Abstract
Background
The authors clarified the causal mechanisms underlying the high prevalence of dental disease encountered in people who habitually use methamphetamine (meth).
Methods
Using a stratified sampling approach, the authors conducted comprehensive oral examinations and psychosocial assessments for 571 study participants who used meth. Three calibrated dentists, who used National Health and Nutrition Examination Survey (NHANES) protocols, characterized the study participants’ dental disease. The authors also collected data related to study participants’ history of meth use and other attributes linked to dental disease.
Results
Study participants who used meth manifested higher rates of xerostomia and caries experience compared with NHANES control participants. Participants who used meth had a higher level of daily consumption of sugary beverages compared with NHANES control participants. Smoking meth did not increase caries experience over other modes of intake. Dental hygiene was a significant determinant of dental health outcomes.
Conclusions
Mode of intake and frequency of meth use have a minimal impact on dental health outcomes. Behaviors, such as sugary beverage consumption and poor oral hygiene, better explain dental health outcomes.
Practical Implications
Having a better understanding of the causal mechanisms of “meth mouth” sets the stage for clinicians to provide more personalized interventions and management of dental disease in people who use meth.
Keywords: Methamphetamine, caries, xerostomia, oral health, statistics
The emergence of methamphetamine (meth) as a commonly used recreational drug has brought greater scrutiny to its health effects.1 A dominant thread of the clinical and media narratives of meth’s negative health consequences has been the description of the rampant dental disease often observed in people who use meth habitually—a condition colloquially referred to as “meth mouth.” Beginning in the early 2000s, results of a growing number of case reports, case-series, and small cohort studies began to portray the meth mouth condition, frequently using extreme, graphic examples to depict the dental aftermath of meth use. The alarming dental imagery became a recurring theme in the media coverage of the meth problem,2 and it was incorporated readily into national antidrug campaigns. For example, the nationally recognized “Faces of Meth” project3 used longitudinal snapshots chronicling facial wasting and dental deterioration to emphasize and dramatize the negative aspects of meth use.4 Yet, the largely anecdotal nature of the dental reports and the general lack of an empirical basis for the claims prompted several researchers5,6 to question the scientific soundness of the meth mouth condition.
To furnish a scientific basis for the dental disease patterns reported, our group carried out systematic, case-control studies involving a broad range of people who used meth.7,8 We clarified the differential rates and patterns of dental disease in people who used meth by means of comparing them with demographically similar control participants selected from participants in the National Health and Nutrition Examination Survey (NHANES).9 A logical next step was to examine the various explanations of the mechanisms of the meth mouth condition.
One proposed mechanism for the increased dental disease is a combination of meth-induced dry mouth (xerostomia) and the frequent sipping of high-sugar soft drinks to relieve the sensation of dry mouth.10,11 An alternate explanation, known as the “contaminant theory,” is that corrosive contaminants in smoked meth cause acid erosion of the enamel12 and accelerate dental caries.13 Using a NHANES cohort as a control group, we posed the following questions:
-
▬
Do people who use meth have higher rates of xerostomia and caries experience compared with demographically similar control participants?
-
▬
Do people who use meth tend to consume more sugary drinks than demographically similar control participants?
-
▬
Does smoking meth produce greater rates of caries and xerostomia than snorting or injecting meth?
-
▬
Are oral health behaviors important dental disease determinants among people who use meth?
METHODS
We and our colleagues9 have previously described the details of the overall study design and settings. In brief, we recruited a broad community sample of 571 people from Los Angeles County in California who used meth over a 2-year period. To accomplish this, we used a stratified sampling protocol that balanced the study participants across meth use patterns. Meth use pattern categories included mild (fewer than 10 days of use in the last 30 days), moderate (10 to 15 days of use in the last 30 days), and heavy use (16 to 20 days of use in the last 30 days). The primary study sites were dental clinics associated with 2 large community health centers: the AIDS Project Los Angeles center, which primarily serves a sociodemographically diverse group of people with human immunodeficiency virus (HIV) and AIDS, and the Mission Community Hospital in Panorama City, CA, in the San Fernando Valley, which caters to a large, underserved migrant population. Approximately 69% of the study participants were recruited from the AIDS Project Los Angeles clinic and the remainder from Mission Community Hospital. We screened potential study participants and admitted them to participate in the study if we determined that they were at least 18 years old, spoke English or Spanish, had described themselves as someone who used meth (as determined by their responses to an extensive 10-year drug history questionnaire) and had used meth in the past 30 days, and were able to undergo a detailed dental examination and psychosocial assessments. We obtained written informed consent using procedures approved by the University of California, Los Angeles (UCLA) Institutional Review Board, and they obtained a certificate of confidentiality from the National Institute on Drug Abuse, National Institutes of Health, to protect participants’ privacy.
Data collection
For this study, 3 experienced dentists, who were trained and calibrated by the national trainer and reference examiner (Bruce Dye) for NHANES, conducted standardized intraoral examinations. An ongoing quality assurance program ensured procedural adherence and the maintenance of high interexaminer and intraexaminer concordances of caries assessments.14 To maximize comparability with national data sets, we chose to use assessments for dental caries status that adhered to NHANES examination protocols.15,16 We recorded the presence and absence of study participants’ teeth and assessed their dental caries at the surface level using Radike criteria17; we determined that we would assess evidence of dental caries visually, by means of using a dental explorer, for each tooth surface. Participants also completed a set of interviewer-facilitated questionnaires covering various behavioral issues, substance use, medications, and dietary attributes linked to the development of dental disease (Appendix, available online at the end of this article).
We assessed the subjective perception of dry mouth by means of using a 4-question inventory described by Fox and colleagues.18 Two of the questions were intended to probe participants’ difficulties with swallowing by means of inquiring about behaviors related to relieving or avoiding oral dryness, 1 item related to the feeling of dryness during eating, and the last item related to the amount of saliva. On the basis of the participant’s “yes” or “no” responses, we assigned each participant to the following groups: no xerostomia (0 positive responses), mild xerostomia (1 positive response), moderate xerostomia (2 positive response), or severe xerostomia (3 or more positive responses). We also collected information related to which dry mouth–inducing medications the participants had taken, including antidepressants, anti-cholinergics, diuretics, and antihypertensives.19 We assessed the impact of the dental disease and dry mouth on the participants’ oral health–related quality of life by means of using their responses to select items from the Oral Health Impact Profile.20
We evaluated dietary intake, particularly the consumption of sugary drinks, using 2 standard dietary assessment methods: the Food Frequency Questionnaire21 and the 24-hour dietary recall.22 In addition to determining the participants’ preferred mode of meth use (for example, smoking, snorting, injecting, or ingesting), we elicited a detailed history of their substance use and behaviors using the UCLA Natural History Interview.23 Finally, we verified the reliability of the drug use reports by carrying out random urine drug tests in a subset of the participants. We collected all data directly on a laptop computer using a Web-based data management system. Built-in logic and data-range checks allowed real-time data verification to protect against invalid data and to ensure the completeness of the data.
Statistical methods
We carried out statistical analyses using SAS, Version 9 (SAS Institute), and publicly available R software.
Main variables
The main outcome variables were the total numbers of decayed, missing, and filled surfaces (DMFS) and decayed, missing, and filled teeth (DMFT). For our analyses with caries experience as the outcome variable, we used DMFT for comparisons between NHANES control participants and participants who used meth, and we used DMFS for all other analyses. We analyzed the number of permanent teeth present (excluding the third molars) as a continuous variable (1–28). We determined the extent of untreated dental caries by calculating the number of decayed surfaces (DS). We also calculated a subcategory of the decayed component (Dx) to indicate the severity of the decay (whether only coronal fragments or residual root tips remained). The main exposure variable was meth use. Meth use patterns included average months of use annually, average frequency of use exceeding 15 days a month, trajectory of use, method of use, changes in method of use, age at which the person first tried meth, and patterns of meth use over the past 30 days. Control variables included age, education, sex, race and ethnicity, smoking status, HIV status, and use of xerogenic medications. Other variables of interest were severity of xerostomia, frequency of toothbrushing, frequency of dental visits, and average daily consumption of sugary beverages (soda, diet soda, coffee, and other sweetened beverages).
We used descriptive summaries to characterize the meth user population. To better understand the difference in prevalence of xerostomia, we implemented direct proportions tests to compare our population who used meth with the control participants from the NHANES 2001 cohort. We performed a propensity score analysis on the sample of participants who used meth and the NHANES control participants using a logistic regression on age, sex, dry mouth status, and race and ethnicity, yielding a sample matched for sex, dry mouth status, race and ethnicity, and age group calipers of 5 years. We used this analysis to mitigate bias attributable to case-mix differences in the distributions of these covariates in the respective samples.24,25 On this matched sample, we conducted a multiple linear regression—controlling for age, race and ethnicity, dry mouth status, and sex—to assess the effect of meth use on DMFT.
To gain insight into the effect of xerostomia on the DMFS measure within the population that used meth, we conducted a multiple linear regression controlling for the following demographic, clinical, and exposure characteristics: age, education, sugary drink intake, sex, race and ethnicity, frequency of dental visits, frequency of toothbrushing, age at which the person first tried meth, smoking status, HIV status, years of meth use (smoking, snorting, injecting, and other), trajectory of use, number of changes in method of use, average months of meth use per year, use of meth more frequently than weekend use only, frequency of meth use in last 30 days, and medication (side effect of dry mouth).
For a more granular perspective, we stratified analyses according to xerostomia level, with descriptive statistics calculated separately within these groups. We used χ2 tests of independence to compare the concentration of certain behaviors and attributes within the different xerostomia groupings. In particular, we examined the method of meth use and average daily consumption of sugary beverages. We conducted a multiple linear regression—controlling for the same demographic, clinical, and exposure characteristics as before—in each xerostomia level to better understand what behaviors were associated with higher levels of DMFS according to the reported severity of xerostomia. To inspect the impact of sugary drinks on the DMFS measure, we conducted a multiple linear regression—controlling for the same demographic, clinical, and exposure variables—with cutoffs for levels of sugary beverage consumption.
To examine the effect of method of meth intake on xerostomia, we performed a logistic regression—controlling for the same demographic, clinical, and exposure characteristics in the previously mentioned multiple linear regression—to determine whether certain methods of meth use increased the odds of reporting xerostomia. We performed a similar analysis using a multiple linear regression to inspect the relationship between smoking meth on the DMFS measure compared with other methods of administering meth. To be comprehensive, we conducted 3 separate analyses—for those who smoked meth exclusively, for those who smoked meth for at least 1 year, and for those who smoked meth for 10 or more years—to assess the impact of smoking meth on the DMFS measure relative to the other methods of use (injection, snorting, and other).
RESULTS
Participant characteristics
Most of our study sample (n ¼ 571) were men (n ¼ 460 [80.7%]), African-American and Hispanic (n = 241 [42.3%] and n = 178 [31.2%], respectively), older than 30 years (mean [standard deviation {SD}] age, 44.5 [9.6] years), and most participants had completed high school (n = 401 [70.4%]) (Table 1). A significant subset of the study participants (n = 147 [25.7%]) were HIV positive. Many of the study participants who used meth were current cigarette smokers (n = 392 [68.8%]) and took xerogenic medications (n = 192 [33.7%]). On the basis of the results of participants’ self-reported histories, 64% of the participants used meth frequently (reported using 15 or more days a month on average; data not shown) and, on average, had used meth for at least 10 of the preceding months (mean [SD], 10.1 [2.1]). The average age (SD) of meth use initiation was 28.5 (10.5) years, and smoking was the most common mode of meth use, with 53% reporting smoking as their exclusive mode (data not shown). Figure 1 captures the efficiency26 of the propensity score matching between the sample of study participants who used meth (n = 569) and the corresponding NHANES control participants (n = 596).
TABLE 1.
Sociodemographics and methamphetamine use patterns.
| VARIABLE | HIV* POSITIVE (N = 147) | HIV NEGATIVE (N = 424) | TOTAL (N = 571) |
|---|---|---|---|
| Age (Y), Mean (SD†) | 45.7 (7.9) | 44.1 (10.1) | 44.5 (9.6) |
| Education, No. (%) | |||
| Less than high school | 43 (29.3) | 126 (29.8) | 169 (29.6) |
| High school | 58 (39.5) | 143 (33.8) | 201 (35.3) |
| More than high school | 46 (31.3) | 154 (36.4) | 200 (35.1) |
| Sex, No. (%) | |||
| Male | 140 (95.2) | 320 (75.6) | 460 (80.7) |
| Female | 7 (4.8) | 103 (24.3) | 110 (19.3) |
| Race and Ethnicity, No. (%) | |||
| Hispanic | 55 (37.4) | 123 (29.1) | 178 (31.2) |
| Black | 57 (38.8) | 184 (43.5) | 241 (42.3) |
| White | 20 (13.6) | 89 (21.0) | 109 (19.1) |
| Asian or other | 15 (10.2) | 27 (6.4) | 42 (7.4) |
| Number of Sugary Drinks per Day, Mean (SD) | 3.9 (3.4) | 3.3 (3.1) | 3.5 (3.2) |
| Methamphetamine Use, Mean (SD) | |||
| Age first tried | 30.1 (10.1) | 27.9 (10.6) | 28.5 (10.5) |
| Years of smoking | 5.6 (6.55) | 6.2 (6.9) | 6.1 (6.85) |
| Years of injecting | 1.2 (3.1) | 1.2 (4.4) | 1.2 (4.1) |
| Years of snorting | 2.4 (5.6) | 2.6 (5.7) | 2.5 (5.7) |
| Years of other | 0.2 (2.4) | 0.4 (2.7) | 0.3 (2.6) |
| Average mo of use annually | 9.9 (2.2) | 10.2 (2.04) | 10.1 (2.1) |
| Times used in last 30 d | 9.8 (7.6) | 10.7 (7.4) | 10.5 (7.4) |
| Frequency of Dental Visits, No. (%) | |||
| More than once per year | 39 (26.5) | 34 (8.0) | 73 (12.8) |
| Once per year | 45 (30.6) | 100 (23.6) | 145 (25.4) |
| Once every 2 years | 22 (14.9) | 74 (17.5) | 96 (16.8) |
| Less than once every 2 years | 41 (27.9) | 215 (50.8) | 256 (44.9) |
| Frequency of Toothbrushing, No. (%) | |||
| Does not brush teeth | 3 (2.0) | 14 (3.3) | 17 (3.0) |
| Less than once per day | 17 (11.6) | 37 (8.7) | 54 (9.5) |
| Between 1 to 3 times per day | 127 (86.4) | 365 (86.3) | 492 (86.3) |
| Other | 0(0) | 7(1.7) | 7(1.2) |
| Cigarette Smoking Status, No. (%) | |||
| Never smoked | 35 (23.8) | 89 (21.0) | 124 (21.7) |
| Former smoker | 20 (13.6) | 34 (8.0) | 54 (9.5) |
| Current smoker | 92 (62.6) | 300 (70.9) | 392 (68.8) |
| Trajectory of Methamphetamine Use, No. (%) | |||
| Increasing | 22 (14.9) | 90 (21.3) | 112 (19.6) |
| Decreasing | 37 (25.2) | 106 (25.1) | 144 (25.2) |
| Maintaining | 88 (59.9) | 227 (53.7) | 315 (55.2) |
| Changes in Methamphetamine Use Method, No. (%) | |||
| One method of use only | 118 (80.3) | 319 (75.4) | 437 (76.5) |
| One change | 18 (12.2) | 61 (14.4) | 79 (13.8) |
| Two changes | 6 (4.1) | 26 (6.1) | 33 (5.8) |
| Three changes | 5 (3.4) | 17 (4.0) | 22 (3.9) |
| Xerostomic Medication, No. (%) | |||
| Yes | 69 (46.9) | 123 (29.1) | 192 (33.7) |
| No | 78 (53.1) | 300 (70.9) | 378 (66.3) |
| Dry Mouth Severity, No. (%)‡ | |||
| 0 | 40 (27.2) | 155 (36.7) | 195 (34.3) |
| 1 | 42 (28.6) | 115 (27.3) | 157 (27.6) |
| 2 | 37 (25.2) | 92 (21.8) | 129 (22.7) |
| 3 | 28 (19.0) | 60 (14.2) | 88 (15.5) |
| Caries Data, Mean (SD) | |||
| Number of decayed, missing, and filled surfaces | 49.2 (34.2) | 43.7 (33.9) | 45.2 (34.1) |
| Number of decayed, missing, and filled teeth | 14.3 (7.2) | 12.8 (7.6) | 13.2 (7.5) |
| Number of decayed surfaces | 5.9 (11.8) | 6.2 (11.8) | 6.2 (12.1) |
| Number of permanent teeth | 23.7 (5.5) | 23.7 (5.7) | 23.7 (5.7) |
HIV: Human immunodeficiency virus.
SD: Standard deviation.
0, no xerostomia (0 positive responses); 1, mild xerostomia (1 positive response); 2 moderate xerostomia (2 positive response); 3 severe xerostomia (3 or more positive responses).
Figure 1.
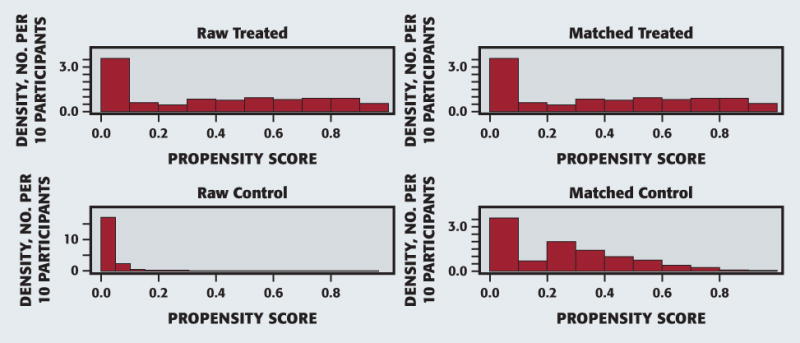
Propensity score matching.
Meth use, caries experience, and xerostomia
In contrast with the proportion of matched NHANES control participants (Table 2) with dry mouth symptoms (n = 632 [7.2%]), xerostomia was significantly more prevalent in the study participants (n = 374 [65.7%]; P < .0001). Most study participants reported mild (n = 157 [27.6%]) or moderate (n = 129 [22.7%]) xerostomia, whereas fewer participants evidenced more marked symptoms (n = 88 [15.5%]). Compared with the propensity score–matched control participants, study participants who used meth had 2.5 more teeth experiencing a caries event (95% confidence interval [CI], 1.66 to 3.39; P < .001). Although dry mouth status was a significant contributor to the severity of the dental caries (as measured by the number of DMFS), the prevalence of dental caries (as measured by the number of DMFT) did not differ significantly between the xerostomia severity levels. The xerostomia severity groups were similar in all sociodemographic and behavioral (dietary and dental) terms. Toothbrushing frequency contributed significantly to the severity of dental caries in each xerostomia severity group. The level of education and use of xerostomic medications (Table 3) varied significantly between xerostomia severity groups (P < .001). Study participants who had higher levels of xerostomia had fewer years of education and reported a higher level of medication use. In addition, years of meth use for each intake mode (by smoking, snorting, injecting, and other) were not significantly different between xerostomia groups nor were the frequency of sugary beverage consumption (data not shown).
TABLE 2.
NHANES* covariates.
| VARIABLE | NHANES (N = 8,917) |
|---|---|
| Age (Y), Mean (SD†) | 45.1 (15.9) |
| Sex, No. (%) | |
| Male | 5,047 (56.6) |
| Female | 3,870 (43.4) |
| Race and Ethnicity, No. (%) | |
| Hispanic | 2,624 (29.4) |
| Black | 2,192 (24.6) |
| White | 3,752 (42.1) |
| Asian or other | 349 (3.9) |
| Number of Sugary Drinks per Day, Mean (SD) | 0.34 (0.4) |
| Dry Mouth Severity, No. (%)‡ | |
| 0 | 8,285 (92.9) |
| 1 | 492 (5.5) |
| 2 | 108 (1.2) |
| 3 | 32 (0.3) |
| Caries Data, Mean (SD) | |
| Number of decayed, missing, and filled teeth | 9.5 (7.9) |
| Number of permanent teeth | 24.6 (7.2) |
NHANES: National Health and Nutrition Examination Survey.
SD: Standard deviation.
0, no xerostomia (0 positive responses); 1, mild xerostomia (1 positive response); 2 moderate xerostomia (2 positive response); 3 severe xerostomia (3 or more positive responses).
TABLE 3.
Xerostomia versus education and medication.
| DRY MOUTH SEVERITY LEVEL* | EDUCATION LEVEL | XEROSTOMIC MEDICATION USE | |||
|---|---|---|---|---|---|
| Less Than High School, No. | High School, No. | More Than High School, No. | No | Yes | |
| 0 | 44 | 65 | 86 | 152 | 43 |
| 1 | 43 | 63 | 51 | 103 | 54 |
| 2 | 53 | 34 | 42 | 70 | 59 |
| 3 | 29 | 38 | 21 | 52 | 36 |
0, no xerostomia (0 positive responses); 1, mild xerostomia (1 positive response); 2 moderate xerostomia (2 positive response); 3 severe xerostomia (3 or more positive responses).
Meth use and sugary drinks
Compared with control participants from the NHANES cohort who averaged 0.3 sugary drinks a day (Table 2), study participants who used meth consumed an average of 3.5 sugary drinks a day (Table 1). Even after adjusting for outliers, the median consumption of 2.7 sugary drinks a day in the study participants was significantly higher than the NHANES cohort (P < .001). On further analysis, we noted that consumption of at least 2 sugary beverages a day, compared with more infrequent consumption, had a significant impact on the caries experience (95% CI, 3.75 to 15.24; P < .001). More exaggerated comparisons (increased thresholds for volume of sugary beverages consumed daily) yielded little difference in expected caries experience.
Mode of meth use and dental caries rates and xerostomia
The logistic regression, controlling for age, race and ethnicity, dry mouth status, and sex, did not indicate any differences in participants’ likelihood of reporting symptoms of xerostomia among the different modes or patterns of meth use. Smoking meth did not significantly increase the odds of having xerostomia over the other modes of use (snorting, injecting, or other). This finding was true even for study participants who reported smoking meth exclusively or for extended periods (10 or more years). We found similar results in the overall multiple linear regression, which indicated a lack of significance between the methods of use and caries experience. We again found similar results for more exaggerated method of use indicators, which included smoking meth exclusively or for extended periods (10 or more years). In addition, the only difference in level of DMFS between methods of meth use was the “other” category, which increased the expected number of DMFS. These results differed from previous analyses, owing to the new definitions for meth use types and the covariates for which the overall multiple linear regression controlled.9 In addition, we investigated using DMFT so that comparisons with NHANES control participants were possible, and we found that study participants 35 years or younger averaged 6.9 DMFT, those who were 36 to 54 years averaged 8.03 DMFT, and 55 years or older averaged 17.01 DMFT, regardless of the method or the reported frequency of meth use (Figure 2). Levels of DMFT were all significantly different between age groups (P < .0001). However, as seen in Table 4, the number of years of meth use increased with participants’ age and were significantly different between the 35-years-or-younger and the 36-to-54-years groups for overall years of use as well as smoking, injecting, and snorting meth individually (P < .0001). However, there was no statistical difference when comparing years of use between the 36-to-54-years and 55-years-or-older groups. There was a significant, but weak, correlation between the number of years smoked and the cumulative caries index, DMFT (r = 0.099; 95% CI, 0.018 to 0.18; P = .02).
Figure 2.
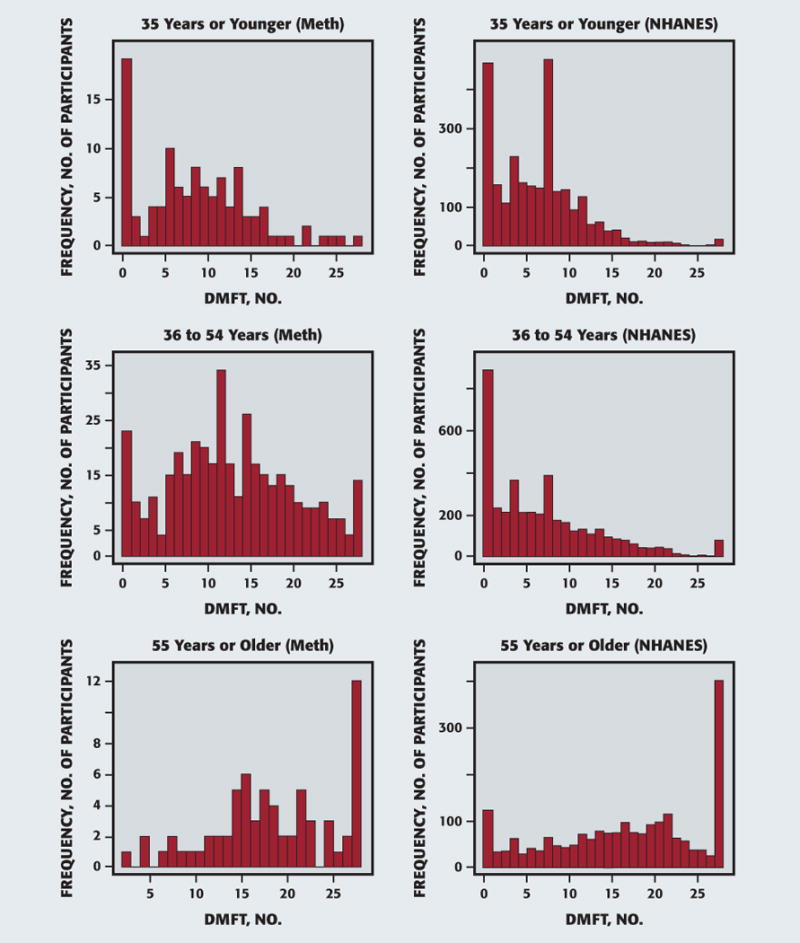
Decayed, missing, and filled teeth by age. DMFT: Decayed, missing, and filled teeth. Meth: Methamphetamine. NHANES: National Health and Nutrition Examination Survey.
TABLE 4.
Years of methamphetamine use by age.
| AGE | MEAN (SD*) | TRIMMED MEAN (MOST EXTREME 10% REMOVED) | MAXIMUM† |
|---|---|---|---|
| 35 Y or Younger (n = 110) | 5.78 (4.10) | 5.3 | 18.0 |
| Years of smoking methamphetamine | 3.69 (3.86) | 3.1 | 17.0 |
| Years of injecting methamphetamine | 0.64 (2.02) | 0.1 | 11.5 |
| Years of snorting methamphetamine | 1.35 (2.90) | 0.6 | 17.0 |
| Years of other method of using methamphetamine | 0.10 (0.70) | 0 | 6.7 |
| 36 to 54 Y (n = 410) | 10.88 (7.90) | 10.0 | 38.5 |
| Years of smoking methamphetamine | 6.51 (6.95) | 5.4 | 33.0 |
| Years of injecting methamphetamine | 1.23 (4.20) | 0.1 | 33.3 |
| Years of snorting methamphetamine | 2.80 (6.10) | 1.2 | 38.5 |
| Years of other method of using methamphetamine | 0.30 (2.53) | 0 | 29.5 |
| 55 Y or Older (n = 53) | 12.79 (10.40) | 11.6 | 46.8 |
| Years of smoking methamphetamine | 7.35 (8.90) | 5.9 | 30.0 |
| Years of injecting methamphetamine | 1.90 (5.97) | 0.3 | 38.7 |
| Years of snorting methamphetamine | 2.80 (6.40) | 1.1 | 25.0 |
| Years of other method of using methamphetamine | 0.80 (4.40) | 0 | 31.0 |
SD: Standard deviation.
Maximum value in sample.
Oral hygiene and dental disease
Most of the participants went to the dentist once every 2 or more years (n = 352) and brushed their teeth 1 to 3 times a day (n = 492). Among the study participants who did not have HIV, 50.8% (n = 215) went to the dentist fewer than once every 2 years. Among the study participants who were HIV positive, we noted that they had similar percentages of going to the dentist more than once per year (n = 39 [26.5%]), once per year (n = 45 [30.6%]), and fewer than once every 2 years (n = 41 [27.9%]), respectively. Oral health behaviors consistently contributed to overall dental health outcomes throughout the analysis. We noted that frequent toothbrushing was associated with an expected decrease of 47.3 surfaces experiencing a caries event (95% CI, 32.7 to 61.9; P < .001; data not shown). This protective effect was the largest, in magnitude, of any effect in the regression analysis. In addition, frequency of toothbrushing remained significant in all previous analyses reported (data not shown).
DISCUSSION
Researchers have attributed the rampant dental caries found in people who habitually used meth to a combination of factors, including xerostomia, frequent sipping of sugary soft drinks to relieve dry mouth symptoms, the corrosive effects of meth contaminants, and poor oral hygiene.12 The results of our study, which involved a large, community sample of people who used meth, provided the following clarifying insights:
-
▬
study participants who used meth had higher rates of xerostomia symptoms and caries experience (DMFT) compared with control participants from the general population;
-
▬
study participants who used meth consumed more sugary drinks daily than control participants from the general population, and even low levels of consumption increase the rate of dental caries;
-
▬
smoking meth did not appear to produce greater rates of caries or xerostomia than in study participants who used another method to use meth;
-
▬
oral health behaviors are important determinants of dental disease in study participants who used meth.
Meth use and xerostomia
Researchers have reported that xerostomia is a prominent side effect of meth use and have attributed it to a vasoconstriction of the salivary gland vasculature and a resultant reduction in salivary flow.27,28 Investigators presented evidence of widespread xerostomia in a study of 119 participants who used multiple types of drugs but who primarily used meth and ecstasy; 95% of participants experienced xerostomia symptoms.29 Although our study involved participants and control participants who represented a wider age range than these studies, our study results confirmed that most study participants who used meth experienced dry mouth symptoms. Whereas fewer than 1 in 10 study participants in the control NHANES group evidenced xerostomia, nearly 7 of 10 of our study participants who used meth reported dry mouth symptoms. Interestingly, the severity of the xerostomia in our study participants was not influenced by the mode or the frequency of meth use, indicating that the intensity of meth use is not linked to its xerostomic side effects. Not surprisingly, the individual use of xerostomic medications was associated consistently with dry mouth symptoms.
It should be noted that the higher prevalence of xerostomia is not unique to people who use meth; rather, it affects all other people who use illicit drugs. The investigators of a cross-sectional study of 58 young adults who used injection drugs found a high prevalence, but no statistical difference, in the occurrence of xerostomia between those who used meth and those who used heroin.30 These findings suggest that poor oral hygiene behaviors and low quality of living (including a poor diet) in people who use substances are more important determinants of xerostomia than the type of illicit drug used. The xerostomia encountered in people who used meth is associated invariably with higher caries rates,12 and the results of our study substantiated that observation. Although the use of illicit drugs can induce hypo-salivation through different mechanisms, a discussion of the xerostomic effects and associated lower quality of life31 should be an important part of the intervention program for people who use meth.
Meth use and sugary beverages
If the surfactant action of saliva is absent or diminished, then speaking, swallowing, and eating can become difficult and result in a person consuming more liquids to mitigate the dry mouth symptoms.32 Researchers have reported that people who have an addiction to meth crave the caffeine and sweetness of drinks like Mountain Dew and consume large amounts of carbonated sugary drinks to quench their thirst.23 Morio and colleagues11 found that, in their pilot study (reported in 2008), 94% of people who used meth (n = 18 people who used meth, n = 18 control participants) stated that they frequently consumed sugary soda compared with 56% of people who did not use meth. In 2016, Murphy and colleagues33 investigated the beverage consumption patterns in our cohort and described that the people who used meth consumed, on average, 35.3 sodas per month. The number of days of meth use over the past 30 days was significantly associated with soda consumption. On further analysis, we found that whereas the people who used meth consumed substantially more sugary beverages than comparable NHANES control participants, the rates of consumption were uniform across the levels of xerostomia among the study participants who used meth. Our findings indicate that people who use meth, as a whole and regardless of xerostomia severity, drink large volumes of sugary beverages daily. Furthermore, we noted a significant difference in the level of caries experience between those who consumed 1 or more sugary beverages daily and those who did not. Increasing soda consumption did not correspondingly increase the caries experience. Our finding suggests that sugary beverages are detrimental to oral health outcomes in people who use meth and that the threshold for affecting the caries experience is quite low. That said, dental disease does not increase substantially with more exposure to sugary beverages. Simply stated, in the presence of poor oral hygiene, people who use meth are susceptible to developing severe dental caries by imbibing just 1 or 2 sugary beverages a day.
Smoking meth and dental disease
In attempting to explain the higher rates of and caries experience found in people who use meth, some investigators have postulated that smoking meth creates an acidic environment in the mouth and contributes to dental caries.12 Others have claimed that the harsh chemicals used in the preparation of meth end up corroding the teeth, especially when a person smokes meth.28 In contrast, Shaner and colleagues10 stated that the “contaminant theory” may be an erroneous explanation for the heightened caries experience in people who use meth. Our data indicate that, contrary to popular perception, smoking meth does not produce greater rates of caries and xerostomia than snorting or injecting meth. Even those who had smoked meth to an extreme extent (study participants who had smoked meth exclusively for over a year, and those who smoked meth frequently for 10 or more years) did not have a significant difference in the caries experience of people who injected, snorted, or administered meth by other means. Furthermore, we found no association between smoking meth and the severity of xerostomia precipitated by meth’s direct effects on the oral mucosa. Thus, our finding does not substantiate the notion of a special association between smoking meth and xerostomia, owing to meth’s direct effects on the oral cavity.
Oral health behaviors in people who use meth
Investigators have suggested that oral health behaviors play a key role in the dental caries observed in people who use meth.12 In particular, sugary soda consumption, poor diet, and infrequent toothbrushing and dental visits have been hypothesized frequently and observed as potential behaviors that can lead to the rampant caries associated with meth mouth.10,11,28 Our results found all of these oral health behaviors to be significant contributors to the observed caries experience, with toothbrushing frequency having the largest protective effect. However, coupled with studies whose results reported no statistical difference in the prevalence of dental disease between people who used meth and people who used heroin,31,32 the frequency of significance of the oral health behaviors throughout the analysis, and the significant difference in level of dental caries between people who use meth and who are 36 to 54 years and people who use meth and who are 55 years or older, despite having a lack of statistical significance in difference of total years of meth use (Table 4), it is likely that oral health behaviors are the primary contributor to the dental caries associated with meth mouth.
Limitations
Our study results necessarily have certain limitations. Although NHANES is a population-based survey, we were working with observational data. Because NHANES is a national sample, any remaining differences between the Los Angeles–based source of meth sample and the broader, geographic frame for NHANES could account for differences in oral health outcomes, as could other uncontrolled factors such as HIV-positive status, which might be considerably higher in the meth sample than in a national population-based survey. In addition, interpreting a between-group difference from propensity score analysis as a causal effect rests on the assumption of strongly ignorable treatment assignments, which would imply that group assignment depends only on variables controlled in the analysis. However, given the magnitude of the effects distinguishing people who use meth from the population-based NHANES sample, we do not believe that plausible alternative models would have led to very different conclusions.
CONCLUSIONS
The “meth mouth” phenomenon has captured the national narrative concerning the affect of meth use on dental health outcomes and has led to a common belief that meth use is associated with a unique and rapid pattern of dental caries. However, many theories related to the reason for this are based on anecdotal evidence and theories about chemical properties. Our results, derived from a convenience sample of 571 community-based people who used meth in the Los Angeles area, substantiate some of these claims but question the merits of others. Our study results did not reveal any evidence to substantiate the narrative that the meth mouth phenomenon is triggered by the direct chemical effects of meth on the oral cavity. Rather, regular consumption of at least 1 sugary beverage a day and poor dental care over a prolonged period seem to be associated with the so-called “meth mouth” state. The elevated rates of xerostomia found in people who use meth also may contribute. The lack of proper oral health behaviors over a long period more likely lead to the observed dental health outcomes of people who use meth. Such oral health behaviors could be enhanced through targeted behavioral interventions and may offer a cost-effective way of minimizing the dental consequences. A better understanding of the mechanisms of meth mouth provides the dental community with a more informed basis for managing dental disease in people who use meth.
Acknowledgments
This study was funded by grant R01 DA025680, awarded to Dr. Vivek Shetty, by the National Institutes of Health (NIH), National Institute on Drug Abuse, which had no role in the design of the study; in the collection, analysis, and interpretation of data; or in the writing of the report. Additional support was funded by grant UL1TR000124, awarded to Dr. Belin, by NIH, and the University of California, Los Angeles, Clinical and Translational Science Institute. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute on Drug Abuse, the NIH, or the Centers of Disease Control and Prevention.
The authors acknowledge the efforts of Mr. Peter Cebezas (interviewer) and Ms. Rachel Fintzy (project director), who were responsible for recruitment, study coordination, and data collection. The authors gratefully acknowledge the participation and support of the study participants as well as the administrative and clinical staff members of the clinics that participated in the study.
The authors prepared the manuscript that led to this article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology Statement.
ABBREVIATION KEY
- DMFS
Decayed, missing, and filled surfaces
- DMFT
Decayed, missing, and filled teeth
- DS
Decayed surface
- Dx
Decayed component
- HIV
Human immunodeficiency virus
- meth
Methamphetamine
- NHANES
National Health and Nutrition Examination Survey
- UCLA
University of California, Los Angeles
Footnotes
SUPPLEMENTAL DATA
Supplemental data related to this article can be found at: http://dx.doi.org/10.1016/j.adaj.2017.02.054.
Disclosure. None of the authors reported any disclosures.
Contributor Information
Jason Clague, Graduate student researcher, School of Dentistry, and a PhD student, Department of Biostatistics, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, CA.
Thomas R. Belin, Professor, Department of Biostatistics, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, CA.
Vivek Shetty, Professor, School of Dentistry, University of California, Los Angeles, Los Angeles.
References
- 1.Donaldson M, Goodchild JH. Oral health of the methamphetamine abuser (published correction appears in Am J Health Syst Pharm. 2006;63 [22]:2180) Am J Health Syst Pharm. 2006;63(21):2078–2082. doi: 10.2146/ajhp060198. [DOI] [PubMed] [Google Scholar]
- 2.Hart CL, Csete J, Habibi D, Global Drug Policy Program . Methamphetamine: fact vs. fiction and lessons from the crack hysteria. Open Society Foundations; Feb, 2014. Available at: www.opensocietyfoundations.org/publications/methamphetamine-dangers-exaggerated. Accessed March 10, 2017. [Google Scholar]
- 3.The faces of meth. Multnomah County Sheriff’s Office; Dec, 2004. Available: www.facesofmeth.us/. Accessed March 10, 2017. [Google Scholar]
- 4.Faces of meth. PBS. Frontline; Feb 14, 2006. Available at: www.pbs.org/wgbh/pages/frontline/meth/body/faces.html. Accessed March 10, 2017. [Google Scholar]
- 5.Murakawa N. Toothless: the methamphetamine “epidemic,” “meth mouth,” and the racial construction of drug scares. Du Bois Review: Social Science Research on Race. 2011;8(1):219–228. [Google Scholar]
- 6.Shafer J. More meth-mouth misinformation. Slate. 2006 May 12; Available at: www.slate.com/articles/news_and_politics/press_box/2006/05/more_methmouth_misinformation.html Accessed March 10, 2017.
- 7.Shetty V, Mooney LJ, Zigler CM, Belin TR, Murphy D, Rawson R. The relationship between methamphetamine use and increased dental disease. JADA. 2010;141(3):307–318. doi: 10.14219/jada.archive.2010.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shetty V, Harrell L, Murphy D, et al. Dental disease patterns in methamphetamine users: findings in a large urban sample. JADA. 2015;146(12):875–885. doi: 10.1016/j.adaj.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shetty V, Harrell L, Clague J, Murphy DA, Dye BA, Belin TR. Methamphetamine users have increased dental disease: a propensity score analysis. J Dent Res. 2016;95(7):814–821. doi: 10.1177/0022034516640478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaner JW, Kimmes NS, Saini T, Edwards P. “Meth mouth”: rampant caries in methamphetamine abusers. AIDS Patient Care and STDS. 2006;20(3):146–150. doi: 10.1089/apc.2006.20.146. [DOI] [PubMed] [Google Scholar]
- 11.Morio KA, Teresa MA, Qian F, Morgan TA. Comparing diet, oral hygiene and caries status of adult methamphetamine users and nonusers: a pilot study. JADA. 2008;139(2):171–176. doi: 10.14219/jada.archive.2008.0133. [DOI] [PubMed] [Google Scholar]
- 12.De-Carolis C, Boyd GA, Mancinelli L, Pagano S, Eramo S. Methamphetamine abuse and “meth mouth” in Europe. Med Oral Patol Oral Cir Bucal. 2015;20(2):e205–e210. doi: 10.4317/medoral.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamamoto DT, Rhodus NL. Methamphetamine abuse and dentistry. Oral Dis. 2009;15(1):27–37. doi: 10.1111/j.1601-0825.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- 14.Dye BA, Harrell L, Murphy DA, Belin T, Shetty V. Performance of a quality assurance program for assessing dental health in methamphetamine users. BMC Oral Health. 2015;15:76. doi: 10.1186/s12903-015-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dye BA, Nowjack-Raymer R, Barker LK, et al. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2003-04. J Public Health Dent. 2008;68(4):218–226. doi: 10.1111/j.1752-7325.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 16.Dye BA, Barker LK, Li X, Lewis BG, Beltran-Aguilar ED. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2005-08. J Public Health Dent. 2011;71(1):54–61. doi: 10.1111/j.1752-7325.2010.00202.x. [DOI] [PubMed] [Google Scholar]
- 17.Radike A. Criteria for diagnosis of dental caries. Proceedings of the Conference on the Clinical Testing of Cariostatic Agents; American Dental Association, Chicago, IL. October 14- 16, 1968; Chicago, IL: ADA Council on Dental Research; 1972. pp. 87–88. [Google Scholar]
- 18.Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. JADA. 1987;115(4):581–584. doi: 10.1016/s0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 19.Ying Joanna ND, Thomson MW. Dry mouth: an overview. Singapore Dental Journal. 2015;36:12–17. doi: 10.1016/j.sdj.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11(1):3–11. [PubMed] [Google Scholar]
- 21.National Institutes of Health, National Cancer Institute, Division of Cancer Control and Population Sciences, Epidemiology and Genomics Research Program. Usual dietary intakes: NHANES food frequency questionnaire. 2015 May; Available at: https://epi.grants.cancer.gov/diet/usualintakes/FFQ.English.June0304.pdf. Accessed March 10, 2017.
- 22.Karvetti RL, Knuts LR. Validity of the 24-hour dietary recall. J Am Diet Assoc. 1985;85(11):1437–1442. [PubMed] [Google Scholar]
- 23.CALDAR UCLA. Natural History Interview (NHI) Available at: http://www.caldar.org/html/natural-history.html. Accessed March 10, 2017.
- 24.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 25.Harrell L, Belin TR, Shetty V. Developing a propensity score protocol for evaluating the oral health consequences of methamphetamine use. Communications in Statistics: Case Studies, Data Analysis and Applications. 2015;1(3):125–135. [Google Scholar]
- 26.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52(1):249–264. [PubMed] [Google Scholar]
- 27.Shaner JW. Caries associated with methamphetamine abuse. J Mich Dent Assoc. 2002;84(9):42–47. [PubMed] [Google Scholar]
- 28.Klasser GD, Epstein J. Methamphetamine and its impact on dental care. J Can Dent Assoc. 2005;71(10):759–762. [PubMed] [Google Scholar]
- 29.McGrath C, Chan B. Oral health sensations associated with illicit drug abuse. Br Dent J. 2005;198(3):159–162. doi: 10.1038/sj.bdj.4812050. [DOI] [PubMed] [Google Scholar]
- 30.Brown C, Krishnan S, Hursh K, et al. Dental disease prevalence among methamphetamine and heroin users in an urban setting. JADA. 2012;143(9):992–1001. doi: 10.14219/jada.archive.2012.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shekarchizadeh H, Khami MR, Mohebbi SZ, Virtanen JI. Oral health behavior of drug addicts in withdrawal treatment. BMC Oral Health. 2013;13:11. doi: 10.1186/1472-6831-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenspan D. Xerostomia: diagnosis and management. Oncology (Williston Park) 1996;10(3 suppl):7–11. [PubMed] [Google Scholar]
- 33.Murphy D, Harrell L, Fintzy R, Vitero S, Gutierrez A, Shetty V. Soda consumption among methamphetamine users in the USA: impact on oral health. Oral Health and Preventitive Dentistry. 2016;14(3):227–234. doi: 10.3290/j.ohpd.a35620. [DOI] [PMC free article] [PubMed] [Google Scholar]


