Summary
AIM:
The aim of this study was to investigate whether an aged systemic environment could impair young cartilage tissue in mice.
METHODS:
Mice differing in age were randomly divided into three groups. Group 1 was the experimental group (Y/O group) consisting of the heterochronic parabiosis model (2-month-old/12-month-old, young/old). Group 2 was the surgical control group (Y/Y group) with the isochronic parabiosis model (2-month-old/2-month-old, young/young). Group 3 consisted of the ageing control mice (2-month-old alone, Y group). Young knee cartilages collected from all three groups at 4 months after surgery were compared. Fluorescence molecular tomography (FMT) was used to confirm whether the two mice in parabiosis shared a common blood circulation at 2 weeks after surgery. The knee joints of young mice were examined radiologically at 4 months after surgery. Histological scoring was assigned to grade the severity of osteoarthritis (OA). Immunohistochemistry and quantitative reverse transcription polymerase chain reaction were used to evaluate OA-related protein expression and gene expression, and chondrocyte proliferation was determined with EdU staining.
RESULTS:
FMT imaging confirmed cross-circulation in the parabiotic pairs. The percentage of EdU-positive chondrocytes in young mice from the Y/O group was significantly lower compared with those of the Y/Y and Y groups (p <0.05 for both). There was no statistically significant difference in the mRNA expression of collagen type II (Col2), collagen type X (Col10), and matrix metalloproteinase 13 (MMP13) among the three groups (P>0.05), but expression of sex-determining region Y box 9 (Sox9) mRNA in young cartilage from the Y/O group was markedly attenuated compared to those in the Y/Y and Y groups (p <0.05 for both). In the Y/O group, mRNA expression of runt-related transcription factor 2 (Runx2) in young cartilage was significantly increased compared to the Y/Y and Y groups (p <0.05 for both). The changes in Col2, Col10, MMP13, Runx2 and Sox9 at the protein level mimicked the alterations found at the mRNA level. Loss of cartilage proteoglycan in young mice from the Y/O group was significantly greater compared to the Y/Y and Y groups (p <0.05 for both), despite the lack of significant difference among the three groups in OARIS and osteophytosis scores.
CONCLUSION:
Heterochronic parabiosis exerts a negative effect on chondrocyte proliferation in the knee cartilage of young mice.
Keywords: osteoarthritis, aging, degeneration, cartilage, heterochronic parabiosis
Introduction
Osteoarthritis (OA) is the leading cause of chronic infirmity in ageing individuals. Age-associated changes that affect joint tissues promote the development of OA [1, 2]. An increase in age has a greater impact on the development of primary OA than other risk factors, including obesity, genetics, sex and anatomical factors [3, 4]. Chondrocytes in the articular cartilage are instrumental for the production and degradation of cartilaginous extracellular matrix. Chondrocyte loss or a decline in chondrocyte proliferation has been associated with the degeneration of aged cartilage [5–7]. Thus it is important to ascertain the factors that promote ageing of the cartilage with the aim to prevent or reduce cartilage dysfunction. Previous reports disclosed that heterochronic parabiosis, which refers to the parabiotic pairing of an older animal with a younger one, could in part reduce the regenerative capacity of young organs and tissues, such as the brain and muscle [8, 9].
Heterochronic parabiosis involves joining two animals of dissimilar ages. The two animals subsequently develop vascular anastomoses and share a single circulatory system. This experimental model provides a method to experimentally evaluate both the systemic consequences on cell and tissue ageing and the development of age-associated ailments [10]. It allows exposure of young tissues to an old systemic environment, which mimics sustained injury and chronic diseases such as primary OA [11]. Nevertheless, it is unknown whether heterochronic parabiosis damages articular cartilage in the younger partner.
OA-like pathological features, although mild, have been detected in the knees of 12-month-old C57/BL6 mice, implying the presence of early OA [12]. More severe pathological changes due to age-related degeneration were detected in the knees of the majority of 16-month-old C57/BL6 mice [13]. The occurrence of pathological changes resembling OA in the knees of mice from 12 to 16 months of age suggests the production of factors such as pro-ageing factors that are harmful to the knees of mice belonging to this age group. In this study, a 2-month-old C57/BL6 mouse was linked with a 12-month-old C57/BL6 mouse for 4 months. We hypothesised that the systemic environment specific to the aged (12-month-old) mouse would damage the cartilage of the younger (2-month-old) mouse. In order to test this hypothesis, we generated a heterochronic parabiosis model in which the blood exchange between the two mice was confirmed with fluorescence molecular tomography (FMT). For the purpose of observing the potential damage of an old systemic environment to young cartilage, we assessed the proliferation of chondrocytes in the young cartilage using 5-ethynyl-2’-deoxyuridine (EdU). We also measured the cartilage content of proteoglycan, as well as gene expression levels and protein levels of cartilage catabolic factors.
Methods and materials
Animals
Male C57BL/6 mice, both aged (12-month-old, O) and young (2-month-old, Y), were obtained from the Experimental Animal Centre, Shanxi Medical University, China. Mice were housed under specific pathogen-free conditions with a 12-hour/12-hour light/dark cycle. All animals were handled and used in accordance with the Guidelines for the Use and Care of Laboratory Animals provided by Shanxi Medical University. All animal experiments were approved by the Institutional Review Board at the Second Hospital of Shanxi Medical University (identification code for project approval: 2013025; project period: January 2013 to December 2018). Heterochronic parabiotic pairs in which 2-month-old male C57BL/6 mice were surgically joined to 12-month-old partners (Y/O, 8 pairs) were allocated to group 1 (experimental group). Isochronic parabiotic pairs with partners of identical age (2-month-old/2-month-old, Y/Y, 5 pairs) were allocated to group 2 (surgery control group), and 2-month-old mice only were allocated to group 3 (ageing control group, Y, 10 mice).
Parabiosis surgery
Parabiosis surgery was as detailed in published papers [9, 14]. Mice in pairs were anaesthetised and shaved, and mirror-image incisions were made at the left and right flanks from the elbow to the hip. The peritoneal openings of the adjacent parabionts were seamed together. Elbow joints of the parabionts were ligated and the skins of the parabionts were stapled together (3.0 prolene, Ethicon, Inc., USA). The knee joints were not joined together in order for the mice to maintain their mobility. Each mouse received subcutaneous injections of Baytril (enrofloxacin; antibiotic) and Buprenex (buprenorphine, as recommended for antinociception) and their subsequent recovery was monitored. For overall health and maintenance behaviour, some characteristics of recovery, including paired weights and grooming behaviour, were determined at various time intervals postoperatively.
Fluorescence molecular tomography
The heterochronic parabiosis model was established 2 weeks after surgery. FMT4000 (PerkinElmer, Boston, MA, USA) was used to confirm that the partners in the model had a common blood circulation. FMT is a noninvasive technique utilising fluorescence, characterised by molecular specificity and sensitivity, and is used for tissue imaging in live animals [15]. ProSense 750-fluorescence agents (PerkinElmer, Boston, MA, USA) become fluorescent upon activation in the presence of cathepsins (cathepsins B, L, S, and plasmin), but do not fluoresce without any activation. ProSense 750FAST was injected into the old mouse in the heterochronic parabiosis model via the tail vein. The young mouse was imaged using FMT4000 24 hours after injection.
Radiography
The knee joints were studied using micro X-ray (Faxitron Bioptics, Lincolnshire, IL, USA) to reveal morphological alterations in the whole knee prior to the euthanasia of the animals. Radiographic grading was accordance with numerical rating scales reported in the literature [16]. The presence and severity of osteophytosis (0–3 scale), osteopenia (not evident in this study) and sclerosis (not evident in this study) were recorded. Subjective grading of osteophytosis was carried out on a scale of 0 to 3 (0 normal, 1 mild, 2 moderate, 3 severe) as judged by severity at the periphery of the knee joint.
Histology
Knee cartilages of young mice from all three groups at 4 months after surgery were collected and compared. On the scheduled day of euthanasia, pentobarbital sodium was administered intraperitoneally to anaesthetise the animals. The right knees were collected for histological examination, EdU staining and immunohistochemical staining. The microscopic scoring of mouse cartilage degeneration followed previously described procedures [17]. Blocks were trimmed to expose the cartilage. A series of frontal sections (5 μm in thickness) across the entire joints were prepared. Three 5-μm thick sections were placed on each slide. Fifteen slides were prepared, harvested at intervals of approximately 80 μm and stained with safranin O / fast green for histological assessment. Intervening sections were stored for EdU and immunohistochemical staining. Degeneration of cartilage at the joint was evaluated with the Osteoarthritis Research Society International (OARSI) scoring system, and proteoglycan depletion was quantified after safranin-O staining with a semi-quantitative scoring system [17]. The OARSI scoring system and the loss of proteoglycan scoring system used in this paper are detailed in table S1 and table S2 in appendix 1. OA severity was expressed as the sum of scores from the medial femoral condyle and medial tibial plateau. A higher score indicates greater impairment of the cartilage. Three blinded researchers independently scored each section.
Immunohistochemistry
Immunohistochemical staining was used for detection of collagen type II (Col2), collagen type X (Col10), matrix metalloproteinase 13 (MMP13), sex-determining region Y box 9 (Sox9) and runt-related transcription factor 2 (Runx2). Samples were incubated for 10 minutes in 3% H2O2 (Sigma-Aldrich, MO, USA) to remove endogenous peroxidase activity. Incubation with 10% diluted goat serum (Solarbio, Beijing, China) was used to minimise nonspecific protein binding. After antigen preparation using Multipurpose Digestive (Boster, Wuhan, China), sections were incubated with primary antibodies against mouse Col2 (Abcam, ab34712, 1:100), Col10 (Abcam, ab58632, 1:50), MMP13 (Abcam, ab39012, 1:100), Sox9 (Abcam, ab26414, 1:50) and Runx2 (Abcam, ab23981, 1:50) at 4°C overnight. The sections were then incubated with a biotinylated secondary antibody (Abcam, ab6721, 1:200) followed by development using a 3,3’-diaminobenzidine chromogen. Images were taken using a Nikon E800 microscope (Nikon, Melville, NY, USA). Positive cells in two different fields of view within a single section were enumerated by a blinded experimenter. The percentage of positive cells was calculated as the ratio of positive cells to total chondrocytes present in the section.
Real-time qRT-PCR
The left knees in each group were collected for mRNA analyses and cartilage samples were collected from the tibia. The samples were ground using a mortar and pestle under liquid nitrogen. Total RNA from the mouse knee joint cartilage was isolated using an RNeasy isolation kit (Cat. No. 74704, Qiagen, Valencia, CA, USA) as previously detailed in the literature [18]. Cartilage samples from two murine tibial plateaus were dissected with a scalpel under a dissection microscope and pooled together. Four pooled samples from each group were analysed. Total RNA (1 μg) was reverse- transcribed to cDNA using the iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). The resulting cDNA (40 ng/μl) served as the template for determining the relative content of mRNA with the QuantiTect SYBR Green PCR kit (QIAGEN, Valencia, CA, USA) using a DNA Engine Opticon 2 Continuous Fluorescence Detection System (MJ Research, Waltham, MA, USA). The primers used are listed in table 1.
Table 1:
Sequences of the primers used in this study.
| Gene | Sequences (5’→3’) | |
|---|---|---|
| Col2 | Forward | AAGGGACACCGAGGTTTCACTGG |
| Reverse | GGGCCTGTTTCTCCTGAGCGT | |
| Col10 | Forward | GCCAGGAAAGCTGCCCCACG |
| Reverse | GAGGTCCGGTTGGGCCTGGT | |
| MMP13 | Forward | GGACCTTCTGGTCTTCTGGC |
| Reverse | GGATGCTTAGGGTTGGGGTC | |
| Sox9 | Forward | CGTGGACATCGGTGAACTGA |
| Reverse | GGTGGCAAGTATTGGTCAAACTC | |
| Runx2 | Forward | CCGCACGCAAACCGCACCAT |
| Reverse | CGCTCCGGCCCACAAATCTC | |
| Gapdh | Forward | GGCAAATTCAACGGCACA |
| Reverse | GTTAGTGGGGTCTCGCTCCTG | |
EdU incorporation
EdU (5 μg/g per day for 7 days) was injected intraperitoneally into the mice during the final week before sacrifice. EdU staining was performed using the protocol provided by the Cell-Light™ Apollo Stain Kit (RIBOBIO, C10371–1, Guangzhou, China). Following deparaffination, the sections were rinsed with a glycine solution for 10 min, permeabilised in phosphate-buffered saline (PBS) containing 0.5% Triton X-100 for 10 min, washed with PBS (3 × 10 min), and incubated at room temperature in the presence of the Apollo reaction cocktail for 30 min. Next, the sections on the slides were permeabilised with 0.5% Triton X-100 in PBS for 10 min and then rinsed with methanol (2 × 5 min). Finally, the slides were incubated with Hoechst at room temperature for 30 min. Nuclear counterstaining was performed using DAPI, and samples were mounted with 50% glycerine. Images were obtained by using fluorescence microscopy. The EdU- and DAPI-positive cells were determined using ImageJ software, and the EdU labeling index (a ratio of the number of EdU-positive cells to the number of DAPI-positive cells) was calculated. The threshold of fluorescence intensity was set according to the range in which these cells of interest can be detected. Two fields of view for each slide were analysed, using two slides from each knee and eight knees from each group.
Data and statistical analyses
Data were expressed as mean ± standard error of the mean (SEM). Statistical analysis was conducted using Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). Data from different groups were analysed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparisons test. Differences were considered statistically significant when the P value was < 0.05.
Results
Confirmation of common circulation in parabiosis model with FMT
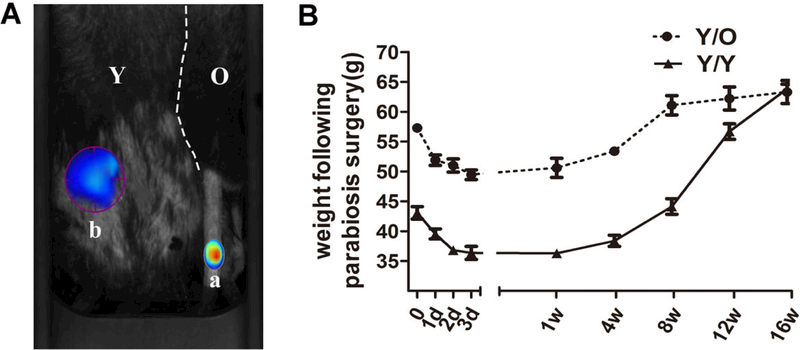
The heterochronic parabiotic pairs (Y/O) and isochronic parabiotic pairs (Y/Y) were used to evaluate the effects of an aged systemic environment on young cartilage. ProSense 750 FAST (100 ng), which is optically silent in its ground state and acquires intense fluorescence after activation by proteases, was injected through the tail vein in one partner of the parabiotic model. A needle-stick injury was created in the other partner of the parabiotic model 2 weeks after the operation. A strong positive signal of ProSense 750 FAST around the site of needle injury was detected (fig. 1A), which indicated that cross-circulation of the parabiotic pair was successfully established in this study. Additionally, the body weight in both groups tended to decline within 2 weeks after the operation, followed by a steady normalisation of the body weight (fig. 1B). Parabiotic pairs were maintained for 16 weeks to ensure adequate exchange of circulatory factors between the partners prior to analysis. The pairs were euthanased 16 weeks after the surgery.
Figure 1:
Common circulation between two mice in the heterochronic parabiosis model. (A) At 2 weeks after parabiosis surgery, the old mouse of the parabiotic pair received an injection of ProSense 750FAST through the tail vein (position a) and ProSense 750FAST was activated around the needling wound in the young mouse of the parabiotic pair (position b), which was imaged with the FMT system 24 hours after injection. ProSense 750FAST agent was activated in the young mouse, which indicated that the mice had developed a shared blood circulation. (B) Absolute weights of the pairs (parabionts) measured at various times during recovery (8 Y/O pairs and 5 Y/Y pairs). Immediately following the surgery, both groups appeared to lose weight, which was followed by steady normalisation of the body weight. FMT = fluorescence molecular tomography; O = old; Y = young
Reduction of proteoglycan in young articular cartilage in an aged systemic environment
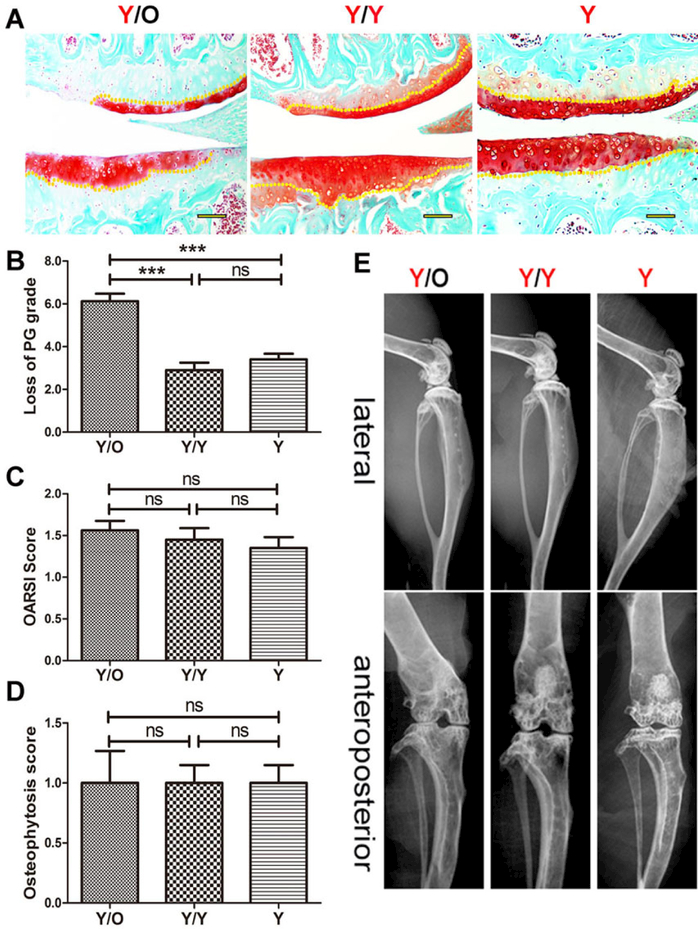
Examination of safranin O staining was conducted to analyse the changes in chondrocyte-derived proteoglycan. The loss of proteoglycan from cartilage in young mice from the Y/O group was significantly greater than that from the Y/Y and Y groups (fig. 2A, B) at 16 weeks post-surgery, which indicated that the aged systemic environment triggered proteoglycan loss in young cartilage. Although OARSI scores in young cartilage from the Y/O group tended to increase compared with those of the Y/Y and Y groups (fig. 2C), differences among the three groups were not statistically significant (p >0.05). In addition, there were no radiographic changes in the knees of young mice among the three groups (fig. 2D, E). These data suggest that an aged systemic environment can negatively affect proteoglycan synthesis before gross damage and radiological changes occur in young articular cartilage.
Figure 2:
Histological and radiographic changes in cartilage of young mice in the Y/O, Y/Y and Y groups. (A) PG decrease in young cartilage from the Y/O group was greater than that from the two other groups and histological scores in the Y/O group were relatively higher. Representative images are shown (8 Y/O mice, 10 Y/Y mice and 10 Y mice were used) Scale bar = 500 μm. (B) Loss of cartilage (proteoglycan grade) in young mice from the Y/O group was significantly greater compared to the Y/Y and Y groups. The area covered from the surface of the cartilage to the yellow dotted lines indicates the proteoglycan content. (C) There was no statistically significant difference in OARSI score among the three groups (p >0.05). (D, E) No significant pathological changes were detected in the micro-X-ray. The presence and severity of osteopenia and sclerosis among the three groups were not evident in this study, and osteophytosis was detected at the margins of the knee joint in young mice from the three groups. There was no statistically significant difference in osteophytosis scores among the three groups (p >0.05). The differences of the proteoglycan content, the Osteoarthritis Research Society International (OARSI) score and the osteophytosis scores among three groups were assessed by one-way ANOVA with Tukey’s post-hoc test.*** p <0.001. Values are presented as mean ± standard error of the mean. ns = not significant; O = old; Y = young
An aged systemic environment induced significant changes in protein levels of Sox9 and Runx2
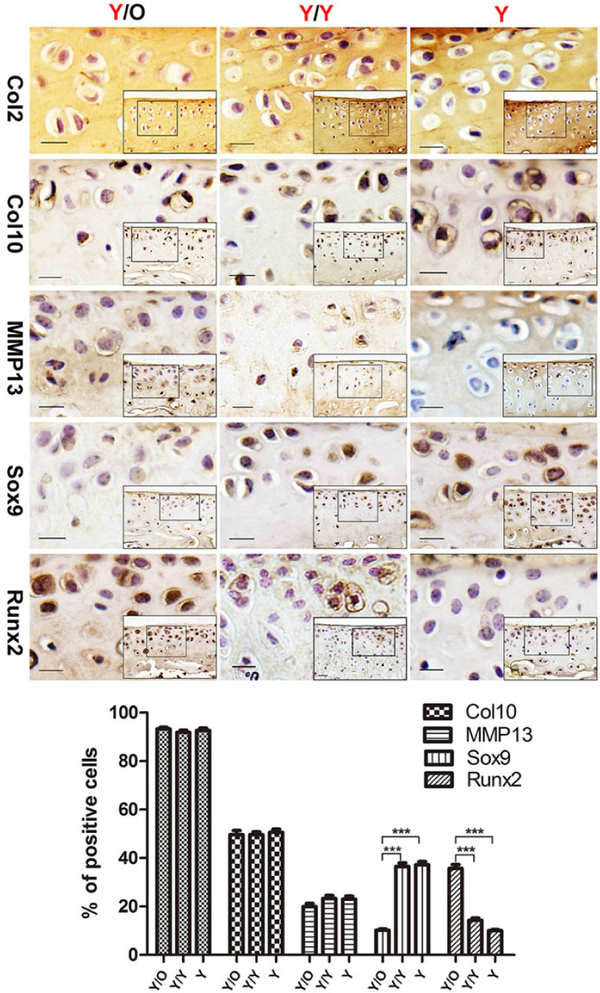
In order to confirm that chondrocytes in young cartilage were impaired under the influence of an aged systemic environment, OA-associated factors were detected by immunohistochemical staining. Although there were no significant changes in the number of Col2/Col10/MMP13-positive chondrocytes among the three groups (fig. 3), there was a marked decrease of Sox9-positive chondrocytes in the young cartilage from the Y/O group compared with the Y/Y and Y groups (fig. 3). In contrast, there was an increase in the number of Runx2-positive chondrocytes following exposure to an aged systemic environment (fig. 3). Immunostaining was absent in the negative control sections (not shown). These results further confirmed that an aged systemic environment adversely affected young articular cartilage.
Figure 3:
Protein expression of Col2, Col10, MMP13, Sox9 and Runx2 in articular cartilage. Young cartilage samples from Y/O, Y/Y and Y groups were analysed with immunohistochemistry for Col2, Col10, MMP13, Sox9 and Runx2. Col2, Col10 and MMP13 levels in young cartilage showed no statistically significant difference among the Y/O, Y/Y and Y groups (p >0.05). However, the level of Sox9 was reduced and the level of Runx2 was increased in young cartilage from the Y/O group compared to the Y/Y and Y groups. Scale bar = 200 μm. (8 Y/O mice, 10 Y/Y mice and 10 Y mice were used). One-way ANOVA with Tukey’s post-hoc test was used to assess the difference in the percentage of positive cells with Col2, Col10, MMP13, Sox9 and Runx2 protein expression among the three groups.*** p <0.001. Values are presented as mean ± standard error of the mean. Col2 = collagen type II; Col10 = collagen type X; MMP13 = matrix metalloproteinase 13; O = old; Runx2 = runt-related transcription factor 2; Sox9 = sex-determining region Y box 9; Y = young
Aged systemic environment induced significant changes in mRNA levels of Sox9 and Runx2
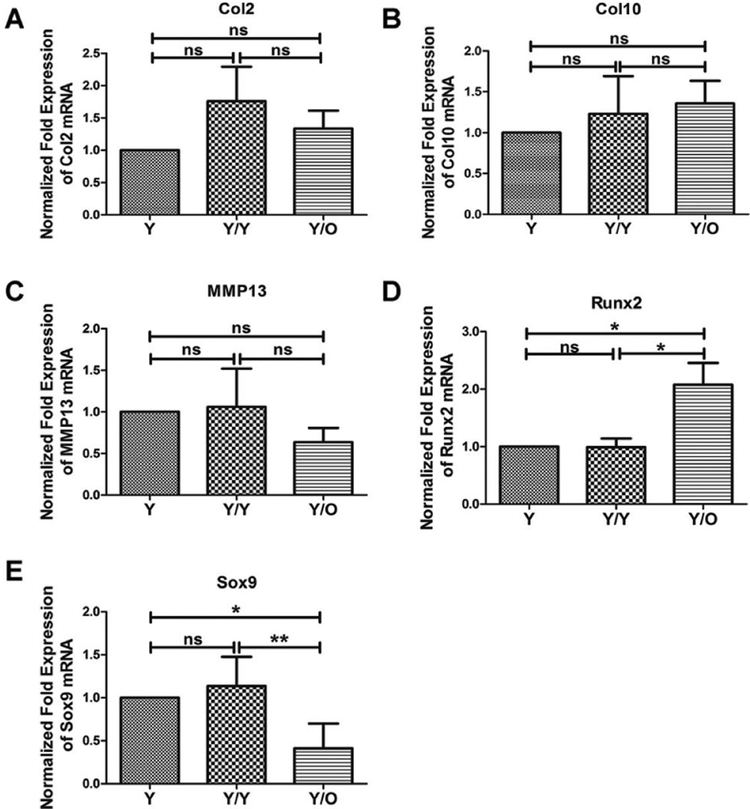
As mentioned above, an aged systemic environment profoundly influenced the protein levels of Sox9 and Runx2. Next, alterations in mRNA levels were examined, and qRT-PCR was used to determine the expression levels of the OA-associated genes. No significant changes were detected in Col2/Col10/MMP13 mRNA levels in young cartilage from the Y/O group compared with those in young cartilage from the Y/Y or Y group (fig. 4A, B, C). However, in an aged systemic environment, the Runx2 mRNA level was significantly upregulated (fig. 4D). In contrast, the Sox9 mRNA level was drastically downregulated in young cartilage in the presence of an aged systemic environment (fig. 4E). These results were in accordance with the protein data described above.
Figure 4:
Messenger RNA expression of cartilage-associated genes in articular cartilage. Expression of Col2, Col10, MMP13, Sox9 and Runx2 was determined with qRT-PCR in the articular cartilage samples and no significant differences in the expression of the Col2, Col10 and MMP13 could be detected when the three groups were compared (A, B, C). However, a significant increase in the expression of Runx2 and a significant decrease of Sox9 were measured in young cartilages from the Y/O group (D, E) (8 Y/O mice, 10 Y/Y mice and 10 Y mice were used). One-way ANOVA with Tukey’s post-hoc test was used to assess the difference in mRNA expression of Col2, Col10, MMP13, Sox9 and Runx2 among the three groups.* p <0.05; ** p < 0.01. Values are presented as mean ± standard error of the mean. Col2 = collagen type II; Col10 = collagen type X; MMP13 = matrix metalloproteinase 13; ns = not significant; O = old; qRT-PCR = quantitative reverse transcription polymerase chain reaction; Runx2 = runt-related transcription factor 2; Sox9 = sex-determining region Y box 9; Y = young
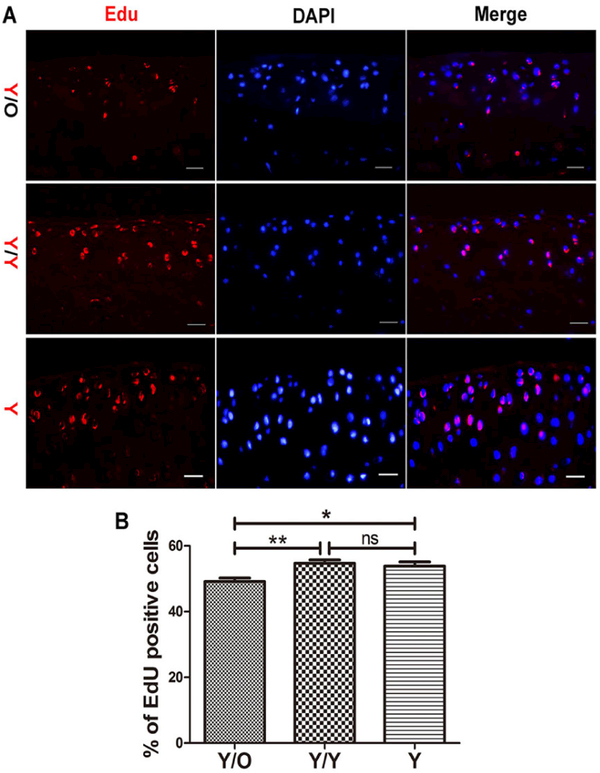
Reduced number of EdU-positive chondrocytes in young cartilage exposed to an aged systemic environment
It was hypothesised that the loss of proteoglycan in young cartilage and the changes in Sox9 and Runx2 levels in young chondrocytes might be attributed, at least in part, to an age-related abatement in the proliferation of chondrocytes. Therefore, the contribution of an aged systemic environment to chondrocyte proliferation was investigated using EdU. Remarkably, the number of EdU-positive chondrocytes was decreased in young cartilage from the Y/O group compared with the Y/Y and Y groups (fig. 5A, B), indicating that an aged systemic environment attenuated the proliferation of chondrocytes.
Figure 5:
Young mice in the Y/O group displayed a lower percentage of EdU-positive chondrocytes. (A) Chondrocyte proliferation decreased in the Y/O group. Pictures are shown with 400 × magnification. Scale bar = 200 μm. (B) The young mice in the Y/O group had significantly lower rates of chondrocyte proliferation compared to the two other control groups (8 mice per group). One-way ANOVA with Tukey’s post-hoc test was used to assess the difference of the percentage of EdU-positive chondrocytes among three groups.* p <0.05, ** p <0.01. Values are presented as mean ± standard error of the mean. EdU = 5-ethynyl-2’-deoxyuridine; O = old; ns, = not significant; Y = young
Discussion
This investigation has described, for the first time, the impact of heterochronic parabiosis on the articular cartilage in young mice. At 2 weeks after surgery, shared circulation was successfully established in the heterochronic parabiosis model, which was in line with the literature [19–21] and was confirmed using FMT examination (fig. 1). Noticeably, the proteoglycan content in cartilage of young mice from the Y/O group was significantly reduced (fig. 2A, C), indicating that the synthetic ability of chondrocytes was repressed in young cartilage following exposure to an aged systemic environment. Although the histological scores tended to be higher in young mice from the Y/O group, the difference in OARSI scores among the three groups did not reach statistical significance (fig. 2A, D), and the effect of aged circulation on the young articular cartilage in heterochronic parabiosis was not apparent upon radiographic inspection at 16 weeks after surgery (fig. 2B, E). In view of the observation that the pathology of cartilage degeneration tends to progress rapidly from a loss of proteoglycan, to mild fibrillation, then focal, broader, and full-thickness loss of noncalcified cartilage [17], we speculate that articular cartilage in young mice from the heterochronic parabiosis model at 16 weeks after surgery begins to undergo pathological changes characteristic of OA due to the loss of proteoglycan as an early sign of the pathological process of OA.
There was a significant downregulation in the expression of Sox9 mRNA and a significant enhancement of Runx2 mRNA in young cartilage from the Y/O group (fig. 4D, E), similar to the immunohistochemical staining results (fig. 4). On the other hand, there were no significant differences in the mRNA and protein expression of Col2, Col10 and MMP13 in young cartilage from the Y-O group compared with the two other groups (fig. 4A, B, C).
Sox9 is a key transcription factor in chondrogenesis and is crucial for chondrocyte differentiation, cartilage formation and maintenance of articular cartilage after birth [22–24]. The histological manifestation of proteoglycan decrease and reduced Sox9 expression in the articular cartilage of young mice from the Y/O group was in line with the study showing that mice devoid of Sox9 did not have obvious OA; however, loss of proteoglycan was histologically evident in cartilaginous tissues [22]. Runx2 induces chondrocyte hypertrophy and promotes the initiation and progression of OA [25]. An increase in Runx2 expression is often associated with reduced Sox9 [26]. Col2 is an important component for maintaining the extracellular matrix and cartilage integrity, and catabolic enzymes such as MMP13 degrade the extracellular matrix during the development of primary OA [2, 27, 28]. Structural damage to cartilage is brought about by increased activity of catabolic enzymes and reduced anabolic synthesis.
The results of Col2, Col10 and MMP13 expression analysis revealed no obvious changes in young cartilage from the Y/O group. This might explain why histological and radiographic examination failed to show significant structural deterioration. One possible explanation for this phenomenon is that young chondrocytes did not suffer extensive damage from exposure to the aged systemic milieu because the 12-month-old mice were not old enough, or the duration of circulatory exchange between the young mouse and old mouse was not long enough. For future studies, older mice will be selected for further investigations of the effect of an aged systemic environment on young articular cartilage. The results presented above suggested that chondrocytes in young cartilage from the heterochronic parabiosis model were indeed adversely affected. We speculate that the observed changes of PG, Sox9 and Runx2 in chondrocytes of young mice in the heterochronic parabiosis model might be related to chondrocyte proliferation.
We further quantified chondrocyte proliferation using EdU, which accurately reflects cell proliferation. The presence of more EdU-positive cells indicates increased cell proliferation. Interestingly, we found that the percentage of EdU-positive chondrocytes in young cartilage from the Y/O group was significantly lower than in the Y/Y and Y groups. The number of EdU-positive chondrocytes in young cartilage from the Y/Y and Y groups showed no statistically significant difference, indicating that the change was not due to surgery (fig. 3). This observation showed that chondrocyte proliferation was reduced in young mice from the heterochronic parabiosis model and suggested that changes to chondrocyte proliferation occurred before gross cartilage change during the process of OA.
In summary, our study demonstrated that an old systemic environment exerts a negative effect on the articular cartilage of young mice, including suppression of chondrocyte proliferation, a reduction in Sox9 expression and proteoglycan content. A limitation in our study is that parabiosis is only shown here as a pro-aging phenomenon, and a proteomics analysis will be applied in our next study to explore the specific pro-aging factors in old mice that are responsible for the effects found on young cartilage. To investigate the possible involvement of soluble factors, parabiosis alone would not be adequate, whereas plasma transfusions [29] could be used to study soluble factors due to removal of the cellular components. Future studies will be required to explore the use of various models (other than parabiosis) to elucidate the pro-aging factors that cause the observed effects on young cartilage.
Acknowledgments
Financial disclosure
The project was supported by the National Natural Science Foundation of China (No. 81572098, 81601949), Programme for International S&T Cooperation Projects of China (No.2015DFA33050) and by the Science Foundation for Shanxi Key Laboratory, China (szddw201601).
Appendix 1. Scoring systems
Table S1:
OARSI scoring system.
| Grade | Osteoarthritic damage |
|---|---|
| 0 | Normal |
| 0.5 | Loss of safranin-O without structural changes |
| 1 | Small fibrillations without loss of cartilage |
| 2 | Vertical clefts down to the layer immediately below the superficial layer and some loss of surface lamina |
| 3 | Vertical clefts/erosion to the calcified cartilage extending to <25% of the articular surface |
| 4 | Vertical clefts/erosion to the calcified cartilage extending to 25–50% of the articular surface |
| 5 | Vertical clefts/erosion to the calcified cartilage extending to 50–75% of the articular surface |
| 6 | Vertical clefts/erosion to the calcified cartilage extending >75% of the articular surface |
Table S2:
Scoring system for loss of proteoglycan.
| Grade | Loss of cartilage proteoglycan |
|---|---|
| 0 | Normal staining of noncalcified cartilage |
| 1 | Decreased but not complete loss of safranin-O staining over 1–100 % of the articular surface |
| 2 | Complete loss of safranin-O staining in the non-calcified cartilage extending to <25% of the articular surface |
| 3 | Complete loss of safranin-O staining in the non-calcified cartilage extending to 25–50% of the articular surface |
| 4 | Complete loss of safranin-O staining in the non-calcified cartilage extending to 50–75% of the articular surface |
| 5 | Complete loss of safranin-O staining in the non-calcified cartilage extending to >75% of the articular surface |
Footnotes
Potential competing interests
The authors declare that no competing interest exists.
References
- 1.Loeser RF. Aging and osteoarthritis. Curr Opin Rheumatol. 2011;23(5):492–6. doi: 10.1097/BOR.0b013e3283494005 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013;25(1):108–13. doi: 10.1097/BOR.0b013e32835a9428 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–69. doi: 10.1016/j.cger.2010.03.001 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):5–15. doi: 10.1016/j.berh.2014.01.004 PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Komori T Cell death in chondrocytes, osteoblasts, and osteocytes. Int J Mol Sci 2016;17(12):2045. doi: 10.3390/ijms17122045 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–34. doi: 10.1002/jcp.21258 PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Rahmati M, Nalesso G, Mobasheri A, Mozafari M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30. doi: 10.1016/j.arr.2017.07.004 PubMed. [DOI] [PubMed] [Google Scholar]
- 8.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–10. doi: 10.1126/science.1144090 PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4. doi: 10.1038/nature10357 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12(3):525–30. doi: 10.1111/acel.12065 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggel A, Coray TW. A revival of parabiosis in biomedical research. Swiss Med Wkly. 2014;144:w13914. doi: 10.4414/smw.2014.13914 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan MF, Ferguson CM, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64(3):705–17. doi: 10.1002/art.33388 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto K, Shishido T, Masaoka T, Imakiire A. Morphological studies on the ageing and osteoarthritis of the articular cartilage in C57 black mice. J Orthop Surg (Hong Kong). 2005;13(1):8–18. doi: 10.1177/230949900501300103 PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659–63. doi: 10.1038/nm.3569 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SW, Wei XC, Zhou JM, Zhang J, Li K, Qian C, et al. Identification of α 2 -Macroglobulin as a Master Inhibitor of Cartilage-Degrading Factors That Attenuates the Progression of Posttraumatic Osteoarthritis. Arthritis Rheumatol. 2014;66(7):1843–53. doi: 10.1002/art.38576 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Wei X, Gao J, Zhao Y, Zhao Y, Guo L, et al. Intra-articular injection of cross-linked hyaluronic acid-dexamethasone hydrogel attenuates osteoarthritis: An experimental study in a rat model of osteoarthritis. Int J Mol Sci. 2016;17(12):411. doi: 10.3390/ijms17040411 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025 PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Wei F, Zhou J, Wei X, Zhang J, Fleming BC, Terek R, et al. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2012;20(7):755–63. doi: 10.1016/j.joca.2012.03.010 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297(5590):2256–9. doi: 10.1126/science.1074807 PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Gibney BC, Chamoto K, Lee GS, Simpson DC, Miele LF, Tsuda A, et al. Cross-circulation and cell distribution kinetics in parabiotic mice. J Cell Physiol. 2012;227(2):821–8. doi: 10.1002/jcp.22796 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellano JM, Palner M, Li SB, Freeman GM, Jr, Nguyen A, Shen B, et al. In vivo assessment of behavioral recovery and circulatory exchange in the peritoneal parabiosis model. Sci Rep. 2016;6(1):29015. doi: 10.1038/srep29015 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22(1):85–9. doi: 10.1038/8792 PubMed. [DOI] [PubMed] [Google Scholar]
- 23.Chavez RD, Coricor G, Perez J, Seo HS, Serra R. SOX9 protein is stabilized by TGF-β and regulates PAPSS2 mRNA expression in chondrocytes. Osteoarthritis Cartilage. 2017;25(2):332–40. doi: 10.1016/j.joca.2016.10.007 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry SP, Liang S, Akdemir KC, de Crombrugghe B. The postnatal role of Sox9 in cartilage. J Bone Miner Res. 2012;27(12):2511–25. doi: 10.1002/jbmr.1696 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12(12):963–73. doi: 10.1016/j.joca.2004.08.008 PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Orfanidou T, Iliopoulos D, Malizos KN, Tsezou A. Involvement of SOX-9 and FGF-23 in RUNX-2 regulation in osteoarthritic chondrocytes. J Cell Mol Med. 2009;13(9B):3186–94. doi: 10.1111/j.1582-4934.2008.00678.x PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacitharan PK, Vincent TL. Cellular ageing mechanisms in osteoarthritis. Mamm Genome. 2016;27(7–8):421–9. doi: 10.1007/s00335-016-9641-z PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Kraan PM, van den Berg WB. Osteoarthritis in the context of ageing and evolution. Loss of chondrocyte differentiation block during ageing. Ageing Res Rev. 2008;7(2):106–13. doi: 10.1016/j.arr.2007.10.001 PubMed. [DOI] [PubMed] [Google Scholar]
- 29.Shytikov D, Balva O, Debonneuil E, Glukhovskiy P, Pishel I. Aged mice repeatedly injected with plasma from young mice: a survival study. Biores Open Access. 2014;3(5):226–32. doi: 10.1089/biores.2014.0043 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]