Abstract
The endoplasmic reticular (ER) protein sigma-1 receptor (Sig-1R) has been implicated in CNS disorders including but not limited to neurodegenerative diseases, depression, amnesia, and substance abuse. Sig-1Rs are particularly enriched in the specific domain where ER membranes make contacts with the mitochondria (MAM). Within that specific domain, Sig-1Rs play significant roles governing calcium signaling and reactive oxygen species homeostasis to maintain proper neuronal functions. Studies showed that the Sig-1R is pivotal to regulate neuroplasticity and neural survival via multiple aspects of mechanism. Numerous reports have been focusing on Sig-1R’s regulatory effects in ER stress, mitochondrial function, oxidative stress and protein chaperoning. In this book chapter, we will discuss the emerging role of Sig-1R in balancing the populations of neuron and glia and their implications in CNS diseases.
Keywords: Glia-neuron interplay, Astrocyte, Axon pathfinding, Axon pruning, Sigma-1 receptor
7.1. Introduction
Neurons are functionally polarized cells extended with neurites. Among neurites, axons are distinct from other dendrites due to their specialization in conducting signal propagation and protein transport in the neural circuit. Axonal guidance and pathfinding are precisely governed during neuronal developments. Failures or malfunction in axonal maintenance, regeneration and target recognition have been implied in the pathogenesis of several CNS disorders such as Alzheimer’s disease, Parkinson’s disease, stroke and spinal cord injuries [1–3].
The axonal pathfinding in the developing nervous system is orchestrated by cytoskeletal element polymerizations as well as the regulation of microtubule-associated proteins and the Rho-GTPases family. In addition, guidance cues and other stimuli such as extracellular signaling proteins also contribute to the precision of axonal pathfindings. These factors include growth factors, matrix glycoproteins, and integrin receptors. Emerging evidence indicates that local axonal translation plays important roles in axonal maintenance [4, 5]. Many local translational mechanisms for mitochondrial proteins are responsible for preventing free radical production and oxidative damage and thus may be contributing to axonal health [5–7]. Recent reports also indicated that mitochondrial biogenesis is not limited to the cell body, but also occurs locally in axons [8–10].
7.2. The Role of Sig-1R in Neurogenesis and Axon Guidance
We recently discovered that the sigma-1 receptor (Sig-1R), an ER chaperone protein that resides in the ER and mitochondrial contacting site (also known as MAM) [11], is essential for neurogenesis in dentate gyrus of adult hippocampus [12] and is pivotal to maintain dendritic arborization via the regulation of mitochondrial functions during neuronal development [13]. In addition, axon extensions are regulated by Sig-1R activities [14,15]. In Sig-1R depleted neurons, the growth cones exhibit reduction in size and in Rac GTPase specific GEF Tiam1 intensities. Sig-1R depletion also caused significant reduction in axonal density as well as decreased mitochondrial number and mobility [15]. These findings further support the important notion of Sig-1Rs in maintaining neuronal survival and their implications in many CNS disorders.
In a primary rat hippocampal neuron model, we employed Sig-1R knockdown (KD) using the AAV transduction. Sig-1R deficiency induces non-neuronal cell proliferation as indicated by DAPI staining. Non-neuronal cell proliferation is an early sign of gliosis, and is usually accompanied by astrocytic activation. Axons were visualized by immuostaining with the α-acetylated tubulin. We noticed that the Sig-1R KD neurons exhibited disoriented axon projections (Fig. 7.1). Wild type (WT) hippocampal neurons displayed structurally organized axon networks and connections, while the axons of the KD neurons established abnormal circular routes and displayed a disoriented phenotype. These findings suggest that Sig-1R deficiency may lead to poor arborization of presynaptic axons and fewer synapse formations. Regressive axon growth is essential to coordinate functional axon connections. Axon pruning occurs constantly during axon pathfinding and elongation. Axons may dislocate and mistarget if left without proper pruning. In addition, aberrant axon pathfinding has been associated with neurological diseases [16]. Though Sig-1Rs have been shown to participate in axon elongations [14, 15], surprisingly, Sig-1R antagonists induced aberrant axon elongation in a primary mouse cortical neuron model. 1 μM BD-1063 significantly increased axon elongations in neurons as indicated by phospho neurofilament immunostaining (Fig. 7.2). Similar results were observed using another Sig-1R specific antagonist haloperidol (data not shown). Perhaps it is too early to conclude that inactivation of Sig-1R enhances axonal activities and elongation. Rather, antagonizing Sig-1Rs may disrupt the well-orchestrated mechanisms that are tightly associated with pruning and guidance. This leads to the hypothesis that Sig-1Rs may be involved in axon guidance/pathfinding as well as in axon pruning and facilitate axon targeting to proper functional areas to form functional synapses.
Fig. 7.1. Sig-1R is required to maintain neuronal polarity.
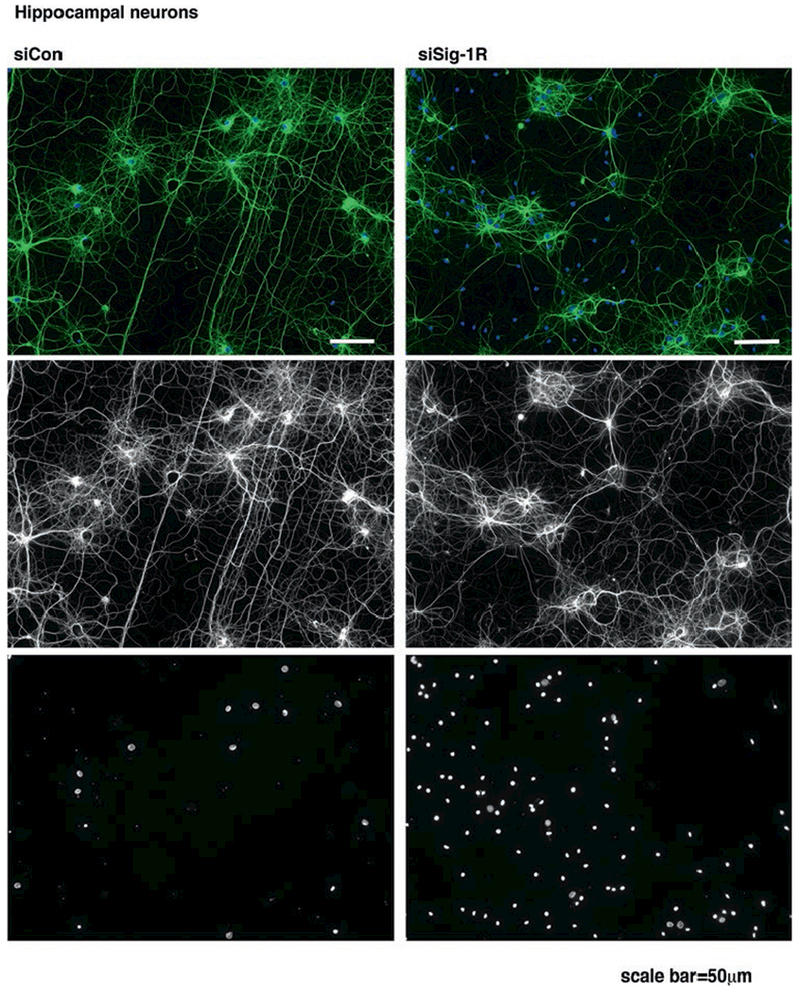
Equal density of cultured hippocampal neurons were infected with an AAV vector expressing a short hair-pin RNA (shRNA) sequence for Sig-1R. Ten days after transduction, neurons were immunostained with the axon marker acetylated alpha tubulin (green). Depletion of Sig-1R disrupts axon polarity and arborization as axons in the Sig-1R KD groups wrapped around neuronal somas and failed to display proper connections. Though the population of neurons in both control and KD groups is similar, Sig-1R KD cultures may be more susceptible to gliosis as indicated by more non-neuronal DAPI staining (blue)
Fig. 7.2. Aberrant axon elongation induced by Sig-1R antagonist.
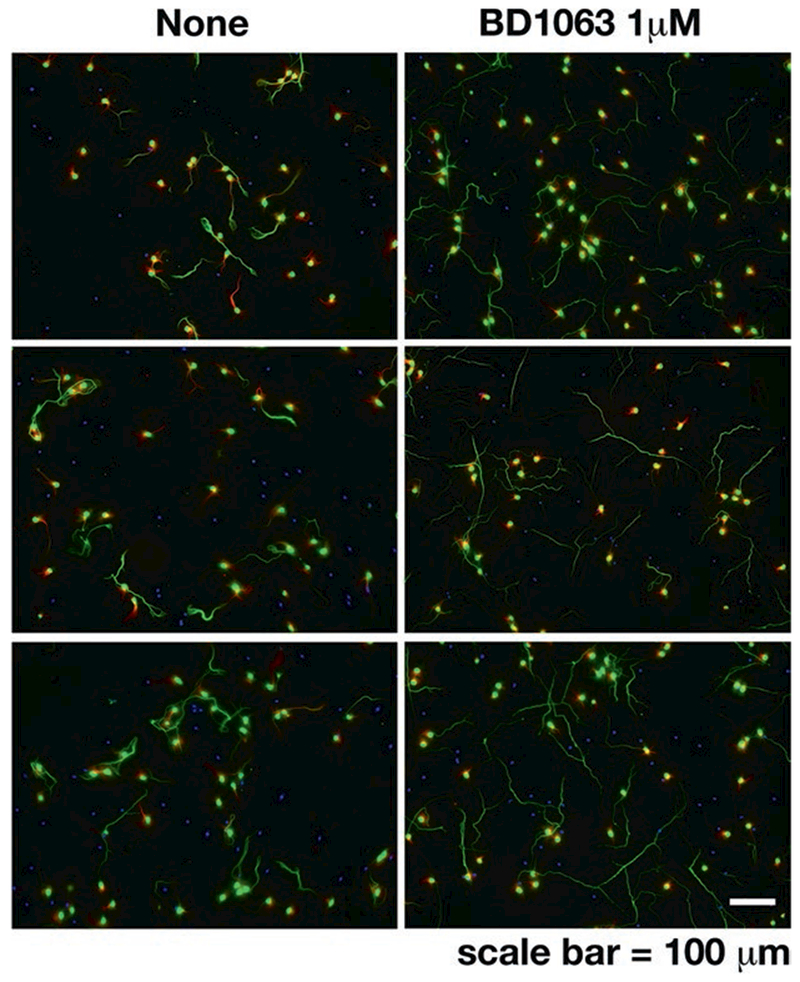
Primary mouse cortical neurons were treated with the Sig-1R antagonist BD-1063 (1 μM) at days in vitro (DIV) 7. Axon lengths were observed at DIV 10 by immunostaining of phospho neurofilament (pNF-H, SMI 31). Neurons treated with BD-1063 (right panels) showed significantly longer axons than the control neurons (left panels)
7.3. Conclusions
Non-neuronal cells are abundant in the central nervous system (CNS) and without doubt participate in axon signaling. Astroglia play important roles and indispensable contributions in many CNS processes including shaping memory formation and recovery from CNS injury. It has been well recognized that the bidirectional astrocyte-neuron communication is part of the axon pruning/pathfinding [17]. A single astrocyte can form synaptic islands by enwrapping a maximum of eight neuron somas and making contact with 300–600 neuronal dendrites [18]. At the synaptic clefts, astrocytes and neurons form the so-called “tripartite synapse” to establish bidirectional communications [19, 20]. Astrocytes can trigger the exocytotic release of gliotransmitters including glutamate, GABA, NMDA receptor co-agonist D-serine and ATP/adenosine, as well as neurotrophic factors [21]. On the other hand, reactive astrocytes can function as the extrinsic inhibition at the lesion site to inhibit axon growth [22, 23]. Sig-1Rs are enriched in astrocytes [24].
Accumulating evidence shows that Sig-1Rs exert regulatory effects on neuropathic pain [25], traumatic brain injury-induced inflammatory responses [26], as well as psychostimulants-induced autophagy [27] and neuroinflammation responses [28] via the astrocytic or microglial activation. Thus, Sig-1Rs may oversee axon guidance/pathfinding via the precise glia-neuron communication networks (Fig. 7.1) as well as govern the functional axon growth via the mechanisms that regulate recessive events (Fig. 7.2). Sig-1R ligands may exert great therapeutic potentials in establishing functional neuronal networks in this regard.
Contributor Information
Shang-Yi Anne Tsai, Cellular Pathobiology Section, Integrative Neuroscience Research Branch, Intramural Research Program, NIDA, NIH, DHHS, Baltimore, MD 21224, USA.
Tsung-Ping Su, Cellular Pathobiology Section, Integrative Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, US Department of Health and Human Services, Baltimore, MD 21224, USA, tsu@intra.nida.nih.gov.
References
- 1.Lin L, Lesnick TG, Maraganore DM, Isacson O (2009) Axon guidance and synaptic maintenance: pre-clinical markers for neurodegenerative disease and therapeutics. Trends Neurosci 32(3):142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubo T, Endo M, Hata K, Taniguchi J, Kitajo K, Tomura S et al. (2008) Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem 105(1):113–126 [DOI] [PubMed] [Google Scholar]
- 3.Lesnick TG, Papapetropoulos S, Mash DC, Ffrench-Mullen J, Shehadeh L, de Andrade M et al. (2007) A genomic pathway approach to a complex disease: axon guidance and Parkinson disease. PLoS Genet 3(6):e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung H, Gkogkas CG, Sonenberg N, Holt CE (2014) Remote control of gene function by local translation. Cell 157(1):26–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG et al. (2007) Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol 178(6):965–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollenbeck PJ, Saxton WM (2005) The axonal transport of mitochondria. J Cell Sci 118(Pt 23):5411–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi P, Wei Y, Zhang J, Gal J, Zhu H (2010) Mitochondrial dysfunction is a converging point of multiple pathological pathways in amyotrophic lateral sclerosis. J Alzheimers Dis 20(Suppl 2):S311–S324 [DOI] [PubMed] [Google Scholar]
- 8.Amiri M, Hollenbeck PJ (2008) Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev Neuropsychol 68(11):1348–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxton WM, Hollenbeck PJ (2012) The axonal transport of mitochondria. J Cell Sci 125(Pt 9):2095–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G (2013) Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep 5(6):1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131(3):596–610 [DOI] [PubMed] [Google Scholar]
- 12.Sha S, Qu WJ, Li L, Lu ZH, Chen L, Yu WF et al. (2013) Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via downregulation of NMDA receptors. CNS Neurosci Ther 19(9):705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai SY, Hayashi T, Harvey BK, Wang Y, Wu WW, Shen RF et al. (2009) Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1xGTP pathway. Proc Natl Acad Sci U S A 106(52): 22468–22473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura Y, Fujita Y, Shibata K, Mori M, Yamashita T (2013) Sigma-1 receptor enhances neurite elongation of cerebellar granule neurons via TrkB signaling. PLoS One 8(10):e75760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai SY, Pokrass MJ, Klauer NR, Nohara H, Su TP (2015) Sigma-1 receptor regulates Tau phosphorylation and axon extension by shaping p35 turnover via myristic acid. Proc Natl Acad Sci U S A 112(21):6742–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Battum EY, Brignani S, Pasterkamp RJ (2015) Axon guidance proteins in neurological disorders. Lancet Neurol 14(5):532–546. doi: 10.1016/S1474-4422(14)70257-1 [DOI] [PubMed] [Google Scholar]
- 17.Eroglu C, Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468(7321):223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG (2007) Synaptic islands defined by the territory of a single astrocyte. J Neurosci 27(24):6473–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halassa MM, Fellin T, Haydon PG (2007) The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13(2):54–63 [DOI] [PubMed] [Google Scholar]
- 20.Zuchero JB, Barres BA (2015) Glia in mammalian development and disease. Development 142(22):3805–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu R, Wang Z, Gou L, Xu H (2015) A cortical astrocyte subpopulation inhibits axon growth in vitro and in vivo. Mol Med Rep 12(2):2598–2606. doi: 10.3892/mmr.2015.3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7(8):617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francardo V, Bez F, Wieloch T, Nissbrandt H, Ruscher K, Cenci MA (2014) Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain 137(Pt 7):1998–2014 [DOI] [PubMed] [Google Scholar]
- 25.Moon JY, Roh DH, Yoon SY, Choi SR, Kwon SG, Choi HS et al. (2014) sigma1 receptors activate astrocytes via p38 MAPK phosphorylation leading to the development of mechanical allodynia in a mouse model of neuropathic pain. Br J Pharmacol 171(24):5881–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong H, Ma Y, Ren Z, Xu B, Zhang Y, Chen J et al. (2015) Sigma-1 receptor modulates neuroinflammation after traumatic brain injury. Cell Mol Neurobiol 36(5):639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao L, Walker MP, Vaidya NK, Fu M, Kumar S, Kumar A (2015) Cocaine-Mediated Autophagy in Astrocytes involves Sigma 1 receptor, PI3K, mTOR, Atg5/7, Beclin-1 and Induces Type II programed cell death. Mol Neurobiol 53(7):4417–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Lv X, Bai Y, Zhu X, Wu X, Chao J et al. (2015) Involvement of sigma-1 receptor in astrocyte activation induced by methamphetamine via upregulation of its own expression. J Neuroinflammation 12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]


