Abstract
Background/purpose
The combined contributions of oncogenes and tumor suppressor genes toward carcino-genesis remain poorly understood. Elucidation of cancer gene cooperativity can provide new insights leading to more effective use of therapies.
Experimental design/Methods
We used somatic cell genome editing to introduce singly and in combination PIK3CA mutations (E545K or H1047R) with TP53 alterations (R248W or knockout), to assess any enhanced cancerous phenotypes. The non-tumorigenic human breast epithelial cell line, MCF10A, was used as the parental cell line, and resultant cells were assessed via various in vitro assays, growth as xenografts, and drug sensitivity assays using targeted agents and chemotherapies.
Result
Compared to single-gene-targeted cells and parental controls, cells with both a PIK3CA mutation and TP53 alteration had increased cancerous phenotypes including cell proliferation, soft agar colony formation, aberrant morphology in acinar formation assays, and genomic heterogeneity. Cells also displayed varying sensitivities to anti-neoplastic drugs, although all cells with PIK3CA mutations showed a relative increased sensitivity to paclitaxel. All cell lines remained non-tumorigenic.
Conclusions
This cell line panel provides a resource for further elucidating cooperative genetic mediators of carcinogenesis and response to therapies.
Keywords: Breast cancer, TP53, PIK3CA, Tumor heterogeneity
Introduction
TP53 and PIK3CA are the two most frequently mutated genes in primary breast cancer and have the highest rate of co-occurrence among the top five mutated genes [1]. In a prior study, 120 primary breast tumors were sequenced and demonstrated that the frequency of mutations in both TP53 and PIK3CA was approximately 5.3% [1]. Although our lab has studied these mutations in isolation using isogenic cell lines derived through genome editing [2–6], several of our recent reports have described oncogene cooperativity when two oncogenic mutations are introduced within an isogenic background in both cancerous and non-cancerous cell lines [6, 7]. However, the cooperative effects of tumor suppressor genes with oncogenes in this context have not been as well studied.
Cancer is a complex and multifaceted disease with fluctuating phenotypes due to both inter- and intratumoral heterogeneity. This inherent variability is due to genomic instability, which generates somatic mutations/alterations that can lead to clonal expansion due to selective pressures such as the microenvironment and anti-cancer therapies [8]. In this study, we paired one of two PIK3CA hotspot mutations, either E545K or H1047R, with either TP53 gene knockout or a common TP53 missense mutation, R248W, in the non-tumorigenic human breast epithelial cell line, MCF10A. We demonstrate that the incorporation of a TP53 alteration with a PIK3CA mutation results in more aggressive cancerous features in vitro, and an increase in genomic instability with resultant cellular heterogeneity, more accurately reflecting the situation in human cancers. In addition, we utilized this panel to show that despite clonal heterogeneity, cells harboring a PIK3CA mutation demonstrated an increased sensitivity to the chemotherapeutic agent paclitaxel. This panel of closely related cell lines provides a tool for modeling tumor heterogeneity and allows us to ascribe phenotypic differences and drug responses to a given mutation or set of mutations, despite clonal differences arising from genomic instability.
Materials and methods
Cell culture
Cell lines were grown in 5% CO2 at 37 °C. The non-transformed human breast epithelial cell line MCF10A [9] and its derivatives were grown in DMEM/F12 (1:1) supplemented with 5% horse serum (Hyclone), EGF at 20 ng/ml, insulin at 10 μg/ml, hydrocortisone at 0.5 μg/ml, and cholera toxin at 0.1 μg/ml. Cell lines with gene-targeted PIK3CA mutations were grown in similar DMEM/F12 media without EGF since these cell lines are EGF independent [5]. All supplements were purchased from Sigma-Aldrich unless otherwise noted.
Gene targeting of PIK3CA mutations
Targeted knockin of PIK3CA exon 9 (E545K) and exon 20 (H1047R) mutations into MCF10A cells already carrying a heterozygous TP53 R248W knockin mutation [10] or MCF10A cells carrying a homozygous TP53 deletion in exon 2 [11] was conducted as previously described [5, 12].
Cell proliferation assays
Exponentially growing cells were washed with Hanks’ Buffered Saline Solution (HBSS) and seeded at a density of 1 × 103 cells/ml in six-well plates. Cell proliferation assays were performed in DMEM/F12 containing 1% charcoal dextran-treated fetal bovine serum with remaining components identical to growth media except that EGF was supplemented at 20, 0.2, or 0 ng/ml as indicated. At days 0, 3, 6, 9, and 12, cells were counted using a Beckman Coulter Vi-Cell XR counter. All cell lines were counted in triplicate.
Matrigel acinar formation assays
Five thousand cells were seeded into eight-well chamber slides containing a solid base layer of Growth Factor Reduced Matrigel (BD Biosciences). Cells were cultured in a 2% Matrigel in DMEM/F12 cell proliferation medium with 1% charcoal dextran-treated fetal bovine serum with varying EGF conditions of 20, 2 or 0 ng/ml as indicated. The assay media was changed every 4 days for 2 weeks.
Anchorage-independent growth in semisolid medium
2 × 104 exponentially growing cells were cast in 3 ml of top-layer medium composed of assay media of varying EGF conditions as described above, and 0.4% UltraPure Agarose (Invitrogen). This mixture was poured on top of a 2 ml bottom layer containing 0.6% agarose in six-well tissue culture plates. Assay media were added to the wells once a week.
Centrosome immunofluorescence
Cells were assayed for centrosome numbers as previously described [4, 10]. Briefly, cells were seeded at 1 × 105 density into eight-well chamber slides and allowed to grow for 2 days. Slides were washed, fixed, and then incubated in ice-cold acetone for 1 min at room temperature. Following several washes, slides were treated with 5% goat serum in 0.3% Triton X-100 for 2 h at room temperature. Slides were then treated with a 1:1000 dilution of rabbit anti-γ-tubulin primary antibody (Sigma, T5192) for 1 h at room temperature. Slides were washed and a 1:100 dilution of goat anti-rabbit IgG-Alexa488 secondary antibody (Life, A11034) was added to the slides for 25 min at room temperature. The slides were then washed and stained with a 1:200 dilution of Texas Red-X-Conjugate Wheat Germ Agglutinin (Life, W21405) for 5 min followed by several washes. A 1× DAPI stain (Sigma, D9542-SMG) was added for 1 min. Slides were mounted with Prolong Gold and imaged using a Nikon fluorescence microscope and NIS-Elements BR 2.30 imaging program.
Fluorescence in situ hybridization (FISH)
Cells were seeded at 3 × 104 density into eight-well chamber slides and allowed to grow to 90% confluency. Cells were fixed overnight in a 10% buffered formalin solution and allowed to dry at bench top the following day. Slides were then treated with 2N HCl for 20 min and treated with the Vysis Pretreatment Kit I as previously described [10]. Briefly, slides were washed with a 2× SSC buffer and incubated in the provided pretreatment buffer at 80 °C for 30 min. Slides were rinsed in dH2O and washed with 2× SSC buffer. The slides were then placed in a protease buffer at 37 °C for 8 min. Pretreated slides were then washed with 2× SSC buffer and fixed in 10% buffered formalin for 10 min. Slides were dehydrated through a series of ethanol baths and allowed to age at room temperature for up to 2 weeks. Slides were then hybridized with various Vysis probes at 95 °C for 5 min and incubated at 37 °C for 48 h. Slides were treated with a 0.3% NP-40 (IGEPAL) at 75 °C, counter stained with DAPI (1:10,000), and sealed with Prolong Gold (Invitrogen, P36930). Slides were imaged using a Nikon fluorescence microscope and NIS-Elements BR 2.30 imaging program.
Immunoblotting
Cells were seeded in six-well plates using EGF-free DMEM/F12 medium with 1% charcoal dextran-treated fetal bovine serum. Cells were harvested after 48 h for protein lysates. Immunoblotting was performed as previously described [7]. Briefly, whole-cell protein extracts prepared in Laemmli sample buffer were resolved by SDS-PAGE using NuPAGE gels (Invitrogen), transferred to Invitrolon PVDF membranes (Invitrogen), and probed with primary antibody followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Antibodies used for this study are listed in Table S1.
Cell cycle analysis
Previously plated cells were washed and resuspended in ice-cold PBS and added dropwise to 70% ethanol. Cells were incubated at −20 °C overnight for ethanol fixation. The following day, cells were pelleted and washed with cold phosphate buffered saline (PBS) and resuspended in propidium iodide (500 μg/ml) in 0.1% Triton in PBS. Cells were analyzed by flow cytometry.
Copy number variance and loss of heterozygosity
The Illumina Human OmniExpress Bead Chip kit (Illumina, WG-312–3001) was used to analyze cell line gDNA for global chromosomal changes, including copy number variance (CNV) and loss of heterozygosity (LOH), when compared to the parental MCF10A cell line as per the manufacturer’s protocol. Analysis was carried out as previously described [13].
Drug sensitivity assays
Preliminary dose–response curves were determined with drugs administered at various concentrations in assay media containing 2 ng/ml EGF. Equivalent concentrations of DMSO were used as vehicle controls. Cells were then analyzed on day 7 using a colorimetric sulforhodamine B (SRB) assay and cell counts as per proliferation assays described above. Drug concentrations (Table S2) were selected for observable responses and cell counting assays were carried out as described above for proliferation assays. Cells were seeded at a density of 3 × 103 cells per well into six-well plates and exposed to drugs at a concentration determined from viability curves. Cells were assayed on day 7. Each cell line was run in triplicate and compared to DMSO vehicle controls. Assays were independently performed at least three times.
In vivo xenograft assays
8- to 10-week-old female athymic nude mice (Harlan Laboratories, Indianapolis, IN) were used for in vivo assays. For xenografts, mice were injected subcutaneously in either flank with 200 μl mixture containing 2 × 106 cells in 20% PBS and 80% Growth Factor Reduced Matrigel (BD Biosciences). Tumor volumes were calculated by multiplying length, width, and height for each individual tumor. All animal experiments were performed in accordance with institutional and The National Institutes of Health Guide for the Care and Use of Laboratory Animals guidelines.
Statistical analysis
All statistical analyses were carried out using GraphPad Prism 6 software. Relative proliferation rates were analyzed by two-way ANOVA. Results from the clonogenic assay, FISH, and centrosome immunofluorescence experiments were compared to control samples using unpaired t-tests.
Results
Genome editing to create a panel of cell lines harboring both a PIK3CA mutation and TP53 alteration
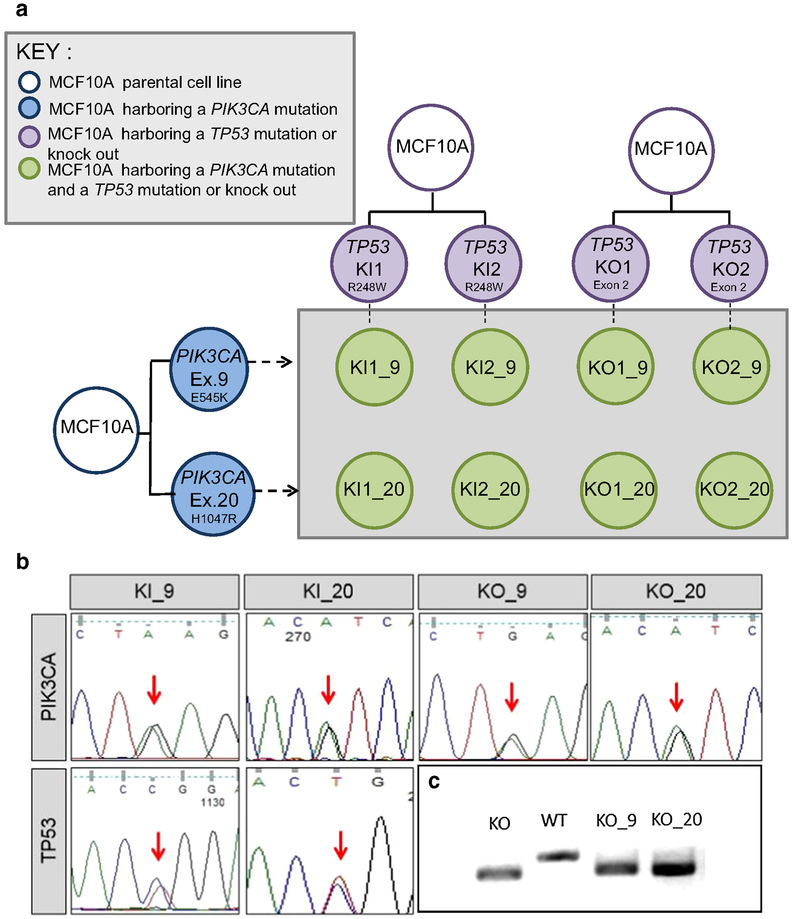
Using AAV-mediated gene targeting, the PIK3CA E545K mutation or the H1047R mutation was incorporated into MCF10A cells already containing either the TP53 R248W mutation or a homozygous deletion, both of which were previously described [10, 11]. A schematic representation of the cell line panel created and used in this study is shown in Fig. 1a. Representative Sanger sequencing traces for each set of clones are shown in Fig. 1b, as well as PCR amplicons demonstrating lack of wild-type alleles for the TP53 knockout gene-targeted clones (Fig. 1c), since sequencing cannot be performed at the deleted locus. Multiple independently derived clones for each set of mutations were created, using independently derived TP53 altered cell lines.
Fig. 1.
Derivation and confirmation of cell lines used in this study. a A panel of isogenic cell lines was developed with both a PIK3CA and TP53 alteration. Single TP53-altered cell lines shown in purple contain either a homozygous deletion/knockout (KO) or a TP53 R248W hotspot mutation/knockin (KI). TP53-altered cell lines were used as the parental cell line and were infected with AAV vectors containing either the PIK3CA E545K mutation in exon 9 (Ex. 9) or the H1047R mutation in exon 20 (Ex. 20) (blue). Double-mutant cell lines were developed (green) from independently derived parental clones. Nomenclature for the derived cell lines indicates the TP53 status and clone number followed by an underscore and the designated PIK3CA mutation. b Representative Sanger sequencing traces confirming mutations. Traces for the PIK3CA and TP53 mutation status for representative double-mutant clones are shown. Equal peaks (indicated by the red arrows) indicate 50:50 allelic ratios demonstrating that the mutation incorporated into one of the two alleles. c TP53 homozygous deletion is detected through deletion of a small portion of genomic DNA in exon 2 as detected by PCR and gel electrophoresis. The lower bands indicate a smaller DNA fragment due to the loss of the deleted DNA. KO = TP53 knockout, WT = TP53 wild-type parental control (MCF10A), KO_9 = TP53 knockout with PIK3CA E545K mutation, KO_20 = TP53 knockout with PIK3CA H1047R mutation
Cells harboring both a PIK3CA and TP53 mutation or knockout exhibit distinct growth phenotypes
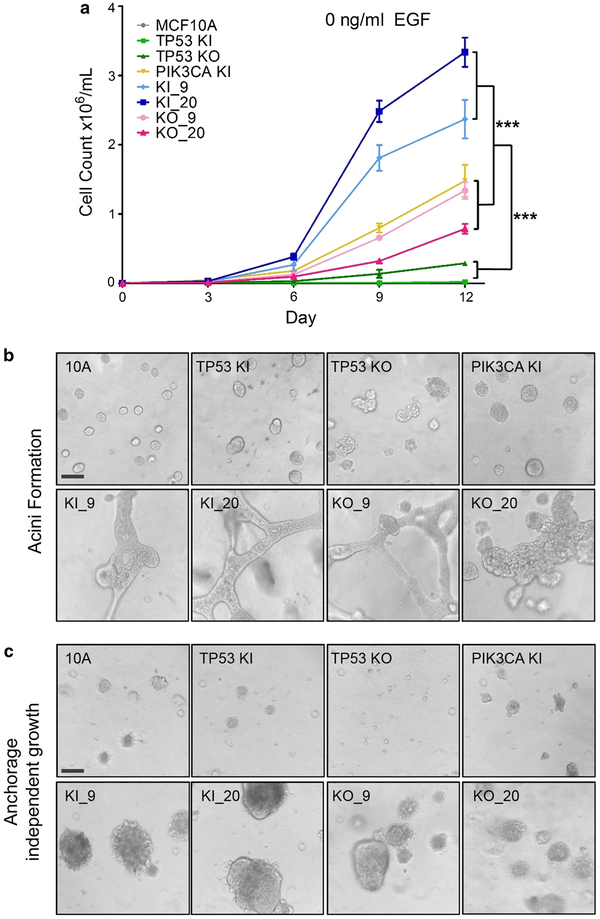
To assess growth phenotypes, proliferation assays were carried out in six-well plates in the absence of epidermal growth factor (EGF). Parental MCF10A cells require EGF to maintain normal proliferation and removal of EGF from the media leads to G1 arrest [9]. We have previously shown that knockin of a PIK3CA hotspot mutation leads to activation of the Phosphoinositide 3 (PI3) kinase pathway and EGF-independent growth [5]. As shown in Fig. 2a, in the absence of EGF, all cell lines harboring a PIK3CA mutation exhibited increased growth compared to parental MCF10A cells or single TP53 knockout or TP53 knockin R248W mutant cells (Fig. 2a). Furthermore, cell lines harboring both a TP53 knockin R248W mutation and a PIK3CA mutation showed a significant growth advantage compared to their TP53 knockout/PIK3CA mutant counterparts, although TP53 knockout/PIK3CA mutant cell lines still proliferated at approximately the same rate as single PIK3CA knockin cells (***p \ 0.001). These results confirm our earlier work and findings by others demonstrating that TP53 missense mutations may impart a gain of function compared to loss of p53 [10, 14].
Fig. 2.
Growth phenotypes of TP53/PIK3CA double-mutant cell lines. a Proliferation assays in no EGF conditions. Double mutants with TP53 KI (R248W) and PIK3CA E545K or H1047R mutations showed a growth advantage over double mutants harboring a TP53 KO mutation (***p value <0.001). Assays were performed in triplicate within each proliferation assay, and each assay was performed at least 4 times. Representative results are shown using multiple independent clones. b TP53/PIK3CA double-mutant cell lines form unique structures in Matrigel. Cells were seeded at equal densities in Matrigel as described in Sect. “Materials and methods”. All double-mutant cells (bottom panels) showed extensive protrusions and irregular morphological transformations. Assays were performedat least 3 times and results shown are representative of multiple independent clones. c TP53/PIK3CA double-mutant cell lines form large colonies in soft agar. All double-mutant cell lines were capable of colony formation in soft agar (bottom panels), while single-mutant and parental cell lines showed minimal to no colony formation (top panels). Assays were performed at least 3 times and results shown are representative of multiple independent clones. MCF10A = parental cells, TP53 KI = MCF10A with TP53 R248W knockin mutation, TP53 KO = MCF10A with TP53 knockout, PIK3CA KI = MCF10A with single PIK3CA E545K knockin, KI_9 = MCF10A with TP53 R248W knockin and PIK3CA E545K knockin, KI_20 = MCF10A with TP53 R248W knockin and PIK3CA H1047R knockin, KO_9 = MCF10A with TP53 knockout and PIK3CA E545K knockin, and KO_20 = MCF10A with TP53 knockout and PIK3CA H1047R knockin. Bars = 100 μm
Double-mutant cell lines exhibit morphological changes and anchorage-independent growth in 3D culture
The panel of cell lines was assayed in Matrigel, a three-dimensional basement membrane culture, which supports acini formation of mammary epithelial cells in vitro. It has been previously shown that the parental MCF10A cell line, TP53, and PIK3CA single-mutant cell lines form normal acini in low doses of EGF [5, 10]. However, cell lines harboring both a PIK3CA mutation and TP53 mutation or knockout showed significant morphological changes, including protrusions at the borders of acini, bridging between neighboring acini, and loss of structural integrity (Fig. 2b). These features mimicked the invasive process characteristic of cancers and suggest that the combination of TP53 alterations with PIK3CA mutations increase transformed properties of cells compared to their single-mutant or knockout counterparts.
In order to assess the effect of combined TP53/PIK3CA alterations on anchorage-independent growth, cells were grown in a soft agar colony formation assay. As with the Matrigel acinar formation assay, all cells were cultured in physiological (0.2 ng/ml) doses of EGF. In agreement with our previously published studies [5, 10], single PIK3CA mutant or TP53 mutant/knockout cells did not exhibit increased colony formation over parental controls. However, the double-mutant cell lines were capable of extensive colony formation, regardless of the PIK3CA mutation or whether cells contained TP53 knockout or knockin of the TP53 R248W mutation (Fig. 2c). These results, along with the abnormal morphology seen in Matrigel assays, confirmed that PIK3CA mutant/TP53-altered cell lines exhibited increased properties of transformation. However, xenograft assays via subcutaneous inoculation in athymic nude mice did not result in tumor formation, suggesting that the combination of PIK3CA mutations with TP53 alterations was not sufficient to fully transform MCF10A cells (data not shown).
Double-mutant cell lines harboring both a TP53 and PIK3CA alteration exhibit a higher rateof centrosome amplification
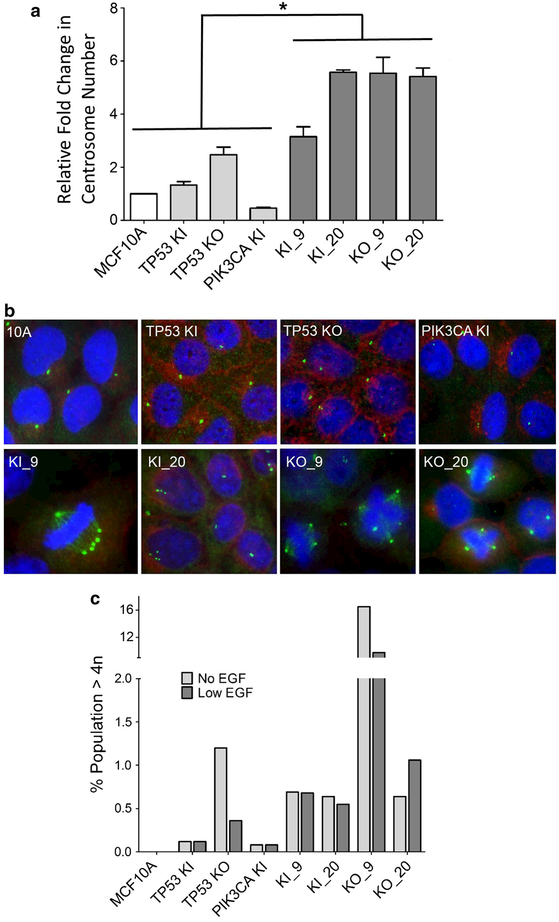
It was previously suggested that loss of functional p53 often coexists with aneuploidy, an increase in centrosomes, and ultimately an increase in genomic instability [15, 16]. However, Bunz et al. demonstrated that inactivation of p53 alone in the mismatch repair-deficient colorectal cancer cell line, HCT-116, was not sufficient for the development of aneuploidy [17]. In order to determine if PIK3CA mutations could cooperate with TP53 alterations in the development of irregular cell division possibly leading to aneuploidy, centrosome amplification was assessed using fluorescence staining and microscopy. As seen in Fig. 3a, all double-mutant cell lines experienced a significant (*p < 0.05) increase in centrosome amplification when compared to parental and single-mutant cell lines. Additionally, cell lines harboring a mutation in PIK3CA and mutation or knockout of TP53 exhibited irregular mitotic bodies due to centrosome amplification (Fig. 3b). Prior work has shown that centrosome amplification can lead to chromosomal mis-segregation and aneuploidy, and is an underlying contributor to loss of cell cycle fidelity and genomic instability [18]. Given these results, we investigated the ploidy of these cell lines using flow cytometry. As shown in Fig. 3c, cell lines with both a PIK3CA mutation and a TP53 mutation or knockout, on average, exhibited a higher percentage of cells with DNA content >4n in both physiologic levels (0.2 ng/ml) and absence of EGF (Fig. 3c). Intriguingly, TP53 knockout cell lines in the absence of EGF demonstrated an increase in polyploidy, with the KO_9 cell line exhibiting almost a tenfold higher percentage of polyploid cells. Clonal variation secondary to disruption of p53 function may explain the heterogeneous results seen in these assays.
Fig. 3.
Double-mutant cell lines exhibit centrosome amplification.a Relative fold change of average centrosome number normalized to parental MCF10A. Double mutants showed statistically significant (*p < 0.05) amplification of centrosomes relative to controls and parental cell lines. b Centrosome amplification determined by immunofluorescence. Representative images for cells are shown. Green represents immunofluorescent staining of γ-tubulin for centrosome identification, while blue identifies nuclear labeling with DAPI. Centrosome counts of one and two are considered normal.c Double mutants show an increase in polyploidy. Cells with >4n were determined using propidium iodide labeling followed by flow cytometry. Results are shown in both the absence and presence of physiologic (0.2 ng/ml) levels of EGF. MCF10A = parental cells, TP53 KI = MCF10A with TP53 R248W knockin mutation, TP53 KO = MCF10A with TP53 knockout, PIK3CA KI = MCF10A with single PIK3CA E545K knockin, KI_9 = MCF10A with TP53 R248W knockin and PIK3CA E545K knockin, KI_20 = MCF10A with TP53 R248W knockin and PIK3CA H1047R knockin, KO_9 = MCF10A with TP53 knockout and PIK3CA E545K knockin, and KO_20 = MCF10A with TP53 knockout and PIK3CA H1047R knockin
TP53 and PIK3CA alterations exhibit an elevated rate of genomic instability
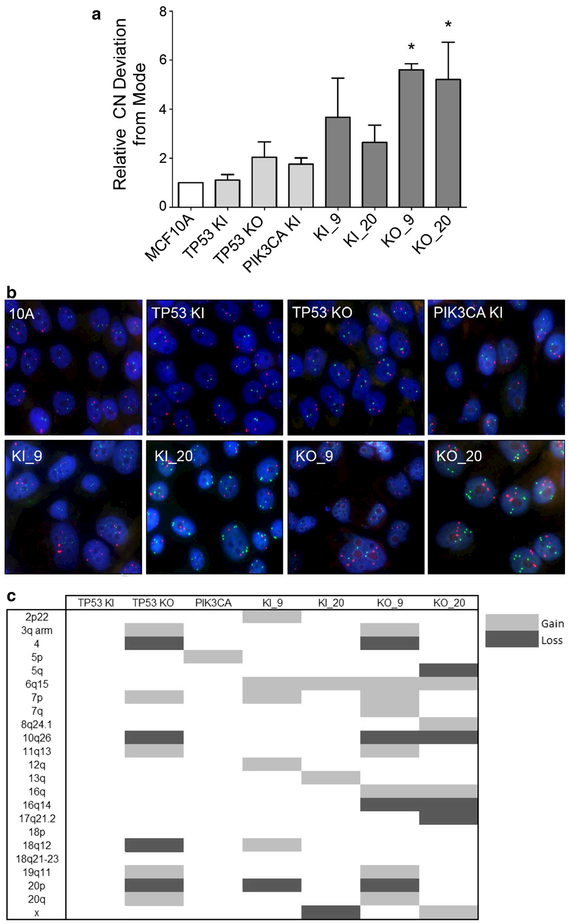
In order to determine if double mutants had increases in genomic instability, Fluorescence In Situ Hybridization (FISH) was performed on the panel of cell lines to assess how often a given gene probe differed from the modal population. Such analyses have been used previously to measure rates of genomic instability [19]. TP53/PIK3CA-altered cells showed varying degrees of increased genomic instability when compared to the parental and single-mutant cell lines. However, this increased rate was relatively small, and only statistically significant in the KO_9 and KO_20 cell lines (*p < 0.05) (Fig. 4a). To determine whether this minor increase in genomic instability could result in tumor heterogeneity, we performed copy number analysis on the panel of cell lines using the Illumina OmniExpress ChIP Array. As shown in Fig. 4c, cell lines harboring both a PIK3CA mutation and a TP53 mutation or knockout showed higher copy number gains and losses. Notably, however, TP53 knockout cells had a number of genomic alterations as we have previously described [10, 11]. The KO_9 and KO_20 clones, which are derived from the TP53 KO shown in Fig. 4c, have unique gains and losses which may be the result of single-cell cloning of the TP53 KO parental line, without contribution from the PIK3CA knockin mutation. Arguing against this is the fact that knockin of a PIK3CA mutation within the TP53 KI cell line (KI_9) also resulted in unique copy number changes relative to the parental TP53 KI or PIK3CA (E545K) cell lines. The results of these copy number analyses, combined with the data from our centrosome and FISH assays, support the notion that PIK3CA mutations may cooperate with TP53 alterations to increase the rate of genomic instability that drives tumor heterogeneity.
Fig. 4.
Double mutants have varying levels of genomic instability.a Relative copy number (CN) deviation from the modal population was determined using fluorescent in situ hybridization (FISH). Three genes (c-MYC, EGFR, BCR) were randomly selected and analyzed with FISH probes. (*p < 0.05). b Representative FISH images for each cell line. EGFR probe (red) and BCR probe (green) with DAPI staining for nucleic acids shown in blue. c CNV data were summarized in the table with representative clones for each set of mutations. All cells were normalized to parental MCF10A. MCF10A = parental cells, TP53 KI = MCF10A with TP53 R248W knockin mutation, TP53 KO = MCF10A with TP53 knockout, PIK3CA KI = MCF10A with single PIK3CA E545K knockin, KI_9 = MCF10A with TP53 R248W knockin and PIK3CA E545K knockin, KI_20 = MCF10A with TP53 R248W knockin and PIK3CA H1047R knockin, KO_9 = MCF10A with TP53 knockout and PIK3CA E545K knockin, and KO_20 = MCF10A with TP53 knockout and PIK3CA H1047R knockin
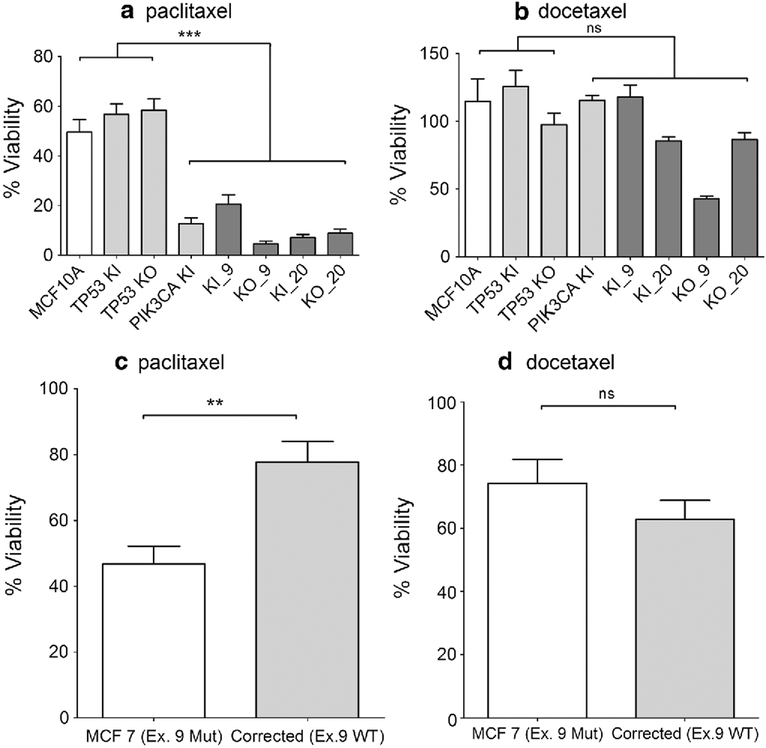
MCF10A cells harboring a PIK3CA mutation are sensitive to paclitaxel regardless of TP53 mutation status
Having established that our cell line panel had inherent genetic instability with resultant heterogeneity akin to human cancers, we next wished to determine whether any predictive markers could be associated with the efficacies of various anti-cancer therapies. In contrast to our past work using isogenic cell lines without inherent genomic instability, this panel of cell lines more closely recapitulates the situation in human cancers where driver mutations exist in all cancer cells, but genomic instability could lead to the emergence of drug-resistant clones. Although this presents opportunities to identify genetic effectors of drug resistance (i.e., a single-drug-resistant cell line/outlier could be compared genetically with the remaining drug sensitive sib clones), we chose initially to focus our efforts on drugs which demonstrated a global anti-proliferative effect across all cell lines with a given mutation. In this manner, there would be greater confidence that any genetic alteration associated with a specific drug response would be a bona fide predictive marker of sensitivity or resistance. We initially tested a number of chemotherapies relevant to breast cancer, including cisplatin, 5-FU, taxanes, and doxorubicin, and demonstrated that only the taxane, paclitaxel, showed increased sensitivity with any cell line that harbored a PIK3CA mutation (Fig. 5a) (data not shown for cisplatin and 5-FU; doxorubicin described below). As a cell line panel, the sensitivity was striking and statistically significant (p < 0.001), regardless of which PIK3CA mutation was present (E545K or H1047R), or whether TP53 was wild-type, heterozygous for R248W, or knocked out. In contrast, the synthetic taxane, docetaxel, did not demonstrate any uniform sensitivity for cells harboring PIK3CA mutations, suggesting that this effect was specific for paclitaxel (Fig. 5b). In order to determine whether these findings were recapitulated in another human breast cancer cell line, we took advantage of a previously described model that employed genome editing to “correct” the PIK3CA E545K mutation back to wild type in the estrogen receptor-positive human metastatic breast cancer cell line, MCF7 [20]. As shown in Fig. 5c and d, parental MCF7 cells with the PIK3CA E545K mutation had increased sensitivity to paclitaxel (p < 0.01), but not docetaxel, compared to MCF7 cells with wild-type PIK3CA. These results suggest that our panel of cell lines could potentially be utilized to assess predictive biomarkers of response to cytotoxic agents despite the tumor heterogeneity that exists within a patient.
Fig. 5.
PIK3CA mutations sensitize cells to paclitaxel. Cells were seeded and analyzed as described in Sect. “Materials and methods”. Results shown are representative examples of multiple independent assays. a Cells were maintained in 2.5 nM paclitaxel in assay media for 1 week (***p < 0.001). b Cells were maintained with 60 pM of docetaxel in assay media for 1 week (ns = not significant). MCF10A = parental cells, TP53 KI = MCF10A with TP53 R248W knockin mutation, TP53 KO = MCF10A with TP53 knockout, PIK3CA KI = MCF10A with single PIK3CA E545K knockin, KI_9 = MCF10A with TP53 R248W knockin and PIK3CA E545K knockin, KI_20 = MCF10A with TP53 R248W knockin and PIK3CA H1047R knockin, KO_9 = MCF10A with TP53 knockout and PIK3CA E545K knockin, and KO_20 = MCF10A with TP53 knockout and PIK3CA H1047R knockin. c Parental MCF7 (Ex. 9 MUT) cells with endogenous mutant PIK3CA E545K and Corrected (Ex.9 WT) MCF7 cells engineered with wild-type-only PIK3CA were maintained in 1.2 nM paclitaxel in assay media for 1 week (**p < 0.01). d Parental MCF7 (Ex. 9 MUT) cells with endogenous mutant PIK3CA E545K and Corrected (Ex.9 WT) MCF7 cells engineered with wild-type-only PIK3CA were maintained in 25 pM of docetaxel in assay media for 1 week (ns = not significant)
PIK3CA mutant/TP53-altered cells exhibit variable responses to drugs
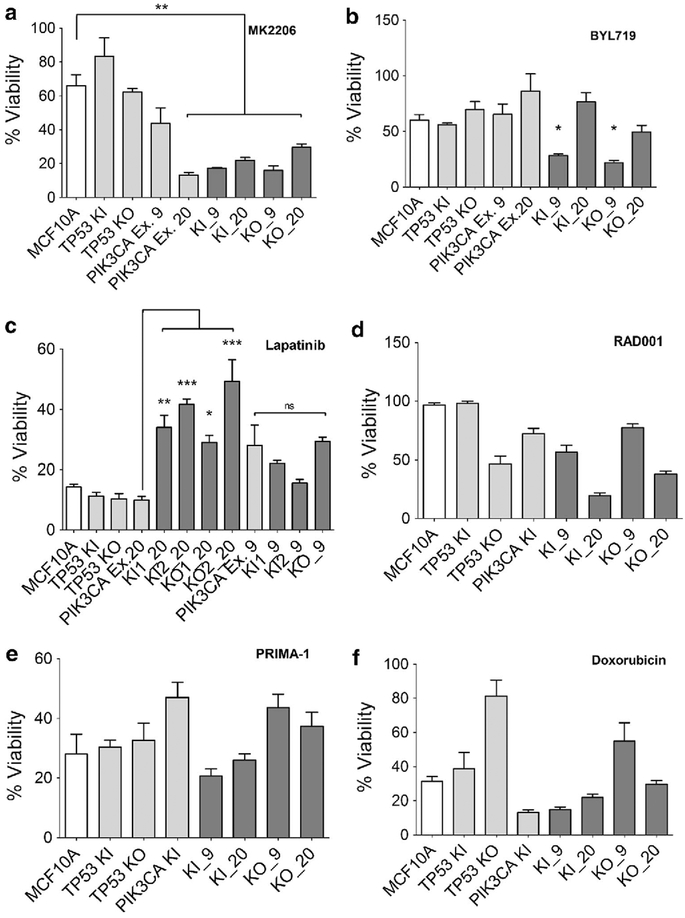
To further demonstrate the utility of our cell line panel, we next exposed cells to additional targeted and non-targeted therapies including MK2206 (AKT inhibitor), BYL719 (PI3 Kinase inhibitor), everolimus (RAD001; mTOR inhibitor), lapatinib (EGFR/HER2 inhibitor), PRIMA-1 (p53 R248W inhibitor), and the chemotherapeutic agent, doxorubicin. In contrast to the prior results with paclitaxel and PIK3CA mutations, all these agents had variable responses across the panel of cell lines. For example, the AKT inhibitor MK2206 did show significant growth inhibitory changes in the majority of cell lines harboring a PIK3CA mutation (**p < 0.01) relative to parental MCF10A and cell lines harboring a single TP53 alteration. However, there was a notable exception in cell lines harboring a single PIK3CA E545K (Ex. 9) mutation (Fig. 6a). Interestingly, the PI3 kinase inhibitor BYL719 did not show consistent results in terms of increased sensitivity in cells harboring a PIK3CA mutation, although notably cells with both a PIK3CA E545K mutation and TP53 alteration appeared to be most sensitive to this compound relative to parental MCF10A (Fig. 6b) (*p < 0.05).
Fig. 6.
Mutational status and variable responses to therapies. Cells were seeded and analyzed as described in Sect. “Materials and methods”. Results shown are representative examples of multiple independent clones and all assays were performed at least three times. Percentage of viable cells shown after 7 days in culture with a MK2206 at 1.5 uM, b BYL719 at 1.5 uM,c lapatinib at 250 nM,d everolimus (RAD001) at250 nM, e PRIMA-1 at 5 uM, and f doxorubicin at 15 nM. MCF10A = parental cells, TP53 KI = MCF10A with TP53 R248W knockin mutation, TP53 KO = MCF10A with TP53 knockout, PIK3CA KI and PIK3CA Ex.9 = MCF10A with single PIK3CA E545K knockin, PIK3CAEx.20 = MCF10A with single PIK3CA H1047R knockin, KI_9 = MCF10A with TP53 R248W knockin and PIK3CA E545K knockin,KI_20 = MCF10A with TP53 R248W knockin and PIK3CA H1047R knockin,KO_9 = MCF10A with TP53 knockout and PIK3CA E545K knockin, andKO_20 = MCF10A with TP53 knockout and PIK3CA H1047R knockin. (*p < 0.05,**p < 0.01, ***p < 0.001)
Other targeted agents revealed responses that may be mutation-specific. For example, cells harboring both a PIK3CA H1047R and TP53 alteration were resistant to the dual EGFR/HER2 kinase inhibitor lapatinib relative to single PIK3CA H1047R (Ex. 20) cells. (*p < 0.05, **p < 0.01 and ***p < 0.001) (Fig. 6c). However, cells with both a TP53 alteration and a PIK3CA E545 K (Ex. 9) mutation did not show relative resistance to lapatinib compared to cells with only a PIK3CA E545K mutation. This is consistent with recent reports that the mechanisms of activation of individual PIK3CA mutants are likely distinct, suggesting drug sensitivity and resistance may also depend on the particular mutation present within a given oncogene [21–23].
For the remaining agents, everolimus, PRIMA-1, and doxorubicin, no consistent patterns were observed in our cell line panel (Fig. 6d–f). Although for each drug there were cell lines that appeared sensitive to these agents, there was no uniformity between clones precluding the ability to ascribe genotype to phenotype. Clonal variability/heterogeneity likely accounts for these differing results, and in many ways this mirrors clinical mixed responses seen in patients with multiple sites of metastases undergoing systemic therapies. Further analysis of clones exhibiting increased resistance or susceptibility may identify additional genetic alterations that could lead to a better understanding of the mechanisms of drug sensitivity and resistance.
Discussion
Although numerous studies have examined the contributions of PIK3CA and TP53 toward carcinogenesis and drug resistance, there have been far fewer studies evaluating the interactions of these two genes and how they may cooperate to increase hallmarks of cancer. Here, we generated and used a panel of relatively isogenic cell lines harboring a PIK3CA mutation and TP53 mutation or deletion in a non-tumorigenic human breast epithelial cell line. Our study provides evidence for oncogene/tumor suppressor gene cooperativity in breast carcinogenesis, as shown in our in vitro assays, and it also demonstrates that this cooperativity can increase genomic instability resulting in greater tumor heterogeneity.
The cell line panel generated in this study provides a platform for examining clonal differences between a set of closely related cell lines harboring the two most common mutations in breast cancer. We found that certain phenotypic changes were the cooperative result of the mutations and alterations introduced within both the PIK3CA and TP53 genes. For example, cells with both a PIK3CA mutation and TP53 alteration demonstrated a significant growth advantage compared to parental controls. Additionally, irregular acini formation and invasive growth in 3D culture were observed only in the presence of mutations/alterations in both TP53 and PIK3CA, regardless of the type of mutation or alteration. The absence of these phenotypes in the single-mutant cell lines suggests that mutations in both pathways are required to observe transformative properties in 3D culture. While the dysregulation of both the TP53 and PIK3CA pathways was enough to confer increased proliferation and irregular growth in 3D culture, this was not sufficient for growth as tumor xeno-grafts. Therefore, we can infer that additional genetic alterations are needed to produce a fully tumorigenic phenotype.
We also demonstrated that PIK3CA mutations and TP53 mutation/deletion can combine to increase genomic instability. Although it is not clear what the mechanism of this may be, there are converging nodal points between these two pathways that may contribute to this. In our hands, the increase in genomic instability was small, though reproducible, and confirmed via analysis of centrosome amplification, DNA ploidy analysis, and FISH. FISH has the advantage of measuring how often a given genomic region deviates in copy number from the modal population, thus measuring the true “rate” of instability rather than the “state” as observed with copy number analysis using a bulk population of genomic DNA. A limitation of this approach is the use of only two or three probes; however, we also showed increases in centrosome amplification in cells with both TP53 alteration and PIK3CA mutation. As demonstrated by us and others, this is a hallmark of chromosome mis-segregation and a possible mechanism leading to genomic instability [10, 24].
It is known that most cancers are initiated by several “driver” genes that are thought to be present in all progeny [25]. However, unique mutations within subclones can emerge as dominant populations at metastatic sites due to clonal selection from either the local microenvironment and/or drug resistance after therapy [26]. Our cell lines employ the stepwise introduction of PIK3CA mutations along with TP53 alterations within the same cellular background giving rise to subclones with genetic variability. This may more accurately reflect the clinical situation in any given patient. Moreover, due to their relatively isogenic nature and the known “driver” gene alterations that have been engineered within these cell lines, we believe that this cell line panel enables us to an observed phenotype to a specific genotype, e.g., paclitaxel sensitivity present in all PIK3CA mutant clones, versus phenotypes that may be due to secondary mutations arising from genomic instability, as was observed for many of the drugs tested in this study. Furthermore, due to the relatively isogenic nature of MCF10A derivatives, clones expressing unique resistant or sensitive properties could be further characterized relative to their sib cell line(s) with opposing phenotypes. We envision that whole genome, exome, and RNA seq studies could be performed to identify secondary mediators of sensitivity/resistance due to clonal heterogeneity, further increasing our understanding of the pathways that can mediate drug resistance and other cancer phenotypes.
Supplementary Material
Acknowledgments
Funding This work was supported by: The Avon Foundation (B.H.P., J.L.), and NIH CA009071 (K.C., B.H.P.), GM007309 (D.J.Z.), CA168180 (R.L.C.), CA167939 (S.C.). We would also like to thank and acknowledge the support of NIH P30 CA006973, the Sandy Garcia Charitable Foundation, the Commonwealth Foundation, The Walsh Foundation, the Santa Fe Foundation, the Breast Cancer Research Foundation, the Health Network Foundation, the ME Foundation, the Augustine Fellowship (W.B.D.), and The Robin Page/Lebor Foundation. None of the funding sources influenced the design, interpretation, or submission of this manuscript.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-017-4147-2) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest BHP is a paid member of the scientific advisory boards of Horizon Discovery, LTD, and Loxo Oncology, and has research contracts with Genomic Health, Inc and Foundation Medicine, Inc. Under separate licensing agreements between Horizon Discovery, LTD, and The Johns Hopkins University, BHP is entitled to a share of royalties received by the University on sales of products. The terms of this arrangement are being managed by the Johns Hopkins University, in accordance with its conflict of interest policies. All other authors declare no potential conflicts.
Ethical approval All animal experiments were performed in accordance with institutional and the National Institutes of Health Guide for the Care and Use of Laboratory Animals guidelines. This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Boyault S, Drouet Y, Navarro C, Bachelot T, Lasset C, Treilleux I, Tabone E, Puisieux A, Wang Q (2012) Mutational characterization of individual breast tumors: TP53 and PI3K pathway genes are frequently and distinctively mutated in different subtypes. Breast Cancer Res Treat 132(1):29–39 [DOI] [PubMed] [Google Scholar]
- 2.Abukhdeir AM, Vitolo MI, Argani P, De Marzo AM, Karakas B, Konishi H, Gustin JP, Lauring J, Garay JP, Pendleton C et al. (2008) Tamoxifen-stimulated growth of breast cancer due to p21 loss. Proc Natl Acad Sci USA 105(1):288–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cidado J, Wong HY, Rosen DM, Cimino-Mathews A, Garay JP, Fessler AG, Rasheed ZA, Hicks J, Cochran RL, Croessmann S et al. (2016) Ki-67 is required for maintenance of cancer stem cells but not cell proliferation. Oncotarget 7(5):6281–6293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochran RL, Cidado J, Kim M, Zabransky DJ, Croessmann S, Chu D, Wong HY, Beaver JA, Cravero K, Erlanger B et al. (2015) Functional isogenic modeling of BRCA1 alleles reveals distinct carrier phenotypes. Oncotarget 6(28):25240–25251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustin JP, Karakas B, Weiss MB, Abukhdeir AM, Lauring J, Garay JP, Cosgrove D, Tamaki A, Konishi H, Konishi Y et al. (2009) Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc Natl Acad Sci USA 106(8):2835–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zabransky DJ, Yankaskas CL, Cochran RL, Wong HY, Croess-mann S, Chu D, Kavuri SM, Red Brewer M, Rosen DM, Dalton WB et al. (2015) HER2 missense mutations have distinct effects on oncogenic signaling and migration. Proc Natl Acad Sci USA 112(45):E6205–E6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang GM, Wong HY, Konishi H, Blair BG, Abukhdeir AM, Gustin JP, Rosen DM, Denmeade SR, Rasheed Z, Matsui W et al. (2013) Single copies of mutant KRAS and mutant PIK3CA cooperate in immortalized human epithelial cells to induce tumor formation. Cancer Res 73(11):3248–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGranahan N, Swanton C (2015) Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 27(1):15–26 [DOI] [PubMed] [Google Scholar]
- 9.Soule HD, Maloney TM, Wolman SR, Peterson WD Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC (1990) Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res 50(18):6075–6086 [PubMed] [Google Scholar]
- 10.Croessmann S, Wong HY, Zabransky DJ, Chu D, Mendonca J, Sharma A, Mohseni M, Rosen DM, Scharpf RB, Cidado J et al. (2015) NDRG1 links p53 with proliferation-mediated centrosome homeostasis and genome stability. Proc Natl Acad Sci USA 112(37):11583–11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss MB, Vitolo MI, Mohseni M, Rosen DM, Denmeade SR, Park BH, Weber DJ, Bachman KE (2010) Deletion of p53 in human mammary epithelial cells causes chromosomal instability and altered therapeutic response. Oncogene 29(33):4715–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konishi H, Lauring J, Garay JP, Karakas B, Abukhdeir AM, Gustin JP, Konishi Y, Park BH (2007) A PCR-based high-throughput screen with multiround sample pooling: application to somatic cell gene targeting. Nat Protoc 2(11):2865–2874 [DOI] [PubMed] [Google Scholar]
- 13.Mohseni M, Cidado J, Croessmann S, Cravero K, Cimino-Mathews A, Wong HY, Scharpf R, Zabransky DJ, Abukhdeir AM, Garay JP et al. (2014) MACROD2 overexpression mediates estrogen independent growth and tamoxifen resistance in breast cancers. Proc Natl Acad Sci USA 111(49):17606–17611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T (2004) Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119(6):847–860 [DOI] [PubMed] [Google Scholar]
- 15.Hollstein M, Sidransky D, Vogelstein B, Harris CC (1991) p53 mutations in human cancers. Science 253(5015):49–53 [DOI] [PubMed] [Google Scholar]
- 16.Hartwell L (1992) Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell 71(4):543–546 [DOI] [PubMed] [Google Scholar]
- 17.Bunz F, Fauth C, Speicher MR, Dutriaux A, Sedivy JM, Kinzler KW, Vogelstein B, Lengauer C (2002) Targeted inactivation of p53 in human cells does not result in aneuploidy. Cancer Res 62(4):1129–1133 [PubMed] [Google Scholar]
- 18.Chan JY (2011) A clinical overview of centrosome amplification in human cancers. Int J Biol Sci 7(8):1122–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lengauer C, Kinzler KW, Vogelstein B (1997) Genetic instability in colorectal cancers. Nature 386(6625):623–627 [DOI] [PubMed] [Google Scholar]
- 20.Beaver JA, Gustin JP, Yi KH, Rajpurohit A, Thomas M, Gilbert SF, Rosen DM, Ho Park B, Lauring J (2013) PIK3CA and AKT1 mutations have distinct effects on sensitivity to targeted pathway inhibitors in an isogenic luminal breast cancer model system. Clin Cancer Res 19(19):5413–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao Y, Wang C, Cao B, Hirsch BM, Song J, Markowitz SD, Ewing RM, Sedwick D, Liu L, Zheng W et al. (2013) Gain of interaction with IRS1 by p110alpha-helical domain mutants is crucial for their oncogenic functions. Cancer Cell 23(5):583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair BG, Wu X, Zahari MS, Mohseni M, Cidado J, Wong HY, Beaver JA, Cochran RL, Zabransky DJ, Croessmann S et al. (2015) A phosphoproteomic screen demonstrates differential dependence on HER3 for MAP kinase pathway activation by distinct PIK3CA mutations. Proteomics 15(2–3):318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Renuse S, Sahasrabuddhe NA, Zahari MS, Chaerkady R, Kim MS, Nirujogi RS, Mohseni M, Kumar P, Raju R et al. (2014) Activation of diverse signalling pathways by oncogenic PIK3CA mutations. Nat commun 5:4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganem NJ, Godinho SA, Pellman D (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460(7252):278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelstein B, Kinzler KW (2004) Cancer genes and the pathways they control. Nat Med 10(8):789–799 [DOI] [PubMed] [Google Scholar]
- 26.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P et al. (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366(10):883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.