly targeting these problems.
In this context, the present study describes the use of innovative technology as an attempt to reveal differences in behaviour in people with vestibular dysfunction compared to normal subjects in daily life activities. Using a very light and precise body-worn sensor that allows for movement recordings in unrestricted and natural conditions, we are aiming for a better understanding of movement differences in patients and their relationship to the perceived handicap in a daily life setting.
Materials and Methods
Subjects
The study group consisted of patients with a diagnosis of vestibular schwannoma (VS) who had undergone a primary surgical resection of their tumour via suboccipital craniotomy and retrosigmoid approach with sectioning of the vestibular nerve at the McGill University Health Center at least six months prior to their participation in the study. Subjects were excluded if their final pathology was not VS and if they were not able to mobilise without assistance. Control patients were recruited from the head and neck surgery, rhinology and general otolaryngology clinics at the McGill University Health Center. One age-, gender- and physical activity level-matched control was recruited for every case. Physical activity level was assessed using the Brief Stanford Physical Activity Screener18 and matching was performed based on a final activity level. Control patients were excluded if they had any history of VS, were evaluated for hearing or balance complaints or reported any dizziness symptoms. Approval for the study was obtained from the Research Ethics Committee at the McGill University Health Center.
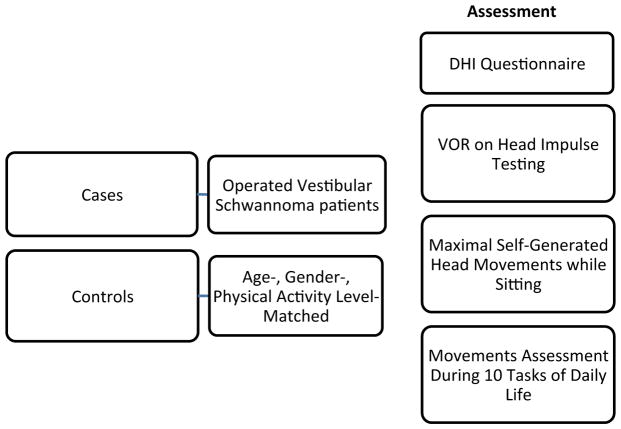
This study involved a one-time participation in four parts: 1) a subjective assessment of symptoms using the Dizziness Handicap Inventory (DHI) questionnaire, 2) vestibulo-ocular reflex (VOR) testing, 3) maximal self-generated head movement measurements and 4) daily tasks performance using a movement sensor. Figure 1 describes a summary of our methodology.
Figure 1.
Methods
Dizziness Handicap Inventory
The DHI is a 25-item validated clinical tool used to document symptomatic complaints and perceived functional disability of individuals with vestibular disorder, with higher scores indicating greater handicap.19 Scores on the DHI range from 0 (no perceived handicap) to 100 (the maximum perceived handicap) and can be further subdivided into functional (36 points), emotional (36 points), and physical subscores (28 points). Validated English and French versions of the questionnaire were available.
VOR Assessment
Passive VOR assessment was performed via horizontal head impulse testing using a head-mounted monocular vestibulo-oculography device, the EyeSeeCam (EyeSeeCam™, Munich, Germany). Following calibration of the light eye-tracker on the left pupil, a single examiner generated horizontal head impulses in both directions while the subjects were instructed to fixate a standardized point in front of them. The passive VOR gains for rotations ipsilateral and contralateral to the lesion were determined by plotting the eye and head velocities in Matlab (MathWorks, Natick, MA). To quantify the VOR gain and phase, we used a dynamic regression model described as:
where is the estimated eye velocity in response to head rotation (Ḣ) and td is the time delay between the eye and head velocity. We found the optimal bias and head velocity gain coefficients to describe the VOR. Note, estimated bias values were not significantly different than zero for either control or patients (p value is p=0.47 for controls and p=0.24 for patients).
Maximal Head Movements Assessment
Maximal self-generated head movement measurements in the pitch and yaw plane while sitting were performed to assess the maximal movement capabilities in both cases and controls when there was no risk of imbalance or falls. The head-mounted EyeSeeCam device was used to record angular head velocities while the subjects were instructed to perform ten full cycles of head rotations in the yaw (left-right) and pitch (up-down) planes. The peak head velocity of each turn and each direction were tabulated, and an average was generated for each subject.
Tasks of Daily Living
Movement performance in daily life was assessed through a standardized protocol of ten tasks set-up within the clinic and aimed at safely challenging the known areas of difficulty in patients with vestibular dysfunction.20 The details of each task are described in Table 1. Head movements were recorded using a micro-electromechanical systems (MEMS) module (iNemo platform, STEVAL-MKI062V2, STMicroelectronics, Geneva, Switzerland). This MEMS module combines three linear accelerometers (recording linear accelerations along the fore-aft, inter-aural and vertical axes) and three gyroscopes (recording angular velocity about pitch, roll and yaw). However, in order to extend the angular velocity range to ±2000 deg/s, we enhanced the MEMS module with a STEVAL-MKI107V2 three axis gyroscope. The data from the six sensors were sampled at 100 Hz and recorded wirelessly on a microSD card. The MEMS module, the battery and the microSD card were regrouped in an extremely light (64g) and small (35×35×15mm) enclosure. Two such enclosures were integrated in an elastic headband specially designed to prevent enclosure movement. The plane spanned by the fore-aft and inter-aural axes of the MEMS module was set parallel to the subject’s Frankfurt plane (i.e., the plane passing through the inferior margin of the orbit to the external auditory meatus). The recording was manually stopped after each task ensuring independent recordings with no inclusion of the transition between tasks.
Table 1.
Details of the tasks of daily living protocol
| Task | Instruction | Materials | Challenge |
|---|---|---|---|
| 1-Bed | The subject is lying flat on the bed; sit up in bed and then stand up from the bed. Barefoot, the subject stretches his/her knees three times. | Flat examining room chair | - Whole body movements - Dynamic balance |
| 2-Pants | Standing up, the subject puts a pair of loose pants one leg at a time. | Extra large scrubs | - Reduction of supporting foot area - Shifting of center of gravity |
| 3-Shoes | Without sitting or kneeling, the subject puts his/her shoes on. * Subjects instructed to wear their most comfortable pair of flat shoes | Subjects’ own shoes | - Head motion - Reduction of supporting foot area |
| 4-Sink | The subject walks 2 meters in as straight line toward the sink and washes his/her face with eyes closed three times | Sink | Absence of vision |
| 5-Walk | Going out of the examining room, the subject walks in the corridor for 15 meters at his or hers most comfortable pace. | ||
| 6-Foam | The subject walks over a foam mattress turns around and walks back over it. | 191cm long, 10 cm high foam mattress | Modification of feet proprioception |
| 7-Dishes | The subject sorts 30 dishes from a bag on the ground into the shelving unit according to color and shape as fast as possible while standing in the middle of the shelving unit. 15 different dishes are already positioned on the shelves to guide the sorting. |
- IKEA 4×4 Expedit shelving unit - 3 plastic cups, bowl and plates of 5 different colors (total 45) |
Rapid alternating head movements |
| 8-Stairs | The subject goes down a series of 7 stairs and back up without holding to the ramp | 7 consecutive stairs | Shifting of center of gravity |
| 9-Weight | The subject carries in one hand a 5 lbs. bag over 5 meters. | A cloth bag with 5lbs of books | Shifting of center of gravity |
| 10- Uphill/Downhill | Subject walks 2 meters over a 15-degree elevation with eyes open, then with eyes closed. Subject turn around and then walks downhill with eyes closed and then with eyes opened without holding onto the wall. | Ground elevation (2 sets of 15 degrees elevation over 2 meters) | - Modification of feet proprioception - Absence of vision |
Data Analysis
For each subject the time taken to complete each task was measured. The dish-sorting task (no. 7), while being the longest, is also the task involving the most head movements. It was therefore selected for an analysis of head turns in the yaw and pitch plane, to determine the maximal velocity generated for comparison with those generated while sitting. The maximal angular velocities were tabulated manually from the raw data graphic depicting movement in the yaw and pitch plane. The number of head turns to complete the dish-sorting task was also counted. Dish-sorting was noted to be an asymmetrical task with the target bag containing the dishes being preferentially placed on the left side across all cases and controls. The count of head turns was therefore reported as the number of head turns ipsilateral or contralateral to the tumour and the number of left or right-sided turns.
For all ten tasks, a systematic analysis of the daily life head movements was performed using a custom-written script in Matlab that provided an average acceleration and velocity generated in all six planes of movement performed by the subjects.
Matched-pairs T-test statistics were used for comparisons of VOR gains, task duration and the generated accelerations/velocities between cases and controls, with a significance level of 0.05. Because DHI scores are not normally distributed, to assess for any relationship between the DHI score among cases and parameters of movement, Spearman’s rho correlations were performed. The JMP 10 statistical software (SAS Institute Inc., Cary, NC) was used for the statistical analyses.
Results
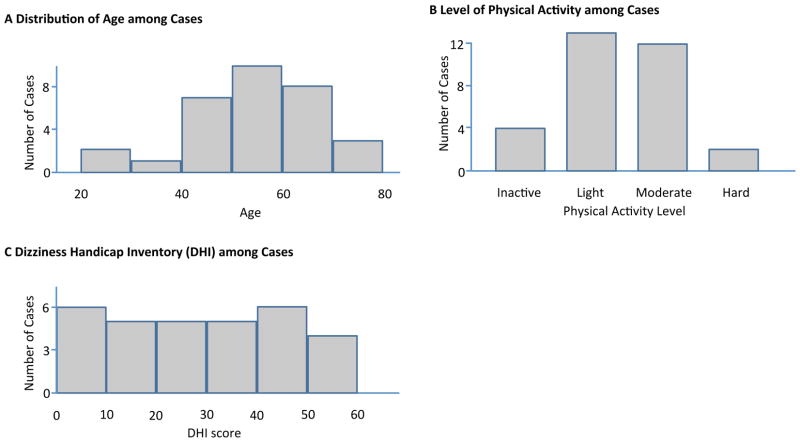
A total of 31 cases and 31 controls were included in the study. There were 15 females and 16 males in each group. Among cases, 11 patients had the VS on the left, and 20 had a right-sided tumour. Figure 2 describes the distribution of age, physical activity level and DHI scores among cases.
Figure 2.
Descriptive Parameters of the Cases
VOR testing
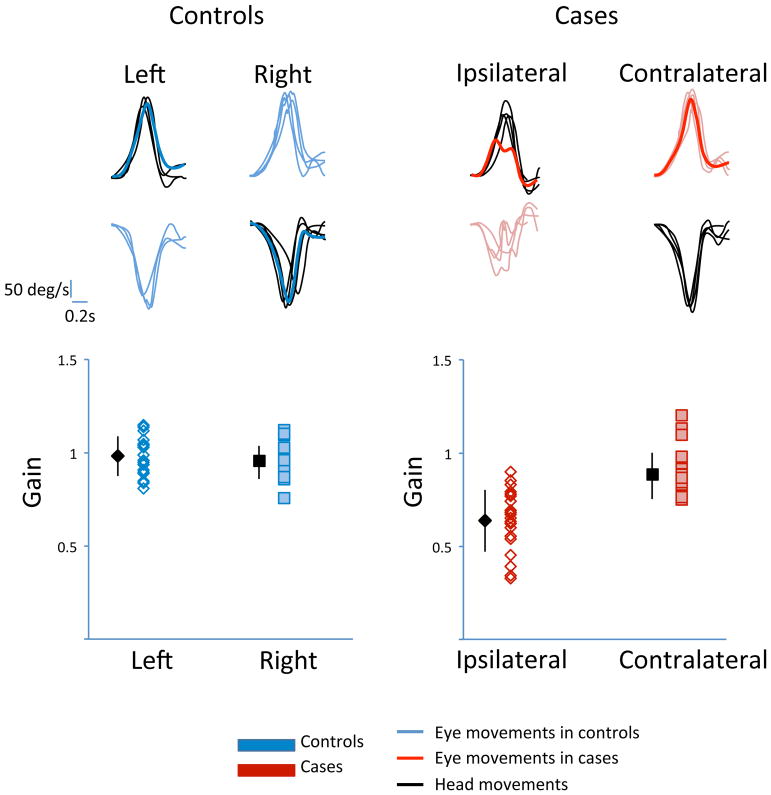
The head impulse stimuli for VOR testing was delivered by a single examiner and had on average a peak velocity of 176.7 +/− 3.8 deg/sec with a duration of 0.33 +/− 0.009 seconds. Figure 3 is an example trace of the head and eye movements during impulse testing in cases and controls, demonstrating symmetrical VORs with a gain of 0.97 in controls while cases had significant weakness ipsilateral to the tumour, compared to their healthy contralateral side. In cases, the mean VOR gain on the ipsilateral side was 0.64 compared to 0.88 on the contralateral side with a mean matched-pair difference of 0.26 (p<0.0001). These results document that controls had symmetrical VOR gains while cases had a unilateral vestibular deficiency.
Figure 3.
Horizontal Vestibulo-Ocular Reflex (VOR) Gains in Cases and Controls
Quantification of head movements during active head-on-body rotations
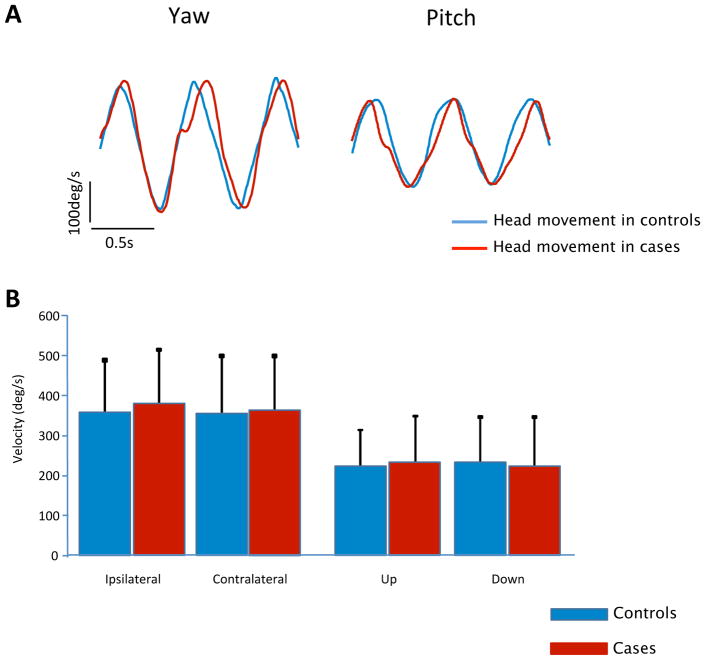
Maximal self-generated head velocities during ten head turns in the pitch and yaw plane while sitting were performed to assess the maximal movement capabilities in both cases and controls when there was no risk of imbalance or falls. Figure 4, describes the results showing no difference between cases and controls. All matched-pairs of subjects have similar maximal movement capabilities while sitting (p value = 0.54 in yaw and 0.92 in pitch), demonstrating that the surgery did not affect the neck musculature of the patients, and that they were able to generate the same maximal head velocities.
Figure 4.
Maximal Self-generated Head Velocity in Yaw and Pitch Planes while Sitting
Quantification of time required to complete everyday tasks
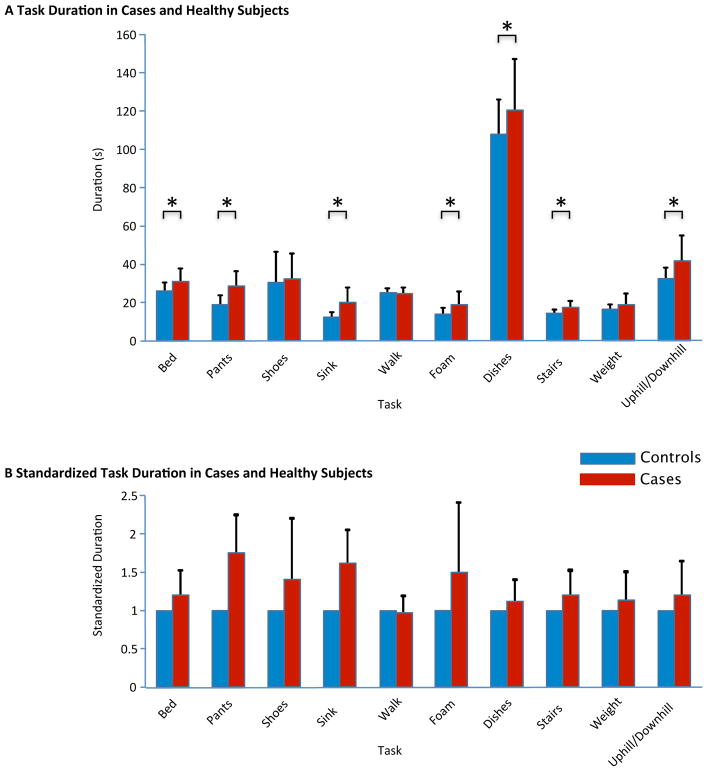
Duration for task completion was the first outcome of interest in the performance of tasks of daily living. Patients with a unilateral vestibular deficit took significantly longer to perform most daily life activities than their age-, gender- and physical-activity-matched peers. The mean durations for each task completion among cases and controls are listed in Table 2 with a visual representation in Figure 5. The biggest relative differences between cases and controls are noticed in Task 4 (washing the face at the sink), Task 2 (putting on pants) and Task 6 (walking on foam). No differences were noted between cases and controls for the time needed to put on shoes (Task 3), walk (Task 5) and walk with a weight (Task 9).
Table 2.
Mean Task Duration in Cases and Controls
| Task | Cases | Controls | 95 CI for mean difference in s | p value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean duration in s | (sdev) | Mean duration in s | (sdev) | |||
| 1-Bed | 31.5 | (6.4) | 26.3 | (4.4) | 2.3–8.2 | 0.001* |
| 2-Pants | 29.7 | (7.0) | 17.8 | (4.3) | 8.9–14.9 | <0.001* |
| 3-Shoes | 31.9 | (13.7) | 29.7 | (17.0) | −4.2–9.6 | 0.4 |
| 4-Sink | 20.6 | (7.4) | 12.8 | (3.0) | 5.4–10.1 | <0.001* |
| 5-Walk | 24.4 | (3.6) | 25.1 | (2.4) | −2.5–1.1 | 0.4 |
| 6-Foam | 18.6 | (7.12) | 13.4 | (3.7) | 2.1–8.4 | <0.002* |
| 7-Dishes | 120.4 | (26.9) | 107.3 | (18.5) | 1.6–24.6 | 0.028* |
| 8-Stairs | 17.7 | (3.4) | 14.2 | (2.3) | 2.1–4.8 | <0.001* |
| 9-Weight | 18.9 | (6.0) | 17.0 | (2.1) | −0.5–4.3 | 0.1 |
| 10-Uphill/Downhill | 41.8 | (13.3) | 33.4 | (4.9) | 3.6–13.1 | <0.001* |
Figure 5.
Duration for Task Completion in Cases and Controls
Quantification of movement features during everyday tasks
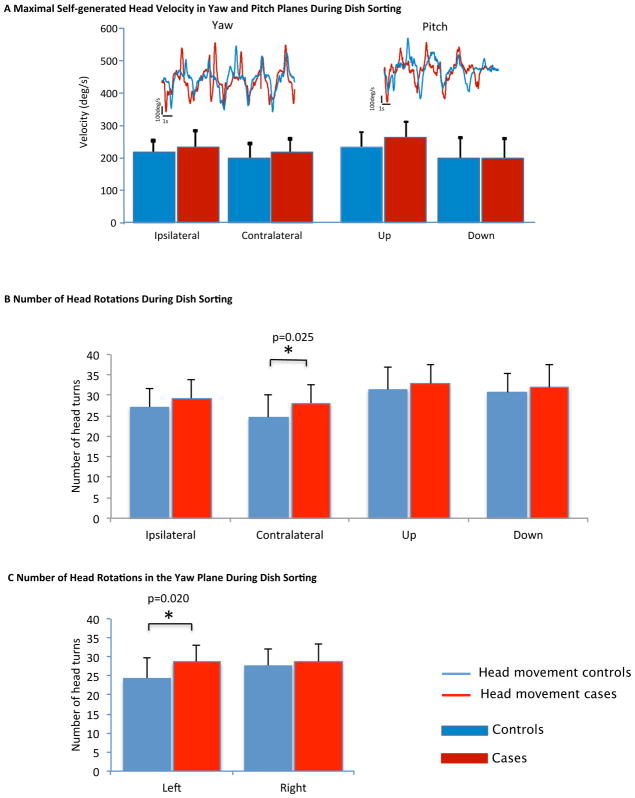
The dish sorting exercise (Task 7) is the task with the most rotational head movements. When looking at the average of the top ten peak velocities in each direction of the yaw and pitch plane generated by the subjects while performing this task, no difference between cases and controls is noted (Figure 6A). The cases took, however, significantly longer to perform the same task. To explain the difference, the number of head turns for task completion was compared. The cases turned their head in the direction contralateral to the lesion significantly more times than controls (4 turns more on average – Figure 6B) (p=0.025) which corresponds, in terms of left /right-sided head turns (Figure 6C), to a significantly higher number of head turns toward the left, where the target container with the dishes to be sorted was located (p=0.02). This could correspond to a potential inefficiency in task performance with more visual confirmation needed to grasp dishes.
Figure 6.
Head Movements During the Dish Sorting Task
When comparing the maximal self-generated head velocities while sitting (Figure 4) to those generated while dish sorting (Figure 6A), in the yaw plane, velocities generated during dish sorting are significantly lower than those generated while sitting. Similar reductions in speed were observed in both cases and controls. In the pitch plane, the velocities generated while sitting are not different from those generated during dish-sorting for both cases and controls. The variation in head movement speed from sitting to standing does not appear to be influenced by the presence of vestibular deficiency.
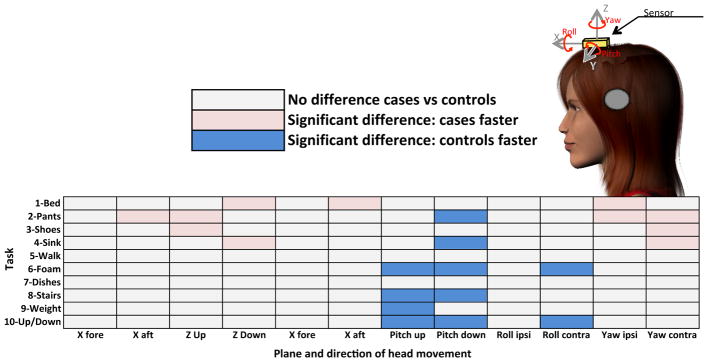
Figure 7 depicts the results of the systematic analysis of all movements of the head in all six dimensions (X, Y, Z, pitch, roll and yaw) for all ten tasks in cases and controls. Condition for which there were differences in the average head velocities recorded for rotational motion, or average head accelerations recorded for translational motion are shown in pink and blue. For translational motion along the X axis (i.e., fore-aft), there was no difference in the average acceleration between cases and controls for all tasks except for getting out of bed (Task 1), where cases had higher accelerations moving backward (aft) than controls. For translational motion along the y axis, (i.e., ipsilateral/contralateral translation), there were also no differences except, when in the “putting on pants” taks (Task 2), where cases had higher accelerations towards the side contralateral to their deficit. For the up and down translation movements (Z axis), cases were again generating higher accelerations during Tasks 1 to 4. In summary, for translational motions during some of the tasks, cases are generating higher accelerations than controls.
Figure 7.
Differences in Head Movements in Cases vs Controls for all Tasks in all Six Dimensions
Similarly, for head rotation around the yaw axis, cases were turning their head faster than controls during Tasks 1 to 4, tasks that on description do not seem to require any specific head turns. For up and down rotations of the head (i.e., pitch plane), controls, however, had faster head movements than cases during the majority of tasks (Task 2,4,6,8,9,10). Similar results were observed for head movements in the roll plane during Task 6 and 10. Overall, there appears to be clustering of the differences within dimensions across all tasks. In the translational motions and yaw head turns, cases are often generating higher accelerations and velocities than controls. Conversely, for pitch and roll head turns, controls are reaching higher velocities. Dimensions of movement are not all affected equally following a unilateral vestibular loss, which might hint at adaptational movement strategies developed by the patients following the injury.
Comparison of subjective and objective measures
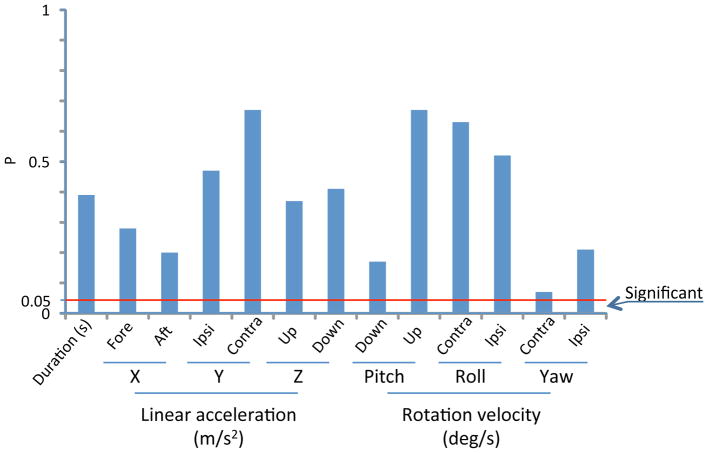
Finally, in the patients with a unilateral vestibular deficit, the relationship between the DHI score and the different parameters of movement measured was assessed to determine if patients’ perceived symptoms are related to their performance. Overall, we found that the DHI score did not correlate with any parameters of movements measured in this study. It did not correlate with the time taken to complete the task, the maximal self-generated head velocities while sitting, the velocities of maximal rotational head movements during dish sorting, the number of head turns during dish sorting, nor the average accelerations and velocities for all tasks in all six planes with the p values of the Spearman’s correlation exceeding the adjusted significance level in all instances. Figure 8 is an example diagram of p-values for the relationship between the total DHI score and parameters of performance during task 2 (putting on pants), demonstrating a lack of significant correlation at all levels. Task 2 was chosen as an example given that large differences in performance were noted between cases and normal subjects.
Figure 8.
P-values of the Spearman’s correlation between total DHI score and parameters of performance during Task 2 (Putting on pants)
Discussion
Patients with a unilateral vestibular deficit move differently than healthy controls during activities of daily life but these differences do not correlate with the patients’ perceived handicap.
More specifically, we first show that there is no difference in the maximal movement capabilities between cases and controls when there is no risk of imbalance or falls, confirming that the surgery undergone by patients with VS did not affect their neck musculature, and in a safe setting, they are still able to generate the same head velocities as controls. Any difference in head movement velocities in a task setting would therefore be the result of a functional adaptation to the task.
Duration for task performance
In a functional setting of daily life activities, individuals with a unilateral vestibular deficit take significantly longer to complete most tasks. This is consistent with patients’ reports of performing many routine daily-life tasks more slowly and more carefully than they did before the onset of their symptoms. 17 Among activities of daily living, the tasks reported most often to be impaired after VS surgery were ladder climbing and night driving, whereas dressing and bathing were seldom problematic.21 Despite the patients’ reports, our performance data demonstrates significantly longer times even for tasks such as putting on pants (Task 2) and face washing (Task 4) suggesting that the impact on performance is probably much wider than what is actually perceived by the patients.
Several authors have documented the impact of vestibular dysfunction on motor tasks. Patients with a vestibular disorder take significantly longer to move bags of beans while sitting,22 they take more time to perform the five times sit to stand test,16 their gait speed is significantly lower in different artificial settings,11 and they take longer to reach for a target with their dominant hand than healthy controls.12 The present study is however the first to our knowledge in which performance during several functional tasks in a daily life setting was assessed for comparisons between patients with unilateral vestibular deficit and matched-controls using body-worn movement sensors. Our results confirm that the lower performance on these short tests as reported by other studies translates to that obtained during most tasks of daily living. Our data, however, did not show any difference in the time required to put on shoes between cases and controls (Task 3), walk (Task 5) and walk with a weight (Task 9). Since there was significant variability of shoe type among both cases and controls, the associated mechanical requirements for putting them on would have also varied. We believe this variability likely explains the lack of association with task performance based on the status of subject’s vestibular system. For the ambulatory tasks, we speculate that simple walking in the hallway and walking with a weight were not challenging enough to show differences between cases and controls.
Dish Sorting Task
The detailed analysis of each head turn during the dish sorting exercise shows that when faced with a practical task, patients with a unilateral vestibular deficit are not rotating their head any slower than the controls. From sitting to sorting dishes, the reduction of the head velocity is comparable between cases and controls. As pointed out previously, cases do, however, take significantly longer to complete the task. One explanation for taking longer to sort the dishes could be the significantly higher number of head turns toward the left performed by the cases than the controls. Indeed, on average, cases performed 28 turns towards the left, where the target bag containing the dishes was located, while controls performed 24 turns to sort out the same 30 dishes. It appears that cases might require more frequent visual confirmation of the target before grasping, while controls were able to store in their memory the location of the target and grasp the dish without requiring visual confirmation each time. The presence of a vestibular deficit as an additional challenge that needs to be taken into account by the central nervous system might have an impact on overall neural processing, attention and task performance strategies beyond the simple effect of vestibular dysfunction on balance and visual stability. Another explanation might be the lower speed of arm movements in patients with unilateral vestibular hypofunction. A study of voluntary upper extremity reach in vestibular deficit patients has shown significantly longer times needed to complete the reaching movement compared to healthy controls irrespective of the direction, associated head movement or body position.12 Arm speed was however not measured in our study.
Movements Across Tasks
In the systematic analysis of all head movements through the custom-written Matlab code, we notice no difference in the average head accelerations or angular velocities generated in all the planes during the dish-sorting task, which is in accordance with pitch and yaw results obtained during the manual analysis of all head turns during that task. When the systematic analysis is extended to all tasks, some differences are noted between cases and controls. Rather than focusing on the magnitude of those differences, the nature of those differences and their clustering might be more informative. It appears that not all planes or directions of movement are affected equally by a vestibular deficit; in some planes cases were faster than controls while in others controls were faster. This difference remains consistent in that plane across different tasks of daily living. For example, in the pitch plane, controls tended to be faster, and there were no instances of cases generating higher pitch rotations in any of the tasks, whereas in yaw, cases were faster and in no tasks were controls turning their head faster than cases.
As mentioned, one noticeable trend is the tendency of cases to generate lower angular velocities in the pitch plane during complex ambulatory tasks such as walking on foam (Task 6), walking with a weight (Task 9), walking uphill/downhill (Task 10) and taking the stairs (Task 8). No such difference was noted during simple walking (Task 5). Pitch rotations are the type of head movements that naturally occurs during gait, and adequate vertical VOR is required to maintain the picture of the surrounding environment stable on the retina. In a study measuring gaze stability in individuals with vestibular dysfunction, poor gaze stability in the pitch plane had the strongest association with measures of gait performance (Timed “Up and Go” and Dynamic Gait Index).23 The trend noted in the present study could be part of an adaptational strategy where subjects with a vestibular deficit would preferentially reduce the up and down wobbling of the head while ambulating in a complex setting to avoid eliciting oscillopsia and maintain there visual stability despite their poor VOR, with minimal effect on their other planes of movement.
Gait speed was not affected during simple walking nor walking with a weight since cases did not take any longer than controls to walk the same distance. Although the average of all fore-aft linear accelerations were not any different between cases and controls in any of the other ambulatory tasks, cases took significantly longer to walk on foam, take the stairs and walk up/downhill. It is possible that their path was not as straight since it has been shown that during active locomotion task subjects with vestibular impairment veer significantly more than normal.24 Their gait was also described as more disorganized. Indeed, in a study of gait in normal and hypovestibular subjects, body acceleration, although not significantly different in amplitude, was more chaotic in patients with less regularity and recurrence patterns noted.25
Task Performance and DHI
The degree of patients’ perceived dizziness and self-reported handicap does not appear to correlate with the parameters of performance in tasks of daily living. Movement assessment is another objective measure that does not correlate with the patients’ perceived symptoms.
In a study by Whitney et al.,16 the DHI score of patients with dizziness of all etiologies was compared with known measures of functional performance, namely the Dynamic Gait Index (DGI), the 5 times sit to stand test (FTSST), the Timed “Up and Go” (TUG) and Gait speed. They concluded that the patients with the greatest perception of handicap were the most functionally impaired which is not supported by our results. A closer look at the data demonstrates that only patients with a vestibular disorder who report scores greater than 60 on the DHI are functionally impaired based on their DGI and FTSST scores. No correlation between DHI score and TUG and Gait Speed was noted, and none of these performance measures showed correlation with DHIs bellow 60. In our study of patients post VS surgery, the median DHI was 30 (mild), and we did not have any patients with a DHI score above 60 (the highest DHI score reported was 56). Our lack of patients with severe symptoms could explain the lack of correlation between performance in tasks of daily living and perceived symptomatology, since the impact on performance is only noticeable in the extreme level of perceived handicap. One might hypothesize that only patients with the most dizziness would be slowing their movements to avoid eliciting symptoms. Our lack of subjects in this symptoms range prevents us from testing this hypothesis. Performing such a study would be challenging since rates of DHI scores above 60 are low, with 6.74% of operated VS patients falling in that range.26 Similar to our results, single leg stance time,27 functional reach distance 27 and the TUG performance28 did not correlate with the DHI scores of patients with peripheral vestibular deficit. Several studies have also tried to assess if self-perception of disability in patients with vestibular disorders is related to impairments in balance. Clinical balance tests such as balance while standing on one-leg and walking in a figure of eight did not correlate with the total DHI score in a population of patients with vestibular dysfunction.29 A more systematic assessment of balance through computed posturography yielded inconsistent results with some studies showing correlation between DHI and sensory organization measures of balance28,30–32 while others reported that there was essentially no relationship between disability and computerized posturography testing.33
Overall, our study joins the vast body of literature that states that it is not possible to accurately predict the degree of disability or handicap that is produced by the disease simply by considering the results of objective testing, even if these are looking directly at the movements in daily life that are directly linked with the handicap. Dizziness induced handicap is by definition a complex phenomenon which takes into account not only the specific impairment, but also the individual’s lifestyle, environment and expectations. Disability is not simply due to the presence of dizziness but is the result of a complex interplay between performance and perception that is depending on patients’ motivation and tolerance of postoperative vestibular symptoms that are all influenced by social, professional, psychological, affective and cognitive factors.
Limitations
The lack of cases with severe postoperative dizziness-related impairment could explain the lack of correlation between DHI and measure of performance. Although unilateral vestibular hypofunction was demonstrated in all cases through horizontal head impulse VOR testing, cerebellar bruising and enema associated with the use of surgical retractor during the retrosigmoid approach to VS could be contributing to the symptoms of disequilibrium reported by the patients.34,35 The same technique was however used in all cases, which limits the impact of cerebellar bruising on the variability noticed.
Conclusions and significance
Overall, this study has shown that vestibular loss affects patients’ movements, which can be measured in an ambulatory setting of daily life activities using body-worn movement sensors. Patients with a unilateral vestibular deficit take longer to complete most tasks of daily living, yet their head movements are not always slower. While they are reducing the pitch rotations of their head during complex ambulatory task likely to avoid oscillopsia, their head movements are in other tasks as fast as those of normal controls. They appear however to adopt different movement strategies, which perhaps in certain instances are less efficient and more disorganised. This could have significant implications in vestibular rehabilitation, where knowledge of the difference between impaired patients and normal subjects allows for more adequate therapy targeting.
The degree of patients’ perceived dizziness and self-reported handicap does not correlate with their performance in tasks of daily living. Movement assessment is another objective measure that does not correlate with the patients’ perceived handicap. The constant discrepancy suggests that it is important to combine both subjective and objective measures of dizziness and disequilibrium during the assessment of patients with vestibular dysfunction.
Future directions
Future study using body-worn movement sensors should target more challenging tasks of daily living that might reveal more subtle adaptational strategies. Monitoring of gaze shifts and active VORs during task performance would add valuable data to explain some of the changes in movement that are noted. Finally, correlation of movement parameters with perhaps a less studied but more task oriented subjective measure, such as the Vestibular Disorder Activity of Daily Living Scale (VADL),36 could potentially find a better correlation between functional objective and subjective measures.
Acknowledgments
Sources of support that require acknowledgment:
T Mijovic - FRSQ – Fonds de Recherche en Santé du Québec – Training Award
Contributor Information
Tamara Mijovic, McGill University Department of Otolaryngology Head and Neck Surgery.
Jerôme Carriot, McGill University Department of Physiology – Vestibular Gaze Control Laboratory.
Anthony Zeitouni, McGill University Department of Otolaryngology Head and Neck Surgery.
Kathleen E Cullen, McGill University Department of Physiology – Vestibular Gaze Control Laboratory.
References
- 1.Saman Y, Bamiou DE, Gleeson M. A contemporary review of balance dysfunction following vestibular schwannoma surgery. The Laryngoscope. 2009 Nov;119(11):2085–2093. doi: 10.1002/lary.20648. [DOI] [PubMed] [Google Scholar]
- 2.Horak FB. Postural compensation for vestibular loss. Annals of the New York Academy of Sciences. 2009 May;1164:76–81. doi: 10.1111/j.1749-6632.2008.03708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meli A, Zimatore G, Badaracco C, De Angelis E, Tufarelli D. Vestibular rehabilitation and 6-month follow-up using objective and subjective measures. Acta oto-laryngologica. 2006 Mar;126(3):259–266. doi: 10.1080/00016480500388885. [DOI] [PubMed] [Google Scholar]
- 4.Cohen HS, Kimball KT, Jenkins HA. Factors affecting recovery after acoustic neuroma resection. Acta oto-laryngologica. 2002 Dec;122(8):841–850. [PubMed] [Google Scholar]
- 5.Bamiou DE, Davies RA, McKee M, Luxon LM. Symptoms, disability and handicap in unilateral peripheral vestibular disorders. Effects of early presentation and initiation of balance exercises. Scandinavian audiology. 2000;29(4):238–244. doi: 10.1080/010503900750022862. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer JB. An evaluation of the relationship among electronystagmographic, audiologic, and self-report descriptors of dizziness. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 1990;247(2):114–118. doi: 10.1007/BF00183180. [DOI] [PubMed] [Google Scholar]
- 7.Bamiou DE, Davies RA, McKee M, Luxon LM. The effect of severity of unilateral vestibular dysfunction on symptoms, disabilities and handicap in vertiginous patients. Clinical otolaryngology and allied sciences. 1999 Feb;24(1):31–38. doi: 10.1046/j.1365-2273.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 8.Perez N, Martin E, Garcia-Tapia R. Dizziness: relating the severity of vertigo to the degree of handicap by measuring vestibular impairment. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2003 Mar;128(3):372–381. doi: 10.1067/mhn.2003.102. [DOI] [PubMed] [Google Scholar]
- 9.O’Neill DE, Gill-Body KM, Krebs DE. Posturography changes do not predict functional performance changes. The American journal of otology. 1998 Nov;19(6):797–803. [PubMed] [Google Scholar]
- 10.Mbongo F, Tran Ba Huy P, Vidal PP, de Waele C. Relationship between dynamic balance and self-reported handicap in patients who have unilateral peripheral vestibular loss. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2007 Oct;28(7):905–910. [PubMed] [Google Scholar]
- 11.Marchetti GF, Whitney SL, Blatt PJ, Morris LO, Vance JM. Temporal and spatial characteristics of gait during performance of the Dynamic Gait Index in people with and people without balance or vestibular disorders. Physical therapy. 2008 May;88(5):640–651. doi: 10.2522/ptj.20070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borello-France DF, Gallagher JD, Redfern M, Furman JM, Carvell GE. Voluntary movement strategies of individuals with unilateral peripheral vestibular hypofunction. Journal of vestibular research : equilibrium & orientation. 1999;9(4):265–275. [PubMed] [Google Scholar]
- 13.Allum JH, Adkin AL. Improvements in trunk sway observed for stance and gait tasks during recovery from an acute unilateral peripheral vestibular deficit. Audiology & neuro-otology. 2003 Sep-Oct;8(5):286–302. doi: 10.1159/000071999. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelmsen K, Nordahl SH, Moe-Nilssen R. Attenuation of trunk acceleration during walking in patients with unilateral vestibular deficiencies. Journal of vestibular research : equilibrium & orientation. 2010;20(6):439–446. doi: 10.3233/VES-2010-0388. [DOI] [PubMed] [Google Scholar]
- 15.Parietti-Winkler C, Gauchard GC, Simon C, Perrin PP. Sensorimotor postural rearrangement after unilateral vestibular deafferentation in patients with acoustic neuroma. Neuroscience research. 2006 Jun;55(2):171–181. doi: 10.1016/j.neures.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Whitney SL, Wrisley DM, Brown KE, Furman JM. Is perception of handicap related to functional performance in persons with vestibular dysfunction? Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2004 Mar;25(2):139–143. doi: 10.1097/00129492-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Cohen HS, Kimball KT, Adams AS. Application of the vestibular disorders activities of daily living scale. The Laryngoscope. 2000 Jul;110(7):1204–1209. doi: 10.1097/00005537-200007000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Piliae RE, Norton LC, Haskell WL, et al. Validation of a new brief physical activity survey among men and women aged 60–69 years. American journal of epidemiology. 2006 Sep 15;164(6):598–606. doi: 10.1093/aje/kwj248. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Archives of otolaryngology--head & neck surgery. 1990 Apr;116(4):424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 20.Uyama K, Takahashi M, Saito A, Okada Y, Tomizawa I, Kanzaki J. Questionnaire evaluation of balance in the performance of everyday activities after acoustic neuroma surgery. Acta oto-laryngologica. Supplementum. 1991;487:91–98. doi: 10.3109/00016489109130452. [DOI] [PubMed] [Google Scholar]
- 21.Chung JH, Rigby PL, Jackler RK, Shah SB, Cooke DD. Socioeconomic impact of acoustic neuroma surgery. The American journal of otology. 1997 Jul;18(4):436–443. [PubMed] [Google Scholar]
- 22.Cohen HS, Gavia JA. A task for assessing vertigo elicited by repetitive head movements. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1998 Sep;52(8):644–649. doi: 10.5014/ajot.52.8.644. [DOI] [PubMed] [Google Scholar]
- 23.Whitney SL, Marchetti GF, Pritcher M, Furman JM. Gaze stabilization and gait performance in vestibular dysfunction. Gait & posture. 2009 Feb;29(2):194–198. doi: 10.1016/j.gaitpost.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen HS. Vestibular disorders and impaired path integration along a linear trajectory. Journal of vestibular research : equilibrium & orientation. 2000;10(1):7–15. [PubMed] [Google Scholar]
- 25.Labini FS, Meli A, Ivanenko YP, Tufarelli D. Recurrence quantification analysis of gait in normal and hypovestibular subjects. Gait & posture. 2012 Jan;35(1):48–55. doi: 10.1016/j.gaitpost.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Tufarelli D, Meli A, Labini FS, et al. Balance impairment after acoustic neuroma surgery. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2007 Sep;28(6):814–821. doi: 10.1097/mao.0b013e31811f40ad. [DOI] [PubMed] [Google Scholar]
- 27.Mann GC, Whitney SL, Redfern MS, Borello-France DF, Furman JM. Functional reach and single leg stance in patients with peripheral vestibular disorders. Journal of vestibular research : equilibrium & orientation. 1996 Sep-Oct;6(5):343–353. [PubMed] [Google Scholar]
- 28.Gill-Body KM, Beninato M, Krebs DE. Relationship among balance impairments, functional performance, and disability in people with peripheral vestibular hypofunction. Physical therapy. 2000 Aug;80(8):748–758. [PubMed] [Google Scholar]
- 29.Kammerlind A, Bergquist Larsson P, Ledin T, Skargren E. Reliability of clinical balance tests and subjective ratings in dizziness and disequilibrium. Advances in Physiotherapy. 2005;7(3):96–107. [Google Scholar]
- 30.Jacobson GP, Newman CW, Hunter L, Balzer GK. Balance function test correlates of the Dizziness Handicap Inventory. Journal of the American Academy of Audiology. 1991 Oct;2(4):253–260. [PubMed] [Google Scholar]
- 31.Jacobson GP, Calder JH. A screening version of the Dizziness Handicap Inventory (DHI-S) The American journal of otology. 1998 Nov;19(6):804–808. [PubMed] [Google Scholar]
- 32.Badke MB, Pyle GM, Shea T, Miedaner J. Outcomes in vestibular ablative procedures. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2002 Jul;23(4):504–509. doi: 10.1097/00129492-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Robertson DD, Ireland DJ. Dizziness Handicap Inventory correlates of computerized dynamic posturography. The Journal of otolaryngology. 1995 Apr;24(2):118–124. [PubMed] [Google Scholar]
- 34.Yamakami I, Uchino Y, Kobayashi E, Yamaura A, Oka N. Removal of large acoustic neurinomas (vestibular schwannomas) by the retrosigmoid approach with no mortality and minimal morbidity. Journal of neurology, neurosurgery, and psychiatry. 2004 Mar;75(3):453–458. doi: 10.1136/jnnp.2003.010827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levo H, Blomstedt G, Pyykko I. Postural stability after vestibular schwannoma surgery. The Annals of otology, rhinology, and laryngology. 2004 Dec;113(12):994–999. doi: 10.1177/000348940411301210. [DOI] [PubMed] [Google Scholar]
- 36.Cohen HS, Kimball KT. Development of the vestibular disorders activities of daily living scale. Archives of otolaryngology-head & neck surgery. 2000 Jul;126(7):881–887. doi: 10.1001/archotol.126.7.881. [DOI] [PubMed] [Google Scholar]