Abstract
DNA editing using CRISPR/Cas has emerged as a potential treatment for diseases caused by pathogenic human DNA viruses. One potential target is HIV-1, which replicates via a chromosomally integrated DNA provirus. While CRISPR/Cas can protect T cells from de novo HIV-1 infection, HIV-1 frequently becomes resistant due to mutations in the chosen single guide RNA (sgRNA) target site. To address this problem, we asked whether an sgRNA targeted to a conserved, functionally critical HIV-1 sequence might prevent the selection of escape mutants. We report that two sgRNAs specific for the HIV-1 transactivation response (TAR) element produce opposite results: the TAR2 sgRNA rapidly selects for mutants that retain TAR function, but are no longer inhibited by Cas9, while the TAR1 sgRNA fails to select any replication competent TAR mutants, most probably because it is targeted to a region of TAR that is disrupted by even minor mutations.
Keywords: CRISPR/Cas, HIV-1 Transactivation response (TAR) element, Tat function, HIV-1 mutants, proviral integration
1. Introduction
CRISPR/Cas-mediated DNA editing, while of prokaryotic origin, can nevertheless be highly efficient in eukaryotic cells (Cong et al., 2013; Mali et al., 2013). As a result, CRISPR/Cas has been proposed as a potential treatment for a wide variety of diseases in humans, including both inborn genetic errors (Cox et al., 2015) and chronic diseases caused by DNA viruses (Kennedy and Cullen, 2017). The latter seems an especially attractive potential application as CRISPR/Cas actually evolved as a prokaryotic adaptive innate immune response to infecting DNA bacteriophage (Barrangou and Marraffini, 2014). Prominent potential targets among pathogenic human viruses include human immunodeficiency virus type 1 (HIV-1) which, while the genetic material found in virions consists of RNA, nevertheless expresses viral gene products in infected cells from a proviral DNA molecule, generated by reverse transcription of the RNA genome, that is integrated into an infected cell chromosome (Kennedy and Cullen, 2017). While there has been particular interest in using CRISPR/Cas as a means of cleaving and inactivating the latent HIV-1 proviruses found in a small number of resting memory T cells in patients on antiretroviral therapy with no detectable replicating HIV-1 (Ebina et al., 2013; Hu et al., 2014; Kaminski et al., 2016; Liao et al., 2015; Wang et al., 2016c; Zhu et al., 2015), CRISPR/Cas has also been shown to effectively protect T cells from de novo infection by HIV-1 (Hu et al., 2014; Kaminski et al., 2016; Lebbink et al., 2017; Liao et al., 2015; Wang et al., 2016b; Wang et al., 2016c). However, it has also been reported that HIV-1 can rapidly evolve resistance to individual HIV-1-specific single guide RNAs (sgRNAs) due to the error prone repair of the proviral cleavage site by the cellular non-homologous end-joining (NHEJ) pathway (Ueda et al., 2016; Wang et al., 2016b; Wang et al., 2016c; Yoder and Bundschuh, 2016). One potential way to address this problem is the simultaneous expression of two or more sgRNAs, which has been reported to reduce the appearance of Cas9 resistant viruses (Lebbink et al., 2017; Wang et al., 2016a). An alternative, but not mutually exclusive, approach, explored here, is to target highly conserved, functionally critical regions of the HIV-1 genome, such as the trans-activation response (TAR) element, which is essential for HIV-1 Tat mediated transcriptional activation and, hence, for HIV-1 replication (Cullen, 1990).
Previous research has generally assumed that Cas9-mediated DNA cleavage either occurs prior to proviral integration, resulting in proviral destruction, or after proviral integration, resulting in indel formation (Ebina et al., 2013; Hu et al., 2014; Kaminski et al., 2016; Lebbink et al., 2017; Liao et al., 2015; Ueda et al., 2016; Wang et al., 2016b; Wang et al., 2016c; Yoder and Bundschuh, 2016; Zhu et al., 2015). Here, we also examine the mechanism underlying the Cas9-mediated inhibition of de novo HIV-1 infection in more detail and report, unexpectedly, that efficient NHEJ-mediated DNA repair also occurs when unintegrated HIV-1 proviruses are cleaved by Cas9.
2. Results
2.1. Efficient inhibition of de novo HIV-1 infection by CRISPR/Cas
Previous results have demonstrated that HIV-1 can rapidly become resistant to Cas9-mediated inhibition due to the acquisition of point mutations or small indels in the region complementary to the single guide RNA (sgRNA) used (Ueda et al., 2016; Wang et al., 2016b; Wang et al., 2016c; Yoder and Bundschuh, 2016). Therefore, we selected target sites within the HIV-1 genome that are highly conserved across viral isolates, in the tat gene and in the transactivation response region (TAR), as targets, as mutational inactivation of either TAR or tat would be predicted to completely block viral gene expression. As controls, we also selected two target sites in the viral gag gene, in the matrix (MA) and capsid (CA) regions, where mutations would not be predicted to affect HIV-1 transcription (Fig. 1A).
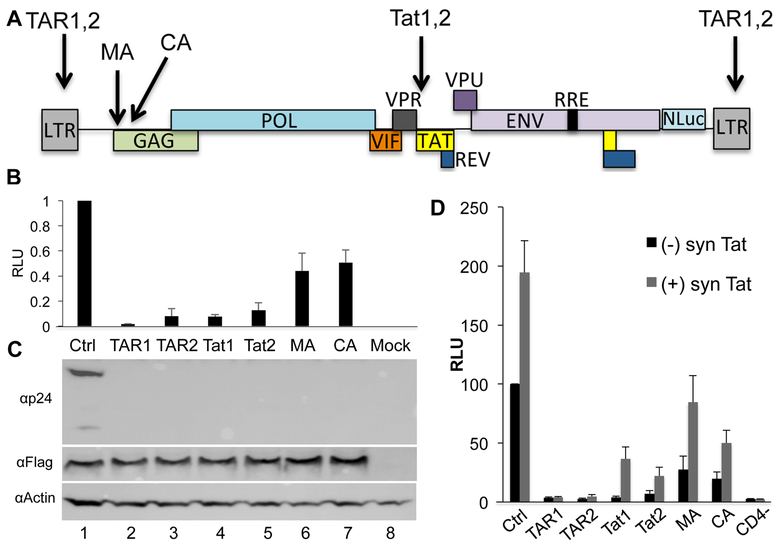
Fig. 1: Efficient Cas9 mediated cleavage of HIV-1-derived indicator constructs.
(A) Schematic of the pNL-NLuc-HXB indicator virus. The viral nef gene was replaced with the NLuc indicator gene. Arrows indicate the positions of sgRNA target sites. (B) 293T cells were co-transfected with an expression plasmid expressing Sp Cas9 and an sgRNA specific for the indicated region of the HIV-1 genome, or a control (Ctrl) non-targeting sgRNA. The cells were also co-transfected with the pNL-NLuc-HXB proviral vector. At 72 hpt, the cultures were harvested and NLuc levels determined. The culture receiving the non-targeting sgRNA (Ctrl) was set at 1.0 and the other results normalized to that value. Average of three biological replicates with SD indicated. RLU: relative light units. (C) In parallel, Western blots were performed to determine the level of Gag and p24 capsid expression in the transfected cells. Flag indicates the Sp Cas9 protein, which bears a FLAG epitope tag, while Actin was used as a loading control. A representative experiment is shown. (D) 293T cells were transfected with expression plasmids encoding CD4 and CXCR4 as well as Sp Cas9 and an sgRNA specific for the indicated region of the HIV-1 genome, or a control (Ctrl) non-targeting sgRNA (black bars). Cells lacking CD4 served as a negative control for non-specific DNA carry over from the virus producer cells. In parallel, 293T cells were co-transfected with these same plasmids as well as a vector expressing a synthetic cDNA (synTat) that encodes wild type HIV-1 Tat protein but lacks the sgRNA target sites present in the WT Tat ORF (grey bars). At 72 hpt, the cells were infected with the replication competent NL-NLuc-HXB indicator virus. A further 72h later, the cells were lysed and NLuc levels determined. All data are normalized to the culture expressing the non-targeting sgRNA in the absence of synTat and are given in RLU. Average of three biological replicates with SD indicated.
Initially, we asked if these sgRNAs would be able to inhibit HIV-1 gene expression and/or Gag protein expression. For this experiment, we co-transfected 293T cells with a plasmid expressing the Streptococcus pyogenes (Sp) Cas9 protein and an sgRNA, as well as the full-length proviral indicator plasmid pNL-NLuc-HXB, in which the viral nef gene has been replaced with the Nano luciferase (NLuc) ORF (Fig. 1A) (Tokunaga et al., 2001). Expression of NLuc is therefore dependent on both Tat and TAR function but, as wild type 293T cells do not express CD4, is not affected by viral spread even though the NL-NLuc-HXB provirus is fully replication competent. As shown in Fig. 1B, we observed a ≥10-fold inhibition of HIV-1 gene expression when using any of the four TAR or tat-specific sgRNAs but only an ~2-fold inhibition when using either gag specific sgRNA. The weak activity of the MA and CA-specific sgRNAs was anticipated as the introduction of mutations into the gag gene, unlike the introduction of mutations into TAR or tat, is not predicted to inhibit HIV-1 transcription. Nevertheless, the MA and CA-specific sgRNAs are clearly effective as both induced a strong reduction in the expression of the HIV-1 Gag protein (Fig. 1C). We note that because the TAR sgRNAs have targets located in both long terminal repeats (LTRs), they might be predicted to be especially efficient in blocking HIV-1 gene expression (Fig. 1A).
The data shown in Fig. 1A-C were generated in transfected cells and we therefore next wished to examine the effect of Sp Cas9 and the various HIV-1-specific sgRNAs on viral infection. For this purpose, we expressed the CD4 and CXCR4 receptors that mediate HIV-1 infection on 293T cells and also engineered these cells to express Sp Cas9 and one of the HIV-1-specific sgRNAs listed in Fig. 1A. We then infected these cells with a stock of the NL-NLuc-HXB indicator virus and measured the induced level of NLuc activity 72 h post-infection. As shown in Fig. 1D, and similar to the data in transfected cells presented in Fig. 1B, we observed a >10-fold inhibition of the expression of the NLuc indicator gene present in NL-NLuc-HXB when the two TAR or the two tat specific sgRNAs were analyzed, with the TAR sgRNAs being particularly effective (3.4% residual NLuc activity with sgRNA TAR1 and 2.6% residual NLuc activity with sgRNA TAR2). In contrast, and as expected, the sgRNAs specific for the gag MA or CA domain were less effective, with ~27% or ~20% residual NLuc activity, respectively. This is again consistent with the hypothesis that Cas9-induced DNA editing within gag should exert a less inhibitory effect on NLuc expression than DNA editing of tat or TAR as mutations introduced into gag, unlike mutations in TAR or tat, should not affect HIV-1 transcription or splicing (Fig. 1A).
The marked inhibition of NLuc expression observed in the HIV-1 infected cells analyzed in Fig. 1D, when sgRNAs specific for tat or TAR were used, indicates that these sgRNAs were able to block HIV-1 transcription either by mutating TAR, which would inhibit HIV-1 transcription in cis, or by mutating tat, which would block HIV-1 transcription in trans (Cullen, 1990). If this hypothesis is correct, then provision of the Tat protein in trans should rescue gene expression from the infecting NL-NLuc-HXB provirus if sgRNAs specific for tat were used but would be ineffective if sgRNA specific for TAR were used. To test this hypothesis, we generated a plasmid, psynTat, that expresses a previously described (Tiley et al., 1990) synthetic tat gene that encodes the wild type Tat protein but uses a different set of synonymous codons, so that the target sites for the Tat1 and Tat2 sgRNAs are no longer present. For this experiment, we again utilized 293T cells transfected with expression plasmids encoding CD4, CXCR4, Sp Cas9 and an HIV-1-specific sgRNA but also introduced the psynTat expression plasmid. The cells were again infected with the NL-NLuc-HXB indicator virus and NLuc levels determined at 72 h post-infection. As predicted, provision of exogenous Tat protein had little or no effect on the level of virally encoded NLuc expression when sgRNAs specific for TAR were used (1.2X increase for sgRNA TAR1, 1.8X increase for sgRNA TAR2) but had a greater effect when sgRNAs specific for tat were used (9.5X increase for sgRNA Tat1 and a 3.1X increase for sgRNA Tat2). Interestingly, provision of Tat protein in cells prior to infection also increased the level of virally encoded NLuc expression even in the absence of any HIV-1-specific sgRNA (1.9X in the Ctrl cells) and similar levels of activation were also observed for the two gag-specific sgRNAs (3.1X for sgRNA MA and 2.5X for sgRNA CA). These results suggest that expression of the Tat protein in cells prior to infection can modestly but significantly increase HIV-1 gene expression. In conclusion, these data are consistent with the expectation that the TAR and tat specific sgRNAs tested are particularly effective because they inhibit HIV-1 LTR-driven transcription and show that this inhibitory effect can be ameliorated for the two tat-specific, but not the two TAR-specific, sgRNAs if the Tat protein is provided in trans.
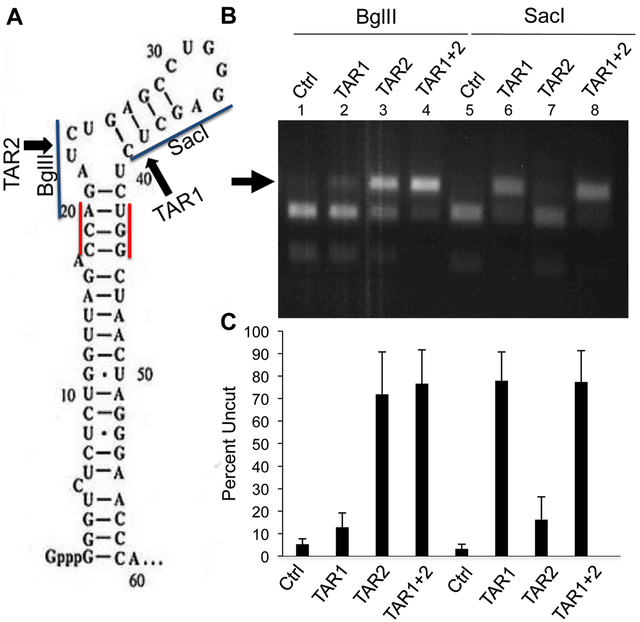
To further confirm that the two TAR-specific sgRNAs TAR1 and TAR2 were indeed inducing mutations that blocked TAR activity, we wished to confirm the existence and identity of the introduced mutations. DNA editing at the predicted Cas9 cleavage site for the TAR1 sgRNA is predicted to disrupt a SacI restriction enzyme site in TAR while Cas9 cleavage induced by the TAR2 sgRNA is predicted to disrupt a BglII restriction enzyme site (Fig. 2A). Therefore, it is possible to quantify the level of gene editing in cells expressing Sp Cas9 and either sgRNA TAR1, or sgRNA TAR2, or both, by PCR across the viral TAR region followed by incubation with either SacI or BglII. As shown in a representative experiment presented in Fig. 2B, and in the compiled data presented in Fig. 2C, we indeed observed a marked increase in the level of TAR DNA that was refractory to cleavage by BglII when the TAR2 sgRNA was expressed in NL-NLuc-HXB-infected cells (from 5.3±2.5% uncut to 72±19% uncut). Interestingly, even though the TAR1 sgRNA is not predicted to cleave within the BglII site present in TAR DNA, but rather 13 bp 3’ to that site (Fig. 2A), expression of the TAR1 sgRNA also reduced the level of cleavage by BglII by ~2–3-fold (Figs. 2B and 2C). Similarly, expression of the TAR1 sgRNA also inhibited SacI cleavage of the TAR DNA recovered from infected cells, with uncut DNA increasing from 3.1±2.2% to 78±13%. Again, sgRNA TAR2, which is predicted to induce Cas9 cleavage 10 bp 5’ to the SacI site in TAR (Fig. 2A), nevertheless also inhibited SacI cleavage by ≥5-fold (Figs. 2B and 2C), thus implying that Cas9 was introducing fairly large deletions in a subset of the cleaved proviruses. Finally, we asked if the simultaneous expression of both sgRNA TAR1 and sgRNA TAR2 would exert a synergistic inhibitory effect. As shown in Fig. 2B, we did see a small increase in the already strong inhibition of TAR cleavage by SacI and BglII when both sgRNAs were present, but this effect was not statistically significant (Fig. 2C).
Fig. 2: Efficient cleavage of DNA encoding the HIV-1 TAR element.
(A) Schematic of the HIV-1 TAR RNA structure with the two Sp Cas9 PAM sequences, the predicted Sp Cas9 cleavage sites and relevant restriction sites indicated (B) The DNA region encoding TAR was recovered by PCR from 293T cells infected with the NL-NLuc-HXB indicator virus, as described in Fig. 1D, that also expressed Sp Cas9 and one or both sgRNAs specific for TAR, as indicated in panel A. DNA editing efficiency was then assessed by cleavage with either BglII or SacI, which cleave at sites that, in wild type TAR, underlie the predicted target sites for the TAR1 (SacI) or TAR2 (BglII) sgRNAs, as indicated in panel A. Therefore, Cas9 cleavage, if followed by the introduction of an indel during NHEJ-mediated repair, would preclude cleavage by SacI and/or BglII. A representative experiment is shown with the percentage of uncut TAR DNA indicated below. (C) Same as panel B except that this bar graph shows the average level of uncut TAR DNA, after incubation with SacI or BglII, from three independent biological replicates with SD indicated.
To more fully characterize the mutations introduced into the HIV-1 TAR element by Sp Cas9 loaded with either sgRNA TAR1 or sgRNA TAR2, or both, we also PCR amplified, cloned and sequenced multiple independent TAR cDNA clones from the NL-NLuc-XB infected cells analyzed in Fig. 2. As shown in Fig. 3A, sequencing of 34 TAR cDNA clones, derived from NL-NLuc-HXB-infected cells expressing Cas9 and sgRNA TAR1, identified 11 clones bearing deletion mutations, 8 clones bearing a single “T” insertion at the predicted Sp Cas9 cleavage site and 1 clone bearing a 2 bp insertion at that site. Similarly, for cells expressing Sp Cas9 and the TAR2 sgRNA, we recovered a total of 31 TAR sequences, of which 15 bore deletions that encompassed the predicted Cas9 cleavage site and 2 clones that had large, 11 bp or 50 bp insertions at that site (Fig. 3B). Finally, in infected cells in which both sgRNA TAR1 and sgRNA TAR2 were expressed, we recovered 35 total TAR cDNA sequences, 15 of which bore large deletions that likely resulted from simultaneous cleavage by Cas9 at both the predicted TAR1 and TAR2 cleavage sites (Fig. 3C). In addition, we also recovered two clones with smaller deletions, two clones with 1 bp or 2 bp missense mutations and two clones with insertions. Overall, therefore, the pattern of mutations observed in the HIV-1 TAR DNA is consistent with what has previously been observed when Cas9 cleavage sites are repaired by NHEJ (Kennedy et al., 2014; Liao et al., 2015; Wang et al., 2016c; Yoder and Bundschuh, 2016; Zhu et al., 2015).
Fig. 3: Characterization of mutations introduced into TAR by Sp Cas9 cleavage.
(A) Sequencing of TAR DNA from cells expressing Sp Cas9 and the TAR1 sgRNA 72 h after infection with the NL-NLuc-HXB indicator virus. At the top, the wild type HIV-1 sequence is shown, with the sgRNA target sequence underlined and the PAM indicated in red. The predicted TAR DNA cleavage site is indicated by an arrow. Missense mutations are indicated in bold and deletions by dashes. (B) Similar to panel A except showing the sequence of TAR DNA recovered from HIV-1 infected cells expressing Sp Cas9 and sgRNA TAR2. (C) Similar to panel A except showing the TAR DNA sequences recovered from HIV-1 infected cells expressing Sp Cas9 and both sgRNA TAR1 and sgRNA TAR2.
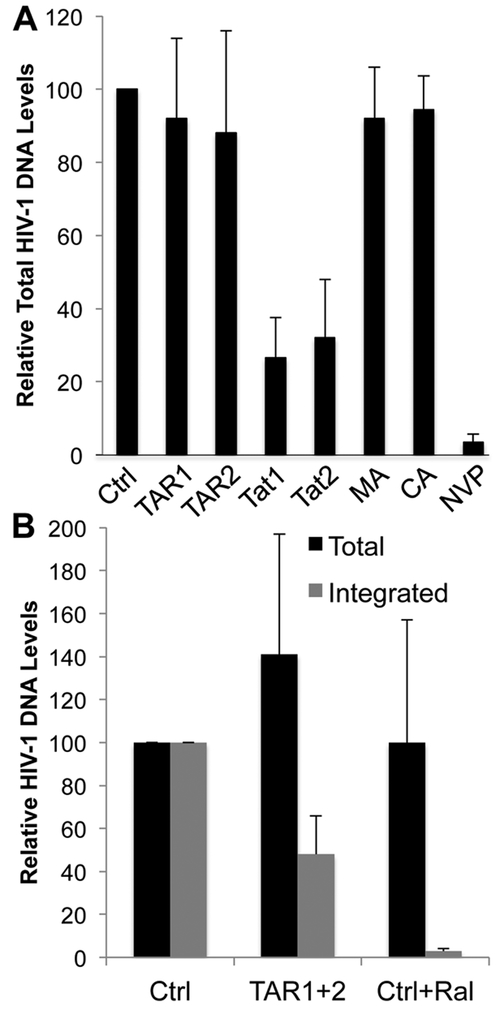
The results presented in Fig. 2 suggest that cleavage by Cas9 may be reducing viral gene expression by two distinct mechanisms. Specifically, the two sgRNAs specific for the MA and CA domains of gag, which are not predicted to affect transcription of the HIV-1 provirus and, hence, should not affect expression of the NLuc indicator gene, nevertheless reduced NLuc expression by ~4-fold. This suggests that Cas9 cleavage prior to proviral integration may be resulting in inhibition of proviral integration and/or proviral destruction. To address this question, we performed qPCR using primers flanking the two sgRNA target sites in tat (Fig. 1A) to determine the level of total and uncleaved DNA in cells infected by NL-NLuc-HXB that express Sp Cas9 and each of the HIV-1-specific sgRNAs (Fig. 4A). Strikingly, both TAR-specific sgRNAs and both gag-specific sgRNAs, despite their clear effectiveness (Fig. 1), nevertheless did not reduce the total level of proviral DNA detected in the infected cells (Fig. 4A). In contrast, both tat-specific sgRNAs, which are predicted to cleave within the segment of the HIV-1 provirus amplified by the TaqMan PCR primers used, revealed a 3–4 fold decline in proviral DNA (Fig. 4A). These data suggest that unintegrated HIV-1 proviruses cleaved by Cas9 are stable, at least up to 72 h post-infection, as shown by the constant PCR signal obtained when sgRNAs specific for TAR or the gag gene were used and proviral levels then analyzed using TaqMan primers specific for the distal tat gene. In contrast, the reduced signal obtained when the sgRNAs specific for tat were used, which are predicted to lie within the amplified tat sequence, show that the majority of these HIV-1 proviral DNA intermediates are in fact cleaved prior to integration and hence unable to integrate or effectively express viral gene products. We note that the observation that the use of TAR-specific sgRNAs does not affect the level of proviral DNA detected using tat-specific PCR primers (Fig. 4A) may suggest that deletion of the body of the provirus by cleavage in both TAR elements is a rare occurrence, though it is also possible that the excised DNA is simply stable.
Fig. 4: Unintegrated HIV-1 proviruses cleaved by Cas9 are stable.
(A) TaqMan qPCR with primers flanking the predicted Sp Cas9 cleavage sites for sgRNAs Tat1 and Tat2, in the HIV-1 tat gene (Fig. 1A), demonstrates that cleaved, unintegrated HIV-1 proviruses are stable. DNA was harvested at 72 hours post-infection with NL-NLuc-HXB. Nevirapine (NVP), an HIV-1 reverse transcriptase inhibitor, was used to control for any HIV-1 DNA carryover from the producer cells. Average of three biological replicates with SD indicated. (B) Alu qPCR was used to determine the effect of expression of the Sp Cas9 protein and both the TAR1 and TAR2 sgRNAs on the level of integrated HIV-1 proviruses. Even though these sgRNAs induce very efficient proviral DNA cleavage by Cas9, as shown in Figs. 1 and 2, the level of integrated HIV-1 DNA is reduced by only ~2-fold. The integrase inhibitor Raltegravir was used as a control for inhibition of proviral integration. Values are given relative to a culture expressing Sp Cas9 and a non-targeting sgRNA (Ctrl), which was set at 100. Average of three biological replicates with SD indicated.
To confirm that Cas9 cleavage is indeed blocking the integration of a significant percentage of the HIV-1 proviruses generated in the infected, Cas9-expressing cells, we next performed Alu-LTR real time nested qPCR (Brussel et al., 2005), a technique which has been shown to accurately quantify integrated HIV-1 proviruses. As shown in Fig. 4B, this experiment revealed that Cas9 cleavage, in this case guided by both sgRNA TAR1 and TAR2, results in a significant diminution in the number of integrated HIV-1 proviruses while the total level of HIV-1 DNA, as determined using the tat-gene specific TaqMan qPCR probe from panel A, again confirmed that total HIV-1 proviral DNA levels were not significantly reduced. As a control, we used the HIV-1 integrase inhibitor Raltegravir which, as expected, almost entirely blocked HIV-1 proviral integration (Rowley, 2008) yet also had little or no effect on the total level of proviral DNA.
2.2. Cleavage and repair of HIV-1 proviruses can occur prior to chromosomal integration
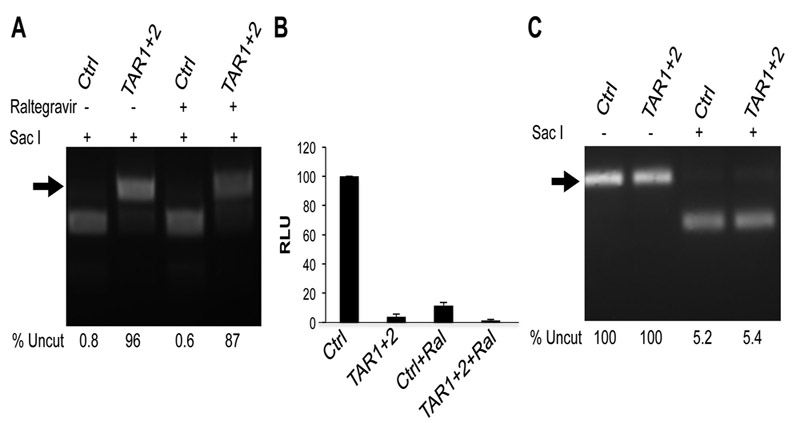
Previously, it has been demonstrated that Cas9, when used in combination with sgRNAs specific for HIV-1, can protect cells from HIV-1 infection and introduce inactivating mutations, such as indels, into the HIV-1 provirus (Hu et al., 2014; Kaminski et al., 2016; Lebbink et al., 2017; Liao et al., 2015; Ueda et al., 2016; Wang et al., 2016b; Wang et al., 2016c; Yoder and Bundschuh, 2016; Zhu et al., 2015). However, the question of whether this mutagenesis occurs before or after integration has not been addressed. The ability of Raltegravir to almost entirely block proviral integration, without significantly affecting proviral DNA levels, (Fig. 4B), allows this question to be answered. Indeed, as shown in Fig. 5A, the unintegrated proviral DNA present in infected cells expressing Cas9 and the sgRNAs TAR1 and TAR2 is edited to essentially the same extent in the presence and absence of Raltegravir, thus clearly demonstrating that DNA editing, that is Cas9 cleavage followed by error-prone DNA repair by NHEJ, is unexpectedly efficient for unintegrated HIV-1 proviruses. This point is further confirmed by the finding that the level of NLuc expression from NL-NLuc-HXB in the presence of Raltegravir—which is low but nevertheless readily detectable—is entirely blocked by Cas9 in the presence of the TAR1 and TAR2 sgRNAs (Fig. 5B).
Fig. 5: Efficient introduction of indels into unintegrated HIV-1 proviruses after Cas9 cleavage.
(A) The level of indel introduction into TAR was assayed as described in Fig. 2 by Sacl cleavage of PCR-amplified TAR sequences recovered from 293T cells expressing Sp Cas9 and the TAR1 and TAR2 sgRNAs in the presence or absence of Raltegravir. (B) Production of NLuc by the infecting NL-NLuc-HXB indicator provirus at 72 h post-infection in cells expressing Sp Cas9 and either a control sgRNA or both the TAR1 and TAR2 sgRNAs in the presence or absence of Raltegravir. Average of three independent experiments with SD indicated. (C) HIV-1 LTRs bearing indels in TAR are largely transcriptionally inactive. RT-PCR was used to amplify DNA encoding TAR from viral mRNAs expressed in HIV-1 infected cells expressing Sp Cas9 and the TAR1 and TAR2 sgRNAs, as shown in panel A. This cDNA was then analyzed for the presence of TAR indels by SacI cleavage, as described in Fig. 2.
Finally, we were curious to know if the low level of NLuc expression observed in NL-NLuc-HXB-infected cells expressing Cas9 and the sgRNAs TAR1 and TAR2 came from the low level of remaining unedited HIV-1 proviruses or was instead due to TAR-independent transcription from the numerous edited proviruses. To this end, we recovered total cellular RNA from cells expressing Cas9 and an irrelevant sgRNA (Ctrl) or the TAR1 and TAR2 sgRNAs, as shown in lanes 1 and 2 of Fig. 5A, and then prepared cDNA. We then amplified the HIV-1 TAR region from the cDNA and cleaved with SacI to determine what percentage of the viral cDNA was resistant to SacI cleavage and hence edited. Despite the fact that 96 +/− 4.4% of the TAR DNA recovered by DNA PCR was edited (Fig. 5A, lane 2), we observed no increase in the level of TAR that was resistant to SacI cleavage when the TAR element was instead recovered from this cDNA preparation (Fig. 5C). Therefore, we conclude that the low level of NLuc expression observed in NL-NLuc-HXB-infected cells expressing Cas9 and the sgRNAs TAR1 and TAR2 (Fig. 5B) arises entirely from the remaining low level of unedited HIV-1 proviruses.
2.3. Stable protection of CD4+ T cells from HIV-1 using Cas9 and the TAR1 sgRNA
Having demonstrated the effective cleavage and inhibition of infecting HIV-1 proviruses in 293T cells, we next wished to extend our analysis to CD4+ T cells and, in particular, we were curious if single sgRNAs specific for the highly conserved HIV-1 TAR element would, unlike sgRNAs targeted to several HIV-1 open reading frames (ORFs), be sufficient to induce stable resistance to infection by HIV-1. For this purpose, we transduced the CD4+ T cell line SupT1 with lentiviral vectors encoding Sp Cas9, the TAR1, TAR2 or MA sgRNA (Fig. 1A), or a control sgRNA, and the puromycin resistance gene. To prevent cleavage of the lentiviral vector by Cas9, we introduced mutations that disrupted both the TAR1 and TAR2 target sites in to both copies of TAR.
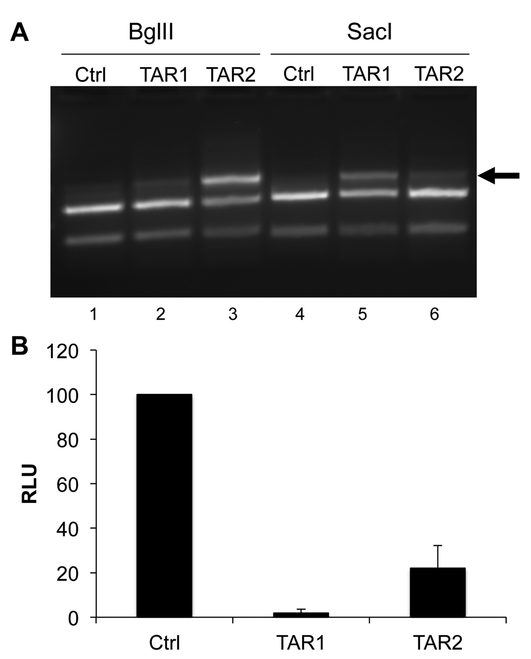
After selection for puromycin resistance, the transduced cells expressing Sp Cas9 and either the control (Ctrl), TAR1 or TAR2 sgRNA were infected with the NL-NLuc-HXB indicator virus and the cells harvested at 24 h post-infection for analysis of indel formation in TAR (Fig. 6A) and NLuc expression (Fig. 6B). As may be observed, even at this short time after HIV-1 infection, the TAR1 and TAR2 guides were clearly introducing indels into TAR at a level sufficient to block cleavage of a readily detectable portion the PCR amplified TAR region by SacI and BglII, respectively (Fig. 6A). Moreover, both the TAR2 sgRNA but also, to an even greater extent, the TAR1 sgRNA were clearly inducing a dramatic inhibition in the expression of the virally encoded NLuc indicator protein in these stably transduced CD4+ T cells (Fig. 6B).
Fig. 6: Cas9 cleavage of infecting HIV-1 proviruses in SupT1 cells.
(A) SupT1 cells stably expressing Sp Cas9 and either a control sgRNA or the TAR1 or TAR2 sgRNA were infected with the NL-NLuc-HXB indicator virus. At 24 h post-infection, cells were harvested and total DNA isolated and used for PCR using the TAR-specific primers described in Fig. 2B followed by cleavage with either SacI or BglII. The arrow indicates the cleavage-resistant TAR DNA sequences that are generated by Cas9 cleavage followed by NHEJ (B) Similar to panel A except that the harvested SupT1 cells were lysed and the level of intracellular NLuc expression determined. The NLuc level observed in SupT1 cells expressing the control sgRNA was set to 100. Average of three biological replicates with SD indicated
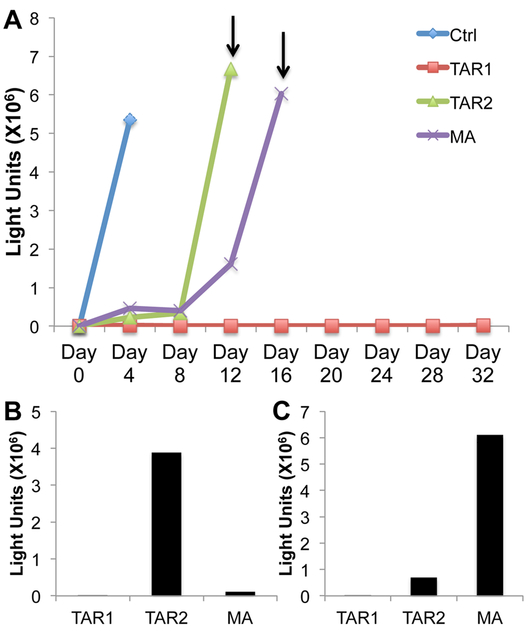
Based on previous work reporting the rapid selection of HIV-1 variants resistant to Cas9 cleavage with a range of sgRNAs primarily targeted to HIV-1 ORFs (Ueda et al., 2016; Wang et al., 2016b; Wang et al., 2016c; Yoder and Bundschuh, 2016), we were interested in whether TAR-specific sgRNAs would also induce resistant HIV-1 mutants. We therefore infected the SupT1 cell lines stably expressing Sp Cas9 and the TAR1, TAR2 or MA sgRNA, or a control non-targeting sgRNA, with the NL-NLuc-HXB indicator virus and then performed a 32-day viral growth curve. For this purpose, the SupT1 cells were passed every four days into fresh media, at which time an aliquot of the cells was harvested for analysis of intracellular NLuc expression levels. As shown in Fig. 7A, and in two independent replicates (Figs. S1 and S2), the NL-NLuc-HXB virus grew rapidly in the control SupT1 cells expressing Sp Cas9 and a non-targeting sgRNA, resulting in the almost complete death of the culture by 4 days post-infection. As expected, we observed a lag in the infected cultures expressing the MA sgRNA, but virus replication was detectable by day 12 in all three replicates with the cells largely dead by day 16. Similarly, we observed the emergence of resistant virus in the culture expressing the TAR2 sgRNA in all three culture experiments, reaching maximal levels between day 12 (Figs. 7A and S1) and day 16 (Fig. S2). However, we did not see emergence of an HIV-1 mutant resistant to the TAR1 sgRNA in any of these three independent virus growth experiments.
Fig. 7: Resistant HIV-1 mutants arise in T cells expressing the TAR2 or MA sgRNA but not in cells expressing the TAR1 sgRNA.
(A) Representative growth curve measuring the level of intracellular NLuc expression seen in SupT1 cells stably expressing the Sp Cas9 protein and a control (Ctrl) or HIV-1 specific sgRNA (MA, TAR1 or TAR2). Cells were infected with a stock of the NL-NLuc-HXB indicator virus on day 0 and then passed into fresh media every four days, at which point an aliquot of 3 × 105 cells was harvested and used to measure intracellular NLuc expression, as indicated. Arrows indicate the times when supernatant media were sampled to detect released, infectious HIV-1. (B) Media sampled from the SupT1 cells in panel A at day 12 were used to infect fresh Cas9-expressing SupT1 cells expressing the cognate sgRNA, as indicated. Cells were then harvested 4 days later and intracellular NLuc levels determined. (C) Same as panel B except using supernatant media harvested at day 16.
2.4. Selection of functional TAR mutants by sgRNA TAR2
To further confirm the presence of resistant viruses in the breakthrough cultures expressing the TAR2 and MA sgRNAs, but not in the culture expressing the TAR1 sgRNA, media were sampled from these cultures at day 12 and day 16, as indicated in Fig. 7A, and used to infect fresh SupT1 cells expressing the cognate sgRNA. As shown in Fig. 7B and 7C, we detected the replication of the NL-NLuc-HXB indicator virus in both the sgRNA TAR2 and MA expressing cells but not in the TAR1 expressing SupT1 cells, by four days post-infection and similar data were also obtained in the other two replicates (Figs. S1B and C and Fig. S2B).
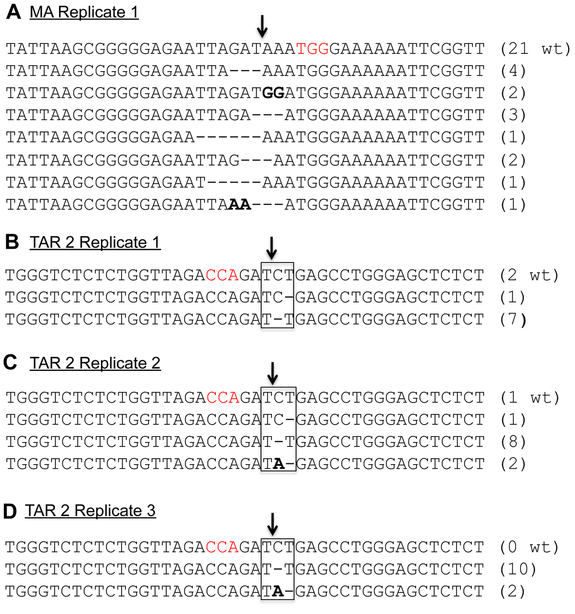
We next wished to define the mutations in the DNA target sites for the sgRNAs TAR2 and MA that conferred the resistance documented in Fig. 7A. For this purpose, we PCR amplified and sequenced the relevant regions of the HIV-1 genome using DNA harvested from the cultures described in Figs. 7B and 7C. In the case of the MA sgRNA resistant cells, we recovered four different 3 bp and one 6 bp deletion mutations in the sgRNA target sequence, as well as a 2bp missense mutation (Fig. 8A). In addition, we recovered one mutant bearing both a 3 bp deletion and a 2 bp missense mutation as well as one 5 bp deletion that is presumably not replication competent. In the case of the viruses resistant to the TAR2 sgRNA, we recovered 7 TAR sequences in which the “C” residue in the TAR “UCU” bulge, which is coincident with the predicted Cas9 cleavage site, was deleted (mutant UΔU). In addition to 2 wild type sequences, we also recovered a TAR sequence in which the 3’ “U” in the “UCU’ bulge was deleted (mutant UCΔ, Fig. 8B). To sample the full spectrum of mutations that induce resistance to the TAR2 sgRNA, we also recovered this region from resistant viruses in the two independent biological replicates shown in Figs.S1 and S2. We again predominantly recovered the UΔU TAR mutant, bearing a deletion of the “C” residue in the TAR “UCU” bulge (18 out of 24 sequences). As minor species, we again recovered the UΔU TAR mutant, lacking the 3’ U residue in the TAR “UCU” bulge (1 read) as well as a third, novel TAR mutant bearing a “UA” bulge in place of the “UCU” bulge (UAΔ, 4 reads) (Figs. 8C and 8D).
Fig. 8: Sequence of Cas9-resistant HIV-1 mutants recovered from SupT1 cells expressing Cas9 and the TAR2 or MA sgRNA.
(A) DNA sequence of mutants in the matrix domain of the HIV-1 gag gene induced by culture in SupT1 cells expressing Sp Cas9 and the MA sgRNA, as shown in Fig. 7. The proviral DNA sequences shown were amplified by PCR and then cloned and sequenced. We recovered 21 clones that retained the wild type (wt) HIV-1 sequence as well as 14 clones bearing the indicated deletion and missense mutations in the targeted region. The PAM used is indicated in red and the predicted Cas9 cleavage site by an arrow (B) Similar to panel A but showing TAR sequences amplified from the HIV-1-infected SupT1 culture shown in Fig. 7B that express Sp Cas9 and the TAR2 sgRNA. (C) Similar to panel B but showing TAR sequences amplified from a second, independent growth experiment shown in Fig. S1A. (D) Similar to panel B but showing TAR sequences recovered from a third, independent growth experiment shown in Fig. S1B.
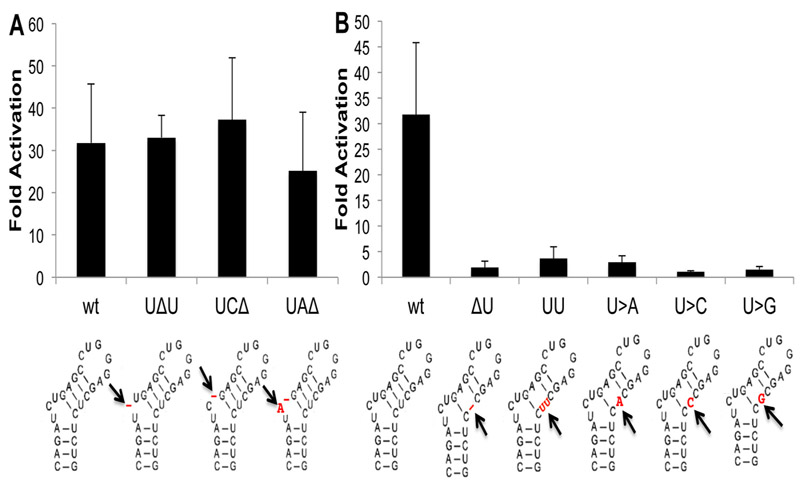
We next wished to ascertain whether the three TAR mutants shown in Fig. 8B, C and D, which were selected upon passage in SupT1 cells expressing Sp Cas9 and the TAR2 sgRNA, retain the ability to be effectively transactivated by the HIV-1 Tat protein. We therefore generated indicator plasmids in which the firefly luciferase (FLuc) indicator gene was expressed under the control of the wild type HIV-1 strain NL4–3 LTR, or LTRs containing each of the three TAR mutations, which were then transfected into Hela cells in the presence or absence of Tat. As shown in Fig. 9A, all three mutants recovered from the cultures expressing the TAR2 sgRNA retained the ability to be effectively transactivated by Tat, thus explaining their rapid emergence upon passage of wild type HIV-1 in T cells expressing the TAR2 sgRNA. But why did the TAR1 sgRNA fail to give rise to resistant mutants? The mechanism that allows such resistant viral mutants to arise relies on the cellular NHEJ machinery to introduce small indels or missense mutations at the Cas9 cleavage site that block further Cas9 cleavage yet do not block the function of the mutated viral sequence (Wang et al., 2016b; Wang et al., 2016c; Yoder and Bundschuh, 2016). We therefore generated the two most conservative indel mutations possible at the TAR1-directed Cas9 cleavage site in TAR (Fig. 2A), the deletion of the coincident U residue (ΔU) or duplication of the U (UU), as well as all three possible missense mutations at this site (U>A, U>C and U>G) and analyzed the ability of these five TAR mutants to support Tat transactivation. As shown in Fig. 9B, all five mutants were in fact non-responsive, thus likely explaining why TAR1 sgRNA resistant HIV-1 mutants were not selected in any of the three viral growth curves (Figs. 7A, S1 and S2).
Fig. 9: Analysis of the biological activity of selected or synthetic TAR mutants.
(A)This panel measures the ability of HIV-1 Tat to activate gene expression from an HIV-1 LTR bearing wild type TAR or bearing one of the three TAR mutations selected in T cells expressing Sp Cas9 and the TAR2 sgRNA, as shown in Fig. 8. TAR function was assessed by co-transfection of HeLa cells with the Tat expression plasmid pcTat and an indicator construct containing the indicated wild type or mutant HIV-1 LTR linked to the FLuc luciferase gene. An RLuc expression plasmid served as an internal control. Data are presented for each indicator plasmid as a multiple of the level of FLuc seen in the absence of Tat, normalized to the RLuc value (B) Similar to panel A, except analyzing the function of the indicated TAR mutations introduced at the predicted Cas9 cleavage site directed by the TAR2 sgRNA. Average of three independent biological replicated with SD indicated. The mutations analyzed are shown below each panel in red.
3. Discussion
Several studies have previously demonstrated that, while expression of Sp Cas9 and sgRNAs specific for the HIV-1 proviral DNA can confer resistance to HIV-1 infection, this resistance is generally transient (Ebina et al., 2013; Hu et al., 2014; Kaminski et al., 2016; Lebbink et al., 2017; Liao et al., 2015; Ueda et al., 2016; Wang et al., 2016a, b; Wang et al., 2016c; Yoder and Bundschuh, 2016; Zhu et al., 2015). This is due to the selection of HIV-1 mutants that bear mutations in the targeted sequence that block Cas9 cleavage yet do not disrupt the replication competence of the resultant HIV-1 mutant (Wang et al., 2016b; Wang et al., 2016c; Yoder and Bundschuh, 2016). This is a particular problem if open reading frames are targeted as missense mutations, as well as short in-frame deletions or insertions, are readily selected if the underlying protein sequence is not essential (Fig. 8A) and can also arise even when it is, due to missense mutations at the wobble position of codons. While the simultaneous expression of several sgRNAs can inhibit the selection of resistant, replication competent HIV-1 mutants (Lebbink et al., 2017; Wang et al., 2016a), we here explore the alternative strategy of targeting highly conserved cis-acting sequences in the HIV-1 genome. Two obvious potential targets are the Rev Response Element (RRE), required for Rev function (Malim et al., 1989), and TAR, required for Tat function (Cullen, 1990), and we decided to concentrate on the latter. Specifically, we focused on two potential Sp Cas9 target sites located towards the apical region of TAR, one of which, guided by the TAR2 sgRNA, was predicted to cleave in the DNA sequence encoding the TAR “UCU” bulge, which is required for Tat binding (Roy et al., 1990), while the second was predicted to cleave in the DNA sequence encoding the short apical stem of TAR, which is known to be critical for TAR function (Berkhout and Jeang, 1991; Delling et al., 1992) (Fig. 2A). Both TAR-specific sgRNAs proved to mediate the effective cleavage of HIV-1 proviral DNAs in infected 293T cells and both were also found to effectively inhibit HIV-1 transcription (Figs. 1) and to introduce a range of indel mutations at the predicted cleavage sites in TAR (Figs. 2 and 3). Similarly, the Sp Cas9 protein, and either TAR-specific sgRNA, also effectively inhibited HIV-1 gene expression when stably expressed in CD4+ T cells and efficiently introducing indels into TAR (Fig.6). However, upon prolonged culture, the two TAR-specific sgRNAs were found to differ in that, while the TAR1 sgRNA conferred stable resistance to HIV-1 replication, the TAR2 sgRNA instead rapidly and consistently selected for resistant HIV-1 mutants that replicated at essentially wild type levels (Figs. 7A, S1A and S2A). Sequencing of the TAR region in the selected TAR2-resistant HIV-1 mutants revealed three distinct mutants in the TAR “UCU” bulge, (“UΔU”, “UCΔ” and “UAΔ”, where Δ is a deleted base, see Fig. 8), all of which proved able to support transactivation by the HIV-1 Tat protein (Fig. 9A). In fact, this result was not unexpected, as it has previously been reported that similar TAR mutants, generated artificially, retain nearly full activity and these results led to the conclusion that only the 5’ “U” residue in the “UCU” bulge is in fact critical for Tat binding and TAR function (Berkhout and Jeang, 1989; Roy et al., 1990). Moreover, the wild type HIV-2 TAR element, which is fully responsive to HIV-1 Tat, actually contains a “UU” bulge at the same location as the “UCU” bulge found in HIV-1 TAR (Berkhout, 1992; Fenrick et al., 1989).
The question then is why didn’t the TAR1 sgRNA also select for a resistant, replication competent TAR mutant? While drug resistant HIV-1 mutants arise due to error prone reverse transcription by the HIV-1 reverse transcriptase, Cas9 resistant mutants generally arise due to mutations introduced by NHEJ-mediated DNA repair at the Cas9 cleavage site and these mutations are generally either small indels or missense mutations (Ueda et al., 2016; Wang et al., 2016b; Wang et al., 2016c; Yoder and Bundschuh, 2016). However, as shown in Fig. 9B, a range of conservative mutations at the predicted TAR1-directed Cas9 cleavage site (single nucleotide deletion or insertion mutations or single nucleotide missense mutations) all block TAR function, thus likely explaining why an HIV-1 resistant to sgRNA TAR1 did not arise. It could be argued that, if a large enough ensemble of viral mutants were tested, a TAR1-resistant HIV-1 mutant might eventually appear. However, the apical stem in TAR is not only required for structural reasons but also contributes to TAR function at the RNA sequence level (Delling et al., 1992), so this is by no means certain. Clearly, if sgRNAs specific for TAR, or other highly conserved regions of the HIV-1 genome, such as the RRE, can be identified that are not subject to the selection of resistant, replication competent HIV-1 mutants then this could be very important. Moreover, there is no reason why these particularly effective sgRNAs could not be used in combination.
In addition to the above, these experiments also led to the identification of novel aspects of the mechanisms underlying the inhibition of de novo HIV-1 infections by Cas9. Specifically, we observed that Cas9 cleavage frequently occurs prior to proviral integration, resulting in a substantial, 3–4-fold reduction in the number of integrated HIV-1 proviruses (Fig. 1D and Fig. 4B). This reduction would likely be far greater except that the cleaved proviral DNA appears to be quite stable (Fig. 4A) and, moreover, is repaired by NHEJ with essentially the same efficiency as integrated proviral DNA (Fig. 5A). This is a surprising result, as one might have anticipated that a cleaved proviral DNA intermediate would rapidly fall apart, thus blocking DNA repair by NHEJ and preventing integration. Presumably, the non-cleaved, LTR-derived ends of the linear proviral DNA intermediate are held together by the integrase protein, though how the internal cleaved DNA ends remain together after Cas9 cleavage, or are brought together to facilitate NHEJ, is not currently apparent. We note that it has recently been reported that DNA breaks introduced into the closed circular DNA genomes expressed by human papillomavirus or hepatitis B virus can be repaired by host cell factors expressed in infected cells (Dong et al., 2015; Mehta and Laimins, 2018), so perhaps the repair of the cleaved HIV-1 DNA proviral intermediate is not totally unexpected. Regardless, these data clearly demonstrate that inhibition of HIV-1 replication by Cas9 results not only from the introduction of indels at the Cas9 cleavage site but also from the partial inhibition of proviral DNA integration.
4. Materials and Methods
4.1. Cell Culture
293T cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 1X antibiotic-antimycotic (Gibco Cell Culture), and 50 µg/mL gentamicin (Life Technologies) at 37°C. SupT1 cells (Ablashi et al., 1995) were grown in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% FBS, 1X antibiotic-antimycotic, and 50 µg/mL gentamicin. Transduced SupT1 cells were maintained in RPMI medium supplemented with 1 µg/mL puromycin.
4.2. Molecular Clones
The previously described pNL-FLuc-HXB vector (Tokunaga et al., 2001) was modified by excision of the Firefly Luciferase ORF, by cleavage with NotI and XhoI, followed by insertion of a cDNA encoding Nano Luciferase (NLuc) (Promega) into the same restriction enzyme sites to generate pNL-NLuc-HXB.
The CMV immediate early (CMV-IE) promoter, the synTat ORF and the genomic rat preproinsulin gene poly(A) addition site were excised from the previously described psTat plasmid (Tiley et al., 1990) and then cloned into pGEM3Zf(+) (Promega) to generate psynTat.
sgRNAs were designed to target proviral DNA sequences encoding the apical region of HIV-1 TAR, the 5’ region of the viral tat gene, and the regions of gag which encode segments of MA and CA, in the proviral clone pNL4–3 (AIDS Reagent #114). The Cas9/sgRNA expression constructs were generated as described previously. Briefly, oligonucleotides containing the 20nt viral target sequences (listed in Supp. Table 1) were annealed and cloned into the Cas9/sgRNA expression vector pX330 (Addgene #42230) using BbsI restriction sites. The terminal 5’ nucleotide of each sgRNA target was replaced with a “G” residue to facilitate transcription from the RNA polymerase III dependent U6 promoter present in pX330.
The lentiviral vector pLC-CMVmut was constructed from the base pLentiCRISPR and pLentiCRISPRV2 plasmids (Sanjana et al., 2014; Shalem et al., 2014). The EFS promoter from LentiCRISPRV2 was replaced with the CMV-IE promoter via EcoRI and XbaI sites. The internal regions of this vector were then cloned into pLentiCRISPR via 5’ and 3’ MluI and NotI sites to generate pLC-CMV, which expresses both a FLAG-tagged version of the Sp Cas9 protein and an sgRNA. Importantly, the pLC-CMV vector was then modified to contain mutated 5’ and 3’ TAR sequences to avoid Cas9 mediated cleavage when paired with sgRNAs targeting TAR, to generate pLC-CMVmut. The oligos used to create these mutations were MutTAR-F and MutTAR-R (Suppl. Table 2). The 20 nt sgRNA targets were fused to the chimeric sgRNA backbone in the lentiviral vector via BsmBI restriction sites.
The HIV-1 LTR indicator plasmid pHIV-FLuc was constructed by excision of the FLuc ORF from pNL-FLuc-HXB by cleavage with NotI and XhoI followed by insertion into the pcDNA3 polylinker via identical NotI and XhoI sites. The wild type HIV-1 LTR was then PCR amplified from pNL-FLuc-HXB as a BamHI-HindIII fragment, which was then inserted 5’ to the FLuc gene via BglII and HindIII sites in pcDNA3, thus removing the CMV-IE promoter. Finally, mutant TAR sequences were introduced through PCR using the BglII site within the LTR sequence and the HindIII site within the pcDNA3 parental vector, with the reverse primer containing the desired mutations.
4.3. Reporter Assays
For analysis of sgRNA efficacy using a transfected proviral clone, 293T cells were plated at 1.25 × 105 cells per well in 12-well plates and transfected using the calcium phosphate method with 1 µg of the Cas9/sgRNA expression plasmid and 100 ng of the proviral clone pNL-NLuc-HXB. Cells were lysed at 72 hours post transfection (hpt) and samples collected for quantification of NLuc activity and also Western blot analysis. For NLuc analysis, samples were harvested, washed twice in phosphate buffered saline (PBS), lysed in Passive Lysis Buffer (Promega) and assayed for NLuc activity using the Nano-Glo Luciferase Assay (Promega).
For analysis of the Tat responsiveness of HIV-1 TAR mutants, 2 × 105 Hela cells were co-transfected using PEI with 250 ng pHIV-FLuc (wild-type or mutant), 20 ng of the pRLucSV40 (Promega) Renilla luciferase (RLuc) expression plasmid as an internal control and 20 ng pcTAT or pCMV as a negative control (total of 1520 ng of DNA). Media were changed 20 hours post transfection and the cells incubated for a total of 48 hours. Cells were then lysed in Passive Lysis Buffer (Promega) and the FLuc and RLuc levels measured using the Dual-Luciferase Reporter Assay System (Promega).
For Western blot analyses, cells were harvested and lysed in SDS-PAGE protein sample buffer supplemented with 2% mercaptoethanol. Lysates were subjected to electrophoresis on 4 to 20% SDS-polyacrylamide gels (Bio-Rad) and transferred onto nitrocellulose membranes. The membranes were then probed in 5% nonfat dry milk–PBS-T (PBS, 0.1% Tween 20, 0.5% bovine serum albumin) with the following antibodies: anti-Flag (catalog #F1804; SigmaAldrich), anti-actin (catalog #MA5–15739; Invitrogen) and anti-p24 (#6458 NIH Aids Reagent) (Simon et al., 1997). The membranes were washed in PBS-T, incubated with a species-specific, horse radish peroxidase-conjugated secondary antibody, and then washed again in PBS-T. The membranes were incubated with a Western Bright Sirius Western blot detection kit (Advansta), and signals captured using GeneSnap (Syngene).
4.4. Synthetic Tat Rescue Assay
293T cells were plated at 2 × 105 cells per well in 6-well plates and transfected using the calcium phosphate method with 2 µg of the Cas9/sgRNA expression plasmid, 200 ng and 800 ng of the CD4 and CXCR4 receptor expression plasmids (Tokunaga et al., 2001), respectively, and 5 ng of psynTat, or pGEM3ZF(+) as a control. In parallel, 10 cm dishes containing 2 × 106 293T cells were transfected with 10 µg of the proviral clone pNL-NLuc-HXB (Tokunaga et al., 2001) using polyethyleneimine (PEI). 72 hours post-transfection, the supernatant from the HIV-1 producer cells was filtered through a 0.45 µM filter (PALL) and used to infect 2 × 105 transfected cells expressing Cas9 and an sgRNA specific for HIV-1. The infected cells were then incubated an additional 72 hours. Cells were washed twice in PBS and then harvested in Passive Lysis Buffer (Promega) and assayed for NLuc activity using the Nano-Glo Luciferase Assay.
4.5. Analysis of HIV-1 replication in Cas9-expressing cells
293T cells were plated at 2 × 105 cells per well in 6-well plates and transfected using the calcium phosphate method (Cullen 1987) with 2 µg of the Cas9/sgRNA expression plasmid, 200 ng and 800 ng of the CD4 and CXCR4 receptor expression plasmids (Tokunaga 2001). In parallel, 2 × 106 293T cells per 10 cm dish were transfected with 10 µg of the proviral indicator clone pNL-NLuc-HXB using PEI. 72 hpt the supernatant medium from the HIV-1 producer cells was filtered through a 0.45 µM filter, treated with DNase I (NEB) for 1 hour at 370C, and then used to infect 2 × 105 Cas9 expressing 293T cells. Similarly, in the case of Cas9-expressing SupT1 cells, 2 × 105 transduced cells were infected with the NL-NLuc-HXB virus in 48-well plates. The infected cells were then incubated for an additional 24 h for 293T and 72 h for SupT1 cells, prior to harvest and analysis of the NLuc expression level.
For analysis of viral replication in the presence of Raltegravir (Sigma Aldrich), a 10 mM stock was initially prepared in DMSO. 293T cells were then pretreated with either Raltegravir (500:1 dilution to give a final concentration of 20µM), or an equal volume of DMSO alone, 24 hours prior to infection. Infections were then performed in the presence or absence of Raltegravir for the duration of the experiment.
For analysis of TAR DNA editing efficiency, DNA was harvested from Cas9- and sgRNA-expressing cells infected with NL-NLuc-HXB using the Quick-DNA Plus Kit (ZYMO). PCR products were generated by nested PCR using primers LTR-F1 and HIV-R1, followed by a second PCR reaction using primers LTR-F1 and HIV-R2 (see Supp. Table 2). PCR products were purified using GeneJet columns (Thermo-Fisher), digested with restriction enzymes BglII or SacI (NEB) and analyzed by agarose gel electrophoresis. Images were captured using GeneSnap and quantified using GeneTools (Syngene). To identify any mutations introduced into TAR by Cas9, PCR primers LTR-F1 and HIV-R1 (Sup. Table 2) were used to isolate segments of the HIV-1 TAR region that were then cleaved with EcoR I and Xho I, cloned into pcDNA3 and then sequenced using the T7 primer.
For RNA analysis, cells were lysed in TRIzol (Thermo Fisher Scientific) and RNA harvested according to the manufacturer’s instructions. The RNA was then converted to DNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). PCR products for analytical digestion were generated by nested PCR using primers TAR-F and HIV-R3, followed by gel purification and a second PCR using primers TAR-F and HIV-R4 (Sup. Table 2). The final DNA product was purified using GeneJet columns (Thermo-Fisher), digested with SacI and then analyzed on a 2% agarose gel. Images were captured using GeneSnap and quantified using GeneTools.
4.6. qPCR Analysis of HIV-1 DNA levels
293T cells were plated at 2 × 105 cells per well in 6-well plates and transfected using calcium phosphate with 2 µg of the Cas9/sgRNA expression plasmid, and 200 ng and 800 ng of the CD4 and CXCR4 expression plasmids (Tokunaga et al., 2001), respectively. Cells were pretreated with Nevirapine (100 µM final) dissolved in DMSO, Raltegravir (20 µM final) dissolved in DMSO, or an equal volume of DMSO alone, for 24 h prior to infection. In parallel, 2 × 106 293T cells in 10 cm dishes were transfected with 10 µg of the proviral clone pNL-NLuc-HXB using the PEI method. 72 h post-transfection, the supernatant from the HIV-1 producer cells was filtered through a 0.45 µM filter, treated with DNase I (NEB) for 1 hour at 37˚C, and used to infect transfected cells. The infected cells were then incubated an additional 72 hours.
DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen). Purified DNA was incubated overnight with DpnI (NEB) and then subjected to qPCR analysis. All quantitative PCRs were performed in triplicate in a StepOnePlus real-time PCR system according to the manufacturer’s instructions. Relative quantification of HIV-1 DNA levels was performed using the ΔΔCT method with β-Actin as an internal control (Livak and Schmittgen, 2001). For experiments analyzing total HIV-1 DNA levels, HIV-1 DNA was PCR amplified with a custom HIV-1 tat TaqMan probe/primer set (ThermoFisher). β-Actin DNA was PCR amplified using a premade TaqMan probe/primer set (ThermoFisher Assay ID Hs01060665_g1).
For Alu-LTR real time nested qPCR experiments, DNA was amplified using a modified version of the nested PCR approach described previously (Brussel et al., 2005). Briefly, an initial PCR using primers ALU1, ALU2, and L-HIV (Sup. Table 2) was performed using DNA isolated from HIV-1 infected cells. After the PCR products were purified using a PCR Kleen kit (Bio-Rad), a second nested qPCR was performed using primers AA55M and L (Sup. Table 2) and the SYBR green master mix (Thermo Fisher Scientific).
4.7. T cell Transductions and Analysis of HIV-1 breakthrough
Lentiviral vectors were prepared by transfecting 2 × 106 293T cells in a 10cm dish with 6 µg of the lentiviral vector pLC-CMVmut, as well as 6 µg and 2.5 µg of the packaging plasmids pCMVR8.74 and pMD2.G, respectively, using PEI (Addgene plasmids #22036 and #12259). Supernatant containing packaged vector was collected 72 hpt and filtered through a 0.45 µM filter. Following filtration, 2 × 106 SupT1 cells were incubated with 2ml of the filtered supernatant at 37°C overnight. Following incubation, the medium was replaced with fresh RPMI medium and cells incubated for 48 h. At this point, the medium was replaced with fresh RPMI medium supplemented with 1 µg/mL of puromycin to allow selection of transduced cells.
The virus NL-NLuc-HXB was prepared by transfecting 2 × 106 293T cells in a 10 cm dish with 10 µg of the proviral indicator clone pNL-NLuc-HXB using PEI. At 72 hpt, the supernatant medium from the HIV-1 producer cells was filtered through a 0.45 µM filter and treated with DNase I (NEB) for 1 hour at 370C. Transduced SupT1 cells were infected at a final density of 3 × 105 cells/mL through addition of 4 ml of media containing cells to 1 ml of media containing NL-NLuc-HXB. Cells were washed in PBS and placed in 5 mL of fresh RPMI medium at 24 hpi.
Infected cells were passaged every 4 days and monitored for HIV-1 replication. At each passage, infected cells were washed in PBS and placed in 5mL of fresh RPMI medium at a density of 3 × 105 cells/ml. Additionally, 3 × 105 infected cells were taken at each passage interval to measure NLuc activity as described above. Finally, 1 ml of virus containing supernatant was used to infect 3 × 105 fresh SupT1 cells expressing the cognate sgRNA at each passage interval to monitor production of resistant virus. Cognate SupT1 infections were assayed for NLuc activity as described 4 days post infection to monitor for breakthrough virus replication. DNA was isolated from the cognate SupT1 infections using the DNeasy Blood and Tissue Kit (Qiagen) once CPE and NLuc activity were clearly detectable. The DNA regions containing either the TAR target sequence or the MA target sequence were PCR amplified using primers LTR-F1 and HIV-R1 for TAR, and gagseq-F and gagseq-R for MA, cloned into pGEM-3Zf+ and sequenced.
Supplementary Material
Acknowledgements
The research described in this manuscript was supported by National Institute of Health grant R01-AI117780 to B.R.C. A.L.M. was supported by NIH grant T32-CA009111.
The following reagent was obtained through the NIH AIDs Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 p24 Gag Monoclonal (#24–3) from Dr. Michael Malim. In addition, we thank Dr. Feng Zhang for the gift of pX330, pLentiCRISPR and pLentiCRISPRV2 and Dr. Didier Trono for the gift of pCMVR8.74 and pMD2.G.
References
- Ablashi DV, Berneman ZN, Kramarsky B, Whitman J Jr., Asano Y, Pearson GR, 1995. Human herpesvirus-7 (HHV-7): current status. Clin Diagn Virol 4, 1–13. [DOI] [PubMed] [Google Scholar]
- Barrangou R, Marraffini LA, 2014. CRISPR-Cas systems: Prokaryotes upgrade to adaptive immunity. Mol Cell 54, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B, 1992. Structural features in TAR RNA of human and simian immunodeficiency viruses: a phylogenetic analysis. Nucleic Acids Res 20, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B, Jeang KT, 1989. trans activation of human immunodeficiency virus type 1 is sequence specific for both the single-stranded bulge and loop of the trans-acting-responsive hairpin: a quantitative analysis. J Virol 63, 5501–5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B, Jeang KT, 1991. Detailed mutational analysis of TAR RNA: critical spacing between the bulge and loop recognition domains. Nucleic Acids Res 19, 6169–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussel A, Delelis O, Sonigo P, 2005. Alu-LTR real-time nested PCR assay for quantifying integrated HIV-1 DNA. Methods Mol Biol 304, 139–154. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F, 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DB, Platt RJ, Zhang F, 2015. Therapeutic genome editing: prospects and challenges. Nat Med 21, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR, 1990. The HIV-1 Tat protein: an RNA sequence-specific processivity factor? Cell 63, 655–657. [DOI] [PubMed] [Google Scholar]
- Delling U, Reid LS, Barnett RW, Ma MY, Climie S, Sumner-Smith M, Sonenberg N, 1992. Conserved nucleotides in the TAR RNA stem of human immunodeficiency virus type 1 are critical for Tat binding and trans activation: model for TAR RNA tertiary structure. J Virol 66, 3018–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Qu L, Wang H, Wei L, Dong Y, Xiong S, 2015. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral Res 118, 110–117. [DOI] [PubMed] [Google Scholar]
- Ebina H, Misawa N, Kanemura Y, Koyanagi Y, 2013. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep 3, 2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrick R, Malim MH, Hauber J, Le SY, Maizel J, Cullen BR, 1989. Functional analysis of the Tat trans activator of human immunodeficiency virus type 2. J Virol 63, 5006–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Kaminski R, Yang F, Zhang Y, Cosentino L, Li F, Luo B, Alvarez-Carbonell D, Garcia-Mesa Y, Karn J, Mo X, Khalili K, 2014. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci U S A 111, 11461–11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski R, Chen Y, Fischer T, Tedaldi E, Napoli A, Zhang Y, Karn J, Hu W, Khalili K, 2016. Elimination of HIV-1 Genomes from Human T-lymphoid Cells by CRISPR/Cas9 Gene Editing. Sci Rep 6, 22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EM, Cullen BR, 2017. Gene Editing: A New Tool for Viral Disease. Annu Rev Med 68, 401–411. [DOI] [PubMed] [Google Scholar]
- Kennedy EM, Kornepati AV, Goldstein M, Bogerd HP, Poling BC, Whisnant AW, Kastan MB, Cullen BR, 2014. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J Virol 88, 11965–11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebbink RJ, de Jong DC, Wolters F, Kruse EM, van Ham PM, Wiertz EJ, Nijhuis M, 2017. A combinational CRISPR/Cas9 gene-editing approach can halt HIV replication and prevent viral escape. Sci Rep 7, 41968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HK, Gu Y, Diaz A, Marlett J, Takahashi Y, Li M, Suzuki K, Xu R, Hishida T, Chang CJ, Esteban CR, Young J, Izpisua Belmonte JC, 2015. Use of the CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells. Nat Commun 6, 6413. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM, 2013. RNA-guided human genome engineering via Cas9. Science 339, 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR, 1989. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature 338, 254–257. [DOI] [PubMed] [Google Scholar]
- Mehta K, Laimins L, 2018. Human papillomaviruses preferentially recruit DNA repair factors to viral genomes for rapid repair and amplification. mBio 9, e00064–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley M, 2008. The discovery of raltegravir, an integrase inhibitor for the treatment of HIV infection. Prog Med Chem 46, 1–28. [DOI] [PubMed] [Google Scholar]
- Roy S, Delling U, Chen CH, Rosen CA, Sonenberg N, 1990. A bulge structure in HIV-1 TAR RNA is required for Tat binding and Tat-mediated trans-activation. Genes Dev 4, 1365–1373. [DOI] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F, 2014. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 11, 783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F, 2014. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JH, Fouchier RA, Southerling TE, Guerra CB, Grant CK, Malim MH, 1997. The Vif and Gag proteins of human immunodeficiency virus type 1 colocalize in infected human T cells. J Virol 71, 5259–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiley LS, Brown PH, Cullen BR, 1990. Does the human immunodeficiency virus Tat trans-activator contain a discrete activation domain? Virology 178, 560–567. [DOI] [PubMed] [Google Scholar]
- Tokunaga K, Greenberg ML, Morse MA, Cumming RI, Lyerly HK, Cullen BR, 2001. Molecular basis for cell tropism of CXCR4-dependent human immunodeficiency virus type 1 isolates. J Virol 75, 6776–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Ebina H, Kanemura Y, Misawa N, Koyanagi Y, 2016. Anti-HIV-1 potency of the CRISPR/Cas9 system insufficient to fully inhibit viral replication. Microbiol Immunol 60, 483–496. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhao N, Berkhout B, Das AT, 2016a. A Combinatorial CRISPR-Cas9 Attack on HIV-1 DNA Extinguishes All Infectious Provirus in Infected T Cell Cultures. Cell Rep 17, 2819–2826. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhao N, Berkhout B, Das AT, 2016b. CRISPR-Cas9 Can Inhibit HIV-1 Replication but NHEJ Repair Facilitates Virus Escape. Mol Ther 24, 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Pan Q, Gendron P, Zhu W, Guo F, Cen S, Wainberg MA, Liang C, 2016c. CRISPR/Cas9-Derived Mutations Both Inhibit HIV-1 Replication and Accelerate Viral Escape. Cell Rep 15, 481–489. [DOI] [PubMed] [Google Scholar]
- Yoder KE, Bundschuh R, 2016. Host Double Strand Break Repair Generates HIV-1 Strains Resistant to CRISPR/Cas9. Sci Rep 6, 29530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Lei R, Le Duff Y, Li J, Guo F, Wainberg MA, Liang C, 2015. The CRISPR/Cas9 system inactivates latent HIV-1 proviral DNA. Retrovirology 12, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.