Abstract
Targeted expression of mammalian biliverdin IXα reductase (BVR), an enzyme that metabolically inactivates linear tetrapyrrole precursors of the phytochrome chromophore, was used to examine the physiological functions of phytochromes in the qualitative short-day tobacco (Nicotiana tabacum cv Maryland Mammoth) plant. Comparative phenotypic and photobiological analyses of plastid- and cytosol-targeted BVR lines showed that multiple phytochrome-regulated processes, such as hypocotyl and internode elongation, anthocyanin synthesis, and photoperiodic regulation of flowering, were altered in all lines examined. The phytochrome-mediated processes of carotenoid and chlorophyll accumulation were strongly impaired in plastid-targeted lines, but were relatively unaffected in cytosol-targeted lines. Under certain growth conditions, plastid-targeted BVR expression was found to nearly abolish the qualitative inhibition of flowering by long-day photoperiods. The distinct phenotypes of the plastid-targeted BVR lines implicate a regulatory role for bilins in plastid development or, alternatively, reflect the consequence of altered tetrapyrrole metabolism in plastids due to bilin depletion.
Light plays an important role in growth and development throughout the life cycle of plants. It regulates a variety of processes, including seed germination, development of chloroplasts, shoot and root growth, and the induction of flowering (Kendrick and Kronenberg, 1994; Fankhauser and Chory, 1997; Jackson and Thomas, 1997). Three discrete classes of photoreceptors have been identified including UV-B, blue/UV-A, and red/far-red photomorphogenetic receptors. Phytochromes, the class of receptors primarily responsible for light absorption in the red/far red region of the light spectrum, are the most widely studied of these photoreceptors (Sage, 1992; Furuya, 1993; Quail et al., 1995; Neff et al., 2000). A family of phytochrome genes has been identified in all angiosperms examined. These include five genes (i.e. phyA–phyE) that exist in Arabidopsis (Clack et al., 1994), perhaps up to six genes in tomato (Alba et al., 2000) and at least three in tobacco (Adam et al., 1997). Phytochrome-deficient mutants have been widely used to determine the discrete photoregulatory activities of individual phytochromes and their roles in light-mediated plant growth and development (Koornneef and Kendrick, 1994). Extensive studies have centered on the model species Arabidopsis for which multiple specific phytochrome mutants are available (for review, see Whitelam and Devlin, 1997). These studies have revealed that each phytochrome possesses distinct, and in some cases, overlapping and/or synergistic/antagonistic physiological roles (Casal, 2000).
Unlike the diversity identified for apoproteins, plants use a single linear tetrapyrrole chromophore, phytochromobilin, for the production of photoactive phytochrome (Terry et al., 1993). It is well established that phytochromobilin is synthesized from heme via the intermediacy of biliverdin IXα (BV) (Terry, 1997). Mutants in both committed steps of the chromophore biosynthesis pathway have been identified in a number of plants species (Terry, 1997). These plants exhibit photomorphogenetic defects throughout their life cycle with phenotypes consistent with deficiencies in multiple phytochrome species. Such mutants have been used to probe the global photosensory functions of phytochromes in plant development (for review, see Koornneef and Kendrick, 1994; Smith, 1995; Terry, 1997). It is interesting that all chromophore-deficient mutants retain some phytochrome responsivity, primarily as mature plants. This observation indicates the leakiness of these mutants, suggesting the presence of alternative pathways for the synthesis of the phytochrome chromophore that likely reflect the existence of multiple, redundant genes encoding key biosynthetic enzymes.
We have previously demonstrated that constitutive expression of the mammalian enzyme biliverdin reductase (BVR) in transgenic Arabidopsis yields plants with altered photomorphogenetic development throughout their life cycle (Lagarias et al., 1997; Montgomery et al., 1999). These analyses revealed BVR-dependent phenotypes to be light dependent, pleiotropic, and dependent upon the subcellular targeting of BVR, results consistent with a loss of multiple phytochrome species. As such, these studies establish the feasibility of using BVR expression to probe the physiological roles of phytochromes in vivo. Using a similar approach, experiments described here explore the global functional roles of phytochromes in the qualitative short-day plant (SDP) tobacco (Nicotiana tabacum cv Maryland Mammoth) for which no phytochrome chromophore-deficient mutants have been reported. The ability to abrogate multiple phytochrome-regulated responses including the photoperiod-dependent inhibition of flowering under long days, conditions that are not usually inductive for flowering in this species, is revealed in this study.
RESULTS
Expression of BVR in Transgenic cv Maryland Mammoth Plants
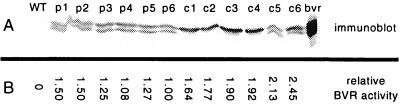
Twelve homozygous, transgenic BVR lines in cv Maryland Mammoth were obtained: six each of plastid (M-pBVR) and cytosolic (M-cBVR) BVR-expressing lines. Relative BVR specific activities of total soluble protein extracts of 10-d-old light-grown seedlings were determined for all lines. As shown in Figure 1, BVR protein levels and enzyme activity were lower in M-pBVR seedlings than in M-cBVR seedlings. The doublet observed on the immunoblot is a consequence of altered processing of the expressed enzyme as previously discussed (Lagarias et al., 1997; Montgomery et al., 1999). The range of BVR expression was not large with the highest expressing M-pBVR lines, M-pBVR1 and M-pBVR2, being 1.5-fold greater than the lowest expressing line, M-pBVR6. In general, the cytosolic lines exhibited slightly greater BVR activity than the plastid-targeted lines with the highest expressing M-cBVR line, M-cBVR6, displaying a 2.5-fold greater expression than M-pBVR6. That BVR expression reduced holophytochrome levels through inducing chromophore deficiency and not by destabilization of apophytochromes was confirmed by immunoblot analyses of phyA and phyB protein levels, which showed no significant differences between BVR transgenic plants and wild type (WT, data not shown).
Figure 1.
BVR protein and enzyme levels in soluble protein extracts from WT and transgenic M-BVR seedlings. A, Immunblot analysis. Transgenic M-BVR (denoted p1–p6 and c1–c6) and wild-type (MMWT) seedlings were grown at 25°C on phytagel medium containing 1% (w/v) Suc for 10 d under Wc illumination of 130 μmol m−2 s−1. Whole seedling soluble protein extracts (20 μg) were used for BVR immunoblot analysis with anti-BVR antibody as described in “Materials and Methods.” A mature BVR standard (Mr approximately 33 kDa) is shown in the far right lane. B, Relative BVR Activity. Seedlings were grown on phytagel medium containing 1% (w/v) Suc for 16 d at 25°C under continuous Gro-lux/Gro-lux Wide Spectrum illumination of 130 μmol m−2 s−1. Whole seedling soluble protein extracts were used for BVR assays as described in “Materials and Methods.” Values were calculated relative to the specific activity for M-pBVR6, which was determined to be 2.86 × 10−3 IU mg−1 protein.
Light-Dependent Hypocotyl and Internode Lengths Are Altered in BVR Transgenic Plants
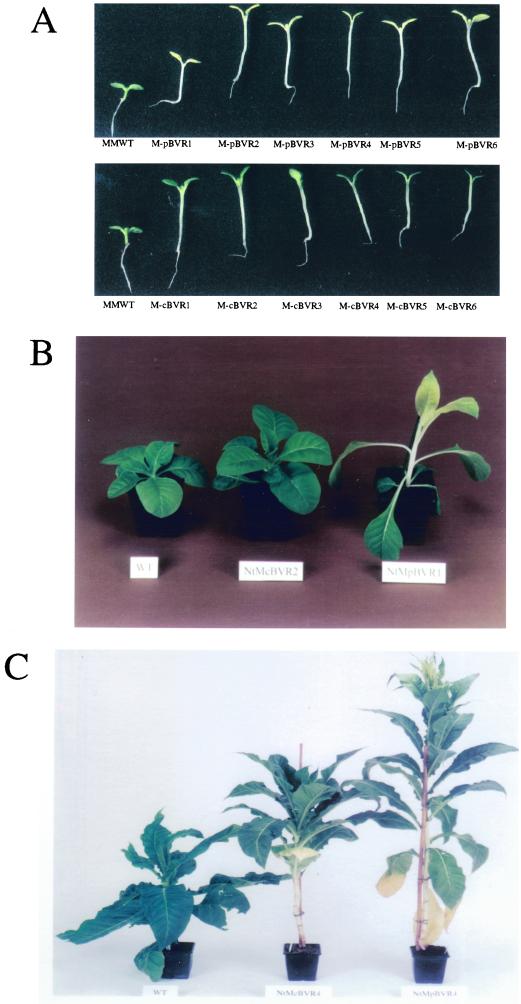
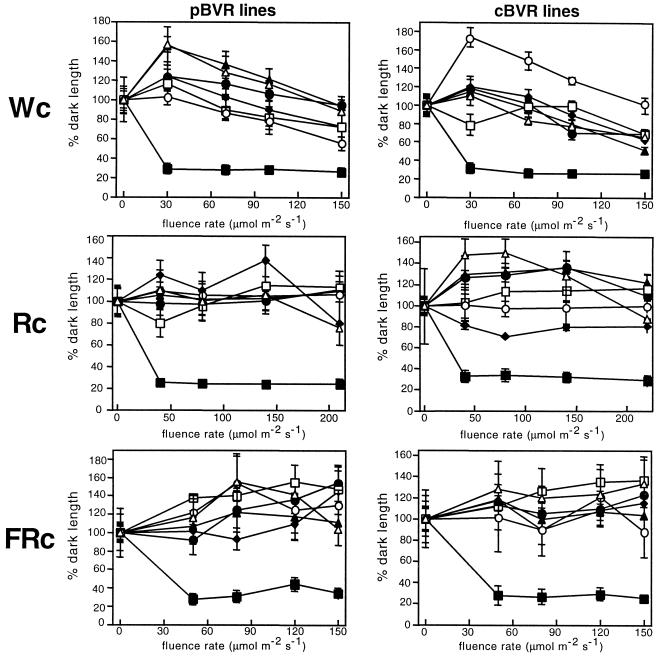
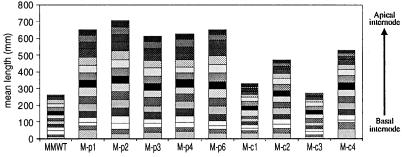
It is well established that phytochrome chromophore-deficient plants exhibit altered light-dependent growth and development throughout their life cycle. Consistent with these observations, M-pBVR and M-cBVR transgenic plants displayed aberrant phenotypes both as seedlings and as mature plants (Fig. 2). Specifically, seedlings displayed elongated hypocotyls under all fluence rates of continuous white (Wc), red (Rc), and far red (FRc) illumination examined (Figs. 2A and 3). This response was qualitatively the same for both plastid- and cytosol-targeted lines (compare Fig. 3, A, C, and E with B, D, and F) as was observed for BVR-expressing transgenic Arabidopsis (Montgomery et al., 1999). When grown in complete darkness, BVR transgenic seedlings on average had slightly longer hypocotyls than WT seedlings (Table I). This effect was more pronounced in the presence of Suc. As mature plants, M-BVR lines exhibited increased plant height with heights of all M-pBVR lines being greater than M-cBVR lines, which in turn were greater than those of WT controls (Fig. 2, B and C). This difference in height was found to be due primarily to an increase in mean internode lengths rather than an increase in total internode number (Fig. 4). This effect was more severe for M-pBVR lines than for M-cBVR lines. Leaf shape and leaf area/length ratios were also altered in M-pBVR lines that exhibited a marked decrease in area/length ratios whereas M-cBVR lines were comparable with WT plants (Fig. 2B and data not shown).
Figure 2.
Light-grown wild-type and BVR transgenic plants. A, cv Maryland Mammoth WT seedlings and transgenic M-BVR lines were grown at 25°C on phytagel medium containing 1% (w/v) Suc for 10 d under Wc illumination of 200 μmol m−2 s−1. Mature WT and representative transgenic M-cBVR and M-pBVR plants were grown under white illumination with a 16-h light/8-h dark photoperiod of 350 μmol m−2 s−1 at 22°C for 4 weeks (B) and 14 weeks (C).
Figure 3.
Hypocotyl lengths of wild-type and transgenic BVR seedlings under Wc, Rc, and FRc illumination. Plastid-targeted BVR lines (left) and cytosolic BVR lines (right) are compared with cv Maryland Mammoth WT seedlings grown at 25°C on phytagel medium containing 1% (w/v) Suc for 10 d under Wc, Rc, and FRc illumination of various fluence rates. Data points represent mean (± sd) of hypocotyl lengths measured for 10 to 25 seedlings and correspond to MMWT (▪), p/c-BVR1 (●), p/c-BVR2 (▴), p/c-BVR3 (♦), p/c-BVR4 (□), p/c-BVR5 (○), and p/c-BVR6 (▵) lines.
Table I.
Mean hypocotyl lengths for dark-grown wild-type and BVR transgenic plants on Suc-enriched or Suc-free media
| Plant Line | Hypocotyl Length
|
|
|---|---|---|
| − Suc | +Suc | |
| mm | ||
| MMWT | 22.5 (±2.6) | 23.1 (±3.8)b |
| M-pBVR1 | 24.6 (±2.9)a | 26.4 (±3.6)b |
| M-pBVR2 | 25.6 (±1.7)a | 29.9 (±3.1)b |
| M-pBVR3 | 27.0 (±1.8)a | 27.1 (±3.3)b |
| M-pBVR4 | 25.5 (±2.1)a | 28.9 (±4.0)b |
| M-pBVR5 | 25.0 (±3.3)a | 26.3 (±2.5)b |
| M-pBVR6 | 27.6 (±2.1)a | 32.8 (±1.4)b |
| M-cBVR1 | 27.3 (±1.2)c | 27.0 (±0.2)d |
| M-cBVR2 | 26.4 (±1.7)c | 29.6 (±2.7)d |
| M-cBVR3 | 21.2 (±3.7)c | 25.0 (±3.3)d |
| M-cBVR4 | 26.9 (±2.4)c | 28.6 (±2.3)d |
| M-cBVR5 | 28.9 (±1.5)c | 29.3 (±1.7)d |
| M-cBVR6 | 24.6 (±2.3)c | 27.8 (±2.4)d |
Seedlings were grown for 15 d at 25°C in continuous darkness. Hypocotyl length values are means (±sd) measured for 10 to 25 seedlings. The average hypocotyl lengths of BVR lines grown in the absence and presence of 1% (w/v) Suc are indicated to the right of the individual values.
Average for six lines = 25.9 (±2.3).
Average for six lines = 28.6 (±3.0).
Average for six lines = 25.9 (±2.1).
Average for six lines = 27.9 (±2.1).
Figure 4.
Mean internode lengths of wild-type and transgenic BVR plants. Transgenic M-pBVR lines, M-cBVR lines, and cv Maryland Mammoth WT plants were grown in controlled environment growth chambers as described in “Materials and Methods.” Individual bars represent the mean of 10 independent measurements of the length of each internode position.
Anthocyanin Levels Are Reduced by BVR Expression
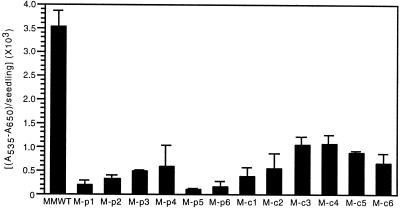
Numerous studies have shown that phytochrome plays a key role in the regulation of anthocyanin biosynthesis in a variety of plant species (Lange et al., 1970; Mancinelli et al., 1991; Kerckhoffs and Kendrick, 1997). In this regard, results from experiments with BVR-expressing Arabidopsis plants definitively established that BVR expression disrupted Suc-stimulated anthocyanin accumulation (Montgomery et al., 1999). Figure 5 shows that anthocyanin accumulation was adversely affected by BVR expression in all M-BVR transgenic seedlings tested with the effect again being greater for M-pBVR expressing lines than for M-cBVR transgenic lines. In contrast with Arabidopsis, Suc did not have a significant inductive effect on anthocyanin accumulation in WT or BVR transgenic cv Maryland Mammoth tobacco plants (data not shown).
Figure 5.
Anthocyanin content of wild-type and transgenic BVR seedlings. Transgenic M-pBVR lines, M-cBVR lines, and cv Maryland Mammoth WT seedlings were grown at 25°C on phytagel medium containing 1% (w/v) Suc for 10 d under Wc illumination of 100 μmol m−2 s−1. Bars represent the mean (± sd) of three independent measurements.
Chlorophyll and Protochlorophyll Accumulation Are Altered by BVR Expression
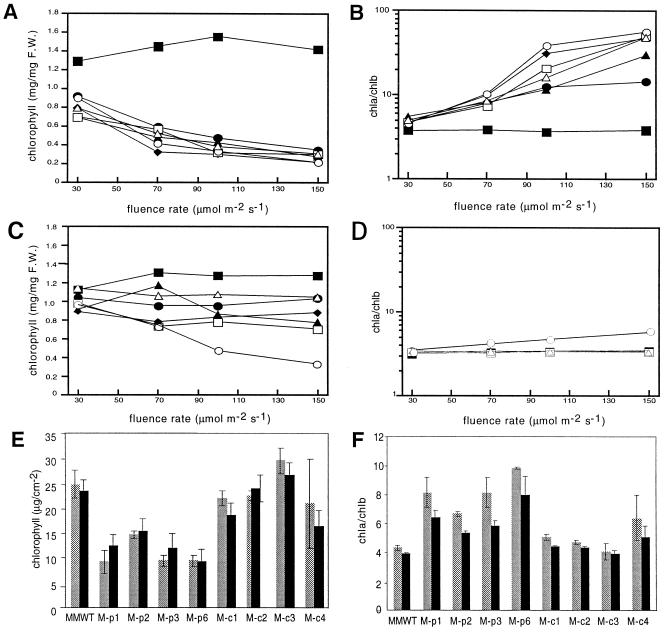
Previous findings have established that chromo-phore-deficient plants are intolerant of elevated light fluence rates (for review, see Terry, 1997). Analysis of the fluence rate-dependent chlorophyll content of M-BVR transgenic seedlings showed that these plants exhibited fluence rate-dependent reductions of total chlorophyll levels, as well as fluence rate-dependent increases in the chlorophyll a/b (Chl a/b) ratios only when BVR was targeted to plastids (Fig. 6). Cytosolic expressing lines also displayed lower levels of chlorophyll, but this effect was not strictly fluence rate-dependent with the notable exception of M-cBVR5. Furthermore, with the exception of the atypical M-cBVR5 line (Fig. 6D, ○), Chl a/b ratios were not affected by cytosolic BVR expression. In mature plants, a significant difference in chlorophyll accumulation was observed only in M-pBVR lines (Fig. 6, E and F).
Figure 6.
Fluence rate-dependent chlorophyll accumulation of wild-type and transgenic BVR seedlings and adult plants. Transgenic M-pBVR lines (A and B) and M-cBVR lines (C and D) were compared with cv Maryland Mammoth WT seedlings grown at 25°C on phytagel medium containing 1% (w/v) Suc for 10 d under Wc illumination of various fluence rates. For mature plants (E and F), chlorophyll measurements were obtained from leaves 5 and 9 of plants grown at 22°C for 40 d under white illumination with a 16-h photoperiod of 350 μmol m−2 s−1. Data points represent the mean obtained from three independent measurements. A, C, and E depict total chlorophyll; B, D, and F depict Chl a/b ratios. The symbol designations are the same as those in Figure 3.
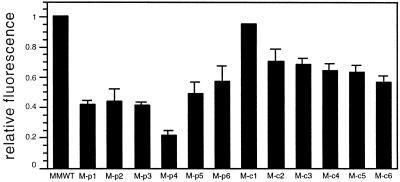
Prior studies have reported a correlation between chromophore-deficiency in plastids and reduced protochlorophyll levels (Terry, 1997; Montgomery et al., 1999). Our laboratory established that in transgenic BVR Arabidopsis plants, protochlorophyll levels were affected only when BVR was expressed in plastids (Montgomery et al., 1999). When examining the effect of BVR expression on protochlorophyll content in M-BVR seedlings, we found that all lines exhibited reduced protochlorophyll levels. However, cBVR expression affected protochlorophyll accumulation to a lesser degree than pBVR expression (Fig. 7).
Figure 7.
Protochlorophyll accumulation in wild-type and transgenic BVR seedling extracts. Transgenic M-pBVR lines and M-cBVR lines were compared with cv Maryland Mammoth WT seedlings grown at 25°C on phytagel medium containing 1% (w/v) Suc for 15 d in darkness. Bars indicate the relative protochlorophyll fluorescence values calculated by normalizing to the fluorescence value of WT seedling extracts (see “Materials and Methods” for details). Values represent the mean (±sd) of three independent measurements.
Carotenoid Accumulation Is Disrupted by Plastid-Targeted Expression of BVR
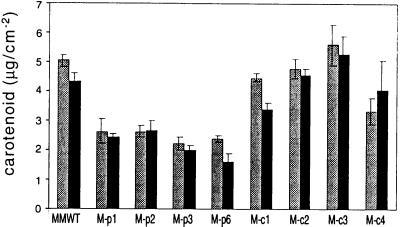
That phytochromes play a role in carotenoid accumulation in angiosperms particularly during the light-dependent transition from etioplast to chloroplast has long been known (Frosh and Mohr, 1980; Rau, 1983). In Arabidopsis, phyA and light stable phytochromes other than phyB appear to be directly involved in the regulation of specific genes in the carotenoid biosynthetic pathway (von Lintig et al., 1997). To assess the potential role of tobacco phytochromes in carotenoid accumulation, we determined the carotenoid content of mature, light-grown M-BVR transgenic plants. Similar to chlorophyll accumulation, a significant reduction in the total carotenoid content was only observed in transgenic plants expressing BVR in plastids (Fig. 8).
Figure 8.
Carotenoid content of wild-type and transgenic BVR plants. Transgenic M-pBVR lines and M-cBVR lines were compared with cv Maryland Mammoth WT seedlings grown at 22°C for 40 d under white illumination with a 16-h photoperiod of 350 μmol m−2 s−1. Bars represent the mean (±se) of three independent carotenoid measurements (see “Materials and Methods” for details) from leaf 5 (gray bars) and leaf 9 (black bars).
Photoperiodic Responses Are Altered in BVR-Expressing cv Maryland Mammoth Plants
It is well recognized that phytochrome mediates floral induction through the perception of photoperiod (Thomas and Vince-Prue, 1997). cv Maryland Mammoth, a qualitative SDP, is one of the most extensively studied of the photoperiod-dependent plants. The inhibitory effect of phytochrome photoactivation during inductive long nights has been well documented. We therefore investigated the effect of photoperiod on the flowering behavior of cv Maryland Mammoth M-BVR lines grown in controlled environment growth chambers at 22°C under defined photoperiods (Fig. 9A). These investigations revealed that BVR expression had no significant effect on days to flowering under inductive short, 8- to 10-h days. When the photoperiod was increased to greater than 13 h, a significant difference was observed between WT and transgenic BVR lines. Under long day conditions, M-pBVR lines again showed a more severe effect than M-cBVR expressing lines with flowering times for all M-BVR lines occurring earlier than WT plants. Two M-pBVR lines in particular, M-pBVR4 and M-pBVR6, flowered at nearly the same time under long days (16 h) as they did under inductive short-day (10.5 h) conditions. M-cBVR lines also exhibited early flowering as compared with WT plants, but all M-cBVR lines showed a photoperiod-induced delay of flowering under long days. By comparison with M-BVR plants, flowering times for WT plants were much more significantly increased when grown under long days.
Figure 9.
Flowering time analyses of wild-type cv Maryland Mammoth and BVR transgenic plants under various photoperiods and temperatures. A, WT cv Maryland Mammoth plants and representative M-pBVR and M-cBVR transgenic lines were grown on a soil mixture in controlled environment growth chambers at 22°C under white illumination of 8-h light/16-h dark (white bars), 10.5-h light/13.5-h dark (light gray bars), 13-h light/11-h dark (dark gray bars), and 16-h light/8-h dark (black bars) photoperiods. Data represent the mean of 10 plants (± se). B, cv Maryland Mammoth WT (shown in front) and transgenic M-pBVR2 plants (in rear) were grown on soil under a long-day photoperiod of 16 h using 150 μmol m−2 s−1 white light in a controlled environment growth chamber at 28°C for 18 weeks.
Although cv Maryland Mammoth is regarded as the “prototypical” qualitative SDP, earlier studies reported flowering under long days at reduced temperatures (Roberts, 1939). At temperatures lower than 25°C, cv Maryland Mammoth is a quantitative SDP with long-day photoperiods delaying rather than preventing floral induction. When grown under long days in the greenhouse at average temperatures of 28°C, cv Maryland Mammoth WT plants showed no evidence of flowering after 253 d, whereas all of the M-pBVR plants had flowered by 120 d (data not shown). That plastid-targeted BVR expression releases the qualitative inhibition of flowering by long-day photoperiods was also supported by flowering experiments at 28°C in long-day growth chambers (Fig. 9B). Flowering of the M-cBVR lines at 28°C under long days was considerably delayed from that of the M-pBVR lines, but to obtain seed the M-cBVR plants were transferred to a short-day growth chamber. A quantitative comparison of the flowering behavior of M-cBVR and M-pBVR lines grown at 28°C under long days remains to be determined.
DISCUSSION
Transgenic cv Maryland Mammoth Plants Expressing Mammalian BVR Are Deficient in Multiple Phytochromes
Transgenic cv Maryland Mammoth plants expressing BVR display phenotypes consistent with phytochrome chromophore deficiency throughout their life cycle independent of subcellular localization of the enzyme to plastids or the cytosol. Phenotypes observed were very similar to those of known chromophore-deficient mutants, e.g. elongated hypocotyls under Wc, Rc and FRc illumination, increased internode lengths, reduced protochlorophyll and chlorophyll content, reduced levels of anthocyanins and carotenoids, and altered photoperiod-dependent flowering (Terry, 1997). Many of these phenotypes are fully consistent with the loss of phyA and phyB photoregulatory activities (Whitelam and Devlin, 1997). Furthermore, the elongated internodes of BVR transgenic plants under Wc illumination are consistent with the loss of phyE photoregulatory activity (Devlin et al., 1998). Studies in Arabidopsis showed that such an elongated internode phenotype was only observed in a background lacking phyA and phyB, providing further support for the BVR-dependent loss of multiple phytochrome activities. In contrast to phytochrome chromophore-deficient mutants, which clearly recover phytochrome-mediated regulation of stem elongation during maturation (Weller et al., 1996; Terry, 1997), the increased internode elongation in the BVR transgenics persists in the mature plant (Fig. 4). The recovery in chromophore-deficient mutants has been attributed to the presence of additional phytochrome chromophore biosynthetic genes (Davis et al., 1999), whereas the constitutive expression of BVR in the transgenics is expected to reduce holophytochrome levels in mature plants as well as seedlings.
With the exception of hypocotyl elongation, which was qualitatively the same for both M-pBVR and M-cBVR lines, the phenotypes observed were always more severe for plastid-targeted BVR lines. This effect did not appear to be due to differences in BVR expression since quantitative levels of enzyme activity were higher for M-cBVR lines than for M-pBVR lines (Fig. 1). This suggests that either plastid-targeted BVR expression is more effective at reducing holophytochrome levels and that various photomorphogenetic responses have different phytochrome “thresholds” or that a plastid deficiency of bilins is responsible for the more exaggerated phytochrome-deficient phenotypes.
BVR Expression Disrupts Accumulation of Chlorophylls and Carotenoids in Transgenic Plants
The accumulation of protochlorophylls, chlorophylls, and carotenoids were all affected by BVR expression. Chlorophyll levels of BVR transgenic plants were always less than WT controls even at low fluences, although the effect was more pronounced for the plastid-targeted lines (Fig. 6). As was seen for Arabidopsis, the reduction of chlorophyll levels and the increased Chl a/b ratios were fluence rate-dependent only when BVR was targeted to plastids (Fig. 6, A and B). The phenotypes observed, however, were not strictly correlated with the level of BVR activity. This suggests that factors other than the level of BVR expression in the plastid may play a role in regulating chlorophyll content. One possibility is that the initial rate of BVR synthesis during early seedling (and plastid) development may vary between lines due to positional effects of BVR transgene incorporation into the genome.
In contrast with that observed for BVR transgenic Arabidopsis plants (Montgomery et al., 1999), protochlorophyll accumulation was reduced independent of the subcellular localization of BVR. Nevertheless, the reduced protochlorophyll phenotypes were on average more severe when BVR was targeted to the plastid. The modest reduction of protochlorophyll levels observed for transgenic M-cBVR plants may reflect higher levels of BVR accumulation in transgenic tobacco by comparison with transgenic Arabidopsis plants. This suggests that the reduced levels of protochlorophyll may result from the combination of reduced global levels of phytochrome, as well as reduced levels of plastid-localized bilins.
In response to the observed reduced levels of chlorophyll in transgenic M-BVR plants and owing to a recognized correlation between the regulation of chlorophyll and carotenoid levels in angiosperms (Hartel and Grimm, 1998), we examined carotenoid levels in mature M-BVR plants. Our results indicate that carotenoid levels are significantly reduced only in plants expressing BVR in plastids. As the site of carotenoid biosynthesis is localized to plastids (for review, see Rau, 1983) and levels of carotenoids are not affected in lines expressing BVR in the cytosol, we propose that carotenoid levels in the plastid may be regulated by bilin metabolism within the plastid compartment.
BVR Expression Alters the Photoperiodic Flowering Response in cv Maryland Mammoth Plants
The flowering behavior of cv Maryland Mammoth is one of the most well characterized for SDPs. It has been proposed that phyB is responsible for the inhibition of flowering under noninductive conditions in WT Arabidopsis and tobacco plants and, based on transgenic experiments, that phyC interacts with the Mammoth gene to inhibit flowering in long days (Halliday et al., 1997; Jackson and Thomas, 1997). This suggests that a lack of phytochromes under long-day photoperiods should release the inhibition of flowering as was observed for transgenic M-BVR plants. In growth chambers maintained at 22°C, cv Maryland Mammoth WT plants displayed a strong delay of flowering under long days, whereas flowering of both M-pBVR and M-cBVR lines was less sensitive to extended photoperiods. Moreover, an almost complete loss of photoperiod sensitivity was observed for specific plastid-targeted BVR transgenic lines. Remarkably, under daylength-extended greenhouse conditions in which WT cv Maryland Mammoth showed no evidence of flowering after 8 months, we were able to observe flowering for plastid-targeted BVR transgenic plants within 4 months. Flowering of the M-cBVR lines was more strongly delayed by long-day photoperiods (than M-pBVR lines) in the greenhouse, and showed no evidence of flowering long after the M-pBVR lines had flowered. The factors contributing to the differences in flowering behavior of WT and BVR transgenic plants in the greenhouse and growth chamber (e.g. temperature, light fluence rate, nutrient/watering regimes, etc.) remain to be addressed in a future investigation. From earlier studies, it is clear that temperature plays an important role (Roberts, 1939).
Are Bilins Regulatory Molecules in the Plastid Compartment?
With the exception of the effect of BVR expression on hypocotyl elongation which was qualitatively the same for M-pBVR and M-cBVR transgenic lines, all of the measured phenotypes in cv Maryland Mammoth tobacco plants were more severely affected when BVR was targeted to plastids. The magnitude of these differences can be divided into three classes. The first consists of small, or negligible, differences that were observed for hypocotyl elongation and anthocyanin accumulation. The second class includes internode elongation and the photoperiodic induction of flowering that showed more significant differences between pBVR and cBVR lines. The final class contains responses such as leaf area/length ratios and chlorophyll and carotenoid accumulation that were solely impaired in plastid-targeted lines, whereas cBVR lines showed virtually no effect of BVR expression. Several alternative hypotheses can account for these observed differences. The first relates to differential phytochrome “thresholds” for various phytochrome-dependent phenotypes. As plastids are the recognized site of chromophore biosynthesis (Terry et al., 1993), the premise that pBVR expression has a greater effect on the accumulation of linear tetrapyrroles may support the hypothesis that at least some of the differences in phenotypes may be directly attributed to different holophytochrome levels in pBVR versus cBVR lines.
A second hypothesis focuses on the fact that some phenotypes, such as chlorophyll and carotenoid accumulation, are only affected in pBVR lines. The observation that cBVR expression has no effect on these phenotypes may be explained by indirect effects on plastid development caused by manipulation of the tetrapyrrole pathway by BVR expression. In this case, the expression of BVR may have the effect of “pulling” tetrapyrroles through the pathway and thereby reducing the pool of chlorophyll precursors. The correlation between chlorophyll and carotenoid accumulation would then account for the simultaneous loss of carotenoids in BVR transgenic plants.
Alternatively, bilins, or a plastid-localized phytochrome, may have a direct signaling effect which influences plastid development. In this regard, it is presumed that in addition to exhibiting a general phytochrome deficiency, chromophore-deficient plants exhibit reduced levels of bilins in plastids. We suggest that at least some aspects of the reported phenotypes for chromophore-deficient mutants are a direct result of these reduced levels of bilins in the plastid compartment and not entirely due to secondary, or indirect, effects of lesions in the chromophore biosynthetic pathway, which would lead to phenomena such as heme feedback inhibition of tetrapyrrole biosynthesis (Terry and Kendrick, 1999). In this regard, previous studies with known chromophore-deficient mutants, with the exception of pea and tomato mutants, have established the ability of exogenously applied BV to “rescue” some aspects of the phytochrome-deficient phenotypes (Parks and Quail, 1991; Kraepiel et al., 1994; Lamparter et al., 1997; Esch and Lamparter, 1998). BV feeding should primarily increase the levels of linear tetrapyrroles in plastids, without necessarily reducing levels of heme, which would be required to overcome the feedback inhibition of tetrapyrrole biosynthesis. Additionally, we have been able to partially restore the defects in protochlorophyll accumulation in dark-grown chromophore-deficient plants by feeding plants a phytochrome chromophore (Montgomery and Lagarias, unpublished data), suggesting that bilin levels are at least partially responsible for regulating the levels of some pigments in plastids.
In summary, the present study has established a definitive perturbation of specific phenotypes only when BVR is expressed in the plastid compartment. These include altered leaf morphology, an intolerance to elevated light fluences, a reduction in carotenoid levels and a severe loss of photoperiodic sensitivity. In conjunction with previous results from BVR-expressing Arabidopsis plants (Lagarias et al., 1997; Montgomery et al., 1999), we believe that these findings suggest that bilins and/or phytochrome molecules have a prominent regulatory role in plastids distinct from their recognized role in the production of the phytochrome chromophore for transport to the cytosol and assembly with apophytochromes.
MATERIALS AND METHODS
Plant Material
Transgenic, plastid-targeted BVR (35S::pBVR) and cytosolic BVR (35S::cBVR) lines in tobacco (Nicotiana tabacum cv Maryland Mammoth) were isolated using an Agrobacterium tumefaciens-mediated leaf disc transformation protocol (Rogers et al., 1988). For these experiments, the previously described 35S::pBVR and 35S::cBVR constructs (Lagarias et al., 1997; Montgomery et al., 1999) in the binary transformation vector pBIB-KAN (Becker, 1990) were used. Six homozygous lines for each construct, designated M-pBVR1 to M-pBVR6 for plastid-targeted BVR lines and M-cBVR1 to M-cBVR6 for cytosolic BVR lines were used in the experiments described here.
Plant Growth Conditions
For seedling phenotype analyses, tobacco seeds were surface sterilized for 15 min with 35% (v/v) ethanol followed by 15 min with 35% (v/v) commercial bleach containing 0.025% (v/v) SDS and rinsed four times with sterile MilliQ (Millipore, Bedford, MA) water. Sterilized seeds were planted in 100 × 25 mm Petri dishes on media containing Murashige and Skoog salts (Gibco-BRL, Gaithersburg, MD), 0% or 1% (w/v) Suc, 0.3% (w/v) phytagel, and adjusted to pH 6.7 with NaOH. Imbibing seeds were cold stratified at 4°C in darkness for 4 d prior to being transferred to appropriate light conditions. Monochromatic light sources used were those previously described (Lagarias et al., 1997; Montgomery et al., 1999).
For experiments with mature plants grown in growth chambers, sterilized seeds were germinated on media containing Murashige and Skoog salts, 2% (w/v) Suc, 0.7% (w/v) agar, and adjusted to pH 5.9. Seeds were germinated at 25°C under white illumination with a 16-h photoperiod of approximately 65 μmol m−2 s−1 from 70-W type 84 fluorescent tubes (Philips, Eindhoven, The Netherlands). Seedlings were then transferred to pots containing a 2:1 mixture of Levington M2 bedding compost and sand, placed under white illumination with a 16-h photoperiod of 85 μmol m−2 s−1, and acclimatized for 14 d at 25°C with 70% humidity. Plants were then transferred to growth chambers with varying photoperiods at 22°C at a fluence rate of 350 μmol m−2 s−1. White light in photoperiod-controlled growth chambers was provided by Philips type 84 fluorescent tubes (Philips, Eindoven, The Netherlands) and incandescent lights.
Long-day photoperiod greenhouse flowering experiments and comparative controlled environment growth chamber experiments were performed using seeds germinated on pots containing a 1:1 mixture of Fison's Sunshine Mix no. 1 (Bellevue, MA) and vermiculite. Plants in the greenhouse were grown under ambient sunlight conditions at an average temperature of 28°C with a daylength extended 16-h photoperiod. Daylength extension was provided by 1,000-W metal halide lamps. Plants in growth chambers for results shown in Figure 9 were grown at 28°C under a long day photoperiod (16-h light/8-h dark) using 150 μmol m−2 s−1 white light provided by cool-white fluorescent tubes (F48FT12/CW/VHO, Sylvania, Danvers, MA).
BVR Enzyme and Immunochemical Analyses
Total soluble protein extracts for enzyme assays and immunoblot analyses were performed as previously described (Montgomery et al., 1999). BVR-specific enzyme activity of soluble protein fractions was measured spectrophotometrically as previously detailed (Lagarias et al., 1997) using the calculations of Kutty and Maines (1984). Protein concentrations were determined by the bicinchoninic acid method (Smith et al., 1985) using bovine serum albumin as a standard. SDS-PAGE and immunoblot analyses were performed as described previously (Lagarias et al., 1997; Montgomery et al., 1999).
Hypocotyl and Internode Length Measurements
Hypocotyl lengths of seedlings grown on Petri plates under defined light conditions were measured by scanning the plant images and quantification performed using MacBAS 2.0 software (Fuji Medical Systems, Stamford, CT). Internode lengths from the basal leaf to the plant apex were measured with a ruler.
Pigment Analyses
Chlorophyll extractions for seedling experiments were performed using N,N-dimethylformamide extracts (Moran, 1982) of 10-d-old seedlings. Chlorophyll concentrations were calculated using extinction coefficients and equations described (Inskeep and Bloom, 1985). For mature plants, relative chlorophyll content was measured for intact leaves using an SPAD-502 chlorophyll meter (Minolta UK, Milton Keynes, UK). Reported values represent an average of readings for 10 independent positions per leaf. Measurements obtained were converted to in vivo chlorophyll concentrations using a calibration curve from 115 leaf tissue samples. Chlorophyll a, chlorophyll b, and carotenoid measurements were obtained for mature leaf tissue samples. Samples were frozen in liquid nitrogen and ground to a fine powder. Samples were homogenized in 80% (v/v) acetone and incubated at −20°C for 1 h. Following centrifugation at 11,000g for 5 min at 4°C, pigment concentrations in the supernatant were determined spectrophotometrically using equations for Chl a and Chl b from Hill et al. (1985) and for carotenoids from Lichtenthaler and Wellburn (1983).
Protochlorophyll extractions were performed under green safe light by immersion of 15-d-old seedlings in N,N-dimethylformamide (Moran, 1982). Protochlorophyll content in the extracts was determined from fluorescence emission curves (560–750 nm) with excitation at 438 nm. Relative fluorescence values were determined by integration of the emission spectra for each sample. Anthocyanins were extracted from intact 10-d-old seedlings using 1% (v/v) HCl in methanol as described (Feinbaum and Ausubel, 1988). Pigments were extracted overnight with agitation at 20°C (Rabino and Mancinelli, 1986). A chloroform-water partitioning was performed (Kerckhoffs et al., 1997) and anthocyanin content in the aqueous phase determined by measuring the A535 minus the A650 (A535–A650).
Footnotes
This work was supported in part by the U.S. Department of Agriculture National Research Initiative Competitive Grant (no. AMD 9801768 to J.C.L.), a Royal Society University Research Fellowship (to M.J.T.), and a CASE Studentship from the Biotechnology and Biological Sciences Research Council (to K.A.F.).
LITERATURE CITED
- Adam E, KozmaBognar L, Schafer E, Nagy F. Tobacco phytochromes: genes, structure and expression. Plant Cell Environ. 1997;20:678–684. [Google Scholar]
- Alba R, Kelmenson PM, Cordonnier-Pratt MM, Pratt LH. The phytochrome gene family in tomato and the rapid differential evolution of this family in angiosperms. Mol Biol Evol. 2000;17:362–373. doi: 10.1093/oxfordjournals.molbev.a026316. [DOI] [PubMed] [Google Scholar]
- Becker D. Binary vectors which allow the exchange of plant selectable markers and reporter genes. Nucleic Acids Res. 1990;18:203. doi: 10.1093/nar/18.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol. 2000;71:1–11. doi: 10.1562/0031-8655(2000)071<0001:pcppii>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of phyD and phyE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Davis SJ, Kurepa J, Vierstra R. The Arabidopsis thaliana HY1 locus, required for phytochrome-chromophore biosynthesis, encodes a protein related to heme oxygenases. Proc Natl Acad Sci USA. 1999;96:6541–6546. doi: 10.1073/pnas.96.11.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Patel SR, Whitelam GC. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1487. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch H, Lamparter T. Light regulation of phytochrome content in wild-type and aphototropic mutants of the moss Ceratodon purpureus. Photochem Photobiol. 1998;67:450–455. doi: 10.1562/0031-8655(1998)067<0450:lropci>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Chory J. Light control of plant development. Annu Rev Cell Dev Biol. 1997;13:203–229. doi: 10.1146/annurev.cellbio.13.1.203. [DOI] [PubMed] [Google Scholar]
- Feinbaum RL, Ausubel FM. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol Cell Biol. 1988;8:1985–1992. doi: 10.1128/mcb.8.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosh S, Mohr H. Analysis of light-controlled accumulation of carotenoids in mustard (Sinapis alba L.) Planta. 1980;148:279–286. doi: 10.1007/BF00380039. [DOI] [PubMed] [Google Scholar]
- Furuya M. Phytochromes: their molecular species, gene families, and functions. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:617–645. [Google Scholar]
- Halliday KJ, Thomas B, Whitelam GC. Expression of heterologous phytochromes A, B or C in transgenic tobacco plants alters vegetative development and flowering time. Plant J. 1997;12:1079–1090. doi: 10.1046/j.1365-313x.1997.12051079.x. [DOI] [PubMed] [Google Scholar]
- Hartel H, Grimm B. Consequences of chlorophyll deficiency for leaf carotenoid composition in tobacco synthesizing glutamate 1-semialdehyde aminotransferase antisense RNA: dependency on developmental age and growth light. J Exp Bot. 1998;49:535–546. [Google Scholar]
- Hill C, Pearson S, Smith A, Rogers L. Inhibition of chlorophyll synthesis in Hordeum vulgare by 3-amino-2,3-dihydrobenzoic acid (gabaculin) Biosci Rep. 1985;5:775–781. doi: 10.1007/BF01119876. [DOI] [PubMed] [Google Scholar]
- Inskeep WP, Bloom PR. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Thomas B. Photoreceptors and signals in the photoperiodic control of development. Plant Cell Environ. 1997;20:790–795. [Google Scholar]
- Kendrick RE, Kronenberg GHM. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1994. [Google Scholar]
- Kerckhoffs LH, Kendrick RE. Photocontrol of anthocyanin biosynthesis in tomato. J Plant Res. 1997;110:141–149. doi: 10.1007/BF02506853. [DOI] [PubMed] [Google Scholar]
- Kerckhoffs LHJ, Schreuder MEL, Van Tuinen A, Koornneef M, Kendrick RE. Phytochrome control of anthocyanin biosynthesis in tomato seedlings: analysis using photomorphogenic mutants. Photochem Photobiol. 1997;65:374–381. [Google Scholar]
- Koornneef M, Kendrick RE. Photomorphogenic mutants of higher plants. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 601–628. [Google Scholar]
- Kraepiel Y, Jullien M, Cordonnier-Pratt MM, Pratt L. Identification of two loci involved in phytochrome expression in Nicotiana plumbaginifolia and lethality of the corresponding double mutant. Mol Gen Genet. 1994;242:559–565. doi: 10.1007/BF00285279. [DOI] [PubMed] [Google Scholar]
- Kutty RK, Maines MD. Hepatic heme metabolism: possible role of biliverdin in the regulation of heme oxygenase activity. Biochem Biophys Res Commun. 1984;122:40–46. doi: 10.1016/0006-291x(84)90436-4. [DOI] [PubMed] [Google Scholar]
- Lagarias DM, Crepeau MW, Maines MD, Lagarias JC. Regulation of photomorphogenesis by expression of mammalian biliverdin reductase in transgenic Arabidopsis plants. Plant Cell. 1997;9:675–788. doi: 10.1105/tpc.9.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamparter T, Esch H, Cove D, Hartmann E. Phytochrome control of phototropism and chlorophyll accumulation in the apical cells of protonemal filaments of wild-type and an aphototropic mutant of the moss Ceratodon purpureus. Plant Cell Physiol. 1997;38:51–58. doi: 10.1093/oxfordjournals.pcp.a029084. [DOI] [PubMed] [Google Scholar]
- Lange H, Shropshire WJ, Mohr H. An analysis of phytochrome-mediated anthocyanin synthesis. Plant Physiol. 1970;47:649–655. doi: 10.1104/pp.47.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. Determination of total carotenoids and chlorophylls a and b in leaf extracts in different solvents. Biochem Soc Trans. 1983;11:591–592. [Google Scholar]
- Mancinelli AL, Rossi F, Moroni A. Cryptochrome, phytochrome, and anthocyanin production. Plant Physiol. 1991;96:1079–1085. doi: 10.1104/pp.96.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery BL, Yeh K-C, Crepeau MW, Lagarias JC. Modification of distinct aspects of photomorphogenesis via targeted expression of mammalian biliverdin reductase in transgenic Arabidopsis plants. Plant Physiol. 1999;121:629–640. doi: 10.1104/pp.121.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with dimethylformamide. Plant Physiol. 1982;69:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Fankhauser C, Chory J. Light: an indicator of time and place. Gene Dev. 2000;14:257–271. [PubMed] [Google Scholar]
- Parks BM, Quail PH. Phytochrome-deficient hy1 and hy2 long hypocotyl mutants of Arabidopsis are defective in phytochrome chromophore biosynthesis. Plant Cell. 1991;3:1177–1186. doi: 10.1105/tpc.3.11.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Rabino I, Mancinelli AL. Light, temperature and anthocyanin production. Plant Physiol. 1986;81:922–924. doi: 10.1104/pp.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau W. Photoregulation of carotenoid biosynthesis. In: Porter JW, Spurgeon SL, editors. Biosynthesis of Isoprenoid Compounds. Chichester, UK: John Wiley & Sons; 1983. pp. 123–157. [Google Scholar]
- Roberts RH. Further studies of the effects of temperature and other environmental factors upon the photoperiodic responses of plants. J Agric Res. 1939;59:699–709. [Google Scholar]
- Rogers SG, Horsch RB, Fraley RT. Gene transfer in plants: production of transformed plants using Ti plasmid vectors. In: Weissbach A, Weissbach H, editors. Methods in Plant Molecular Biology. New York: Academic Press; 1988. pp. 423–436. [Google Scholar]
- Sage LC. Pigment of the Imagination: A History of Phytochrome Research. San Diego: Academic Press; 1992. [Google Scholar]
- Smith H. Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- Smith PK, Krohn RI, Hemanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olsen BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Terry MJ. Phytochrome chromophore-deficient mutants. Plant Cell Environ. 1997;20:740–745. [Google Scholar]
- Terry MJ, Kendrick RE. Feedback inhibition of chlorophyll synthesis in the phytochrome chromophore-deficient aurea and yellow-green-2 mutants of tomato. Plant Physiol. 1999;119:143–152. doi: 10.1104/pp.119.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MJ, Wahleithner JA, Lagarias JC. Biosynthesis of the plant photoreceptor phytochrome. Arch Biochem Biophys. 1993;306:1–15. doi: 10.1006/abbi.1993.1473. [DOI] [PubMed] [Google Scholar]
- Thomas B, Vince-Prue D. Photoperiodism in Plants. San Diego: Academic Press; 1997. [Google Scholar]
- von Lintig J, Welsch R, Bonk M, Giuliano G, Batschauer A, Kleinig H. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. Plant J. 1997;12:625–634. doi: 10.1046/j.1365-313x.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- Weller JL, Terry MJ, Rameau C, Reid JB, Kendrick RE. The phytochrome-deficient pcd1 mutant of pea is unable to convert heme to biliverdin IXα. Plant Cell. 1996;8:55–67. doi: 10.1105/tpc.8.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Devlin PF. Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 1997;20:752–758. [Google Scholar]