Abstract
Sudden cardiac death (SCD) accounts for approximately one-third of all deaths among patients with non-ischaemic cardiomyopathy (NICM). Implantable cardioverter-defibrillator (ICD) therapy has been the primary intervention for managing individuals at high risk for SCD. However, individual ICD trials in the NICM population have failed to demonstrate a mortality benefit with prophylactic ICD implantation. Current guidelines recommend ICD implantation in NICM patients with symptomatic heart failure and a left ventricular ≤35% and are based on meta-analyses of multiple trials that span three decades and include the recent Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischaemic Heart Failure on Mortality (DANISH) trial. These pooled analyses report a significant reduction in all-cause mortality with ICD implantation compared with medical therapy alone. In addition, each of these trials has demonstrated consistently a reduction in the risk of SCD compared with medical therapy alone. As a result, a refined approach of risk stratification that selects patients at the highest risk for SCD may lead to a significant improvement in ICD efficacy. In this clinical review, we first discuss the evolution of clinical trials that have evaluated ICDs in the NICM population. We then highlight some key markers of arrhythmia risk that hold promise in personalizing risk stratification for SCD.
Keywords: Non-ischaemic cardiomyopathy, Implantable cardioverter-defibrillator, Sudden cardiac death, Risk prediction , Risk stratification , Clinical trials
Introduction
Sudden cardiac death (SCD) is an important public health problem that accounts for 15–20% of the total annual mortality in industrialized nations.1,2 Coronary heart disease has been implicated in the majority of SCD cases. In approximately 25% of SCDs, coronary artery disease (CAD) is absent, and either non-ischaemic cardiomyopathies (NICM) or primary electrical disorders are implicated as the causative factor.2 Sudden cardiac death, which is a spontaneous condition often resulting from ventricular arrhythmias, accounts for ∼35% of all deaths among patients with NICM.3,4 Implantable cardioverter-defibrillator (ICD) therapy has been the mainstay for managing individuals at risk of SCD. However, in the NICM population, individual ICD trials have failed to demonstrate a mortality benefit with prophylactic ICD implantation. Recommendations for ICD implantation were initially based on the survival benefit observed in a mixed population of both ischaemic and non-ischaemic patients5 and a meta-analysis of several non-ischaemic cohorts included in ICD clinical trials.6 Given the limited data surrounding ICD benefit in the NICM population, the contemporary Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischaemic Heart Failure on Mortality (DANISH) was conducted. The DANISH study did not demonstrate a reduction in mortality with prophylactic ICD implantation; however, similar to other primary prevention trials in NICM populations,3,4 ICDs reduce the risk of SCD. As a result, a refined approach of risk stratification that selects patients at the highest risk for SCD may lead to a significant improvement in ICD efficacy.
In this clinical review, we first discuss the evolution of clinical trials that have evaluated ICDs in the NICM population. We will then highlight some key markers of arrhythmia risk that hold promise in personalizing risk stratification for SCD.
Evidence for primary prevention of sudden cardiac death in non-ischaemic cardiomyopathy
Multiple primary prevention trials have evaluated the benefit of ICDs in the NICM population. An overview and comparison of each trial’s design, characteristics, and outcomes are provided in Table 1 and Figure 1. Initial studies including the Cardiomyopathy Trial (CAT)7 and the Amiodarone vs. Implantable Cardioverter-Defibrillator (AMIOVIRT) trial8 each enrolled slightly more than 100 NICM participants with a LVEF ≤ 30–35% and randomized them to ICD vs. pharmacologic therapy. No mortality benefit was observed in either study. The larger Defibrillators in Non-ischaemic Cardiomyopathy Treatment Evaluation (DEFINITE) Study3 then evaluated the impact of ICDs in 458 NICM participants with both non-sustained ventricular tachycardia (NSVT) and a LVEF ≤ 35%. Most individuals in this trial were treated with optimal medical therapy including beta-blockers and angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB). Over a median follow-up period of 26 months, the total mortality was 6.2% in the medical only group, and there was a relative 35% decrease in overall mortality in the ICD group, a difference that did not achieve statistical significance. Because of an overestimation of the control group mortality and subsequent compromise in statistical power, none of these initial trials demonstrated a significant benefit to ICD therapy.
Table 1.
Study details of clinical trials evaluating implantable cardioverter-defibrillator use in non-ischaemic cardiomyopathy
| CAT | AMIOVIRT | DEFINITE | SCD-HeFT | COMPANION | DANISH | |
|---|---|---|---|---|---|---|
| No. of patients randomized | 104 | 103 | 458 | 2521 | 1520 | 1116 |
| No. of patients with NICM (%) | 104 (100) | 103 (100) | 458 (100) | 792 (47.3) | 682 (44.0) | 1116 (100) |
| Year of publication | 2002 | 2003 | 2004 | 2005 | 2004 | 2016 |
| Trial details | ||||||
| Design | ICD vs. OMT | ICD vs. OMT | ICD vs. amiodarone | ICD vs. amiodarone vs. placebo | OMT vs. CRT-P vs. CRT-D | ICD vs. OMT |
| Inclusion criteria | LVEF ≤ 30%, NYHA II–III | LVEF ≤ 35%; DCM; NSVT; NYHA I–III | LVEF ≤ 35%; DCM; NSVT or PVCs; NYHA I–III | LVEF ≤ 35%; NYHA II–III | NYHA III–IV; QRS duration ≥120 ms | LVEF ≤ 35%; NT-pro-BNP >200 pg/mL; NYHA II–IV |
| Duration of follow-up (months) | 66 ± 26.4 (mean) | 24 ± 14.4 (mean) | 29 ± 14.4 (mean) | 45.5 (median) | 14.8–16.0 (median) | 67.6 (median) |
| Primary endpoint | All-cause mortality | All-cause mortality | All-cause mortality | All-cause mortality | All-cause mortality/ hospitalization | All-cause mortality |
| Baseline characteristics | ||||||
| Mean age (years) | 52 | 59 | 58 | 60 | 67 | 64 |
| Male (%) | 86 | 71 | 71 | 77 | 68 | 73 |
| HTN (%) | n/a | 63 | n/a | 56 | n/a | 32 |
| Diabetes (%) | n/a | 34 | 23 | 31 | 41 | 19 |
| Medication | ||||||
| Beta-blocker (%) | 4 | 51.5 | 86 | 69 | 67 | 92 |
| ACE inhibitor/ARB (%) | 94% | 85 | 97 | 94 | 89 | 96 |
| MRA (%) | n/a | 20 | NR | 20 | 55 | 59 |
| Number with CRT (%) | 0 | 0 | 0 | 0 | 100% | 58 |
| Main result | ||||||
| ICD therapy did not reduce mortality, P = 0.55 | ICD therapy did not reduce mortality, P = 0.80 | ICD therapy resulted in 35% RR reduction, P = 0.08 | ICD therapy resulted in 23% RR reduction in all-cause mortality, P = 0.007 | CRT-D therapy resulted in a 36% RR reduction in mortality P = 0.001 | ICD therapy did not reduce mortality P = 0.28 | |
ACEI, angiotensin-converting enzyme inhibitor; AMIOVIRT, Amiodarone vs. Implantable Defibrillator Randomized Trial; ARB, angiotensin receptor blocker; CAT, Cardiomyopathy Trial; COMPANION, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure trial; CRT, cardiac resynchronization therapy; DANISH, Danish Study to Assess the Efficacy of ICDs in Patients with Non-Ischaemic Systolic Heart Failure on Mortality; DEFINITE, Defibrillators in Non-Ischaemic Cardiomyopathy Treatment Evaluation; HTN, Hypertension; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist, n/a, not available; NICM, non-ischaemic cardiomyopathy; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association; OMT, optimal medical therapy; PVCs, premature ventricular contractions; RR, relative risk; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial; Y, Year.
Figure 1.
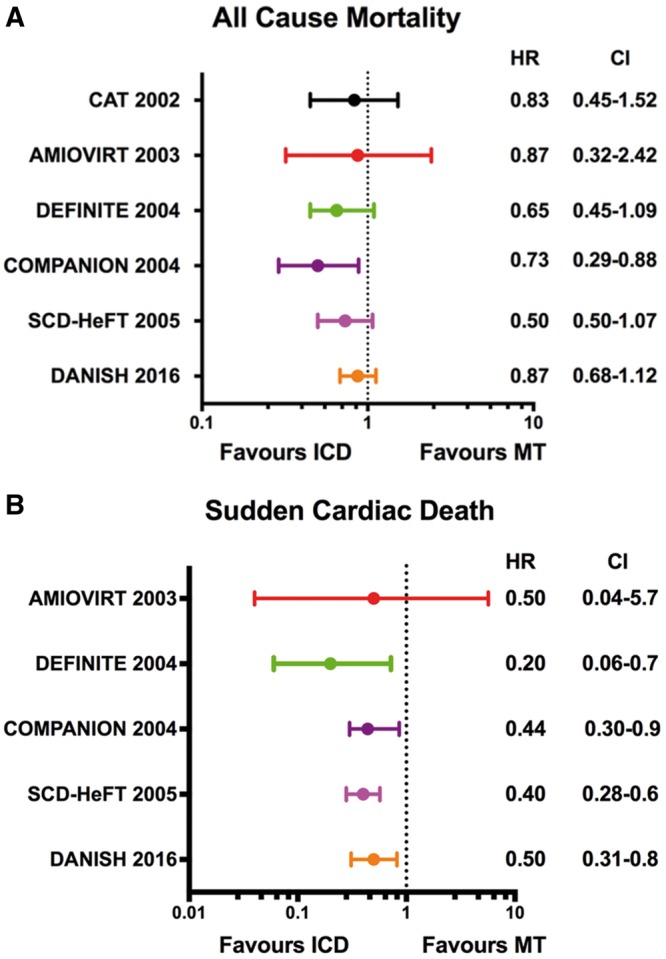
(A) All-cause mortality among primary prevention trials. (B) Sudden cardiac death among primary prevention trials. For each randomized trial, the point estimates of the hazard ratio for individual studies are represented by circle with 95% confidence intervals shown as bars. AMIOVIRT, Amiodarone vs. Implantable Defibrillator Randomized Trial; CI, confidence interval; CAT, Cardiomyopathy Trial; COMPANION, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure trial; DANISH, Danish Study to Assess the Efficacy of ICDs in Patients with Non-Ischaemic Systolic Heart Failure on Mortality; DEFINITE, Defibrillators in Non-Ischaemic Cardiomyopathy Treatment Evaluation; HR, hazard ratio; ICD, implantable cardioverter-defibrillator; MT, medical therapy; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
These initial studies were followed by the large multicentre Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) that randomly assigned 2521 symptomatic individuals with either ischaemic (n = 1310) or non-ischaemic (n = 1211) heart failure and LVEF ≤ 35% to placebo, amiodarone, or ICD.5 Compared with placebo, the ICD group was associated with a 23% relative risk reduction and an absolute decrease of 7.2% in all-cause mortality after 5 years of follow-up; however, this benefit was not statistically significant in the stratified analysis of NICM participants [hazard ratio (HR) 0.73, 95% confidence interval (95% CI) 0.50–1.07; P = 0.06]. In 2005, after the publication of SCD-HeFT, a pooled analysis of NICM participants from the primary prevention trials in Table 1 demonstrated a statistically significant 31% reduction (P = 0.002) in all-cause mortality for ICD relative to medical therapy.6 This analysis formed the basis for the European Society of Cardiology Guidelines’ recommendation on prophylactic ICD implantation for symptomatic dilated cardiomyopathy patients with a LVEF ≤ 35%.9
The DANISH trial was conducted in response to the limited evidence surrounding ICD benefit in the chronic, NICM population. The trial enrolled individuals with New York Heart Association (NYHA) Class II or III heart failure symptoms with a LVEF ≤ 35% not due to CAD. In addition, it is the first ICD trial to require an elevated level of N-terminal pro-brain natriuretic peptide (NT-pro BNP) despite optimal medical therapy.4 Randomization in this trial involved the assignment of 556 participants to ICD and 560 individuals to standard clinical care. In both the interventional and control groups, the NT-pro BNP levels were markedly elevated (median value 1244 pg/mL in the ICD group and 1110 pg/mL in the medical group), and the majority of participants received cardiac resynchronization therapy (CRT). After a median follow-up of 67.6 months, all-cause mortality had occurred in 21.6% of the ICD group and 23.4% in the control group (HR 0.87, 95% CI 0.68–1.12; P = 0.28). In a pre-specified analysis from DANISH, survival benefit from ICD implantation was seen only in individuals ≤70 years of age.10 Participants ≤70 years had a 30% reduction in total mortality in the ICD arm compared with standard medical therapy. Among individuals >70 years of age, the rate of non-sudden deaths was nearly twice as high compared with the younger population, and no mortality benefit was seen with ICD implantation. As a result of this age interaction, the widespread use of cardioprotective therapies including CRT, and overall lack of mortality benefit to prophylactic ICD implantation, clinicians are evaluating critically the evolution of heart failure management, ICD implantation, and SCD risk stratification in NICM.
The advent of CRT has resulted not only in improved morbidity from heart failure but also a reduction in mortality events. In the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure trial (COMPANION), both CRT with pacemaker function only (CRT-P) and CRT with defibrillator (CRT-D) demonstrated similar reductions in mortality risk.11,12 Further, the Cardiac Resynchronization-Heart Failure (CARE-HF) study also demonstrated that CRT pacing alone reduced mortality in NICM patients when compared with medical therapy only.13 These findings have been supported by a recent, multicentre, observational study of 5307 European patients, who had an indication for CRT. The addition of primary prevention, defibrillator therapy over CRT pacing only is beneficial in well-selected patients with ischaemic cardiomyopathy but does not convey a significant survival benefit in NICM patients.14 Understanding how best to leverage defibrillator therapy in the selected patients with CRT pacing remains an important area for investigation.
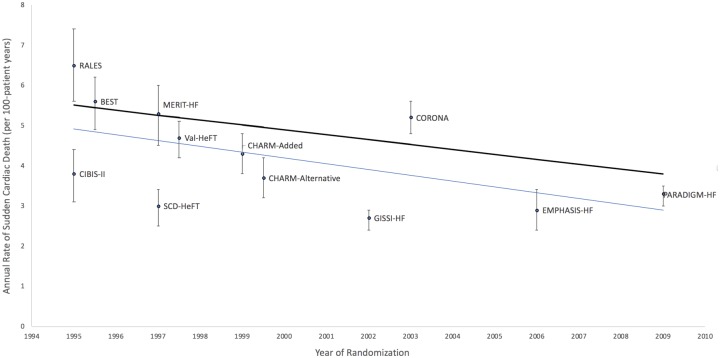
Recently, the Heart Rhythm Society guidelines have continued to recommend prophylactic ICD implantation in patients with NICM, LVEF ≤35%, and NYHA Class II–III heart failure despite optimal medical therapy.15 Partial justification for recommending prophylactic ICD implantation in NICM is provided by multiple meta-analyses, which span three decades and now include the DANISH study. These studies report a significant reduction in all-cause mortality with ICD implantation compared with medical therapy alone.16–19 Regardless of whether DANISH was evaluated in context of the five other primary prevention ICD trials from Table 1 or only those trials that did not include CRT, the pooled analyses demonstrate that prophylactic ICD implantation in NICM results in an ∼25% relative risk reduction in all-cause mortality compared with standard therapies only. These pooled analyses, however, do not account adequately for the evolution and benefit of heart failure therapies. The annualized mortality rate in the control arm of DANISH was ∼4.2% compared with 7–8% in the other NICM, ICD trials. Similarly, in a recent analysis of 12 heart failure clinical trials spanning nearly two decades and comprising over 40 000 participants, investigators have demonstrated a 44% reduction in SCD rates (Figure 2).20 Annual SCD rates decreased from 6.5% in the earliest trials to 3.3% in one of the most recent heart failure trials, Prospective Comparison of Angiotensin-Neprilysin Inhibition with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF). This decline paralleled the increasing use of evidence-based pharmacotherapies such as beta-blockers, ACE inhibitors, ARBs, mineralocorticoid receptor blockers, and CRT.21,22 Further, the proportion of SCD relative to overall mortality did not change across trials indicating that the falling rates of SCD were in line with the downward trend in the overall death rates. Overall improvements in heart failure therapeutics and the recent DANISH study should force clinicians to consider how these data may affect decision-making for prophylactic ICD implantation in a NICM patient.
Figure 2.
Declining rates of sudden cardiac death in heart failure trials. The black line reflects the unadjusted rates of sudden cardiac death. The blue line is based on the linear regression of the annualized rate of sudden cardiac death in each trial group with the randomization year and group as covariates.9 BEST, Beta-Blocker Evaluation of Survival Trial; CHARM, Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity; CIBIS-II, Cardiac Insufficiency Bisoprolol Study II; CORONA, Controlled Rosuvastatin Multinational Trial in Heart Failure; EMPHASIS-HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; GISSI-HF, Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca Heart Failure Trial; MERIT-HF, Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure; PARADIGM-HF, Prospective Comparison of Angiotensin-Neprilysin Inhibition with ACE Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure; RALES, Randomized Aldactone Evaluation Study; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial; Val-HeFT, Valsartan Heart Failure Trial.
Future directions: potential markers and approach for arrhythmia risk stratification
Non-ischaemic cardiomyopathies remain a heterogeneous set of conditions, and optimal ICD use will rely on identifying individuals who have an increase in SCD risk without a concomitant rise in the risk for non-arrhythmic death. Implantable cardioverter-defibrillators have consistently demonstrated a reduction in SCD.3,4,19,23 However, the current approach of utilizing a low LVEF for ICD decision-making has poor sensitivity and specificity for identifying a group at increased SCD risk.24–27 In ICD patients who had a LVEF ≤ 35% and dilated cardiomyopathy, 80% did not have any interventions for VT/VF during a 5-year follow-up period.5,28 In addition, a decision based on LVEF only does not account for the competing risk of non-sudden cardiac death and non-cardiac death. Various risk stratification approaches such as the Seattle Heart Failure Model, which has been validated in multiple cohorts, have attempted to differentiate arrhythmic risk in order to maximize the benefit of ICD therapy.29 In particular, the Seattle Heart Failure Model is a multivariable risk score that incorporates routine clinical variables including age, gender, NYHA class, weight, ejection fraction, and specific laboratory parameters to predict both all-cause and cause-specific mortality in heart failure patients. As a result, the model can identify patient groups who have different levels of benefit from ICD therapy.29 This score can also predict the mode of death including either SCD vs. pump failure.30 Individuals with demographic and clinical factors corresponding to male sex, younger age, and the absence of several other comorbid conditions had a disproportionate likelihood of dying suddenly than by other modes of death such as pump failure.
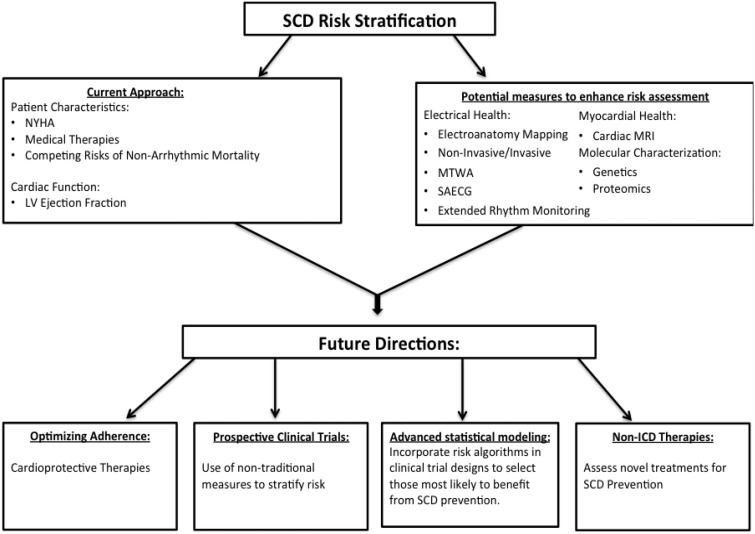
In addition to more sophisticated approaches to SCD risk modelling, advances in cardiac imaging, biologic markers, and non-invasive electrophysiologic testing have the potential to provide insight into arrhythmia risk stratification (Figure 3). Future studies that incorporate traditional measures of risk stratification with novel ones have the potential to enhance the benefits of prophylactic ICD implantation.
Figure 3.
Future model for sudden cardiac death risk stratification. ICD, implantable cardioverter-defibrillator; LV, left ventricular; MRI, magnetic resonance imaging; MTWA, microvolt T-wave alternans; NYHA, New York Heart Association; SAECG, signal-averaged electrocardiogram; SCD, sudden cardiac death.
Myocardial substrate
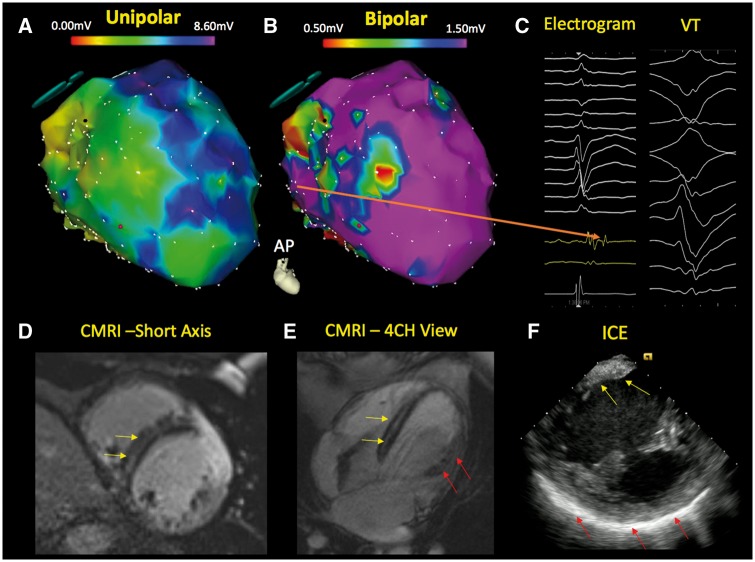
The remodelling process in NICM patients is characterized by fibrosis, and the presence of myocardial scar results in tissue heterogeneity that can provide a substrate for ventricular arrhythmias (Figure 4).31–33 Late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (CMR) reflects myocardial fibrosis34 and correlates closely with histopathological scar. Multiple studies have demonstrated strong associations between the presence and degree of myocardial scar and arrhythmic events.35–39 A meta-analysis of 9 studies that included 1488 NICM patients with a mean LVEF of 37% reported that LGE was present in 38% of patients.40 Patients with LGE had increased risk of SCD or ventricular arrhythmias (odds ratio 5.32; P < 0.00001) compared with those without LGE. Specifically, the annualized event rates for SCD/aborted SCD were 6.0% in those with LGE vs. 1.2% in those without. These data may lead to clinical trials that incorporate cardiac MRI into additional risk stratification approaches for identifying not only those at the highest risk for SCD but also a subset of NICM patients with a LVEF ≤ 35%, who are unlikely to benefit from ICD implantation. Cardiac MRI may also provide an opportunity to identify high risk, non-ischaemic patients, who do not meet indication for ICD implantation based on LVEF alone. Recently, the presence of mid-wall LGE on cardiac MRI in 399 non-ischaemic cardiomyopathy patients with an LVEF ≥ 40% was associated with a nine-fold increased risk of SCD/aborted SCD.41 Over a median follow-up period of 4.6 years, the risk of SCD/aborted SCD in those with mid-wall LGE was 17.8% compared with 2.3% in those without LGE. The rate of SCD in this population with mild left ventricular dysfunction and LGE compares to the SCD rate observed in ICD trials with LVEF ≤ 35% and provides additional rationale for the CMR-Guide (ClinicalTrials.gov identifier no. NCT01918215) randomized trial, which aims to evaluate the efficacy of ICD therapy in patients with more mild to moderate reductions in LVEF and LGE. In clinical practice, current guidelines support the use of cardiac MRI to guide ICD decision-making for non-ischaemic conditions such as cardiac sarcoidosis.15 Conduction abnormalities and ventricular arrhythmias can occur in sarcoid patients with relatively normal left ventricular function, and prophylactic ICD implantation is recommended for those who have evidence of myocardial scar by MRI even when the LVEF > 35%.
Figure 4.
Electrophysiologic imaging of a non-ischaemic cardiomyopathy patient with ventricular tachycardia. The electroanatomical map of the left ventricle demonstrates a significantly abnormal endocardial unipolar voltage map (A—modified antero-posterior view) and a limited bipolar abnormality (B) in the septum. The unipolar electroanatomic map can provide insight regarding the presence of intramyocardial scar when the bipolar voltage map is relatively normal. Late potentials are also observed in this region during sinus rhythm (C—orange arrow). Cardiac magnetic resonance imaging demonstrates mid myocardial septal scar (yellow arrow) in the short axis (D) and four-chamber view (E). The patient’s intracardiac echocardiography (F) depicts regions of brightness in the septum (yellow arrow) and lateral wall (red arrow). CMRI, cardiac magnetic resonance imaging; ICE, intracardiac echocardiography; VT, ventricular tachycardia.
Electroanatomic mapping (EAM) has also expanded our knowledge regarding the distribution of myocardial scarring seen in NICM (Figure 4). Electroanatomic mapping assesses the intrinsic myocardial health by measuring local electrical properties. Abnormalities on endocardial EAM are strongly associated with regions of scar detected through LGE-CMR. In contrast to ischaemic cardiomyopathies, NICM have different scar patterns that are often represented as patchy areas and commonly involve the epicardial and mid-myocardial layers of the heart.42–45 Identification of the underlying substrate with EAM can provide prognostic insight in patients with newly diagnosed NICM.46,47 Greater scar percentage and wider native QRS duration are strong predictors of mortality in NICM patients.32,48–51 Ongoing and future studies will assess whether electrophysiologic phenotyping utilizing EAM can enhance risk stratification beyond LVEF and possibly even CMR. This area holds potential promise as non-invasive methods for EAM are utilizing body surface mapping, which can be performed with minimal risk to patients.52–56
Biologic markers
The identification of an arrhythmogenic substrate is particularly meaningful in situations where fatal arrhythmias occur before myocardial dysfunction. Genetic mutations that are associated with a loss of integrity in the sarcolemma, cytoskeleton, sarcomere or intercellular links could disrupt ion channel function, and result in cardiac arrhythmias. More than 60 genes have been implicated in contributing to dilated cardiomyopathy, and some of these are strongly associated with the added risk of fatal arrhythmias (Table 2).57–64 Recent HRS guidelines have highlighted how genetic information can be combined with clinical features for risk stratification and ICD decision-making.15 In particular, Lamin A/C mutations, which have been implicated in familial cardiomyopathies and subtypes of muscular dystrophy, are also associated with cardiac conduction disease and SCD. Implantable cardioverter-defibrillator implantation is recommended in mutation carriers who also have at least two of the following features: NSVT, LVEF <45%, non-missense mutation, and male sex. Further, although not traditionally categorized as a dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy (ARVC) is a commonly inherited NICM. At least eight genes have been implicated in ARVC, and mutations in many of these genes result in impaired desmosomal function. Approximately 35% of mutation positive individuals ultimately develop progressive disease expression characterized by either a major arrhythmic event such as SCD or heart failure.65,66 As such, electrocardiographic and MRI screening are recommended in mutation carriers to identify patients at early, subclinical stages of the disease process.15
Table 2.
Genetic mutations associated with dilated cardiomyopathy and cardiac arrhythmias
| Gene | Function | Findings/Notes |
|---|---|---|
| Lamin A/C (LMNA) | Encodes structural proteins that provide stability to the nuclear envelope. | Mutations are involved in 8% of familial and 2% of sporadic DCM. They are associated with cardiac conduction disease.57,58 In one small study, LMNA mutation carriers, who had cardiac conduction disease requiring permanent pacemaker, had a high risk for ventricular arrhythmias and benefitted from ICD implantation.59 |
| Phospholamban (PLN) | Transmembrane protein in the sarcoplasmic reticulum that plays a key role in calcium homeostasis. | In 403 mutation carriers, 20% of individuals had incident ventricular arrhythmias after a median follow-up of 42 months.60 |
| Desmin (DES) | Intermediate filament protein that is expressed in skeletal, cardiac, and smooth muscle and helps to form the cytoskeletal network. | The majority of mutation carriers are reported to have cardiac conduction disease and/or arrhythmias.61 The most common abnormality reported was high degree atrioventricular block. |
| SCN5A | Cardiac sodium channel gene that is responsible for the fast depolarization of the myocardium and maintenance of impulse conduction in the heart. | Mutations found in 1.7% of DCM families.62 Two-thirds of these SCN5A mutations localized to the highly conserved, transmembrane segments suggesting a shared mechanism of disruption of the voltage-sensing mechanism of this channel and DCM. |
DCM, dilated cardiomyopathy.
Circulating cardiac biomarkers offer another potential opportunity to assess arrhythmia risk. At present, only selected candidate biomarkers that reflect inflammation, neurohormonal activation, and cardiac injury have been evaluated as predictors of ventricular arrhythmias and sudden cardiac death. In the Prospective Observation Study of Implantable Cardioverter Defibrillators (PROSe-ICD), which is a cohort of 1189 patients with systolic dysfunction who underwent primary prevention ICD implantation, elevations in N-terminal pro-brain natriuretic peptide, C reactive protein, and troponin T performed poorly in predicting the likelihood of an appropriate ICD shock.67 These markers, however, enhanced identification of individuals that had an increased risk of non-SCD. Similarly, in a population-based analysis from the Cardiovascular Health Study, the addition of these three biomarkers to a 12-variable clinical risk score did not enhance the prediction of SCD.68 Based in part on these studies, the candidate biomarker approach is unlikely to capture the complexity of SCD. Instead, recent developments in large-scale proteomics have provided a less biased approach to identifying important biomarkers that may be important in various diseases.69–71 Protein expression integrates the effects of genetics with environmental influences such as lifestyle habits, comorbidities and medications. This type of agnostic approach has demonstrated improvements in the risk prediction of a global cardiovascular endpoint69 and should be assessed rigorously for the risk of ventricular arrhythmias in NICM.
Non-invasive electrophysiologic parameters
Non-sustained ventricular tachycardia
Multiple studies have evaluated the independent association between NSVT on cardiac monitoring and total mortality.72,73 Limited studies, however, have evaluated rigorously the presence of NSVT and arrhythmic death. The GESICA study, which evaluated the effect of low-dose amiodarone on mortality in patients with advanced heart failure, contained a stratified randomization procedure in which all participants underwent Holter monitoring prior to randomization.74 The study, which included a majority of NICM patients, demonstrated a higher incidence of SCD in the NSVT group (relative risk 2.77, 95% CI 1.78–4.44) compared to those without NSVT.75 The subsequent higher risk of total mortality in the NSVT group was driven by this higher risk of SCD and was independent of amiodarone use.
Signal-averaged electrocardiogram
Delayed activation in small regions of the ventricle can be detected using signal-averaged electrocardiogram (SAECG). These abnormalities are common findings in regions of scar since the parallel orientation of myocardial fibres are disrupted and lead to areas of slow ventricular conduction.76 In sinus rhythm, delayed ventricular activation or the presence of late potentials are detectable at more cardiac sites in patients with sustained VT compared to those without VT.77 Abnormalities in the SAECG are also associated with increased mortality and an increased risk of arrhythmic events.78,79 In prospective analyses, investigators have demonstrated that patients with abnormal SAECG had a higher likelihood of having either sustained VT or cardiac arrest.80,81 These studies also demonstrated that dilated cardiomyopathy patients with a normal SAECG have an excellent prognosis with adverse outcomes related to progressive HF and not arrhythmic complications.
Microvolt T-wave alternans
Microvolt T-wave alternans (MTWA) or significant fluctuations in the T-wave amplitude at high heart rates reflect abnormalities in repolarization and are associated with ventricular arrhythmias.82 Multiple clinical studies and meta-analyses have evaluated MTWA as a predictor of arrhythmic events in non-ischaemic populations.83–85 The findings across these studies are consistent and summary estimates suggest a positive predictive value of only 21% and a negative predictive value of 95% after approximately 20 months of follow-up. Clinically, this NPV could classify a patient with absent MTWA as low arrhythmic risk; however, it is unlikely for MTWA to identify those who would benefit the most from ICD therapy.
Conclusions
Complications from ventricular arrhythmias are a critical issue in patients with NICM, and the ICD remains the primary intervention for SCD prevention. The recent DANISH study has challenged the efficacy of ICD implantation; however, multiple meta-analyses of primary prevention trials of ICD use in the NICM population have demonstrated clinical efficacy. It is interesting to consider whether risk stratification beyond LVEF and heart failure symptoms will help to identify patients who are either eligible or can be safely excluded from prophylactic ICD implantation. The current lack of prospective data will rightfully limit a more widespread approach that incorporates risk stratification beyond LVEF in the NICM population. Although observational studies and subgroup analyses of large clinical trials suggest the benefits of refining the ‘at risk’ population, the application of advanced statistical methods and novel risk markers will require clinicians, biostatisticians, and government and industry stakeholders to partner on the thoughtful design of future clinical trials. Only then are we likely to enhance and implement methods for both arrhythmia risk stratification and SCD prevention.
Acknowledgements
The authors would like to thank Ms Gabriela Daszewska-Smith for her technical assistance in preparing this manuscript.
Funding
RACP Bushells Travelling Fellowship and an Early Career Fellowship from the National Health and Medical Research Council of Australia to R.P.; Practitioner Fellowship from the National Health and Medical Research Council of Australia and by the National Heart Foundation of Australia to P.S.; and National Institutes of Health [U01 DK108809 to R.D.].
Conflict of interest: P.S. reports having served on the advisory board of Biosense-Webster, Medtronic, St Jude Medical, Boston Scientific and CathRx. P.S. reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Biosense-Webster, Medtronic, St Jude Medical, and Boston Scientific. P.S. reports that the University of Adelaide has received on his behalf research funding from Medtronic, St Jude Medical, Boston Scientific, Biotronik and Sorin.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 2. Deo R, Albert CM.. Epidemiology and genetics of sudden cardiac death. Circulation 2012;125:620–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH, Defibrillators In Non-Ischemic Cardiomyopathy Treatment Evaluation I.. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151–2158. [DOI] [PubMed] [Google Scholar]
- 4. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S; DANISH Investigators. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 5. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH.. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 6. Desai AS, Fang JC, Maisel WH, Baughman KL.. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. Jama 2004;292:2874–2879. [DOI] [PubMed] [Google Scholar]
- 7. Bansch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, Block M, Gietzen F, Berger J, Kuck KH.. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation 2002;105:1453–1458. [DOI] [PubMed] [Google Scholar]
- 8. Strickberger SA, Hummel JD, Bartlett TG, Frumin HI, Schuger CD, Beau SL, Bitar C, Morady F; AMIOVIRT Investigators. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia–AMIOVIRT. J Am Coll Cardiol 2003;41:1707–1712. [DOI] [PubMed] [Google Scholar]
- 9. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ.. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 10. Elming MB, Nielsen JC, Haarbo J, Videbæk L, Korup E, Signorovitch J, Olesen LL, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp-Pedersen C, Pehrson S, Køber L, Thune JJ.. Age and outcomes of primary prevention implantable cardioverter-defibrillators in patients with nonischemic systolic heart failure. Circulation 2017;136:1772–1780. [DOI] [PubMed] [Google Scholar]
- 11. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM; Comparison of Medical Therapy, Pacing, and Defibrillation In Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 12. Bristow MR, Saxon LA, Feldman AM, Mei C, Anderson SA, DeMets DL.. Lessons learned and insights gained in the design, analysis, and outcomes of the COMPANION trial. JACC Heart Fail 2016;4:521–535. [DOI] [PubMed] [Google Scholar]
- 13. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L.. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J 2006;27:1928–1932. [DOI] [PubMed] [Google Scholar]
- 14. Barra S, Boveda S, Providencia R, Sadoul N, Duehmke R, Reitan C, Borgquist R, Narayanan K, Hidden-Lucet F, Klug D, Defaye P, Gras D, Anselme F, Leclercq C, Hermida JS, Deharo JC, Looi KL, Chow AW, Virdee M, Fynn S, Le Heuzey JY, Marijon E, Agarwal S; French-UK-Sweden CRT Network. Adding defibrillation therapy to cardiac resynchronization on the basis of the myocardial substrate. J Am Coll Cardiol 2017;69:1669–1678. [DOI] [PubMed] [Google Scholar]
- 15. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SC, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL.. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Golwala H, Bajaj NS, Arora G, Arora P.. Implantable cardioverter-defibrillator for nonischemic cardiomyopathy: an updated meta-analysis. Circulation 2017;135:201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al-Khatib SM, Fonarow GC, Joglar JA, Inoue LYT, Mark DB, Lee KL, Kadish A, Bardy G, Sanders GD.. Primary prevention implantable cardioverter defibrillators in patients with nonischemic cardiomyopathy: a meta-analysis. JAMA Cardiol 2017;2:685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fauchier L, Bisson A, Clementy N.. ICD implantation in patients with nonischemic heart failure. N Engl J Med 2017;376:89–90. [DOI] [PubMed] [Google Scholar]
- 19. Kolodziejczak M, Andreotti F, Kowalewski M, Buffon A, Ciccone MM, Parati G, Scicchitano P, Uminska JM, De Servi S, Bliden KP, Kubica J, Bortone A, Crea F, Gurbel P, Navarese EP.. Implantable cardioverter-defibrillators for primary prevention in patients with ischemic or nonischemic cardiomyopathy: a systematic review and meta-analysis. Ann Intern Med 2017;167:103–111. [DOI] [PubMed] [Google Scholar]
- 20. Shen L, Jhund PS, Petrie MC, Claggett BL, Bariera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Swedberg K, Tavazzi L, Zannad F, Zile MR, McMurray JJV.. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 21. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C.. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 23. Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, Boehmer JP, Carlson MD, Frantz RP, McNulty SE, Rogers JG, Anderson J, Johnson GW, Walsh MN, Poole JE, Mark DB, Lee KL, Bardy GH.. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation 2009;120:2170–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O'Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ.. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 2010;122:2335–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, Maron BJ, Page RL, Passman RS, Siscovick D, Stevenson WG, Zipes DP; American Heart Association; American College of Cardiology Foundation; Heart Rhythm Society. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death. A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J Am Coll Cardiol 2008;52:1179–1199. [DOI] [PubMed] [Google Scholar]
- 26. Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ.. Out-of-hospital cardiac arrest—the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J 2003;24:1204–1209. [DOI] [PubMed] [Google Scholar]
- 27. Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS.. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 2006;47:1161–1166. [DOI] [PubMed] [Google Scholar]
- 28. Ezekowitz JA, Rowe BH, Dryden DM, Hooton N, Vandermeer B, Spooner C, McAlister FA.. Systematic review: implantable cardioverter defibrillators for adults with left ventricular systolic dysfunction. Ann Intern Med 2007;147:251–262. [DOI] [PubMed] [Google Scholar]
- 29. Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, Maggioni AP, Anand I, Poole-Wilson PA, Fishbein DP, Johnson G, Anderson J, Mark DB, Bardy GH.. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation 2009;120:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, Carson PE, Maggioni AP, Mann DL, Pitt B, Poole-Wilson PA, Levy WC.. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation 2007;116:392–398. [DOI] [PubMed] [Google Scholar]
- 31. Wu TJ, Ong JJ, Hwang C, Lee JJ, Fishbein MC, Czer L, Trento A, Blanche C, Kass RM, Mandel WJ, Karagueuzian HS, Chen PS.. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. J Am Coll Cardiol 1998;32:187–196. [DOI] [PubMed] [Google Scholar]
- 32. Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM.. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol 2004;43:1834–1842. [DOI] [PubMed] [Google Scholar]
- 33. Bogun FM, Desjardins B, Good E, Gupta S, Crawford T, Oral H, Ebinger M, Pelosi F, Chugh A, Jongnarangsin K, Morady F.. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol 2009;53:1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ.. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004;43:2260–2264. [DOI] [PubMed] [Google Scholar]
- 35. Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ.. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol 2012;60:408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernandez-Armenta J, Berruezo A, Mont L, Sitges M, Andreu D, Silva E, Ortiz-Perez JT, Tolosana JM, de Caralt TM, Perea RJ, Calvo N, Trucco E, Borras R, Matas M, Brugada J.. Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace 2012;14:1578–1586. [DOI] [PubMed] [Google Scholar]
- 37. Lehrke S, Lossnitzer D, Schob M, Steen H, Merten C, Kemmling H, Pribe R, Ehlermann P, Zugck C, Korosoglou G, Giannitsis E, Katus HA.. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart 2011;97:727–732. [DOI] [PubMed] [Google Scholar]
- 38. Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK.. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 39. Muser D, Santangeli P, Pathak RK, Castro SA, Liang JJ, Magnani S, Hayashi T, Garcia FC, Hutchinson MD, Supple GE, Frankel DS, Riley MP, Lin D, Schaller RD, Desjardins B, Dixit S, Callans DJ, Zado ES, Marchlinski FE.. Long-term outcomes of catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. Circ Arrhythm Electrophysiol 2016;9:e004333.. [DOI] [PubMed] [Google Scholar]
- 40. Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M.. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging 2014;7:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Halliday BP, Gulati A, Ali A, Guha K, Newsome S, Arzanauskaite M, Vassiliou VS, Lota A, Izgi C, Tayal U, Khalique Z, Stirrat C, Auger D, Pareek N, Ismail TF, Rosen SD, Vazir A, Alpendurada F, Gregson J, Frenneaux MP, Cowie MR, Cleland JGF, Cook SA, Pennell DJ, Prasad SK.. Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation 2017;135:2106–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hutchinson MD, Gerstenfeld EP, Desjardins B, Bala R, Riley MP, Garcia FC, Dixit S, Lin D, Tzou WS, Cooper JM, Verdino RJ, Callans DJ, Marchlinski FE.. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar S, Barbhaiya C, Nagashima K, Choi EK, Epstein LM, John RM, Maytin M, Albert CM, Miller AL, Koplan BA, Michaud GF, Tedrow UB, Stevenson WG.. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol 2015;8:87–93. [DOI] [PubMed] [Google Scholar]
- 44. Sasaki T, Miller CF, Hansford R, Zipunnikov V, Zviman MM, Marine JE, Spragg D, Cheng A, Tandri H, Sinha S, Kolandaivelu A, Zimmerman SL, Bluemke DA, Tomaselli GF, Berger RD, Halperin HR, Calkins H, Nazarian S.. Impact of nonischemic scar features on local ventricular electrograms and scar-related ventricular tachycardia circuits in patients with nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2013;6:1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haqqani HM, Tschabrunn CM, Tzou WS, Dixit S, Cooper JM, Riley MP, Lin D, Hutchinson MD, Garcia FC, Bala R, Verdino RJ, Callans DJ, Gerstenfeld EP, Zado ES, Marchlinski FE.. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization, and implications. Heart Rhythm 2011;8:1169–1176. [DOI] [PubMed] [Google Scholar]
- 46. Pathak RK, Garcia FC.. Ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Card Electrophysiol Clin 2017;9:99–106. [DOI] [PubMed] [Google Scholar]
- 47. Muser D, Santangeli P, Castro SA, Pathak RK, Liang JJ, Hayashi T, Magnani S, Garcia FC, Hutchinson MD, Supple GG, Frankel DS, Riley MP, Lin D, Schaller RD, Dixit S, Zado ES, Callans DJ, Marchlinski FE.. Long-term outcome after catheter ablation of ventricular tachycardia in patients with nonischemic dilated cardiomyopathy. Circ Arrhythm Electrophysiol 2016;9:e004328.. [DOI] [PubMed] [Google Scholar]
- 48. Frankel DS, Tschabrunn CM, Cooper JM, Dixit S, Gerstenfeld EP, Riley MP, Callans DJ, Marchlinski FE.. Apical ventricular tachycardia morphology in left ventricular nonischemic cardiomyopathy predicts poor transplant-free survival. Heart Rhythm 2013;10:621–626. [DOI] [PubMed] [Google Scholar]
- 49. Cassidy DM, Vassallo JA, Miller JM, Poll DS, Buxton AE, Marchlinski FE, Josephson ME.. Endocardial catheter mapping in patients in sinus rhythm: relationship to underlying heart disease and ventricular arrhythmias. Circulation 1986;73:645–652. [DOI] [PubMed] [Google Scholar]
- 50. Hsia HH, Marchlinski FE.. Characterization of the electroanatomic substrate for monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy. Pacing Clin Electrophysiol 2002;25:1114–1127. [DOI] [PubMed] [Google Scholar]
- 51. Hsia HH, Callans DJ, Marchlinski FE.. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation 2003;108:704–710. [DOI] [PubMed] [Google Scholar]
- 52. Dubois R, Shah AJ, Hocini M, Denis A, Derval N, Cochet H, Sacher F, Bear L, Duchateau J, Jais P, Haissaguerre M.. Non-invasive cardiac mapping in clinical practice: application to the ablation of cardiac arrhythmias. J Electrocardiol 2015;48:966–974. [DOI] [PubMed] [Google Scholar]
- 53. Intini A, Goldstein RN, Jia P, Ramanathan C, Ryu K, Giannattasio B, Gilkeson R, Stambler BS, Brugada P, Stevenson WG, Rudy Y, Waldo AL.. Electrocardiographic imaging (ECGI), a novel diagnostic modality used for mapping of focal left ventricular tachycardia in a young athlete. Heart Rhythm 2005;2:1250–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Y, Cuculich PS, Zhang J, Desouza KA, Vijayakumar R, Chen J, Faddis MN, Lindsay BD, Smith TW, Rudy Y.. Noninvasive electroanatomic mapping of human ventricular arrhythmias with electrocardiographic imaging. Sci Transl Med 2011;3:98ra84.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rudy Y. Noninvasive electrocardiographic imaging of cardiac resynchronization therapy in patients with heart failure. J Electrocardiol 2006;39:S28–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ploux S, Lumens J, Whinnett Z, Montaudon M, Strom M, Ramanathan C, Derval N, Zemmoura A, Denis A, De Guillebon M, Shah A, Hocini M, Jais P, Ritter P, Haissaguerre M, Wilkoff BL, Bordachar P.. Noninvasive electrocardiographic mapping to improve patient selection for cardiac resynchronization therapy: beyond QRS duration and left bundle branch block morphology. J Am Coll Cardiol 2013;61:2435–2443. [DOI] [PubMed] [Google Scholar]
- 57. Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio A, Mannarino S, Gambarin F, Favalli V, Grasso M, Agozzino M, Campana C, Gavazzi A, Febo O, Marini M, Landolina M, Mortara A, Piccolo G, Vigano M, Tavazzi L, Arbustini E.. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol 2008;52:1250–1260. [DOI] [PubMed] [Google Scholar]
- 58. van Berlo JH, de Voogt WG, van der Kooi AJ, van Tintelen JP, Bonne G, Yaou RB, Duboc D, Rossenbacker T, Heidbüchel H, de Visser M, Crijns HJGM, Pinto YM.. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: do lamin A/C mutations portend a high risk of sudden death? J Mol Med 2005;83:79–83. [DOI] [PubMed] [Google Scholar]
- 59. Meune C, Van Berlo JH, Anselme F, Bonne G, Pinto YM, Duboc D.. Primary prevention of sudden death in patients with lamin A/C gene mutations. N Engl J Med 2006;354:209–210. [DOI] [PubMed] [Google Scholar]
- 60. van Rijsingen IA, van der Zwaag PA, Groeneweg JA, Nannenberg EA, Jongbloed JD, Zwinderman AH, Pinto YM, Dit Deprez RH, Post JG, Tan HL, de Boer RA, Hauer RN, Christiaans I, van den Berg MP, van Tintelen JP, Wilde AA.. Outcome in phospholamban R14del carriers: results of a large multicentre cohort study. Circ Cardiovasc Genet 2014;7:455–465. [DOI] [PubMed] [Google Scholar]
- 61. van Spaendonck-Zwarts KY, van Hessem L, Jongbloed JD, de Walle HE, Capetanaki Y, van der Kooi AJ, van Langen IM, van den Berg MP, van Tintelen JP.. Desmin-related myopathy. Clin Genet 2011;80:354–366. [DOI] [PubMed] [Google Scholar]
- 62. McNair WP, Sinagra G, Taylor MR, Di Lenarda A, Ferguson DA, Salcedo EE, Slavov D, Zhu X, Caldwell JH, Mestroni L; Familial Cardiomyopathy Registry Research Group. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol 2011;57:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hershberger RE, Siegfried JD.. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 2011;57:1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taylor MR, Fain PR, Sinagra G, Robinson ML, Robertson AD, Carniel E, Di Lenarda A, Bohlmeyer TJ, Ferguson DA, Brodsky GL, Boucek MM, Lascor J, Moss AC, Li WL, Stetler GL, Muntoni F, Bristow MR, Mestroni L; Familial Dilated Cardiomyopathy Registry Research Group. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J Am Coll Cardiol 2003;41:771–780. [DOI] [PubMed] [Google Scholar]
- 65. Groeneweg JA, Bhonsale A, James CA, Te Riele AS, Dooijes D, Tichnell C, Murray B, Wiesfeld AC, Sawant AC, Kassamali B, Atsma DE, Volders PG, de Groot NM, de Boer K, Zimmerman SL, Kamel IR, van der Heijden JF, Russell SD, Jan Cramer M, Tedford RJ, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Hauer RN, Calkins H.. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet 2015;8:437–446. [DOI] [PubMed] [Google Scholar]
- 66. Te Riele AS, James CA, Groeneweg JA, Sawant AC, Kammers K, Murray B, Tichnell C, van der Heijden JF, Judge DP, Dooijes D, van Tintelen JP, Hauer RN, Calkins H, Tandri H.. Approach to family screening in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Eur Heart J 2016;37:755–763. [DOI] [PubMed] [Google Scholar]
- 67. Cheng A, Zhang Y, Blasco-Colmenares E, Dalal D, Butcher B, Norgard S, Eldadah Z, Ellenbogen KA, Dickfeld T, Spragg DD, Marine JE, Guallar E, Tomaselli GF.. Protein biomarkers identify patients unlikely to benefit from primary prevention implantable cardioverter defibrillators: findings from the Prospective Observational Study of Implantable Cardioverter Defibrillators (PROSE-ICD). Circ Arrhythm Electrophysiol 2014;7:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Deo R, Norby FL, Katz R, Sotoodehnia N, Adabag S, DeFilippi CR, Kestenbaum B, Chen LY, Heckbert SR, Folsom AR, Kronmal RA, Konety S, Patton KK, Siscovick D, Shlipak MG, Alonso A.. Development and validation of a sudden cardiac death prediction model for the general population. Circulation 2016;134:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, Sterling DG, Williams SA.. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. Jama 2016;315:2532–2541. [DOI] [PubMed] [Google Scholar]
- 70. Ngo D, Sinha S, Shen D, Kuhn EW, Keyes MJ, Shi X, Benson MD, O’Sullivan JF, Keshishian H, Farrell LA, Fifer MA, Vasan RS, Sabatine MS, Larson MG, Carr SA, Wang TJ, Gerszten RE.. Aptamer-based proteomic profiling reveals novel candidate biomarkers and pathways in cardiovascular disease. Circulation 2016;134:270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Smith JG, Gerszten RE.. Emerging affinity-based proteomic technologies for large-scale plasma profiling in cardiovascular disease. Circulation 2017;135:1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gradman A, Deedwania P, Cody R, Massie B, Packer M, Pitt B, Goldstein S.. Predictors of total mortality and sudden death in mild to moderate heart failure. Captopril-Digoxin Study Group. J Am Coll Cardiol 1989;14:564–570. [DOI] [PubMed] [Google Scholar]
- 73. Hofmann T, Meinertz T, Kasper W, Geibel A, Zehender M, Hohnloser S, Stienen U, Treese N, Just H.. Mode of death in idiopathic dilated cardiomyopathy: a multivariate analysis of prognostic determinants. Am Heart J 1988;116:1455–1463. [DOI] [PubMed] [Google Scholar]
- 74. Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R.. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet 1994;344:493–498. [DOI] [PubMed] [Google Scholar]
- 75. Doval HC, Nul DR, Grancelli HO, Varini SD, Soifer S, Corrado G, Dubner S, Scapin O, Perrone SV.. Nonsustained ventricular tachycardia in severe heart failure. Independent marker of increased mortality due to sudden death. GESICA-GEMA Investigators. Circulation 1996;94:3198–3203. [DOI] [PubMed] [Google Scholar]
- 76. Gardner PI, Ursell PC, Fenoglio JJ Jr, Wit AL.. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation 1985;72:596–611. [DOI] [PubMed] [Google Scholar]
- 77. Simson MB, Untereker WJ, Spielman SR, Horowitz LN, Marcus NH, Falcone RA, Harken AH, Josephson ME.. Relation between late potentials on the body surface and directly recorded fragmented electrograms in patients with ventricular tachycardia. Am J Cardiol 1983;51:105–112. [DOI] [PubMed] [Google Scholar]
- 78. Gomes JA, Cain ME, Buxton AE, Josephson ME, Lee KL, Hafley GE.. Prediction of long-term outcomes by signal-averaged electrocardiography in patients with unsustained ventricular tachycardia, coronary artery disease, and left ventricular dysfunction. Circulation 2001;104:436–441. [DOI] [PubMed] [Google Scholar]
- 79. Gold MR, Bloomfield DM, Anderson KP, El-Sherif NE, Wilber DJ, Groh WJ, Estes NA 3rd, Kaufman ES, Greenberg ML, Rosenbaum DS.. A comparison of T-wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol 2000;36:2247–2253. [DOI] [PubMed] [Google Scholar]
- 80. Mancini DM, Wong KL, Simson MB.. Prognostic value of an abnormal signal-averaged electrocardiogram in patients with nonischemic congestive cardiomyopathy. Circulation 1993;87:1083–1092. [DOI] [PubMed] [Google Scholar]
- 81. Galinier M, Albenque JP, Afchar N, Fourcade J, Massabuau P, Doazan JP, Legoanvic C, Fauvel JM, Bounhoure JP.. Prognostic value of late potentials in patients with congestive heart failure. Eur Heart J 1996;17:264–271. [DOI] [PubMed] [Google Scholar]
- 82. Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS.. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 1999;99:1385–1394. [DOI] [PubMed] [Google Scholar]
- 83. Gehi AK, Stein RH, Metz LD, Gomes JA.. Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: a meta-analysis. J Am Coll Cardiol 2005;46:75–82. [DOI] [PubMed] [Google Scholar]
- 84. Grimm W, Christ M, Bach J, Muller HH, Maisch B.. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation 2003;108:2883–2891. [DOI] [PubMed] [Google Scholar]
- 85. Hohnloser SH, Ikeda T, Bloomfield DM, Dabbous OH, Cohen RJ.. T-wave alternans negative coronary patients with low ejection and benefit from defibrillator implantation. Lancet 2003;362:125–126. [DOI] [PubMed] [Google Scholar]