Abstract
Background
Adverse cardiovascular events have been linked with PM2.5 exposure obtained primarily from air quality monitors, which rarely co-locate with participant residences. Modeled PM2.5 predictions at finer resolution may more accurately predict residential exposure; however few studies have compared results across different exposure assessment methods.
Methods
We utilized a cohort of 5679 patients who had undergone a cardiac catheterization between 2002–2009 and resided in NC. Exposure to PM2.5 for the year prior to catheterization was estimated using data from air quality monitors (AQS), Community Multiscale Air Quality (CMAQ) fused models at the census tract and 12km spatial resolutions, and satellite-based models at 10km and 1km resolutions. Case status was either a coronary artery disease (CAD) index >23 or a recent myocardial infarction (MI). Logistic regression was used to model odds of having CAD or an MI with each 1-unit (µg/m3) increase in PM2.5, adjusting for sex, race, smoking status, socioeconomic status, and urban/rural status.
Results
We found that the elevated odds for CAD>23 and MI were nearly equivalent for all exposure assessment methods. One difference was that data from AQS and the census tract CMAQ showed a rural/urban difference in relative risk, which was not apparent with the satellite or 12km-CMAQ models.
Conclusions
Long-term air pollution exposure was associated with coronary artery disease for both modeled and monitored data.
Keywords: Particulate matter, air pollution, cardiovascular disease, epidemiology, exposure assessment
1. Introduction
Most previous epidemiology studies that associate PM2.5 concentrations with adverse cardiopulmonary outcomes have used data from central site monitors to characterize ambient exposures fine particulate matter (PM2.5). These studies assume measurements at a single site are representative of air quality over a larger area. Further, monitoring networks are usually placed in highly populated urban areas; thus, the measurements may not accurately reflect exposure for rural populations. More sophisticated emerging exposure estimation approaches may better characterize air pollution exposure and overcome some of these limitations.
The Community Multiscale Air Quality (CMAQ) is a publicly available modeling system that combines information from a meteorological model, an emissions model, and simulation of chemical and physical processes to predict air pollutant concentrations at 12-km grids throughout the United States. Recent models have “fused” (defined here as the integration of different data sources) 12km CMAQ simulated and ground-based measured pollutant concentrations with resulting predictions at the census tract level (Berrocal et al. 2010) or 12km spatial resolution (Friberg et al. 2016). Several recent epidemiological studies have used these fused models to characterize PM2.5 exposure in relation to birth outcomes (Gray et al. 2014; Warren et al. 2016) asthma symptoms, (Mirabelli et al. 2016; Sacks et al. 2014) and pediatric emergency visits (Xiao et al. 2016).
Recent epidemiological studies have also incorporated satellite information on aerosol optical depth (AOD) into air pollution models to better characterize exposure. In order to address missing data due to cloud coverage and help model fit, these satellite-based air pollution models often calibrate the aerosol optical depth (AOD) retrievals with data from ground monitoring stations. Several recent epidemiological studies have used AOD retrievals calibrated with monitoring data at a 10km spatial resolution (Lee et al. 2012; van Donkelaar et al. 2010). Hyder et al. (2014) applied satellite-based estimates developed by Lee et al. (2012) to examine the effect of using combined ground-based and satellite-derived measurements in examining associations with birth outcomes. They found that the satellite-based models, calibrated with data from monitors had an overall better fit compared to using the ground-based measurements alone. Further, Kloog et al. (2011) calibrated AOD retrievals with ground-based measurements, but additionally incorporated land use terms and meteorological variables. Studies have used these estimates to assess associations with myocardial infarctions, mortality, and birth outcomes (Kloog et al. 2012; Kloog et al. 2013; Madrigano et al. 2013). Recent satellite-based estimates have been developed at finer spatial resolutions using the multiangle implementation of atmospheric correction (MAIAC) AOD retrieval algorithm (Chudnovsky et al. 2014; Di et al. 2016; van Donkelaar et al. 2016). In addition to ground-based and satellite-derived measurements, several of these models used a hybrid approach that incorporated information on land use terms and data from chemical transport models to estimate ambient pollutant concentrations. Jerrett et al. (2016) recently compared several model approaches for estimating PM2.5 concentrations, and found that the 1km satellite-based models that were calibrated with ground-based measurements and incorporated land use terms, had an overall better model fit than using remote sensing data alone. Further, Chudnovsky et al. demonstrated that the correlation between PM2.5 and AOD decreased significantly as AOD resolution was degraded and also indicated large spatial variability in particle concentration at a sub-10 km scale (Chudnovsky et al. 2013).
In the current paper, we compare five different data sources used to assign exposure in assessing associations between long-term PM2.5 exposure and adverse cardiovascular outcomes. First, we used direct measurements from central site air quality monitors, a common approach in many epidemiological studies. Next, we used CMAQ simulated values fused with ground-based measurements at the census tract and 12km grid spatial resolutions. Finally, we used 10km and 1km satellite-based models calibrated with monitoring data. We use the year prior to study visit as our long-term exposure window, as several previous epidemiological studies have done (Hoek et al. 2013).
Using data from these five sources, we assessed associations between long-term PM2.5 exposure and measures of cardiovascular disease in a cohort of cardiac catheterization patients residing in North Carolina. Specific measures of cardiovascular disease included the coronary artery disease (CAD) severity index and myocardial infarction (MI). We evaluated the robustness of the associations with PM2.5 exposure estimates from the five different sources. We chose to focus on long-term exposure averages for our comparison analyses as chronic PM2.5 exposure has been most strongly related to severity of atherosclerosis in previous studies (Brook et al. 2010). The study additionally assesses whether the PM2.5-CAD association differs by urban/rural status across the different exposure assignment approaches.
2. Materials and methods
2.1 Study Population
Study participants came from the CATHeterization GENetics (CATHGEN) study, a large cohort of 9334 participants primarily from North Carolina presenting to the Duke University Medical Center Cardiac Catheterization Laboratory from 2001 to 2010, inclusive (Kraus et al. 2015). Participants underwent a cardiac catheterization and coronary angiography for suspected coronary artery disease. Intake clinical information was obtained from an intake questionnaire and medical records at the time of catheterization. All subjects received and signed informed consent prior to enrollment; CATHGEN has been approved by the Duke University Institutional Review Board.
Exposure data for the 10km satellite-based PM2.5 estimates were available from January 1, 2002 through December 31, 2009, thus exposure data for all exposure assignment approaches were restricted to this timeframe for comparability across exposure metrics. Average PM2.5 concentrations for the 365-day time-period prior to each participant’s catheterization date was used as the exposure metric for each study participant in North Carolina. Therefore, patients were included in the current analysis if they resided in North Carolina and their catheterization procedure was performed from January 1, 2003 through December 31, 2009. Residential addresses were obtained from medical records and geocoded for the 5679 study participants who resided in NC and had a catheterization that occurred between 2003 and 2009.
2.2 Outcome Ascertainment
The Coronary Artery Disease (CAD) index was used to measure severity of coronary artery disease (Bart et al. 1997). The index ranges from 0 to 100 and is a risk indicator of events due to coronary atherosclerosis. A higher CAD index corresponds with an increased risk of ischemic events due to atherosclerosis. A binary measure of CAD was constructed, identifying individuals with a CAD index >23, representing having at least one hemodynamically significant lesion (>75% luminal stenosis) in one epicardial coronary artery. There were 610 individuals who underwent a therapeutic intervention and thus did not have a full catheterization, therefore the total sample size for the CAD index outcome is 5069 participants.
We additionally assessed whether participants experienced an MI within a year prior to their catheterization. Participants were considered to be cases for the MI analyses if they had a documented MI in their medical records within a year prior to their catheterization visit. There were 5679 participants who had full MI outcome information available.
2.3 Exposure Assessment
Five different exposure assignment approaches were used: data from a) central site air quality monitors; CMAQ fused predictions at the b) census tract level (CMAQ-PHASE), and c) 12km spatial resolution (CMAQ-DF); and satellite-based predictions at a d) 10km and e) 1km spatial resolution. We used primary residence as indicated at the time of the most recent catheterization. Patients’ geocoded addresses were matched to the nearest EPA air quality monitor location, centroid of the 2000 census tract location (CMAQ-PHASE estimates), or centroid of the nearest 12km, 10km, and 1km grid locations (CMAQ-DF, Satellite 10km, and Satellite 1km). Daily PM2.5 exposure at the primary residence was estimated for the year prior to the catheterization and averaged over this period to estimate the annual average PM2.5 exposure. Some participants underwent multiple catheterization events during the study period. For those individuals, the most recent catheterization visit was linked with exposure data.
2.3.1 Central Site Monitored Data
PM2.5 monitor data (daily average in µg/m3) were obtained from the Environmental Protection Agency’s (EPA) Air Quality System (AQS) Data Mart for the years 2002 to 2009 for the state of North Carolina (EPA 2016c). This network of monitors measures ambient PM2.5 concentrations either daily or every 1 in 3 days. We used the closest air quality monitor to each participant’s residence that was active for the entire year prior to the participant’s catheterization date and had >90% available PM2.5 data. Monitored ozone data, which was available from April – October, was obtained to use in multipollutant models.
2.3.2 EPA CMAQ PHASE Downscaler Model
The CMAQ model combines input from a meteorological model and an emissions model with simulation of chemical and physical processes to describe pollutant transformation, transport and fate (EPA 2016a). A recently developed fusion model was created that uses a Bayesian space-time downscaler approach to combine CMAQ 12km gridded output with monitored data across the US (EPA 2016b). This approach uses a weighted linear combination of the different data sources within a Bayesian framework. Thus, the probability of a calculated coefficient is updated daily, and the weighting is dependent on the estimated uncertainty of each of the data sources. The term “downscaler” refers to the scaling of the areal grid-cell CMAQ output to the point-level air monitoring data, with resulting outputs at the census tract level. Daily predictive surfaces of PM2.5 (daily average in µg/m3) were used for the years 2002 through 2009 for the 2000 and 2010 US census tract centroid locations. More detailed information on this downscaler model has been described previously (Berrocal et al. 2010).
2.3.3 CMAQ-Observation Data Fusion Model
CMAQ-Observation Data Fusion (CMAQ-DF) is another method that fuses ground base monitored observations and chemical transport model (CTM) simulations (Friberg et al. 2016). The CMAQ model fields are used in conjunction with observations in a multi-step process, which has been described in detail previously (Huang et al. 2017). The final outputs capture spatially detailed information by the air quality model, as well as the coarser scale spatial and fine scale temporal information from the observations. The approach was applied from 2002 to 2010 over North Carolina (USA) to develop the spatiotemporal fields of 24hr-average PM2.5 concentrations used here, but has also been used to provide PM2.5 species and gaseous pollutant concentration fields as well.
2.3.4 10km Satellite-based Model
Daily PM2.5 concentrations were estimated at a 10km spatial resolution for the state of North Carolina using recently developed statistical prediction models (Chudnovsky et al. 2012; Lee et al. 2011; Lee et al. 2012). These models estimate exposure using two main stages: calibration with monitored data followed by cluster analysis. For the first stage, satellite-based AOD data were used to estimate ground-level PM2.5 concentrations for days when satellite data were available. A daily calibration approach using a mixed effects model was then applied to control for the inherent day-to-day variability in the AOD-PM2.5 relationship. Next, cluster analysis was applied using AOD and PM2.5 ground monitoring data to predict PM2.5 concentrations on days when satellite data are not available due to the presence of clouds or snow (Lee et al. 2011). More detailed information on the prediction model has been described previously (Chudnovsky et al. 2012; Lee et al. 2011; Lee et al. 2012).
2.3.5 1km Satellite-based Model
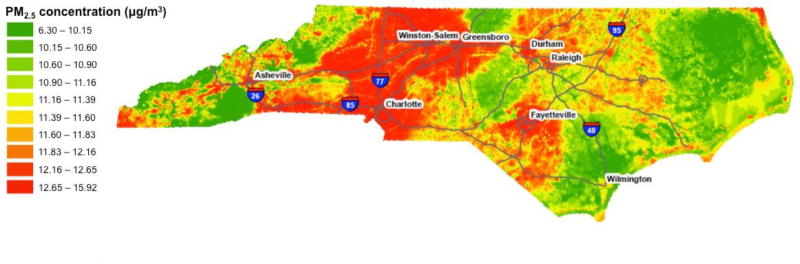
We also assessed the use of PM2.5 concentrations estimated at a 1km × 1km spatial grid resolution, available from a previously published and verified hybrid model (Di et al. 2016). The hybrid prediction model incorporated satellite-based AOD measurements, simulation outputs from a chemical transport model (GEOS-Chem), land-use terms (population density, road density, NDVI, elevation etc.), meteorological variables (temperature, wind speed, humidity, etc.) and other ancillary data sets (e.g., climate types, vertical profile of PM2.5). The hybrid model used a neural network to calibrate all the predictors to monitored PM2.5 and was trained and validated with ten-fold cross-validation. Detailed information on this model has been described previously (Di et al. 2016). Figure 1 shows the annual average 1km PM2.5 (µg/m3) concentrations for the state of North Carolina for the years 2002–2009, using input from the 1km model. A description of each of the five exposure assessment methods are contained in Table 1.
Figure 1.
Annual average 1×1 km satellite-based PM2.5 concentrations (µg/m3) for the state of North Carolina from 2002–2009.
Table 1.
Description of the included exposure assessment methods
| Exposure metric |
Description | Main data inputs |
Additional data inputs |
Spatial resolution |
|---|---|---|---|---|
| Monitor | Monitored PM2.5 data from the EPA's air quality system data mart | Monitor data | Nearest monitor | |
| CMAQ-PHASE | Bayesian space-time downscaler model was used to fuse monitored data with 12km CMAQ data | CMAQ and monitor data | Census tract | |
| CMAQ-DF | CMAQ-observation data fusion (DF) was used to combine monitored data with chemical transport model quality fields | CMAQ and monitor data | 12km | |
| Satellite 10km | Satellite AOD data calibrated with monitored data | Remote sensing and monitor data | 10km | |
| Satellite 1km | Hybrid approach that combines satellite AOD data calibrated with monitored data and GEOS-chem predictions | GEOS-Chem, remote sensing and monitor data | Meteorological variables and land-use terms | 1km |
Abbreviations: AOD, aerosol optical depth; CMAQ, Community Multi-scale Air Quality; DF, data fusion; EPA, Environmental Protection Agency;
2.4 Confounders and Effect Measure Modifiers
Covariates were chosen based upon past associations with air pollution exposure and cardiovascular outcomes. Covariates of interest included: age, sex, race/ethnicity, body mass index (BMI), smoking status, area level attained education, urban/ rural status, history of hypertension, and history of diabetes. Participants were considered smokers if they smoked ≥ 10 cigarettes/day currently or had quit smoking ≥ 10 cigarettes/day within the past 5 years.
Data from the 2000 U.S. Census was used to characterize each participant’s area level educational attainment, (U.S. Census Bureau 2000) which was the main SES indicator using in this study. We defined area level educational attainment as the percentage of individuals in the block group without a high school education. Participants were assigned to block groups and census tracts based on their address at catheterization visit. Educational attainment is a commonly used SES measure and is established as a strong predictor of cardiovascular disease (Havranek et al. 2015). Previous studies have found area level education to be related to PM2.5 concentrations (Hajat et al. 2013).
Rural-Urban Commuting Area Codes (RUCAs) at the census tract level were used to characterize each participant’s urban/rural status. These codes use data from the 2000 decennial census urbanized area and urban cluster definitions to describe each U.S. census tract’s degree of urbanicity (Rural Health Research Center 2009). Urban census tracts were those defined as a metropolitan area core (primary flow within an urbanized area).
2.5 Statistical Analyses
We first reported descriptive statistics comparing the PM2.5 exposure assignment approaches. We then compared individual minimum and maximum differences of exposure assessment approaches across individuals to assess extent of variability between exposure models. Finally, we compared correlations between the annual averages of each of the PM2.5 exposure assignment methods to assess the extent of agreement among the exposure assessment approaches.
Logistic regression analysis was used to estimate odds ratios (OR) and 95% confidence intervals (CI) associated with a CAD index >23 or MI for each 1-µg/m3 increase in annual average PM2.5. Models were adjusted for sex, race/ethnicity, smoking status, area level attained education, and urban/rural status. We assessed associations in single and multipollutant models. Multipollutant models were additionally adjusted for seven-month average ozone concentrations using monitored ozone data during the warm season (April to October).
An additional objective of the current analysis was to assess whether urban/rural status modifies the association between long-term PM2.5 exposure and CAD. Therefore, we included an interaction term between continuous levels of PM2.5 for each of the exposure metrics and urban/rural status. We then compared these models with the main effects model without interaction terms. The Likelihood Ratio Test (LRT) was used to assess potential effect modification and a cutoff of p<0.10 was used to indicate presence of modification. Statistical analyses were performed using SAS version 9.3 (Cary, NC).
3. Results
The characteristics of the CATHGEN study population for this analysis are shown in Table 2. There were 5,679 individuals who met the requirement of NC residence and had a catheterization between 2003–2009; however, there were 610 who did not have outcome information available for the CAD index, resulting in a final sample size of 5,069. There were 2,491 (49%) participants who had a CAD index score >23, indicating presence of significant CAD, and 704 (12%) had an MI within a year of their catheterization visit. The majority of the participants were male (61%), non-Hispanic white (73%), and were either overweight or obese (77%).
Table 2.
Characteristics of the CATHGEN study population, N (%).
| Total Cohort | Urbana | Rural | |
|---|---|---|---|
| Total | 5679 | 2436 | 3243 |
| CVD outcomes | |||
| CAD >23b* | 2491 (49) | 950 (45) | 1541 (52) |
| Recent MI* | 704 (12) | 239 (10) | 465 (14) |
| Age at time of enrollment in years (mean ± SD) | 60.8 ± 12.1 | 61.1 ± 12.2 | 60.6 (12.0) |
| Sex | |||
| Male | 3471 (61) | 1487 (61) | 1984 (61) |
| Female | 2208 (39) | 949 (39) | 1259 (39) |
| Body mass index (kg/m2) | |||
| <18.5 (Underweight) | 80 (1) | 30 (1) | 50 (2) |
| 18.5–24.9 (Normal weight) | 1187 (21) | 507 (21) | 680 (21) |
| 25.0–29.9 (Overweight) | 1987 (35) | 881 (36) | 1106 (34) |
| ≥30.0 (Obese) | 2399 (42) | 1006 (42) | 1393 (43) |
| Missing | 26 | 12 | 14 |
| Racec* | |||
| Non-Hispanic white | 4146 (73) | 1722 (71) | 2424 (75) |
| African American | 1204 (21) | 637 (26) | 567 (17) |
| Other | 329 (6) | 77 (3) | 252 (8) |
| History of smoking* | |||
| Yes | 2664 (47) | 1025 (42) | 1639 (51) |
| No | 3015 (53) | 1411 (58) | 1604 (49) |
| History of diabetes | |||
| Yes | 1660 (29) | 696 (29) | 964 (30) |
| No | 4019 (71) | 1740 (71) | 2279 (70) |
| History of hypertension | |||
| Yes | 3882 (68) | 1647 (68) | 2235 (69) |
| No | 1797 (32) | 789 (32) | 1008 (31) |
| Neighborhood educational attainmentd* | |||
| Low | 2290 (40) | 542 (22) | 1748 (54) |
| High | 3389 (60) | 1894 (78) | 1495 (46) |
| Neighborhood median home value ($)* | |||
| <82,700 | 1400 (25) | 228 (9) | 1172 (36) |
| 82,700–118,000 | 1403 (25) | 477 (20) | 926 (29) |
| 118,000–166,500 | 1436 (25) | 656 (27) | 780 (24) |
| ≥166,500 | 1414 (25) | 1052 (44) | 362 (11) |
| Missing | 26 | 23 | 3 |
Abbreviations: CAD, coronary artery disease; CVD, cardiovascular disease; MI, myocardial infarction.
Urban status was defined as living in a metropolitan urban core census tract.
Binary measure of CAD (>23 CAD index). The total sample size for the CAD outcome is 5,069.
Other race/ethnicity includes Native American, Hispanic, Asian, and unknown.
Low educational attainment includes those who live in block groups where ≥25% of males and females have less than a high school education.
Indicates p<0.05 for differences between urban/rural status.
There were slightly more participants that lived in rural areas versus urban areas (N=3,243 versus N=2,436). Additionally, rural participants had a higher prevalence of coronary artery disease and MIs compared to urban participants. Urban participants tended to live in areas of higher attained education and with higher median home values. There were also significant urban/rural differences for race/ethnicity and smoking status (Table 2).
Table 3 shows the distribution of exposure estimates for each of the exposure assessment methods. In general, there were fairly similar distributions across the exposure assessment methods, which may be because the satellite and CMAQ values were calibrated to monitored values. Mean annual average PM2.5 levels ranged from 12.32 to 12.79 µg/m3, with the 1km model showing the smallest mean annual average PM2.5 level. The monitored and CMAQ-PHASE results showed the most variation with SDs of 1.22 and 1.27, respectively. In general, PM2.5 levels decreased in time from 2002 to 2009 for all exposure assessment methods, as seen in Supplemental Table 1. Additionally, PM2.5 levels were significantly higher in urban areas across exposure assignment methods (Supplemental Table 2).
Table 3.
Annual average PM2.5 (ug/m3) levels for CATHGEN participants.
| Mean (SD) | Min | 25th percentile |
Median | 75th percentile |
Max | IQR | |
|---|---|---|---|---|---|---|---|
| PM2.5 Monitor | 12.76 (1.22) | 6.56 | 12.34 | 12.97 | 13.6 | 16.04 | 1.26 |
| PM2.5 CMAQ-PHASE | 12.79 (1.27) | 6.79 | 12.49 | 13.05 | 13.57 | 19.56 | 1.08 |
| PM2.5 CMAQ-DF | 12.55 (1.03) | 8.29 | 12.25 | 12.78 | 13.17 | 17.32 | 0.91 |
| PM2.5 Satellite 10km | 12.38 (0.90) | 8.57 | 12.15 | 12.50 | 12.94 | 14.35 | 0.79 |
| PM2.5 Satellite 1km | 12.32 (1.10) | 6.92 | 11.80 | 12.50 | 13.08 | 16.50 | 1.28 |
Abbreviations: CMAQ, Community Multi-scale Air Quality; DF, data fusion; EPA, Environmental Protection Agency; IQR, interquartile range; Min, minimum; Max, maximum; SD, standard deviation.
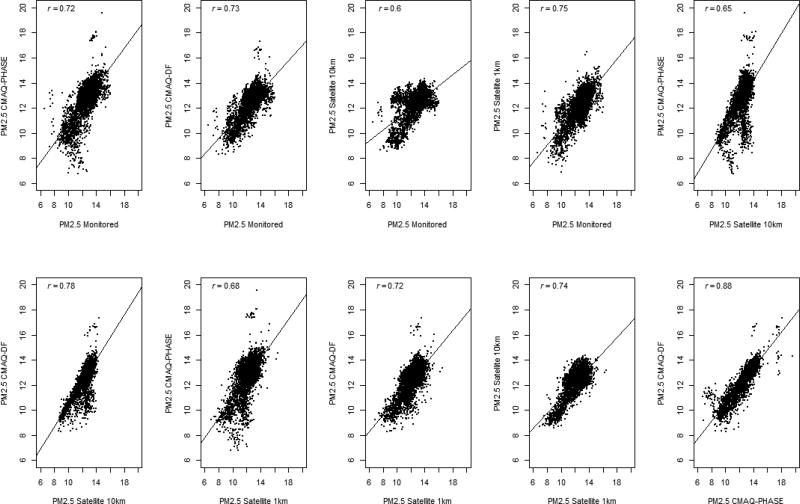
Correlations between the annual averages of each of the PM2.5 exposure assessment methods are shown in Figure 2. There were strong positive correlations between the PM2.5 monitored and modeled data, with coefficients ranging from 0.60 to 0.88. The highest correlation was between the PHASE and DF CMAQ models, with a coefficient of 0.88. The CMAQ-PHASE model was slightly less well correlated with the satellite models, though it had a strong correlation with the monitor data (0.72). When conducting pairwise comparisons of the variation between the exposure assessment averages, the CMAQ-PHASE and CMAQ-DF models showed the least amount of variation in averages (Supplemental Table 3). Participants’ annual average exposure levels were highly correlated in each of the pairwise comparisons, and did not differ by CAD or MI outcome status (Supplemental Figures 1 and 2).
Figure 2.
Correlation plots comparing the different exposure assessment methods.
The adjusted odds ratios for CAD and recent MI in relation to the PM2.5 exposure metrics are shown in Table 4. There were positive associations seen with all of the PM2.5 exposure methods. Associations between a 1-µg/m3 increase in annual average PM2.5 and CAD ranged from an OR of 1.04 (95%CI: 0.99–1.10) for the monitored data to 1.13 (95%CI: 1.06–1.21) for the 10km satellite-based PM2.5 estimates. The 10km results were slightly strongest in magnitude, though overall results were all did not differ across exposure metrics. For the PM2.5-MI analyses, the monitored and CMAQ-PHASE results were slightly more precise and the CMAQ results were slightly strongest in magnitude (CMAQ-DF OR: 1.22, 95%CI: 1.11–1.33; CMAQ-PHASE OR: 1.20, 95%CI: 1.11–1.29), and again results were comparable across exposure assessment methods.
Table 4.
Odds ratios and 95% confidence intervals for the associations between 1-ug/m3 increase in PM2.5 and select CVD outcomes. Results are shown for single and multipollutant models.
| CAD index >23 | MI in prior year | |||
|---|---|---|---|---|
|
|
|
|||
| Single pollutant models |
Multipollutanta models |
Single pollutant models |
Multipollutant models |
|
|
|
||||
| PM2.5 Monitor | 1.04 (0.99, 1.10) | 1.07 (1.01, 1.13) | 1.19 (1.10, 1.29) | 1.19 (1.10, 1.28) |
| PM2.5 CMAQ-PHASE | 1.07 (1.02, 1.13) | 1.08 (1.03, 1.14) | 1.20 (1.11, 1.29) | 1.21 (1.12, 1.30) |
| PM2.5 CMAQ-DF | 1.10 (1.04, 1.17) | 1.14 (1.07, 1.22) | 1.22 (1.11, 1.33) | 1.21 (1.10, 1.33) |
| PM2.5 Satellite 10km | 1.13 (1.06, 1.21) | 1.14 (1.06, 1.22) | 1.17 (1.06, 1.29) | 1.17 (1.06, 1.29) |
| PM2.5 Satellite 1km | 1.09 (1.03, 1.15) | 1.12 (1.06, 1.18) | 1.16 (1.07, 1.26) | 1.17 (1.07, 1.27) |
Abbreviations: CAD, coronary artery disease; CMAQ, Community Multi-scale Air Quality; DF, data fusion; EPA, Environmental Protection Agency; MI, myocardial infarction. Models are adjusted for sex, smoking status, race, area level attained education, and urban/rural status.
Multipollutant models are additionally adjusted for monitored warm season (April – October) ozone averages.
We investigated associations in multipollutant models to assess if annual average ozone levels confounded the PM2.5-CAD associations. Results were similar for multipollutant models adjusted for ozone (Table 4). For the PM2.5-CAD associations overall results increased in strength and precision when PM2.5 concentrations were adjusted for ozone concentrations. PM2.5-MI results were less influenced by adjustment for ozone concentrations.
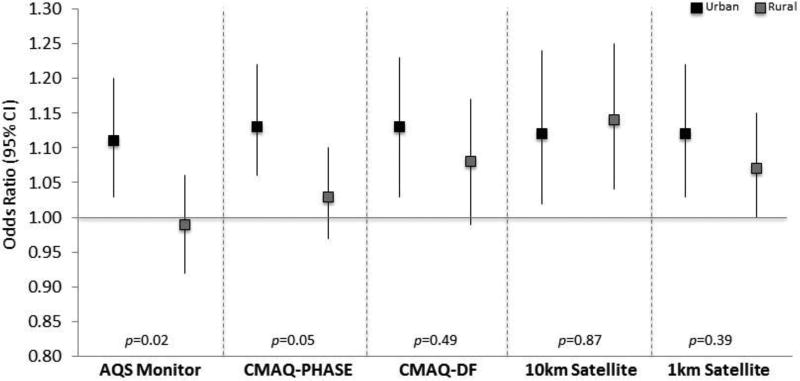
We additionally assessed whether urban/rural status modified PM2.5-CAD associations. Figure 3 shows the results for the modification by urban/rural status. We found significant modification by urban/rural status for the monitored (p=0.02) and CMAQ-PHASE (p=0.05) models. For the monitored data there was an OR of 1.11 (95% CI: 1.03, 1.20) for those living in urban areas and an OR of 0.99 for those living in rural areas (95% CI: 0.92, 1.06). For the CMAQ-PHASE models, there was an OR of 1.13 (95% CI: 1.06, 1.22) for those living in urban areas and an OR of 1.03 for those living in rural areas (95% CI: 0.97, 1.10). PM2.5-CAD associations did not differ by urban/rural status for the CMAQ-DF or the satellite-based models.
Figure 3. Modification of the PM2.5-CAD association by urban/rural status.
Results are shown for each of the five exposure assessment methods. Black squares represent urban participants and grey squares represent rural participants. Vertical lines represent 95% confidence intervals.
4. Discussion
Epidemiologic studies have reported associations between long-term PM2.5 and adverse cardiovascular outcomes. However, exposure assessment methods vary by study, making the results difficult to compare across studies. In this paper, we examined the association among adverse cardiovascular outcomes and PM2.5 concentrations obtained using five different approaches: direct measurements taken from air quality monitors, derived measurements taken from two different models that use emissions inventories as the primary basis for calculating PM2.5 concentrations, and two different models that primarily use AOD measurements obtained from satellites at either 10 km or 1 km resolution.
We observed positive associations among long-term PM2.5 exposure and severity of coronary artery disease as measured by the CAD index. We additionally found consistent associations between long-term PM2.5 exposure and prevalence of a recent MI. These findings were consistent across all five of the exposure assignment approaches. This was not surprising since the annual average PM2.5 values for the various approaches shown in Table 3 were very similar, and there were strong positive correlations of the annual averages between the various exposure assignment methods. These findings increase confidence that the association between PM2.5 and coronary artery disease is robust and not due to measurement error or an anomaly in one of the exposure assignment methods.
In this study, PM2.5-CAD associations were similar among monitored and modeled estimates of exposure. When using data from central site air quality monitors, we make the assumption that measurements at a single site are representative of air quality over a larger area. In theory, one might have expected that increased resolution (10km vs 1km for satellite or census tract vs 12km for CMAQ) would result in less measurement error and more robust associations with health end points. However, this was not the case. More advanced modeling techniques may also bring additional uncertainty into the resulting estimates and interpretation of results (Baxter et al. 2013). Further, individuals are not only exposed at their place of residence, and thus increased spatial resolution may not adequately capture an individual’s complete time-activity pattern of exposure. Additionally, increased resolution may reduce measurement error for spatially heterogeneous pollutants such as carbon monoxide and nitrogen oxides (NOx), but potentially less so for more homogenous pollutants with less spatial variation such as PM2.5 and ozone (Baxter et al. 2013). Therefore, improvements in exposure assessment may be more meaningful for more spatially heterogeneous pollutants (Sellier et al. 2014). However, associations among short-term PM2.5 exposure and health end points may benefit from increased resolution. Previous studies have observed that air quality monitors adequately capture individual long-term PM2.5 averages, while monitors less adequately capture short-term averages (Baxter et al. 2013; Ebelt et al. 2000; Suh and Zanobetti 2010). Finally, PM2.5 in North Carolina is dominated by emissions derived from mobile sources. Some of these models may perform differently in regions where multiple sources contribute to PM2.5.
We did not observe differences between the outcomes. Both the CMAQ and satellite-based models used ground-based measurements to optimize estimates, which may explain some of the similarity in the results across exposure assessment methods. First, the CMAQ 12km gridded output was fused with ground-based measurements, with resulting estimates generated for each census tract (CMAQ-PHASE model) or 12km grid (CMAQ-DF model) centroid. In addition, both of the satellite-based models were calibrated to ground-based measurements. The 1km hybrid estimates also incorporated data from a chemical transport model (GEOS-Chem), as well as meteorological data and land use terms. Recent studies have shown the importance of calibrating remote sensing data to ground-based measurements (Jerrett et al. 2016).
There have been a limited number of previous studies comparing exposure assessment methods in their associations with human disease. Associations between PM2.5 estimated by dispersion or Land-Use Regression (LUR) models and lung function in children were generally consistent for both exposure models (Wang et al. 2015). Adverse pregnancy outcomes were generally associated with PM2.5 measured at ambient monitors, LUR, and CALINE 4, though the size of the estimate depended on both temporal and spatial variations that were incorporated into the exposure assessments (Wu et al. 2011). Sellier et al. (2014) reported consistent associations between infant birth weight and PM10 measured by monitors and calculated by two dispersion models and a LUR model. Another recent study compared findings from several PM2.5 models; they observed stronger associations with cardiovascular mortality using the satellite-based models, particularly when land use terms and monitored data were incorporated into the models (Jerrett et al. 2016).
Because CATHGEN participants reside in both urban and rural locations across North Carolina, we were able to assess modification of the PM2.5-CAD association by urban/rural status using rural-urban commuting area codes at the census tract level to characterize each participant’s urban/rural status. When using the monitored and CMAQ-PHASE exposure assignment methods, we found stronger associations for those participants living in urban census tracts than those for rural residents. There are several plausible explanations for these findings. Air quality monitors are primarily located in heavily populated urban areas; thus, there is more likely to be greater exposure misclassification for those participants living in rural areas further away from monitors (Bravo et al. 2012). Recent studies have shown stronger associations between PM2.5 exposure and mortality for urban participants when using data from air quality monitors (Lee et al. 2016). For the CMAQ-PHASE models, the home addresses were tethered to census tract centroids. Census tracts are much larger in rural than in urban areas, which may potentially increase exposure misclassification more for rural participants. A recent study found stronger associations between PM2.5 and cardiovascular hospitalizations for those living in the most urban counties, when using CMAQ downscaler data (Bravo et al. 2017). Therefore, rather than true urban/rural exposure-response differences, it is possible that our monitored and CMAQ-PHASE model findings were due to more accurate exposure measurement for the urban participants (Sarnat et al. 2010). There were no differences among rural and urban participants for the satellite-based models or CMAQ-DF models. Therefore, these models adequately capture PM2.5 exposures for urban as well as rural participants. These findings additionally show the utility of using satellite-based models and CMAQ models at a finer spatial resolution, to assess health effects of air pollution for both urban as well as rural participants.
The current study is not without limitations. Cardiac catheterization patients represent a selective population and therefore results may not be generalizable to the general population. Additionally, because of their diagnosis, many of the Cathgen participants were taking multiple medications at the time of the study visit, which could modify or confound PM2.5 associated health outcomes. Unfortunately, we did not have a complete record of medication usage. We controlled for area level attained education and urban/rural status in our analyses, which are both related to area level air pollution exposure, however we were unable to account for individual level SES indicators. Further, it’s possible that we may have incorrectly classified smoking status, however we did not have information on number of cigarettes smoked per day. Despite these limitations, the current study has several strengths. It is the first study to associate adverse CV outcomes in a population with indications for cardiac complications, who may thus be more susceptible to air pollution exposures, with PM2.5 concentrations obtained from five different exposure assignment methods at different spatial resolutions. We used the coronary artery disease index as our main measure of coronary artery disease. CATHGEN additionally has an adequate sample size and spatial variability throughout the state to conduct our main and modification analyses by urban/rural status.
5. Conclusions
In summary, we found associations among long-term PM2.5 exposure using five different air-quality exposure methods and both CAD and acute coronary events (MI). Our study compared results from both monitored and modeled data, while making use of both satellite and CMAQ-based models at different spatial resolutions. The findings were robust to multi-pollutant models. Overall our results were fairly similar across exposure assessment methods, for both the CAD and MI. Further, we found modification by rural/urban status for the monitored and CMAQ-PHASE exposure assessment methods. Future analyses should consider the comparison of exposure metrics for short-term analyses and multi-pollutant models, as results may vary by study design, pollutant of interest, geographical location, and length of exposure.
Supplementary Material
Acknowledgments
We would like to acknowledge and thank all CATHGEN staff and participants for making this work possible. This research was supported by intramural research funding by the US EPA; Health Effects Institute (Research Agreement #4946- RFPA10-3/14-7); National Institute of Environmental Health Sciences (award number T32ES007018); EPA grants RD834799 and RD83587201; and an appointment to the Internship/Research Participation Program at Office of Research and Development (National Health and Environmental Effects Research Laboratory), U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA.
DISCLAIMER
The research described in this article has been reviewed by the Environmental Protection Agency and approved for publication. The contents of this article do not necessarily represent Agency policy nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Human Study Approval (from page 6 of the manuscript): All subjects received and signed informed consent prior to enrollment; CATHGEN has been approved by the Duke University Institutional Review Board.
References
- Bart BA, Shaw LK, McCants CB, Jr, Fortin DF, Lee KL, Califf RM, et al. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. Journal of the American College of Cardiology. 1997;30:1002–1008. doi: 10.1016/s0735-1097(97)00235-0. [DOI] [PubMed] [Google Scholar]
- Baxter LK, Dionisio KL, Burke J, Ebelt Sarnat S, Sarnat JA, Hodas N, et al. Exposure prediction approaches used in air pollution epidemiology studies: Key findings and future recommendations. Journal of exposure science & environmental epidemiology. 2013;23:654–659. doi: 10.1038/jes.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal VJ, Gelfand AE, Holland DM. A spatio-temporal downscaler for output from numerical models. Journal of agricultural, biological, and environmental statistics. 2010;15:176–197. doi: 10.1007/s13253-009-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo MA, Fuentes M, Zhang Y, Burr MJ, Bell ML. Comparison of exposure estimation methods for air pollutants: Ambient monitoring data and regional air quality simulation. Environmental research. 2012;116:1–10. doi: 10.1016/j.envres.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo MA, Ebisu K, Dominici F, Wang Y, Peng RD, Bell ML. Airborne fine particles and risk of hospital admissions for understudied populations: Effects by urbanicity and short-term cumulative exposures in 708 u.S. Counties. Environmental health perspectives. 2017;125:594–601. doi: 10.1289/EHP257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Chudnovsky AA, Lee HJ, Kostinski A, Kotlov T, Koutrakis P. Prediction of daily fine particulate matter concentrations using aerosol optical depth retrievals from the geostationary operational environmental satellite (goes) J Air Waste Manag Assoc. 2012;62:1022–1031. doi: 10.1080/10962247.2012.695321. [DOI] [PubMed] [Google Scholar]
- Chudnovsky AA, Kostinski A, Lyapustin A, Koutrakis P. Spatial scales of pollution from variable resolution satellite imaging. Environ Pollut. 2013;172:131–138. doi: 10.1016/j.envpol.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Chudnovsky AA, Koutrakis P, Kloog I, Melly S, Nordio F, Lyapustin A, et al. Fine particulate matter predictions using high resolution aerosol optical depth (aod) retrievals. Atmos Environ. 2014;89:189–198. doi: 10.1016/j.atmosenv.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Kloog I, Koutrakis P, Lyapustin A, Wang Y, Schwartz J. Assessing pm2.5 exposures with high spatiotemporal resolution across the continental united states. Environ Sci Technol. 2016;50:4712–4721. doi: 10.1021/acs.est.5b06121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebelt ST, Petkau AJ, Vedal S, Fisher TV, Brauer M. Exposure of chronic obstructive pulmonary disease patients to particulate matter: Relationships between personal and ambient air concentrations. J Air Waste Manag Assoc. 2000;50:1081–1094. doi: 10.1080/10473289.2000.10464166. [DOI] [PubMed] [Google Scholar]
- EPA. Community multi-scale air quality (CMAQ) modeling system for air quality management. 2016a Available: http://www.epa.gov/air-research/community-multi-scale-air-quality-cmaq-modeling-system-air-quality-management.
- EPA. Fused air quality surfaces using downscaling tool for predicting daily air pollution. 2016b Available: http://www.epa.gov/air-research/fused-air-quality-surfaces-using-downscaling-tool-predicting-daily-air-pollution.
- EPA. Air Quality System Data Mart. 2016c Available: http://aqsdr1.epa.gov/aqsweb/aqstmp/airdata/download_files.html.
- Friberg MD, Zhai X, Holmes HA, Chang HH, Strickland MJ, Sarnat SE, et al. Method for fusing observational data and chemical transport model simulations to estimate spatiotemporally resolved ambient air pollution. Environ Sci Technol. 2016;50:3695–3705. doi: 10.1021/acs.est.5b05134. [DOI] [PubMed] [Google Scholar]
- Gray SC, Edwards SE, Schultz BD, Miranda ML. Assessing the impact of race, social factors and air pollution on birth outcomes: A population-based study. Environmental health : a global access science source. 2014;13:4. doi: 10.1186/1476-069X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O'Neill MS, et al. Air pollution and individual and neighborhood socioeconomic status: Evidence from the multi-ethnic study of atherosclerosis (mesa) Environmental health perspectives. 2013;121:1325–1333. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, et al. Social determinants of risk and outcomes for cardiovascular disease: A scientific statement from the american heart association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. Long-term air pollution exposure and cardio- respiratory mortality: A review. Environmental health : a global access science source. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Hu Y, Zheng J, Yuan Z, Russell AG, Ou J, et al. A new combined stepwise-based high-order decoupled direct and reduced-form method to improve uncertainty analysis in pm2.5 simulations. Environ Sci Technol. 2017 doi: 10.1021/acs.est.6b05479. [DOI] [PubMed] [Google Scholar]
- Hyder A, Lee HJ, Ebisu K, Koutrakis P, Belanger K, Bell ML. PM2.5 exposure and birth outcomes: Use of satellite- and monitor-based data. Epidemiology. 2014;25:58–67. doi: 10.1097/EDE.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Turner MC, Beckerman BS, Pope CA, 3rd, van Donkelaar A, Martin RV, et al. Comparing the health effects of ambient particulate matter estimated using ground-based versus remote sensing exposure estimates. Environmental health perspectives. 2016 doi: 10.1289/EHP575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Koutrakis P, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved pm2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45:6267–6275. [Google Scholar]
- Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy pm2.5 exposure, premature birth and birth weight in massachusetts. Environmental health : a global access science source. 2012;11:40. doi: 10.1186/1476-069X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Ridgway B, Koutrakis P, Coull BA, Schwartz JD. Long- and short-term exposure to pm2.5 and mortality: Using novel exposure models. Epidemiology. 2013;24:555–561. doi: 10.1097/EDE.0b013e318294beaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WE, Granger CB, Sketch MH, Jr, Donahue MP, Ginsburg GS, Hauser ER, et al. A guide for a cardiovascular genomics biorepository: The cathgen experience. Journal of cardiovascular translational research. 2015 doi: 10.1007/s12265-015-9648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Liu Y, Coull BA, Schwartz J, Koutrakis P. A novel calibration approach of modis aod data to predict pm2.5 concentrations. Atmos Chem Phys. 2011;11:7991–8002. [Google Scholar]
- Lee HJ, Coull BA, Bell ML, Koutrakis P. Use of satellite-based aerosol optical depth and spatial clustering to predict ambient pm2.5 concentrations. Environmental research. 2012;118:8–15. doi: 10.1016/j.envres.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Koutrakis P, Coull B, Kloog I, Schwartz J. Acute effect of fine particulate matter on mortality in three southeastern states from 2007–2011. Journal of exposure science & environmental epidemiology. 2016;26:173–179. doi: 10.1038/jes.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigano J, Kloog I, Goldberg R, Coull BA, Mittleman MA, Schwartz J. Long-term exposure to pm2.5 and incidence of acute myocardial infarction. Environmental health perspectives. 2013;121:192–196. doi: 10.1289/ehp.1205284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli MC, Vaidyanathan A, Flanders WD, Qin X, Garbe P. Outdoor pm2.5, ambient air temperature, and asthma symptoms in the past 14 days among adults with active asthma. Environmental health perspectives. 2016;124:1882–1890. doi: 10.1289/EHP92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rural Health Research Center. [accessed July 16, 2016];Rural-urban commuting area codes (rucas) 2009 Available: http://depts.washington.edu.libproxy.lib.unc.educ/uwruca/
- Sacks JD, Rappold AG, Davis JA, Jr, Richardson DB, Waller AE, Luben TJ. Influence of urbanicity and county characteristics on the association between ozone and asthma emergency department visits in north carolina. Environmental health perspectives. 2014;122:506–512. doi: 10.1289/ehp.1306940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat SE, Klein M, Sarnat JA, Flanders WD, Waller LA, Mulholland JA, et al. An examination of exposure measurement error from air pollutant spatial variability in time-series studies. Journal of exposure science & environmental epidemiology. 2010;20:135–146. doi: 10.1038/jes.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellier Y, Galineau J, Hulin A, Caini F, Marquis N, Navel V, et al. Health effects of ambient air pollution: Do different methods for estimating exposure lead to different results? Environment international. 2014;66:165–173. doi: 10.1016/j.envint.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Suh HH, Zanobetti A. Exposure error masks the relationship between traffic-related air pollution and heart rate variability. Journal of occupational and environmental medicine. 2010;52:685–692. doi: 10.1097/JOM.0b013e3181e8071f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. Summary files 1 and 3. 2000 Available: http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml.
- van Donkelaar A, Martin RV, Brauer M, Kahn R, Levy R, Verduzco C, et al. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: Development and application. Environmental health perspectives. 2010;118:847–855. doi: 10.1289/ehp.0901623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, et al. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ Sci Technol. 2016;50:3762–3772. doi: 10.1021/acs.est.5b05833. [DOI] [PubMed] [Google Scholar]
- Wang M, Gehring U, Hoek G, Keuken M, Jonkers S, Beelen R, et al. Air pollution and lung function in dutch children: A comparison of exposure estimates and associations based on land use regression and dispersion exposure modeling approaches. Environmental health perspectives. 2015;123:847–851. doi: 10.1289/ehp.1408541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JL, Stingone JA, Herring AH, Luben TJ, Fuentes M, Aylsworth AS, et al. Bayesian multinomial probit modeling of daily windows of susceptibility for maternal pm2.5 exposure and congenital heart defects. Statistics in medicine. 2016;35:2786–2801. doi: 10.1002/sim.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wilhelm M, Chung J, Ritz B. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environmental research. 2011;111:685–692. doi: 10.1016/j.envres.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Liu Y, Mulholland JA, Russell AG, Darrow LA, Tolbert PE, et al. Pediatric emergency department visits and ambient air pollution in the u.S. State of georgia: A case-crossover study. Environmental health : a global access science source. 2016;15:115. doi: 10.1186/s12940-016-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.