Abstract
We present the case of an HIV-negative patient clinically diagnosed with relapsing-remitting MS who achieved significant disease improvement on Combivir (zidovudine/lamivudine). Within months of treatment, the patient reported complete resolution of previously unremitting fatigue and paresthesiae, with simultaneous improvements in lesion burden detected by MRI. All improvements have been sustained for more than 3 years. This response may be related to the action of AZT as a known inhibitor of EBV lytic DNA replication, suggesting future directions for clinical investigation.
Keywords: multiple sclerosis, Epstein-Barr virus
Case Report
The epidemiological link between multiple sclerosis (MS) and the Epstein-Barr virus (EBV) is well-established1, but the role of EBV in MS pathology and clinical progression remains controversial. Classic anti-herpesviral drugs, such as acyclovir and valacyclovir, have no significant clinical benefit in MS2, and are similarly ineffective for infectious mononucleosis, known to be caused by primary EBV infection3. Here we report a case of a patient with severe MS who experienced a dramatic improvement after treatment with Combivir (zidovudine/lamivudine). Zidovudine is known to effectively inhibit EBV (and no other herpesviruses) in vitro4, but has never been tested in a randomized clinical trial of MS.
In September 2014, a 25-year old female student presented to her university’s health services clinic complaining of progressive inability to feel her right leg for two months. She also felt severe fatigue and worsening bilateral leg pain aggravated by walking. On examination, she had bilateral lower extremity numbness and extensor plantar response on the right side.
MRI revealed multiple small brain lesions. Additional lesions were detected in the right peripheral cord at the C4 and C6 vertebral levels, with gadolinium enhancement at C4. The thoracic cord contained extensive lesions spanning T7–T11 as follows: T7–T8 measuring 8 mm, T9–T10 measuring 7 mm, T11 superiorly measuring 1.3 cm, and T11 inferiorly measuring 1.2 cm.
The patient recalled a long history of relevant clinical symptoms including: sudden change in vision in the right eye at age 13, an episode of unilateral lower extremity weakness at 17, bilateral leg numbness at 20, and upper extremity weakness at 24 causing her to drop a pot of boiling water leading to second degree burns. All symptoms had lasted at least 24 hours. While the patient had sought medical care throughout this time, she was instructed each time to wait for improvement and return to the clinic if symptoms did not go away on their own. Since each symptom resolved, no follow-up occurred. Medical attention was only sought at presentation because of decline without improvement in right leg numbness, which was atypical compared to prior numbness, which had disappeared on its own.
Serological testing performed at presentation was negative for HIV, HBV, HCV, HSV1/2, syphilis, lyme, and anti-AQP4 antibody. Subsequently, the patient also tested negative for HTLV1/2 and CMV. No EBV testing was performed at presentation. EBV serology was later performed on July 31, 2017 and showed the following: EBNA1 IgG of 191, VCA IgM negative and IgG >600, EA-D IgG negative. There was no history of infectious mononucleosis. After MRI imaging, her primary care provider referred her to the MS clinic at Brigham and Women’s Hospital, where she was diagnosed with clinically definite relapsing remitting MS. CSF was not examined because the patient met the diagnostic criteria for RRMS based on the McDonald criteria (2010).
In October, she was started on glatiramer acetate injections. While on glatiramer acetate, she was sleeping more than 16 hours a day without feeling rested, developed increasingly disabling pain in her arms and legs, and could not walk more than 100 feet without needing to sit down. On December 2, 2014, a 3-day course of corticosteroids was initiated for suspicion of a relapse, but decline continued.
Because of this clinical decline and local skin reactions, she stopped treatment with glatiramer acetate in December. Repeat MRI performed on January 9, 2015 (Figure) showed a new lesion in the cervical spine at C3 (Supplemental Figure) as well as more pronounced lesions at C4 and C6. The patient, a medical student, after reading a case report of MS with sustained resolution of symptoms on HAART5, independently started therapy with Combivir (zidovudine 300mg/lamivudine 150mg) twice daily. During this time, she was under medical supervision in accordance with standard of care and well-established guidelines for Combivir treatment.
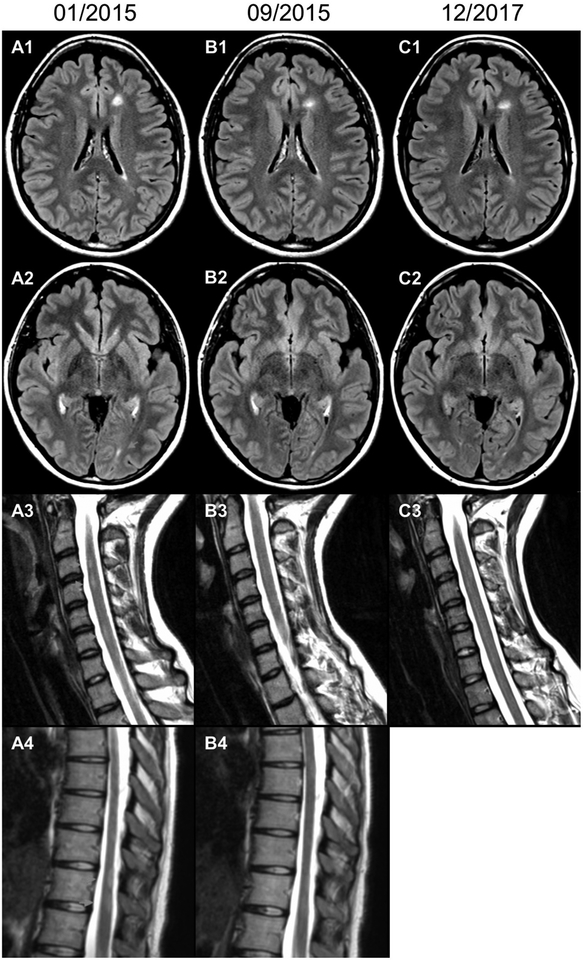
Figure.
Axial T2-FLAIR MRI images of the brain (A1–C1, A2–C2) immediately prior to, 8.5 months after, and 35 months after starting Combivir. Sagittal T2-weighted MRI images of the cervical (A3–C3) and thoracic cord (A4, B4) before and after starting Combivir. MRI of the thoracic cord at 35 months was not performed. All imaging was done without gadolinium contrast on 12/2017 due to lack of clinical symptoms.
A few days after starting Combivir, she noticed dramatic improvement in fatigue. After 2 months, she had gradual improvement in numbness and pain in her arms and legs. Her neurologic exam on March 24, 2015 (11 weeks after starting Combivir) was normal. After 9 months, she had minimal numbness in her feet, only noticeable after walking for long stretches. She could go jogging for the first time in years.
Repeat MRI was performed on September 22, 2015 (after 8.5 months of Combivir). The previously described cervical and thoracic cord abnormalities were significantly less distinct compared to prior study (Figure). The brain MRI abnormalities remained stable with no change on subsequent imaging. The patient has now been on Combivir for more than 3 years. During this time, she has experienced no new symptoms, and all described improvements, including complete cessation of MS-related fatigue, have been sustained to date. Follow-up MRIs performed on December 7, 2016 and December 6, 2017 (Figure) revealed no new focal lesions.
Discussion
Multiple cases of patients with HIV and MS who experienced indefinite remission or resolution of MS symptoms on HAART regimens have been reported in the literature5–7. These have raised an important question of whether or not it is HIV infection that modulates MS or if treatment with HAART could directly impact MS. We report this case of an HIV-negative patient on antiretroviral therapy to support the possibility that antiretroviral drugs may directly affect MS. In particular, this case supports careful examination of drugs in the class of nucleoside/nucleotide analogues.
Addressing the question of mechanism is critical to guide future clinical studies in MS and most importantly, to inform drug selection for these studies. Several mechanisms have been proposed for the impact of HAART on MS. These mechanisms include treatment of a human endogenous retrovirus by inhibition of an endogenous reverse transcriptase5, and chemical similarity of the fumaric acid component of tenofovir disoproxil fumarate with dimethylfumarate6,7.
We propose a different mechanism. Given the link between EBV and MS, it is possible that nucleoside analogues could have a direct effect on EBV, a dsDNA virus, by inhibiting lytic EBV DNA replication. Zidovudine, a component of Combivir, is known to inhibit EBV DNA replication. However, any mechanism must account for the lack of clinical efficacy of acyclovir-class drugs in MS and infectious mononucleosis. Acyclovir drug metabolism is different from drug metabolism of antiretroviral nucleoside analogues because acyclovir requires a viral kinase for phosphorylation. Bypassing this requirement facilitates the accumulation of active drug intracellularly. This unique feature of antiretroviral nucleosides may be important during low-level viral replication or for pre-treatment during a period of viral latency prior to reactivation in the CNS.
To account for the other reported cases of HIV and MS with improvement on HAART, this theory would require the activity of other nucleoside analogues on lytic EBV DNA replication. We suggest this should be tested in vitro, and that the results be used to inform drug selection for a future clinical trial. Meanwhile, it should be emphasized that neither Combivir nor other anti-retroviral drugs have been as of yet proven to be effective treatments for MS.
Supplementary Material
References
- 1.Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8(11):602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman J, Zabriskie J, Plank C, et al. A randomized clinical trial of valacyclovir in multiple sclerosis. Mult Scler. 2005;11(3):286–295. doi: 10.1191/1352458505ms1185oa. [DOI] [PubMed] [Google Scholar]
- 3.Andersson J, Britton S, Ernberg I, et al. Effect of acyclovir on infectious mononucleosis: A double-blind, placebo-controlled study. J Infect Dis. 1986;153(2):283–290. doi: 10.1093/infdis/153.2.283. [DOI] [PubMed] [Google Scholar]
- 4.Lin JC, Zhang ZX, Smith MC, Biron K, Pagano JS. Anti-human immunodeficiency virus agent 3’-azido’-3’-deoxythymidine inhibits replication of Epstein-Barr virus. Antimicrob Agents Chemother. 1988;32(2):265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruszak H, Brew BJ, Giovannoni G, Gold J. Could antiretroviral drugs be effective in multiple sclerosis? A case report. Eur J Neurol. 2011;18(9). [DOI] [PubMed] [Google Scholar]
- 6.Skarlis C, Gontika M, Katsavos S, Velonakis G, Toulas P, Anagnostouli M. Multiple sclerosis and subsequent human immunodeficiency virus infection: A case with the rare comorbidity, focus on novel treatment issues and review of the literature. In Vivo (Brooklyn). 2017;31(5):1041–1046. doi: 10.21873/invivo.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalkley J, Berger JR. Multiple sclerosis remission following antiretroviral therapy in an HIV-infected man. JNeurovirol. 2014;20(6):640–643. doi: 10.1007/s13365-014-0288-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.