Synopsis
For decades, colorectal screening strategies have been largely driven by static features, particularly polyp size. Although cross-sectional features of polyp size, morphology, and location are important determinants of clinical relevance prior to histology, they lack any dynamic information on polyp growth rates. CT colonography allows for in vivo surveillance of colorectal polyps, providing volumetric growth rates that are providing new insights into tumorigenesis. Existing cross-sectional and longitudinal data on colorectal polyps will be reviewed, with an emphasis on how these features may impact clinical relevance and patient management.
Keywords: Colorectal cancer, Colorectal polyps, CT colonography, Virtual colonoscopy, Optical colonoscopy
Introduction
It is widely accepted that colorectal cancers (CRC) generally derive from once benign dysplastic colorectal polyps. However, it is also true that colorectal polyps are a highly prevalent human condition, affecting the majority of adults according to recent endoscopic screening data. As such, the vast majority of colorectal polyps behave in a benign fashion and will of course never develop into cancer. Furthermore, although the precise timing and sequence of events for progression to cancer have not yet been fully elucidated, the typical dwell time for this infrequent transformation is likely a decade or more, whether via the classic adenoma-carcinoma sequence or the serrated polyp pathway. When taking all these factors into consideration, CRC can clearly be effectively prevented through the detection and removal of benign dysplastic colorectal polyps, but also that a strategy of universal polypectomy is an inefficient approach, leading to excessive resource utilization, costs, and complications. At the other end of the spectrum, tests that primarily target cancer detection ignore the larger benefit of cancer prevention. Between these two extremes, a more rational CRC screening approach that targets large and/or growing polyps and early cancers likely represents a more clinically efficacious and cost-effective strategy.
A large and ever-growing volume of data exists surrounding static cross-sectional features of colorectal polyps such as lesion size, morphology, and location – and their relationship to underlying histologic features. Much of this data comes from large colonoscopic databases, where detected polyps are removed without any knowledge of their preceding growth rates. With the emergence of CT colonography (CTC) as an attractive CRC screening tool, we have the ability to follow polyps longitudinally prior to resection. This dynamic in vivo investigation also provides unique opportunities for studying the natural history of polyps, such as correlating growth rates with underlying polyp histology and genetic alterations. These insights could ultimately inform future strategies for screening and surveillance, as well as fuel novel theories on tumor evolution.
Cross-sectional (Static) Polyp Data: Size, Morphology, and Location
Static polyp features including lesion size, morphology, and anatomic location have long served as the major determinants of clinical significance. Of these, polyp size is likely the single most important consideration, as it directly correlates with important histologic features such as high-grade dysplasia (HGD) and invasive cancer.1,2 However, polyp morphology and segmental location can both further enhance classification and risk stratification, as discussed below after polyp size considerations. Implicit in this discussion on static polyp features is ensuring sensitive detection by both CTC and OC, as this represents the only means for preventing polyp progression.
Given the extremely high prevalence of sub-cm colorectal polyps and their potential influence on screening algorithms, it is critical to first focus attention on this subset. Sub-cm polyps are further subdivided into diminutive (≤5 mm) and small (6-9 mm) size categories. Although abundant prior cumulative evidence has demonstrated very low rates of HGD and exceedingly low (or even nonexistent) rates of cancer among sub-cm polyps,1–3 a recent study by Ponugoti et al4 has further crystalized these findings. This report on >40,000 sub-cm colorectal polyps resected at optical colonoscopy (OC) has substantially increased our cumulative experience. From prior observational series, rates of HGD and invasive cancer typically ranged from 0.5-0.8% and 0-0.5% for small polyps, respectively, and would be even lower for diminutive lesions.2 The large study by Ponugoti et al4 essentially doubles the cumulative data on sub-cm polyps. In this study, rates of HGD and invasive cancer among diminutive conventional adenomas was 0.3% and 0%, respectively, and 0.8% and 0% for small conventional adenomas, respectively. Reported rates of advanced histology (villous component, HGD, or invasive cancer) are somewhat higher, typically around 0.3-1.2% for diminutive adenomas and 2.9-5.3% for small adenomas, respectively. However, as witnessed by the very low rates of HGD and cancer, sub-cm advanced adenomas are therefore almost exclusively determined by a prominent villous component, which is of more debatable immediate clinical relevance. The actual prevalence of advanced diminutive and small polyps (as the largest advanced lesion) has been estimated at 0.1-0.3% and 0.3-0.6%, respectively.5
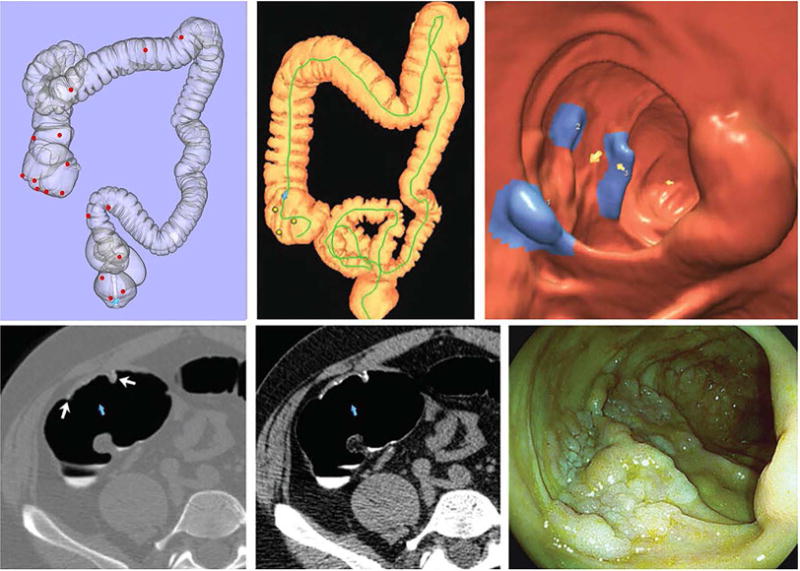
From the preceding discussion, it can be concluded that colorectal polyps under 10 mm in size can be considered to behave in a benign fashion– but what about larger polyps? According to more recent data, including our own experience with CTC, large polyps measuring 1-2 cm harbor invasive cancer in only about 1% of cases (or less).1,2 This percentage is considerably lower than the traditional quote of 5-10% cancer risk that was based on older surgical literature. For colorectal masses (3 cm or larger), rates of HGD and invasive cancer rise precipitously with lesion size. However, in our CTC screening experience, approximately 50% of OC-confirmed and resected colorectal lesions ≥3 cm prove to not be invasive cancers, most often tubulovillous adenomas (TVA), villous adenomas, and serrated polyps (sessile serrated and traditional subtypes). Carpet lesions (flat superficially spreading tumors) comprise a substantial proportion of these benign but potentially pre-malignant “masses”, as their large linear size belies their relatively limited tumor bulk in terms of volume (Figure 1).6 This has been indirectly supported by a large OC database of large superficial colorectal lesions (mean size, 3.7 cm), where the rate of submucosal invasion was only 7.6%.7
Figure 1. Carpet lesions (flat superficially spreading tumors).
Three-dimensional CTC map (upper row, left) shows anatomic locations (one red dot for each patient) of colorectal carpet lesions detected at CTC in our experience. Except for two cases, all carpet lesions were located in the proximal right colon or rectosigmoid colon.
The remaining images are from a cecal carpet lesion detected at CTC screening in a 50-year-old man. Three-dimensional colon map (upper row, middle) and 3D endoluminal CTC view (upper row, right) show three CAD marks in the cecum (yellow dots and blue regions with arrows, respectively), which identify focal areas of a broad 3.5-cm carpet lesion. The lesion is located across from the normal-appearing ileocecal. Transverse 2D images in polyp window (lower row, left) and soft-tissue window (lower row, middle) confirm a flat soft-tissue lesion (arrows). Note the etching of positive oral contrast material on the surface of the lesion, which is better seen on soft tissue windowing. The lesion was confirmed at same-day colonoscopy (lower row, right) and proved to be a tubulovillous adenoma without high-grade dysplasia after laparoscopic right hemicolectomy.
From Pickhardt PJ, Lam VP, Weiss JM, et al. Carpet Lesions Detected at CT Colonography: Clinical, Imaging, and Pathologic Features. Radiology 2014;270:435-43; with permission.
Beyond polyp size, lesion morphology has also long been recognized as an important static determinant of clinical significance. For example, pedunculated polyps tend to be larger in size relative to sessile polyps, on average, and more often have tubulovillous or villous architecture at histologic evaluation. For flat (nonpolypoid) lesions, however, controversy and confusion exist in terms of their clinical relevance and rate of growth. To clarify, “flat” is somewhat of a misnomer as these lesions are typically raised slightly at the edges in a plaque-like manor and only rarely truly flush with the surrounding mucosa.8–10 At colonoscopy, most of the so-called aggressive flat lesions are mixed lateral spreading tumors characterized by a typically large sessile nodular component emerging from a flatter carpet-like lesion. Since the invasive cancer component tends to be associated with the polypoid portion, these lesions would be readily identified at CTC as sessile lesions in the first place. Furthermore, because a very small subset of these flat lesions might be more aggressive in nature, especially those with a central depression, some have assumed that all flat lesions are therefore more aggressive in general. However, our experience has been quite the opposite, with flat lesions acting more benign and indolent in nature (especially for their typically large linear size, as noted above) compared with their polypoid counterparts.6,10 Because flat lesions are less conspicuous at both OC and CTC, they are more likely to be missed on initial evaluation, leading to the appearance of a rapidly growing new lesion as opposed to the true case. As discussed in the dynamic section below, large flat lesions missed at initial evaluation often show little or no progression at follow-up CTC examinations. Increased awareness and improved techniques at both CTC and OC are leading to the detection of more and more flat colorectal lesions, many of which are right-sided serrated lesions.11,12
Given the recent strides in the awareness and understanding of serrated polyps and their associated alternative pathway to cancer,11–14 these lesions deserve separate consideration. Sessile serrated polyps (SSPs; also referred to as “sessile serrated adenomas” or SSP/As), previously mischaracterized as hyperplastic polyps without malignant potential, may ultimately account for up to 20-25% of sporadic colorectal cancers.14 The serrated pathway of carcinogenesis is characterized by mutations in BRAF and epigenetic methylation that silence mismatch repair genes and lead to cancers with microsatellite instability.11,14 The time course for this transformation is many years, possibly longer than that of the adenoma-carcinoma sequence (natural history behavior at CTC is discussed below). This is supported by a recent colonoscopy series of large SSPs (mean size, 2.9 cm), where the rate of invasive cancer was only 3.9%; another 3.3% showed high-grade cytologic dysplasia without invasive cancer. 20 SSPs tend to be right-sided in location and flat in morphology, and were likely a common cause of screen failures at OC (and CTC) dowing to their often subtle appearance.15,16 Fortunately, many of these SSP lesions exhibit a mucus cap on their surface, which allows for improved detection at both OC and CTC (Figure 2). Luminal contrast agents (particularly barium), however, are needed to leverage this advantage at CTC by preferentially coating the lesional surface.11,12 This contrast surface coating phenomenon at CTC is critical for improved detection of flat lesions in general, whether serrated or adenomatous (Figure 1).17,18 Lack of barium tagging (and possibly also lack of reader awareness) in a recent Dutch trial19 might account for the decreased detection of SSPs relative to OC. Our experience in CTC detection of SSPs (and the less common traditional serrated adenoma) appears to be different.11 Previous OC studies have also described markedly variable rates in SSP detection.16 In general, simple awareness of the subtle nature of SSPs is the first critical step in their detection at either OC or CTC. In addition, it has been recently shown that cytologic dysplasia in large SSP/As is usually associated with a sessile polypoid component that would be readily identifiable at CTC.20
Figure 2. Sessile serrated polyps (SSPs) detected at screening CTC.
Three different patients with right-sided SSPs (each row corresponds to one patient) detected at screening CTC. Optical colonoscopy images (left images in each row) depict the flat, subtle nature of these large right-sided polyps. CTC 3D endoluminal images show the corresponding appearance that led to detection and same-day referral for polypectomy at colonoscopy. Notice that the polyps are slightly more prominent and protruding at CTC compared with the OC. Transverse 2D CTC images (right images) demonstrate how the 3D appearance represents a combination of the flat polyp and the overlying adherent tagging contrast agent (arrows). Magnified images (insets on lower 2 rows) better depict the subtle soft-tissue thickening underneath the overlying contrast cap.
From Kim DH, Matkowskyj KA, Lubner MG, et al. Serrated Polyps at CT Colonography: Prevalence and Characteristics of the Serrated Polyp Spectrum. Radiology 2016;280:455-63; with permission.
A wide variety of novel OC techniques and approaches are also providing for improved detection and clinical management of colorectal polyps.21 Improvements in bowel preparation, such as split regimen dosing of the cathartic agent, may seem mundane but one randomized trial showed a 35% increase in advanced adenoma detection with the split regimen.22 Endoscopist factors include scope withdrawal time and retraining in newer techniques.21,23 Most advances relate to improvements in endoscope technology and technique.21 Wider lens angles and, more recently, lenses that are lateral to the tip of the scope (full spectrum endoscopy – or FUSE) help to close the gap on the mucosal coverage advantage enjoyed by CTC.24 A number of OC add-on devices have been fashioned to address perhaps the greatest challenge at OC: detection of right-sided lesions, especially those behind folds (Figure 3).25,26 These include a variety of fitted caps, cuffs, or rings on the scope tip to flatten folds, as well retrograde camera angles to image behind folds. Finally, a number of approaches aim to improve OC detection of flat lesions, especially those in the right colon.21 Dye-spraying chromoendoscopy can improve detection of subtle flat lesions, but is generally considered too cumbersome to perform on a routine basis for screening. Electronic processing of white light, as with narrow band imaging (NBI), provides a form of virtual chromoendoscopy, but its additive value for polyp detection is less clear.
Figure 3. Malignant right-sided polyp in ascending colon missed at initial colonoscopic evaluation.
Three-dimensional colon map (right) shows the location of a large polyp in the ascending colon at CTC; 3D endoluminal CTC view shows the large polypoid lesion on or adjacent to a fold. This patient was enrolled in the DoD CTC screening trial. This polyp was not found at initial colonoscopy immediately following CTC. However, after segmental unblinding of the CTC results, the polyp was eventually found after several attempts to reposition the instrument because of repeated slippage in this region. Invasive adenocarcinoma was confirmed at surgery.
From Pickhardt PJ, Nugent PA, Mysliwiec PA, et al. Location of adenomas missed by optical colonoscopy. Annals of Internal Medicine 2004;141:352-9; with permission.
Historically, wide variations in polyp detection rates among endoscopists have been associated with OC,23 particularly when compared with CTC performance.27 The aforementioned measures for improving OC have presumably helped close this gap. Considerable emphasis has recently been placed on the adenoma detection rate (ADR) by endoscopists, which can be considered a proxy measure for examination quality.28 However, a more relevant measure would be the advanced adenoma detection rate, since the vast majority of OC-detected adenomas will be diminutive in size and of doubtful clinical significance. Although higher ADRs at screening OC are associated with a reduction in CRC risk,29 excessive focus on this statistic alone might shift attention away from clinically relevant lesions to those of little or no importance. Finally, resect and discard strategies may help alleviate some of the cost burden related to diminutive lesions.30 However, molecular analysis of even these diminutive lesions may identify patients at increased risk for ultimately developing colorectal cancer and consequently this analysis could impact individualized screening intervals.
Longitudinal (Dynamic) Polyp Data: Growth Rates and Natural History
Despite the wealth of data on static polyp features that correlate with increased risk, the fate of resected polyps had they been left in place cannot be inferred. Although it is clear from the existing cross-sectional data that sub-cm polyps behave in an overwhelmingly benign fashion, in vivo longitudinal follow-up with at least two time points is required to actually determine growth rates and progression.
The natural history of colorectal polyps, especially those under 10 mm in size, has become a highly relevant clinical issue. Although largely missed and ignored by stool-based tests and generally removed when detected by OC, the situation is quite different for CTC, which allows for the opportunity of selective referral for polyp removal.31 This important filtering function minimizes unnecessary resource utilization and complications related to removal of pseudodisease.32 Overall screen detection rates of non-diminutive polyps (≥6 mm) have been shown to be similar in prospective CTC-OC trials.32,33 In contrast, performance drops off for CTC detection of diminutive lesions. While it is undoubtedly highly efficacious and cost-effective to refer all large CTC-detected polyps to OC, isolated diminutive lesions (≤5 mm) are actually intentionally ignored at CTC, given the absence of cancer and low rates of high-grade dysplasia, as discussed above.34,35 For small polyps (6-9 mm) detected at CTC, however, the appropriate management is less certain, and may depend upon a number of factors, including patient preference and health condition. While we know from the preceding static data that these small polyps are invariably benign, the likelihood of ultimate progression to cancer is quite low, but certainly not zero. Based on both clinical and economic modeling, one could make a plausible argument for either immediate polypectomy or CTC surveillance,34–36 choices which were reflected in the first major guidelines governing CTC.37 As such, the CTC practice of in vivo surveillance of unresected lesions is providing new insights into the natural history of colorectal polyps. However, before delving into this unique CTC experience, a brief review of the cumulative data on polyp growth rates based on older colorectal modalities is warranted.
Prior to CTC surveillance, a number of older endoscopy and barium enema studies reported on experiences with small unresected polyps. Although these studies were limited in their ability to precisely localize and measure polyps, a fair amount of preliminary data on polyp natural history exists from these older longitudinal trials. Some of these studies were published over 50 years ago. When considered together as a group, all of these longitudinal studies have repeatedly shown the benign, indolent nature of unresected sub-cm colorectal polyps.
In a longitudinal study using barium enemas to follow unresected colorectal polyps, Welin et al38 showed exceedingly slow growth rates by studying 375 unresected polyps over a mean interval of 30 months. In Norway, Hofstad et al39 performed serial colonoscopy on unresected 189 sub-cm polyps, finding that only one (0.5%) lesion eclipsed the 10-mm threshold after a one-year time interval. At the 3-year follow-up mark, most polyps in this study remained stable or even regressed in size, with an overall tendency to net regression amongst small (5–9 mm) polyps.40 The authors of this endoscopic trial concluded that following unresected 5-9 mm polyps for 3 years was a safe practice. Other longitudinal studies using flexible sigmoidoscopy have also demonstrated the stability of small polyps over time.41–43 In one study that used serial sigmoidoscopy to follow polyps measuring up to 15 mm in size over a 3-5 year period, Knoernschild41 reported a significant increase in polyp size in only 4% of patients. In a classic study by Stryker et al44 using barium enema for surveillance, the cumulative 5-year and 10-year risks of cancer related to large colorectal polyps (≥10 mm) left in place were less than 3% and 10%, respectively. Although routine polypectomy remains indicated for large colorectal lesions, the relatively indolent nature suggested by this study matches the static data for 1-2 cm polyps discussed above. In the National Polyp Study, the high observed adenoma detection rates noted at surveillance, in conjunction with the low observed colorectal cancer incidence, was thought to be explainable (indirectly) only by the regression of adenomas.45 More recently, a longitudinal colonoscopy study by Togashi et al46 followed 412 diminutive polyps over an average interval of 3.6 years in patients who had previously undergone CRC resection. At the final OC examination, 74% were stable or decreased in size, 15% were increased in size, and 11% could not be identified. Of 88 resected polyps, histology showed neither HGD nor cancer in any case.
Although these longitudinal endoscopic and barium enema studies provide some reassurance of the benign course of sub-cm polyps, detail is lacking regarding polyp growth rates and clinical implications. These shortcomings are largely addressed by CTC surveillance. CTC is an ideal tool for polyp monitoring, as it allows for both precise localization and size assessment. Confirming whether a detected polyp is in the exact same location over serial studies is difficult with endoscopy but rather straightforward with CTC, where confident determination of specific localization is possible. Beyond improved linear size measurement, CTC also has the distinct advantage of volumetric assessment, which greatly amplifies interval changes in polyp size compared with linear measurement.47 Because the initial standard of care was to refer all CTC-detected polyps measuring ≥6 mm to OC for polypectomy, we undertook a prospective natural history trial to follow small (6-9 mm) polyps at CTC.48
This ongoing polyp natural history trial was initiated at the University of Wisconsin (UW) in 2004, with the National Naval Medical Center (NNMC) in Bethesda, MD joining soon after. Complementary protocols provided histology-rich data in the NNMC arm, whereby all polyps were resected after one-year CTC surveillance, and longer follow-up intervals in the UW arm, where only progressing polyps were typically referred for polypectomy. Consenting patients with only small polyps (6-9 mm in size, 1-2 in number) detected at initial CTC screening were enrolled for in vivo CTC surveillance. Progression, stability, and regression of polyps at followup CTC were based on a 20% volumetric change per year from baseline (ie, 20% or more growth for progression, –20% or more reduction for regression; and stability when annual change is <20% in either direction). Results from the initial 8-year period of investigation involved 243 adults (mean age, 57.4 years; 106 [37%] women) with 306 small colorectal polyps. The mean surveillance interval was 2.3 ± 1.4 years (range 1–7 years). In total, 68 (22%) of the 306 polyps progressed by the above volumetric criteria (Figure 4), 153 (50%) were stable, and 85 (28%) regressed, including an apparent total resolution of the polyp in 32 (10%) cases (Figure 5).
Figure 4. Interval progression of small colorectal polyps in two patients undergoing CTC surveillance.
3D colon map from CTC (A) showing the location of a small sigmoid polyp (arrow, red dot), which measured 7.8 mm at the index screening examination (B). Polyp segmentation for volume measurement is shown on both 3D and 2D (inset) views of B. At follow-up CTC 1 year later (C), the polyp grew only 0.8 mm in linear size but showed a 50% increase in volume (to 205 mm3). The lesion proved to be a tubulovillous adenoma after polypectomy at same-day colonoscopy (inset).
3D colon map (D) in a second patient showing the location of three small polyps in the right colon (arrows, red dots). 3D images from the index CTC (E) and surveillance CT colonography 16 months later (F) show a small sessile polyp in the proximal transverse colon that increased from 6.0 mm to 8.0 mm, but increased in volume by 203% (153% per year). Similar growth was seen with the two cecal polyps (not shown). The polyp in the transverse colon was a tubular adenoma, whereas the cecal lesions were both tubulovillous adenomas.
From Pickhardt PJ, Kim DH, Pooler BD, et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol 2013;14:711-20; with permission.
Figure 5. Polyp regression at computed tomography colonography (CTC) surveillance.
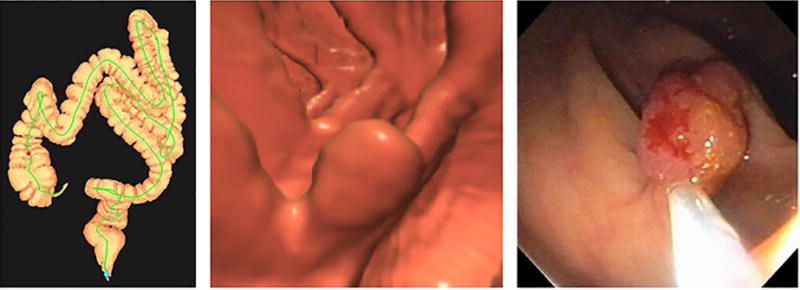
3D colon map (A) showing the location of a 6.2 mm polyp in the descending colon (arrows, red dot) detected at screening CTC (B). Surveillance CTC two years later (C) showed no interval change in size. By the time of continued surveillance (D), 6.4 years after the initial CTC, the polyp had completely resolved. Detection of the small polyp on the intermediate CT colonography in 2007 essentially excludes the possibility of a false-positive interpretation.
From Pickhardt PJ, Kim DH, Pooler BD, et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol 2013;14:711-20; with permission.
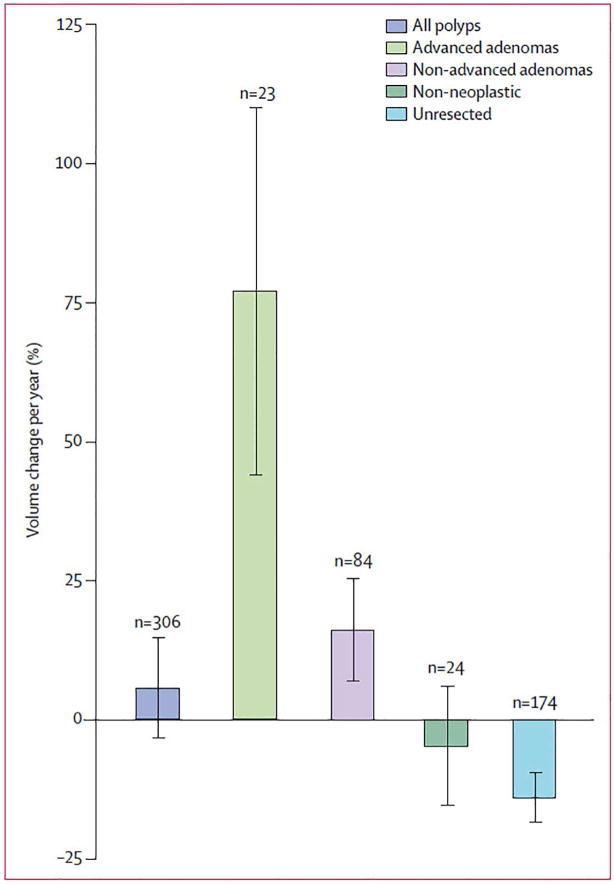
At the time of submission, polyp histology had been established in 131 lesions that were confirmed and removed at colonoscopy. Of the 23 proven advanced adenomas, 21 (91%) progressed in size; the other two advanced adenomas both had positive growth rates (8%/year) but were less than the +20%/year defining threshold. In comparison, 31 (37%) of 84 proven non-advanced adenomas progressed in size, and only 15 (8%) of 198 other resected lesions (p<0.0001) progress. Of note, serrated polyps represented a major subset of this the “other” category, but these lesions were not yet recognized as such. The odds ratio for a growing polyp at CTC surveillance to become an advanced adenoma was 15.6 (95% CI: 7.6–31.7) compared with 6–9 mm polyps detected and removed at initial CTC screening (without surveillance). Polyp volume showed a mean 77% annual increase for the 23 proven advanced adenomas, compared with a 16% annual increase for 84 proven non-advanced adenomas, and a 13% annual decrease for all other polyps, including serrated, non-neoplastic, and unresected polyps (p<0.0001) (Figure 6). An absolute polyp volume of more than 180 mm3 at surveillance CTC was predictive of advanced neoplasia with a sensitivity of 92% (22 of 24 polyps), specificity of 94% (266 of 282 polyps), positive-predictive value of 58% (22 of 38 polyps), and negative-predictive value of 99% (266 of 268 polyps).
Figure 6. Polyp growth according to histological subgroup.
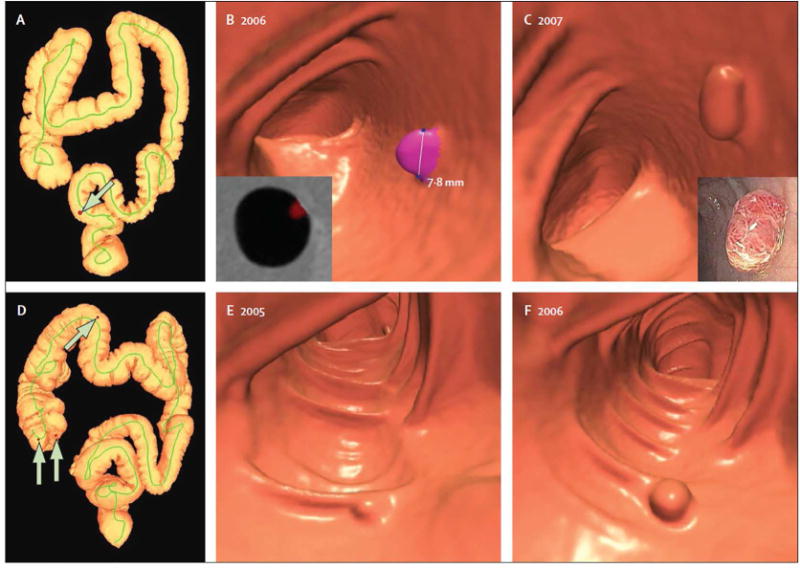
Polyp growth categories are shown according to the definition 20% volume change per year as progression or regression. Note overall mean growth in adenomas, especially those that are advanced.
From Pickhardt PJ, Kim DH, Pooler BD, et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol 2013;14:711-20; with permission.
Changes in linear size of polyps also correlated with histology in this trial, albeit the magnitude of these changes was blunted relative to the amplified volumetric changes (Figure 4). In general, linear measurement lead to an increased proportion of lesions categorized as stable. For example, the percentage of advanced adenomas categorized as stable by the three different linear size criteria (changes of <1 mm per year, <10% per year, and <25% total) ranged from 38% to 58%, compared with only 8–12% categorized as stable for three volumetric criteria (changes of <20% per year, <15 mm3 per year, and <30% total). Only 5% of all 6-9 mm polyps exceeded 10 mm in size at final CTC follow-up. Lesion morphology also correlated with future growth pattern, with 45% of pedunculated polyps showing growth, compared with 21% of sessile polyps and 8% of flat lesions (p<0.0001). Again, our experience with flat lesions argues against a more aggressive nature.
A second longitudinal study of small colorectal polyps utilizing CTC surveillance was published two years later by a Dutch group.49 A total of 70 patients with one or two 6–9 mm colorectal polyps identified at the index CTC underwent surveillance a mean 3.3 years later. Of these, 57 patients underwent subsequent colonoscopy with polypectomy of 68 polyps. Defining progression as a ≥30% increase in polyp volume, the investigators found that after 3 years, 35% of polyps progressed in size, 38% remained stable, and 27% regressed, with apparent resolution in 14%. Advanced histology was present in 47% of the progressing polyps, 21% of stable polyps, and none of the regressing polyps. None of the resected polyps harbored HGD or CRC. Overall, these results are in concert with our natural history trial.48
Relatively little longitudinal work has focused on diminutive colorectal lesions (≤5 mm) in size. This reality is due in part to the difficulty of confidently identifying these lesions on index and follow-up studies, but also the fact that if small (6-9 mm) polyps are safe to follow, diminutive lesions would be as well. The Japanese colonoscopic surveillance by Togashi et al46 described above showed that, even high-risk CRC patients, diminutive lesions are of little or no immediate concern. In reviewing our own experience with routine 5-10 year CTC screening after initial negative screening in 1429 adults,15 we were able to indirectly assess the natural history of diminutive lesions. Because potential isolated diminutive lesions (ie, without associated polyps ≥6 mm) constitute a negative CTC screening exam, the 5-year CTC routine screening follow-up effectively represents a surveillance study for these highly prevalent diminutive lesions. Given the acceptably low rate of positive non-diminutive findings at followup screening (lower than the initial round of CTC screening), this study provided further evidence that non-reporting of isolated diminutive lesions at CTC screening is a valid clinical approach. Not surprisingly, we were able to identify some diminutive lesions that progressed to non-diminutive polyps (Figure 7). Interestingly, of the non-diminutive lesions missed at initial CTC screening but detected at follow-up, many were large flat, right-sided serrated lesions. The natural history of serrated lesions has not been well established, and has been the source of ongoing debate. In our CTC experience to date, these flat serrated lesions tend to show a very indolent course, with little or no growth seen in most cases, even at five or more years (Figure 8). In general, flat lesions appear to portend a lower risk for future advanced neoplasia, an observation noted by the Pathologist from the National Polyp Study.50 Given our ever-expanding experience with serrated lesions at CTC, we specifically intend to study their natural history in the near future.
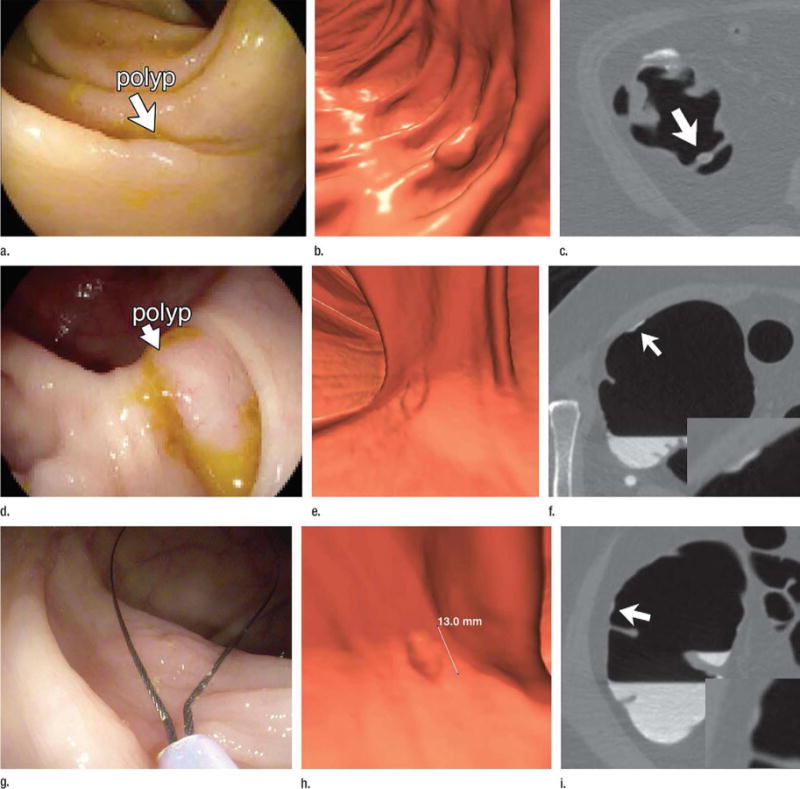
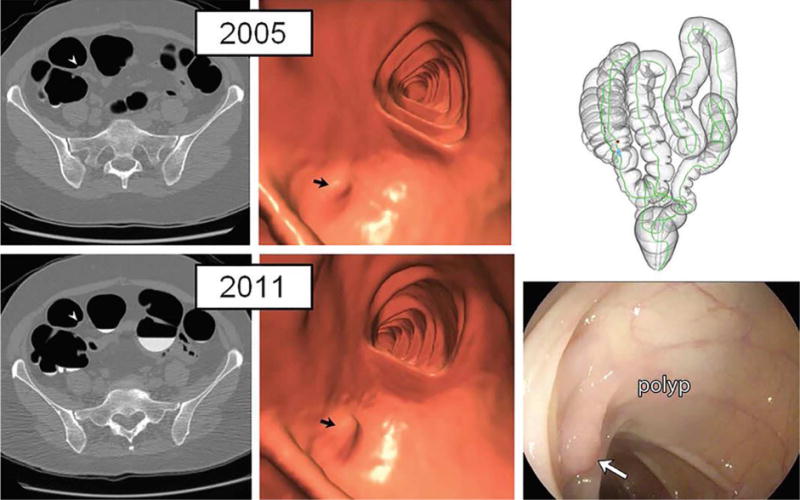
Figure 7. Diminutive polyp at initial CT colonography screening that grew to small size at follow-up screening 6 years later in an asymptomatic woman (61 years old at initial screening).
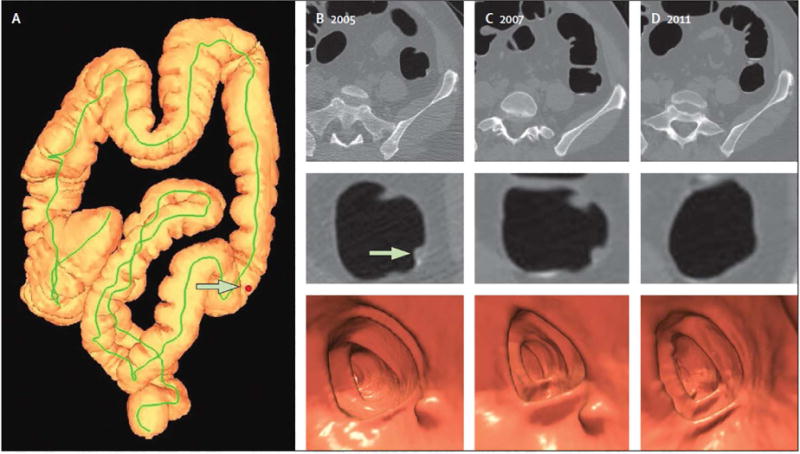
Top: two-dimensional (2D) (left) and three-dimensional (3D) (middle) images from the initial CTC screening in 2005 show a diminutive lesion (arrowhead for 2D, arrow for 3D) measuring less than 5 mm in the proximal transverse colon. The specific colonic location is indicated on the colon map (right) by the red dot. We do not report isolated diminutive lesions at CT colonography screening.
Bottom: 2D (left) and 3D (middle) images from repeat CT colonography screening in 2011 show that the sessile polyp has grown in the intervening 6 years, now measuring 7 mm (arrowhead for 2D, arrow for 3D). The polyp was confirmed (arrow) and removed at same-day colonoscopy (right) and proved to be a tubular adenoma at pathologic evaluation. The vast majority of diminutive lesions do not progress to non-diminutive size.
From Pickhardt PJ, Pooler BD, Mbah I, et al. Colorectal Findings at Repeat CT Colonography Screening after Initial CT Colonography Screening Negative for Polyps Larger than 5 mm. Radiology 2017;282:139-48; with permission.
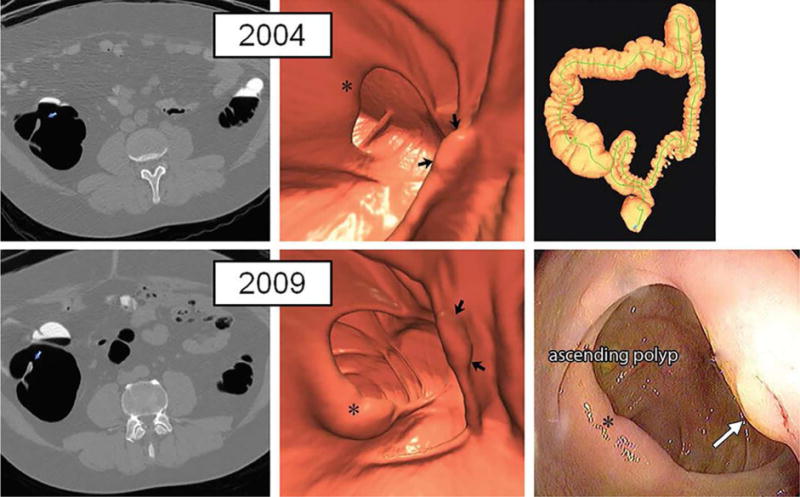
Figure 8. Large right-sided flat serrated lesion missed at initial CTC screening that was detected at follow-up screening 5 years later in an asymptomatic man (50 years old at initial screening).
Top: two-dimensional (2D) (left) and three-dimensional (3D) (middle) images from the initial CT colonography screening in 2004 show a subtle flat lesion (arrows) was missed in the ascending colon just distal to the ileocecal valve (*). The specific colonic location is indicated on the colon map (right) by the red dot. Little or no contrast material coating of the polyp surface is seen. Bottom: 2D (left) and 3D (middle) images from repeat CT colonography screening in 2009 show the same flat lesion (arrows), which measured 12 mm without significant change in size from 2004. The lesion now demonstrates subtle contrast coating, which increases conspicuity and reader confidence. The polyp was confirmed (arrow) and removed at same-day colonoscopy (right) and proved to be a sessile serrate polyp at pathologic evaluation. The asterisk indicates ileocecal valve.
From Pickhardt PJ, Pooler BD, Mbah I, et al. Colorectal Findings at Repeat CT Colonography Screening after Initial CT Colonography Screening Negative for Polyps Larger than 5 mm. Radiology 2017;282:139-48; with permission.
We have also assessed the theoretical cost-effectiveness of immediate polypectomy versus 3-year CTC surveillance for small and diminutive polyps detected at CTC screening.34,36 If all detected diminutive lesions at CTC screening were referred for OC polypectomy, an estimated 2,352 lesions would need to be resected to prevent one cancer over 10 years, at a cost of $464,407 per life-year gained. Without any intervention, we estimated that the 5-year CRC death rate for patients with unresected 6-9 mm polyps was 0.08%, which already represents a seven-fold decrease from the 0.56% 5-year colorectal cancer death rate in the general screening population, most of whom do not harbor non-diminutive polyps. Therefore, for patients with 6-9 mm polyps detected at CTC screening, the exclusion of large polyps (≥10 mm) already confers a very low CRC risk. For the concentrated cohort with a small polyp, the death rate was further reduced to 0.03% with the CTC surveillance strategy and 0.02% with immediate colonoscopy referral. However, for each additional cancer-related death prevented with immediate polypectomy versus CTC follow-up, 10,000 additional colonoscopy referrals would be needed, resulting in 10 perforations and an exorbitant incremental cost-effectiveness ratio of $372,853. These modeling simulations further support the practices of CTC surveillance for small polyps, and for non-reporting of diminutive lesions.
The issue of “interval cancers” has received considerable attention in the recent GI literature, and relates to a discussion on polyp natural history. An interval cancer may be defined as CRC diagnosed after a screening or surveillance exam in which no cancer was detected and before the date of the next recommended exam.51 Possible explanations for interval CRCs following a negative OC (or CTC) exam would include missed lesions, incompletely resected lesions, and rapidly-growing new lesions. It is generally accepted that the majority of interval cancers following OC represent missed lesion at the index colonoscopy.52 Most interval cancers are right-sided, some of which are likely related to the alternate serrated pathway. Although early on this has led some to hypothesize that SSP/As might therefore be fast growing,53 it appears more likely that these lesions were repeatedly missed.16 Issues relating to decreased protection from right-sided cancer at OC are well established.54,55 In general, CTC has advantages over OC for evaluating the right colon (whereas the opposite may be true for the left colon), given its ability to distend the right colon and evaluate “behind” folds (Figures 3 and 9).25,56 Our experience with interval cancers following negative CTC suggest that the rates are lower relative to OC.15,57
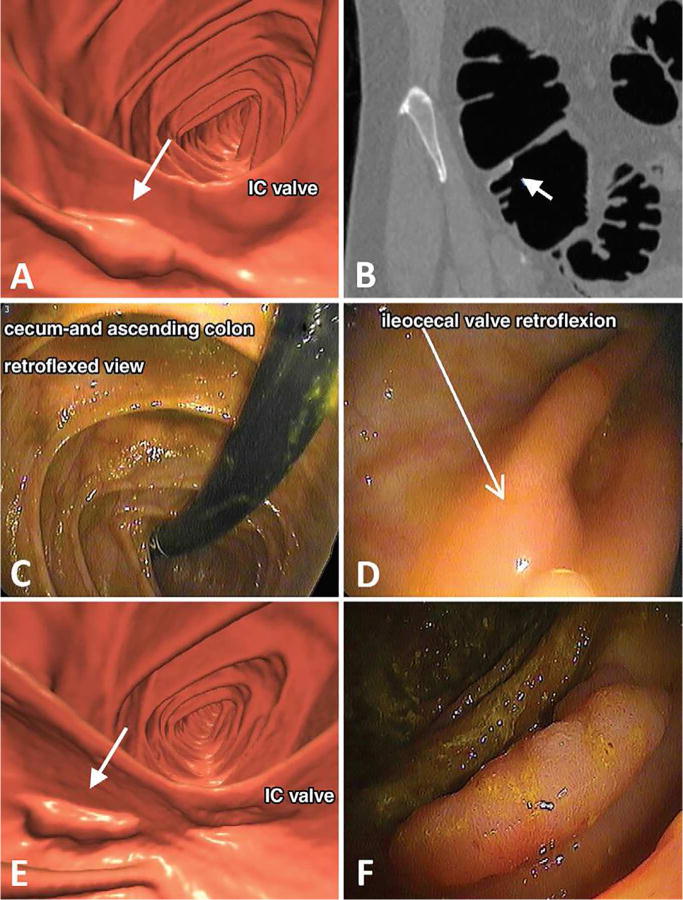
Figure 9. Large advanced tubular adenoma missed at initial non-blinded OC after prospective detection at initial CT colonography screening.
A, Three-dimensional (3D) endoluminal CTC view shows a 2-cm sessile polyp (arrow) detected behind a cecal fold adjacent to the ileocecal (IC) valve. B, Coronal two-dimensional (2D) CTC image confirms a true mucosal-based polyp (arrow). A diminutive rectal lesion (not shown) was also detected and incidentally noted in the CT colonography report. C, D, Retroflexed images from same-day OC referral show the (C) cecum and ascending colon and (D) dedicated evaluation of the ileocecal valve fold (arrow in D). The polyp was not found despite previous knowledge of specific location and thorough inspection. The diminutive rectal lesion was confirmed and proved to be a hyperplastic polyp. The OC report recommended follow-up OC in 5 years. E, Because of the standard expert discordant review process, repeat CT colonography with same-day OC, if needed, was recommended, and repeat CT colonography 9 months later shows the 2-cm polyp (arrow). F, Repeat OC performed by a different gastroenterologist confirms the large polyp behind a fold, which was resected and proved to be a tubular adenoma, advanced according to size criteria (10 mm).
From Pooler BD, Kim DH, Weiss JM, et al. Colorectal Polyps Missed with Optical Colonoscopy Despite Previous Detection and Localization with CT Colonography. Radiology 2016;278:422-429; with permission.
In summary, the cumulative evidence on natural history shows that polyp growth assessment at CTC surveillance is a useful biomarker for determining the clinical importance of small polyps, and that a 3-year interval is reasonable. Furthermore, diminutive lesions can safely be ignored for at least five years, and large lesions should generally be referred for polypectomy, even though their immediate risk is relatively small unless mass-like in appearance. This reinforces the initial recommendations made when the C-RADS classification system was first conceived.37 Advanced adenomas, the primary target of CRC prevention, generally show more rapid growth than non-advanced adenomas, whereas most other small polyps remain relatively stable or regress. Flat lesions tend to be less aggressive but should eventually be removed when large in size. Collectively, these findings might allow for less invasive surveillance strategies, reserving polypectomy for lesions that show substantial or rapid growth. Further research is needed to regarding the ultimate fate of unresected small polyps without significant growth at initial follow-up. To that end, we now have another 5-6 years of additional polyp follow-up data, with CTC surveillance on over 750 total polyps, which we are currently in the process of further analyzing.
Radiogenomic Correlation and Novel Tumorigenesis Theories
The long-held classical view on CRC formation is that tumors arise via the gradual stepwise accumulation of mutations.58,59 At each step, a new mutation was thought to generate a more advantageous sub-clone that would outcompete less-fit clones in a Darwinian fashion. Under such a stepwise or linear evolution model, all tumors should have the potential to progress from a benign to malignant state, typically over a decade or two, and the resulting cancers should be relatively homogeneous, based on natural selection of fitness. However, emerging technologies and “big data” solutions that allow for rapid analysis of DNA, RNA, and proteins are revealing vast amounts of intra-tumoral heterogeneity.60 In concert, more recent experience with CTC surveillance consisting of multiple follow-up exams has allowed us to see that colorectal polyps demonstrate a variety of growth behaviors. Some polyps will grow and progress, others may grow for a period of time and then remain static in size, whereas some may grow for a while and then regress below our limits of detection. Mutational analysis of polyps with varying fates by CTC has revealed sub-clonal mutations that must have arisen very early on before the polyp was of a detectable size (Figure 10).61 In addition, other investigators have uncovered epigenetic methylation alterations early on in benign and even non-neoplastic colonic tissue.62 These findings indicate that some precursor lesions might be “born to be bad”,63 where early molecular events may dictate later tumor growth and progression. These new observations render the classic stepwise model of tumor evolution inadequate to explain the degree of molecular and phenotypic intra- and inter-tumoral heterogeneity observed in CRC tumorigenesis. In response, new evolutionary theories of tumorigenesis have been proposed, which may provide new insights into tumor formation and progression to invasive disease.
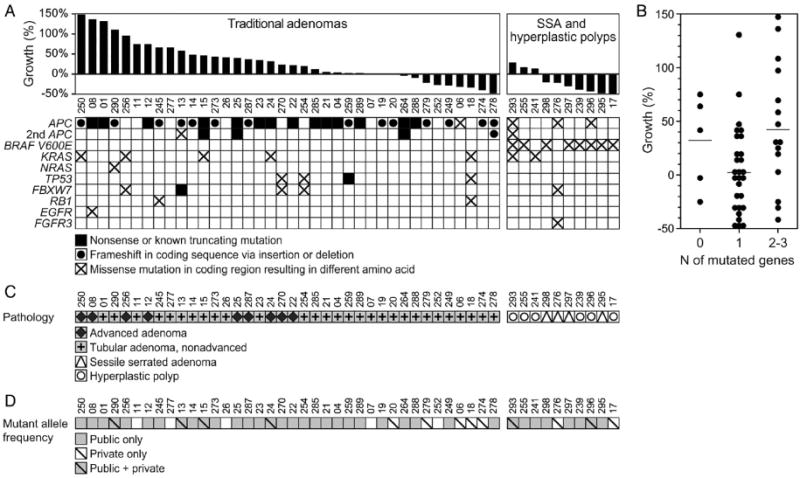
Figure 10. Small polyps often carried multiple pathogenic mutations.
(A) Mutation profile of polyps with known growth fates is shown. Only well-annotated, known pathogenic variants are included. (B) Small polyps had 0–3 pathogenic mutations. Horizontal lines represent the mean (p-value=0.044). The difference between polyps with one mutation and those with two or more was significant. (C) The pathology of polyps with known growth fates (A) compared with mutation frequency. (D) The mutations can be classified as public, that is, clonal with an adjusted allele frequency of ≥30% or private, that is, subclonal with an adjusted allele frequency of 5%–30%. Small polyps with only private mutation(s) tended to regress. Private only versus public only and public and private were significantly different.
From Sievers CK, Zou LS, Pickhardt PJ, et al. Subclonal diversity arises early even in small colorectal tumours and contributes to differential growth fates. Gut 2017;66:2132-40; with permission.
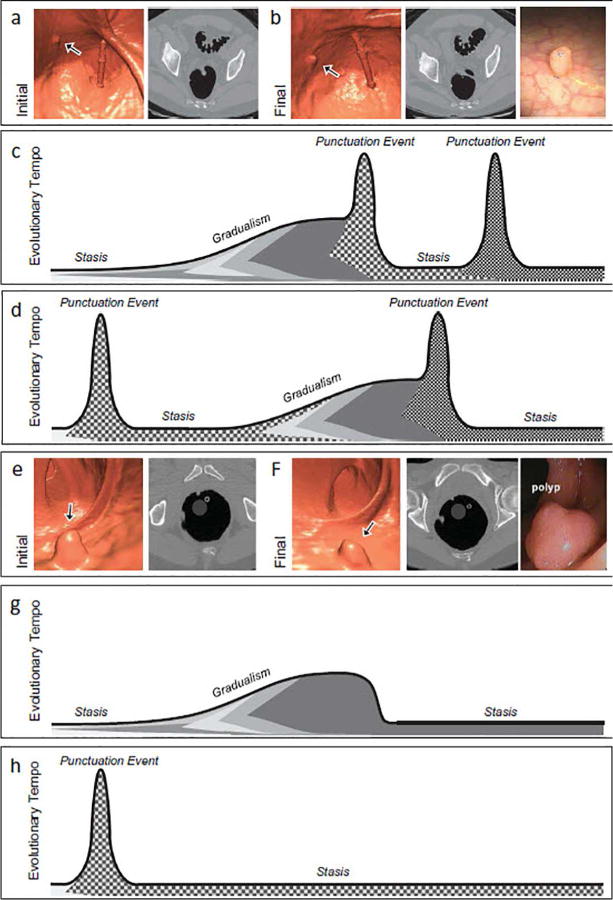
Two novel theories on colorectal tumorigenesis have been proposed in response to these newer observations, namely, the “big bang” and “punctuated equilibrium” models of tumor evolution.60,64 The big bang theory asserts many mutations and copy number alterations are generated within the first few neoplastic cell divisions leading to the development of an adenoma. Once all the necessary driver mutations are acquired, cancers grow from a single expansion of a diverse population of tumor cells, characterized by neutral evolution instead of Darwinian survival. This model helps to account for the presence of intra-tumoral heterogeneity not explained by the stepwise growth model, which arises as a function of time, and not as a function of increased fitness. As such, more recently acquired mutations will be at lower frequencies, often undetectable by bulk genomic methodologies. This possibility also might help explain why drug-resistant sub-clones are undetectable prior to treatment in the clinical setting. The big bang growth model also allows for variable tumor growth fates. More recently, the punctuated equilibrium theory was advanced as a more comprehensive model of tumor evolution. This model seeks to better explain how tumors evolve, including latent periods and gradual change, punctuated by rapid periods of transformation (Figure 11). Three evolutionary phases are at work in this model: stasis, gradualism, and punctuation events. The stasis phase is characterized by the neutral accrual of passenger mutations, resulting in the stable phenotype. The gradualism phase is most similar to the classic stepwise growth model, in which molecular changes are acquired in a sequential manner, under natural selection, resulting in a quantifiable impact on the phenotype. Finally, punctuation events are derived from the big bang growth model, whereby periods of genomic instability result in many simultaneous molecular changes, often leading to dramatic phenotypic changes. These punctuation events are not restricted to genetic mutations, but may also include epigenetic and transcriptional alternations that result in a phenotypic change.
Figure 11. The cancer punctuated equilibrium model of colon tumor evolution better explains the variability of colorectal polyp growth.
CTC images from the initial (a) and final (b) scans from a patient with polyp that had an annual volumetric growth rate of 59% that was followed over 2.1 years prior to polypectomy. Black arrows point to the polyp that was followed longitudinally. (c) and (d) are possible evolutionary trajectories for a growing polyp. Shading under the line represent levels of intratumoral heterogeneity with punctuation events creating the greatest amount of heterogeneity. Tumorigenesis may begin with a punctuation event or periods of stasis and gradualism, a second punctuation event may provide enough molecular diversity allowing for malignant transformation. (e) and (f) are CTC images from the initial and final scans from a patient with polyp that had an annual volumetric growth rate of −33% that was followed over 0.9 years prior to polypectomy. (g) and (h) are possible evolutionary trajectories for a regressing polyp. Tumor regression may occur with the emergence of a negative or immunogenic phenotype acquired during a period of gradualism or via a punctuation event.
From Sievers CK, Grady WM, Halberg RB, et al. New insights into the earliest stages of colorectal tumorigenesis. Expert Rev Gastroenterol Hepatol 2017;11:723-9; with permission.
The inclusion of these three mechanisms of evolutionary change into this punctuated equilibrium model of CRC development appears to better explain the variability of polyp growth patterns at CTC and intra-tumoral heterogeneity. The clinical observation of different growth fates at CTC cannot be accounted for in the classical step-wise acquisition model, especially for static and regressing lesions. CTC also has the potential to assess intra-tumoral heterogeneity in vivo through texture analysis. Although typically applied to malignancy, either the primary cancer or metastatic foci,65,66 CT texture analysis appears to be feasible even for benign colorectal polyps.67,68 In summary the punctuated equilibrium model of tumor evolution does seem to better fit what has been observed on both the molecular and gross tumor levels. However, the precise timing of these mechanisms during tumorigenesis and evolution, as well as the specific dynamics between multiple clones, remains unknown. By harnessing our unique CTC polyp surveillance experience, we hope to further investigate these questions. Specifically, we plan to further study our ongoing CTC-based cohort, correlating growth rates with whole exome sequencing, transcriptional profiling, and methylation profiling of the polyps when ultimately resected. With a greater understanding of polyp growth and progression behavior, screening intervals may increase or decrease for patients with certain low-risk or high-risk molecular characteristics. Additionally, a deeper understanding of colorectal tumor evolution might allow for more precise and less-invasive screening strategies in the future.
Conclusion
Substantial cross-sectional or static data exist on polyp features that correlate with increased cancer risk, but are limited by the lack of longitudinal information. Cumulative natural history data from in vivo polyp surveillance have repeatedly shown the benign nature of sub-cm colorectal polyps, rendering diminutive lesions of little or no clinical relevance and demonstrating that in vivo surveillance of small (6-9 mm) polyps is a rational approach on both clinical and economic grounds. In terms of polyp management, this supports the CTC mantra of “ignore the tiny, watch the small, and remove the large”. Emerging longitudinal polyp data from CTC surveillance and novel models of tumor evolution are challenging the classical theories of tumorigenesis. Further radiogenomic investigation should provide even further insights into cancer formation, which may one day alter clinical management strategies.
Key Points.
Sub-centimeter colorectal polyps are highly prevalent in adults.
Sub-centimeter colorectal polyps are invariably benign, and the vast majority will never develop into cancer.
Polyp size is an important determinant of clinical relevance and management.
Polyp growth rates provide further insight into natural history and clinical significance.
CT colonography is unique among screening tools by allowing for accurate assessment of volumetric growth rates, which are likely tied to underlying genetic and epigenetic alterations.
Acknowledgments
This work was supported in part by NIH NCI grant 1R01 CA220004-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Pickhardt is co-founder of VirtuoCTC; advisor to Check-Cap and Bracco; and shareholder in SHINE, Elucent, and Cellectar Biosciences; Dr. Kim is co-founder of VirtuoCTC and shareholder in Elucent and Cellectar Biosciences.
References
- 1.Pickhardt PJ, Hain KS, Kim DH, Hassan C. Low rates of cancer or high-grade dysplasia in colorectal polyps collected from computed tomography colonography screening. Clin Gastroenterol Hepatol. 2010;8:610–5. doi: 10.1016/j.cgh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Pickhardt PJ, Kim DH. Colorectal cancer screening with CT colonography: key concepts regarding polyp prevalence, size, histology, morphology, and natural history. American Journal of Roentgenology. 2009;193:40–6. doi: 10.2214/AJR.08.1709. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp Size and Advanced Histology in Patients Undergoing Colonoscopy Screening: Implications for CT Colonography. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponugoti PL, Cummings OW, Rex DK. Risk of cancer in small and diminutive colorectal polyps. Dig Liver Dis. 2017;49:34–7. doi: 10.1016/j.dld.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Hassan C, Pickhardt PJ, Kim DH, et al. Systematic review: distribution of advanced neoplasia according to polyp size at screening colonoscopy. Aliment Pharmacol Ther. 2010;31:210–7. doi: 10.1111/j.1365-2036.2009.04160.x. [DOI] [PubMed] [Google Scholar]
- 6.Pickhardt PJ, Lam VP, Weiss JM, Kennedy GD, Kim DH. Carpet Lesions Detected at CT Colonography: Clinical, Imaging, and Pathologic Features. Radiology. 2014;270:435–43. doi: 10.1148/radiol.13130812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess NG, Hourigan LF, Zanati SA, et al. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology. 2017;153:732–42 e1. doi: 10.1053/j.gastro.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. Jama-Journal of the American Medical Association. 2008;299:1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt PJ, Levin B, Bond JH. Screening for nonpolypoid colorectal neoplasms. JAMA: the journal of the American Medical Association. 2008;299:2743. doi: 10.1001/jama.299.23.2743-a. author reply -4. [DOI] [PubMed] [Google Scholar]
- 10.Pickhardt PJ, Kim DH, Robbins JB. Flat (Nonpolypoid) Colorectal Lesions Identified at CT Colonography in a US Screening Population. Academic Radiology. 2010;17:784–90. doi: 10.1016/j.acra.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Lubner MG, Cahoon AR, Pooler BD, Pickhardt PJ. Flat Serrated Polyps at CT Colonography: Relevance, Appearance, and Optimizing Interpretation. Radiographics. 2017;170110 doi: 10.1148/rg.2018170110. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Matkowskyj KA, Lubner MG, et al. Serrated Polyps at CT Colonography: Prevalence and Characteristics of the Serrated Polyp Spectrum. Radiology. 2016;280:455–63. doi: 10.1148/radiol.2016151608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine - A morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124:380–91. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 14.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–30. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 15.Pickhardt PJ, Pooler BD, Mbah I, Weiss JM, Kim DH. Colorectal Findings at Repeat CT Colonography Screening after Initial CT Colonography Screening Negative for Polyps Larger than 5 mm. Radiology. 2017;282:139–48. doi: 10.1148/radiol.2016160582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and Variable Detection of Proximal Colon Serrated Polyps During Screening Colonoscopy. Clinical Gastroenterology and Hepatology. 2011;9:42–6. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Hinshaw JL, Lubner MG, del Rio AM, Pooler BD, Pickhardt PJ. Contrast coating for the surface of flat polyps at CT colonography: a marker for detection. European Radiology. 2014;24:940–6. doi: 10.1007/s00330-014-3095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor SD, Summers RM, Choi JR, Pickhardt PJ. Oral contrast adherence to polyps on CT colonography. Journal of Computer Assisted Tomography. 2006;30:51–7. doi: 10.1097/01.rct.0000191686.35968.f1. [DOI] [PubMed] [Google Scholar]
- 19.Ijspeert JEG, Bevan R, Senore C, et al. Detection rate of serrated polyps and serrated polyposis syndrome in colorectal cancer screening cohorts: a European overview. Gut. 2017;66:1225–32. doi: 10.1136/gutjnl-2015-310784. [DOI] [PubMed] [Google Scholar]
- 20.Burgess NG, Pellise M, Nanda KS, et al. Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps. Gut. 2016;65:437–46. doi: 10.1136/gutjnl-2014-308603. [DOI] [PubMed] [Google Scholar]
- 21.Hassan C, Repici A. Recent Advances in Diagnostic Colonoscopy for Colorectal Cancer Screening: An Update for Radiologists. American Journal of Roentgenology. 2017;209:88–93. doi: 10.2214/AJR.17.17863. [DOI] [PubMed] [Google Scholar]
- 22.Radaelli F, Paggi S, Hassan C, et al. Split-dose preparation for colonoscopy increases adenoma detection rate: a randomised controlled trial in an organised screening programme. Gut. 2017;66:270–7. doi: 10.1136/gutjnl-2015-310685. [DOI] [PubMed] [Google Scholar]
- 23.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. New England Journal of Medicine. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 24.Pickhardt PJ, Taylor AJ, Gopal DV. Surface visualization at 3D endoluminal CT colonography: Degree of coverage and implications for polyp detection. Gastroenterology. 2006;130:1582–7. doi: 10.1053/j.gastro.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 25.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Annals of Internal Medicine. 2004;141:352–9. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 26.Pooler BD, Kim DH, Weiss JM, Matkowskyj KA, Pickhardt PJ. Colorectal Polyps Missed with Optical Colonoscopy Despite Previous Detection and Localization with CT Colonography. Radiology. 2015;150294 doi: 10.1148/radiol.2015150294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pooler BD, Kim DH, Hassan C, Rinaldi A, Burnside ES, Pickhardt PJ. Variation in diagnostic performance among radiologists at screening CT colonography. Radiology. 2013;268:127–34. doi: 10.1148/radiol.13121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan C, Repici A. Defeating Cancer by Boosting the Adenoma Detection Rate: The Circle of Life. Gastroenterology. 2017;153:8–10. doi: 10.1053/j.gastro.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Kaminski MF, Wieszczy P, Rupinski M, et al. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology. 2017;153:98–105. doi: 10.1053/j.gastro.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Hassan C, Pickhardt PJ, Rex DK. A Resect and Discard Strategy Would Improve Cost-Effectiveness of Colorectal Cancer Screening. Clin Gastroenterol H. 2010;8:865–9. doi: 10.1016/j.cgh.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Pickhardt PJ. Emerging stool-based and blood-based non-invasive DNA tests for colorectal cancer screening: The importance of cancer prevention in addition to cancer detection. Abdom Radiol. 2016 doi: 10.1007/s00261-016-0798-4. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. New England Journal of Medicine. 2007;357:1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 33.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 34.Pickhardt PJ, Hassan C, Laghi A, et al. Small and diminutive polyps detected at screening CT colonography: a decision analysis for referral to colonoscopy. AJR Am J Roentgenol. 2008;190:136–44. doi: 10.2214/AJR.07.2646. [DOI] [PubMed] [Google Scholar]
- 35.Pickhardt PJ, Hassan C, Laghi A, Zullo A, Kim DH, Morini S. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: the impact of not reporting diminutive lesions. Cancer. 2007;109:2213–21. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 36.Pickhardt PJ, Hassan C, Laghi A, et al. Clinical management of small (6- to 9-mm) polyps detected at screening CT colonography: a cost-effectiveness analysis. AJR Am J Roentgenol. 2008;191:1509–16. doi: 10.2214/AJR.08.1010. [DOI] [PubMed] [Google Scholar]
- 37.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: a consensus proposal. Radiology. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 38.Welin S, Youker J, Spratt JS., Jr The Rates and Patterns of Growth of 375 Tumors of the Large Intestine and Rectum Observed Serially by Double Contrast Enema Study (Malmoe Technique) Am J Roentgenol Radium Ther Nucl Med. 1963;90:673–87. [PubMed] [Google Scholar]
- 39.Hofstad B, Vatn M, Larsen S, Osnes M. Growth of Colorectal Polyps - Recovery and Evaluation of Unresected Polyps of Less-Than 10 Mm, 1 Year after Detection. Scand J Gastroenterol. 1994;29:640–5. doi: 10.3109/00365529409092485. [DOI] [PubMed] [Google Scholar]
- 40.Hofstad B, Vatn MH, Andersen SN, et al. Growth of colorectal polyps: redetection and evaluation of unresected polyps for a period of three years. Gut. 1996;39:449–56. doi: 10.1136/gut.39.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knoernschild HE. Growth Rate and Malignant Potential of Colonic Polyps: Early Results. Surg Forum. 1963;14:137–8. [PubMed] [Google Scholar]
- 42.Hoff G, Foerster A, Vatn MH, Sauar J, Larsen S. Epidemiology of Polyps in the Rectum and Colon - Recovery and Evaluation of Unresected Polyps 2 Years after Detection. Scand J Gastroenterol. 1986;21:853–62. doi: 10.3109/00365528609011130. [DOI] [PubMed] [Google Scholar]
- 43.Bersentes K, Fennerty B, Sampliner RE, Garewal HS. Lack of spontaneous regression of tubular adenomas in two years of follow-up. American Journal of Gastroenterology. 1997;92:1117–20. [PubMed] [Google Scholar]
- 44.Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, Maccarty RL. Natural-History of Untreated Colonic Polyps. Gastroenterology. 1987;93:1009–13. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 45.Loeve F, Boer R, Zauber AG, et al. National Polyp Study Data: Evidence for regression of adenomas. Int J Cancer. 2004;111:633–9. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 46.Togashi K, Shimura K, Konishi F, et al. Prospective observation of small adenomas in patients after colorectal cancer surgery through magnification chromocolonoscopy. Diseases of the Colon & Rectum. 2008;51:196–201. doi: 10.1007/s10350-007-9106-2. [DOI] [PubMed] [Google Scholar]
- 47.Pickhardt PJ, Lehman VT, Winter TC, Taylor AJ. Polyp volume versus linear size measurements at CT colonography: Implications for noninvasive surveillance of unresected colorectal lesions. American Journal of Roentgenology. 2006;186:1605–10. doi: 10.2214/AJR.05.0760. [DOI] [PubMed] [Google Scholar]
- 48.Pickhardt PJ, Kim DH, Pooler BD, et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol. 2013;14:711–20. doi: 10.1016/S1470-2045(13)70216-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nolthenius CJT, Boellaard TN, de Haan MC, et al. Evolution of Screen-Detected Small (6-9 mm) Polyps After a 3-Year Surveillance Interval: Assessment of Growth With CT Colonography Compared With Histopathology. American Journal of Gastroenterology. 2015;110:1682–90. doi: 10.1038/ajg.2015.340. [DOI] [PubMed] [Google Scholar]
- 50.O’Brien MJ, Winawer SJ, Zauber AG, et al. Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin Gastroenterol Hepatol. 2004;2:905–11. doi: 10.1016/s1542-3565(04)00392-1. [DOI] [PubMed] [Google Scholar]
- 51.Sanduleanu S, le Clercq CMC, Dekker E, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64:1257–U203. doi: 10.1136/gutjnl-2014-307992. [DOI] [PubMed] [Google Scholar]
- 52.Adler J, Robertson DJ. Interval Colorectal Cancer After Colonoscopy: Exploring Explanations and Solutions. American Journal of Gastroenterology. 2015;110:1657–64. doi: 10.1038/ajg.2015.365. [DOI] [PubMed] [Google Scholar]
- 53.Jass JR. Serrated route to colorectal cancer: back street or super highway? J Pathol. 2001;193:283–5. doi: 10.1002/1096-9896(200103)193:3<283::AID-PATH799>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 54.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection From Right- and Left-Sided Colorectal Neoplasms After Colonoscopy: Population-Based Study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 55.Bressler B, Paszat LF, Vinden C, Li C, He JS, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: A population-based analysis. Gastroenterology. 2004;127:452–6. doi: 10.1053/j.gastro.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 56.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection–systematic review and meta-analysis. Radiology. 2011;259:393–405. doi: 10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim DH, Pooler BD, Weiss JM, Pickhardt PJ. Five year colorectal cancer outcomes in a large negative CT colonography screening cohort. European Radiology. 2012;22:1488–94. doi: 10.1007/s00330-011-2365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muto T, Bussey HJR, Morson BC. Evolution of Cancer of Colon and Rectum. Cancer. 1975;36:2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 59.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal tumor development. New England Journal of Medicine. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 60.Sievers CK, Grady WM, Halberg RB, Pickhardt PJ. New insights into the earliest stages of colorectal tumorigenesis. Expert Rev Gastroenterol Hepatol. 2017;11:723–9. doi: 10.1080/17474124.2017.1330150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sievers CK, Zou LS, Pickhardt PJ, et al. Subclonal diversity arises early even in small colorectal tumours and contributes to differential growth fates. Gut. 2017;66:2132–40. doi: 10.1136/gutjnl-2016-312232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo Y, Wong CJ, Kaz AM, et al. Differences in DNA methylation signatures reveal multiple pathways of progression from adenoma to colorectal cancer. Gastroenterology. 2014;147:418–29 e8. doi: 10.1053/j.gastro.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sottoriva A, Kang H, Ma Z, et al. A Big Bang model of human colorectal tumor growth. Nature genetics. 2015;47:209–16. doi: 10.1038/ng.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cross WCH, Graham TA, Wright NA. New paradigms in clonal evolution: punctuated equilibrium in cancer. Journal of Pathology. 2016;240:126–36. doi: 10.1002/path.4757. [DOI] [PubMed] [Google Scholar]
- 65.Lubner MG, Stabo N, Lubner SJ, et al. CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdominal imaging. 2015;40:2331–7. doi: 10.1007/s00261-015-0438-4. [DOI] [PubMed] [Google Scholar]
- 66.Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V. Assessment of Primary Colorectal Cancer Heterogeneity by Using Whole-Tumor Texture Analysis: Contrast-enhanced CT Texture as a Biomarker of 5-year Survival. Radiology. 2013;266:177–84. doi: 10.1148/radiol.12120254. [DOI] [PubMed] [Google Scholar]
- 67.Hu YF, Liang ZR, Song BW, et al. Texture Feature Extraction and Analysis for Polyp Differentiation via Computed Tomography Colonography. Ieee Transactions on Medical Imaging. 2016;35:1522–31. doi: 10.1109/TMI.2016.2518958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song BW, Zhang GP, Lu HB, et al. Volumetric texture features from higher-order images for diagnosis of colon lesions via CT colonography. International Journal of Computer Assisted Radiology and Surgery. 2014;9:1021–31. doi: 10.1007/s11548-014-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


