Abstract
Background.
Laboratory tasks to delineate anxiety disorder features are used to refine classification and inform our understanding of etiological mechanisms. The present study examines laboratory measures of response inhibition, specifically the inhibition of a pre-potent motor response, in clinical anxiety. Data on associations between anxiety and response inhibition remain inconsistent, perhaps because of dissociable effects of clinical anxiety and experimentally manipulated state anxiety. Few studies directly assess the independent and interacting effects of these two anxiety types (state v. disorder) on response inhibition. The current study accomplished this goal, by manipulating state anxiety in healthy and clinically anxious individuals while they complete a response inhibition task.
Method.
The study employs the threat-of-shock paradigm, one of the best-established manipulations for robustly increasing state anxiety. Participants included 82 adults (41 healthy; 41 patients with an anxiety disorder). A go/nogo task with highly frequent go trials was administered during alternating periods of safety and shock threat. Signal detection theory was used to quantify response bias and signal-detection sensitivity.
Results.
There were independent effects of anxiety and clinical anxiety on response inhibition. In both groups, heightened anxiety facilitated response inhibition, leading to reduced nogo commission errors. Compared with the healthy group, clinical anxiety was associated with excessive response inhibition and increased go omission errors in both the safe and threat conditions.
Conclusions.
Response inhibition and its impact on go omission errors appear to be a promising behavioral marker of clinical anxiety. These results have implications for a dimensional view of clinical anxiety.
Keywords: Anxiety, anxiety disorders, behavioral inhibition, go/nogo, threat of shock
Introduction
The National Institute of Mental Health Research Domain Criteria (RDoC) initiative seeks to uncover transdiagnosis, biobehavioral dimensions related to specific neurobiological processes, as quantified with laboratory tasks (Insel et al. 2010). From this perspective, anxiety disorders are conceptualized as extreme variants of normal functioning. A key question therefore is to determine the extent to which adaptive and maladaptive responses to threats vary as a function of clinical and non-clinical variations in anxiety (Robinson et al. 2015). The present study examines the effects of both experimentally induced and clinical anxiety on one such response, behavioral inhibition or response inhibition, operationally defined as the inhibition of pre-potent motor responses (Bari & Robbins, 2013) (as opposed to cognitive inhibition, the inhibition of thoughts, emotions and perception, which is impaired in clinical anxiety; Eysenck et al. 2007).
Works in rodents led Gray to suggest that anxiety activates the behavioral inhibition system, which triggers a set of defensive responses, including inhibition of pre-potent responses (i.e. response inhibition) (Gray & McNaughton, 2000). Others proposed that excessive engagement of the behavioral inhibition system in humans contributes to clinical anxiety (Quay, 1997; Gray & McNaughton, 2000; Epstein et al. 2001; Sylwan, 2004). However, few studies have examined how experimentally induced and clinical anxiety relates to laboratory measures of response inhibition in humans. Moreover, the limited data that do exist are conflicting, with studies finding inconsistent effects of clinical anxiety, trait anxiety, and manipulations in state anxiety (Geen, 1985; Daugherty et al. 1993; Hagopian & Ollendick, 1994; Oosterlaan & Sergeant, 1996; Kooijmans et al. 2000; Karch et al. 2008; Li et al. 2009; Righi et al. 2009; Sehlmeyer et al. 2010; Neo et al. 2011; Robinson et al. 2013a; Forster & Lavie, 2014; Wright et al. 2014).
The sustained response to attention task is a go/nogo (GNG) paradigm that generates a measure of response inhibition (Helton, 2009; Peebles & Bothell, 2004). This paradigm requires a motor response to frequent ‘go’ target stimuli but not rare ‘nogo’ stimuli, to which a response is withheld. Such withholding of a pre-potent response generates a prototypical index of response inhibition (Helton, 2009; Bari & Robbins, 2013). Consistent with Gray’s model, we recently showed that heightened state anxiety induced by threat of shock facilitates response inhibition during GNG, i.e. it reduced nogo commission errors (failure to inhibit the pre-potent response) without affecting go response time (Robinson et al. 2013a). We also found, in a recent replication and extension of this finding, a positive correlation between state anxiety and go omission errors; individuals with the highest elevation in state anxiety showed improved nogo accuracy but also impaired go accuracy (Grillon et al. 2016). These results suggest that high state anxiety is associated with an overall inhibition of motor responding, a hypothesis that was confirmed in a subsequent reanalysis of these data using signal detection theory to quantify changes in response bias (Snodgrass & Corwin, 1988; McVay & Kane, 2009).
These data show that heightened state anxiety induced by threat of shock drives a transient response inhibition tendency with beneficial and detrimental effects depending on the nature of the response (i.e. improved nogo responses, impaired go responses). However, these data emerged only from variations in normal anxiety. This raises questions on the relationships among variations in response inhibition, threat-induced state anxiety and clinical levels of anxiety. Although data in children find some evidence of associations between response inhibition and clinical anxiety (Daugherty et al. 1993; Oosterlaan and Sergeant, 1996; Kooijmans et al. 2000), little is known about this link in adults (Beutel et al. 2010; Thomas et al. 2016).
Investigations of the effect of trait anxiety on response inhibition can inform predictions. Most studies found no behavioral effect of trait anxiety in GNG (Karch et al. 2008; Righi et al. 2009; Sehlmeyer et al. 2010) or stop-signal tasks (Li et al. 2009; Neo et al. 2011). However, there is indirect evidence that when state anxiety is elevated, high trait anxiety is also associated with increased nogo accuracy, but at the expense of go accuracy (Geen, 1985; Hagopian & Ollendick, 1994). These results suggest that state anxiety and trait anxiety drive a response inhibition tendency along a continuum from ‘normal’ to ‘abnormal’. Given that clinical anxiety is characterized by both high state and trait anxiety, one could then hypothesize that response inhibition would be excessive in clinical anxiety.
To test this possibility, we compared the effect of threat of shock during GNG performance in individuals with clinical anxiety and in healthy participants. We focused on generalized anxiety disorder (GAD) and social anxiety disorder (SAD), two conditions with an overly sensitive behavioral inhibition system (Rosenbaum et al. 1991; Gray & McNaughton, 2000; Morgan et al. 2009; Roelofs et al. 2009; Maack et al. 2012). We expected both heightened state anxiety and clinical anxiety to shift response bias (McVay & Kane, 2009; Snodgrass & Corwin, 1988) away from the pre-potent go response (towards nogo response, i.e. response inhibition tendency), but to a greater extent in clinical anxiety. Excessive response inhibition in clinical anxiety could be a disease characteristic. One would then expect increased response inhibition in the patients compared with the healthy participants in both the safe and the threat conditions. Alternatively, excessive response inhibition in the patients could be state-dependent, i.e. arising from elevated state anxiety during threat processing. If so, excessive response inhibition in the patients v. healthy participants would be seen only during shock anticipation.
Because heightened state anxiety facilitates perceptual processes (Baas et al. 2006; Cornwell et al. 2007), we also expected signal detection to be facilitated by the threat of shock. Tasks such as GNG usually promote a speed–accuracy trade-off, with slower reaction time (RT) being correlated with better nogo accuracy (Peebles & Bothell, 2004). However, our previous results showed that heightened state anxiety improved nogo accuracy without affecting go RT, i.e. independently of a speed–accuracy trade-off (Robinson et al. 2013b). To control for individual differences in RT, a skill index was also computed (nogo accuracy/go-trial RT) (Saucedo Marquez et al. 2013; Seli, 2016; Seli et al. 2016). This index provides a measure of efficiency by accounting for both response speed and accuracy.
Method
Participants
Participants included 41 medication-free patients (34 female) and 41 healthy participants (34 female). Following an initial telephone screen, participants visited the National Institutes of Health for comprehensive screening by a clinician. The patients had a diagnosis of GAD (n = 14), SAD (n = 7) or GAD and co-morbid SAD (n = 20) and no other current Axis I psychiatric disorders, or past psychosis as assessed by the DSM-IV-TR (American Psychiatric Association, 2000). The healthy participants had no current or past history of any Axis I psychiatric disorders as assessed by SCID-I/NP (non-patient edition). Exclusion criteria for all participants consisted of any interfering acute or chronic medical conditions and positive urine drug screen. All participants gave written informed consent approved by the National Institute of Mental Health Combined Neuroscience Institutional Review Board. The study was registered at clinical-trials.gov (no. NCT00055224).
Overview
We used a procedure modeled after one of our previous studies (Grillon et al. 2016), as described in Fig. 1. Briefly, subjects participated in task (GNG) and no-task (see below) conditions during periods of threat of shocks and periods of safety when no shock could be administered. This resulted in a 2 (healthy participants, anxiety patients) × 2 (task, no task) × 2 (safe, threat) design. Acoustic startle stimuli used to produce a startle response, operationally defined as an eyeblink reflex, were regularly delivered throughout testing to assess subjects’ defensive reactivity. Subjects’ anxiety was assessed via retrospective reports.
Fig. 1.
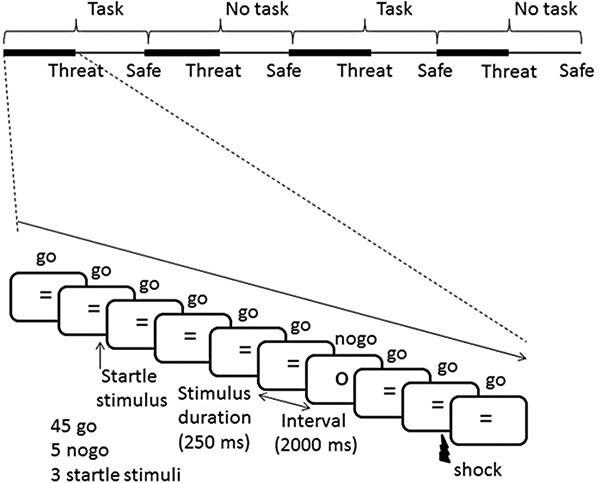
Schematic description of stimulus presentation (sequence 2). There were four sequences of predetermined order of stimulus presentation (see text). Each sequence consisted of eight blocks with alternating blocks of safe and threat conditions. Sequences started with either two task blocks or two no-task blocks followed by two blocks of the alternating task condition (i.e. task → no task → task → no task, or no task → task → no task → task). Each subject was presented with two sequences of stimulus order. Each block consisted of 45 go, five nogo and three acoustic startle stimuli. In addition, one shock was given in two out of the four threat blocks per sequence. Finally, at the end of each block, subjects had to retrospectively rate their anxiety and select one type of thought with choices of task-related thoughts, task-unrelated/threat-unrelated thoughts (non-threat TUTs) and threat-related thoughts (threat TUTs) (see online Supplementary material).
Procedure
Shortly after participants’ arrival on the testing day, a startle habituation procedure was conducted. For this, two electrodes were attached under the left eye to record the eyeblink/startle reflex. Nine startle stimuli were then delivered every 18–25 s to reduce initial startle reactivity. This was followed by a shock work-up procedure that set the shock intensity at a level that was uncomfortable but not painful. Shock levels (Table 1) did not differ significantly between the two groups (t80 = 0.8, N.S., d = 0.2). The GNG task was then initiated.
Table 1.
Demographic information and shock intensity
| Age, years | State anxietya | Trait anxietya | BDI score | Shock intensity, mA | |
|---|---|---|---|---|---|
| Healthy participants | 30.0 (1.2) | 25.9 (1.1) | 28.8 (1.1) | 0.8 (0.3) | 2.9 (0.2) |
| Anxiety patients | 28.1 (1.2) | 43.6 (1.8) | 52.3 (1.5) | 8.7 (1.3) | 2.8 (0.1) |
Data are given as mean (standard error).
BDI, Beck Depression Inventory (Beck & Steer, 1987).
State portion and trait portion of the State-Trait Anxiety Inventory (Spielberger, 1983).
GNG and control tasks
During the GNG and the control tasks, stimuli were presented on a monitor. In the GNG task, participants were asked to respond to frequent (91%) ‘go’ stimuli (‘=‘) by pressing the ‘2’ on the keypad of a computer keyboard and to withhold their response to infrequent (9%) ‘nogo’ stimuli (‘O’). They were asked to focus on speed and accuracy equally. In the control task, frequent ‘*’ (90%) and infrequent ‘#’ (10%) stimuli were presented and participants were asked to look passively at the screen. In both the GNG and control tasks, these stimuli were randomly distributed and were presented for 250 ms at a rate of one every 2000 ms. Stimuli were presented on a blank screen and were not followed by a mask. A correct go hit was a response recorded during these 2000 ms to a go trial. Similarly, a correct nogo omission was a no response during the same period to a nogo trial.
A total of four sequences of stimulus presentation, with each sequence consisting of eight blocks, were created: (1) sequence 1 (Fig. 1): no task (threat then safe), task (threat then safe), no task (threat then safe), task (threat then safe); (2) sequence 2 was similar to sequence 1 but no-task and task conditions were reversed: (3) sequence 3 was similar to sequence 1 but threat and safe were reversed: (4) sequence 4 was similar to sequence 1 but no task and task, and safe and threat were reversed. Each participant was presented with one of the following two sequences (1 and 2, 2 and 1, 4 and 3, or 3 and 4) with approximately equal numbers of subjects per sequence pairs. In each block, the frequent stimuli (‘=‘ or ‘*’) were presented on 45 occasions while the infrequent stimuli (‘O’ or ‘#’) occurred five times for a total of 720 (45 × 8 blocks × 2 sequences) go trials and (5 × 8 × 2) 80 nogo trials over the two sequences. Each block lasted 100 s (50 × 2000 ms).
Startle stimuli, shocks, and threat condition
The first block of each sequence was preceded by three startle stimuli to further reduce initial startle reactivity. Subsequently, three startle stimuli separated by 22–30 s were delivered in each block to assess participants’ anxiety. Startle stimuli always occurred between two go trials, and go trials that followed a startle stimulus were not included in the analysis. A shock was delivered in two of the four threat blocks in each sequence, just prior to the last go trial, which was not included in the analysis (for a total of four shocks). Participants were informed that shock could be administered only in the threat condition and never in the safe condition. The safe and threat conditions were signaled by a blue and red border on the monitor, respectively.
Subjective anxiety and thought probes
At the end of each safe and threat block of a sequence, subjects were asked to report their level of anxiety during the preceding block on an analog scale ranging from 1 ‘not at all anxious’ to 10 ‘extremely anxious’.
Tasks such as GNG are also used to explore task-related thoughts and task-unrelated thoughts (Seli, 2016). At the end of each block, just prior to assessing subjective anxiety, participants were asked to indicate whether they were focused on the task (task-related thoughts) or whether they were mind-wandering with task-unrelated, whether threat-related or threat-unrelated, thoughts. In this study, as in our past study (Robinson et al. 2013a), we found no reliable correlations between task-unrelated thoughts and GNG performance. Consequently, the thought probe methodology and the results are presented in the online Supplementary material.
Stimulation and physiological responses
Stimulation and recording were controlled by a commercial system (Contact Precision Instruments, UK). Presentation of the visual stimuli was controlled by E-Prime. The acoustic startle stimulus was a 40-ms duration 103-dB (A) white noise presented via headphones. The eyeblink reflex was recorded with two electrodes placed under the left eye and a ground electrode placed on the left arm. The electromyographic (EMG) eyeblink signal was amplified with bandwidth set to 30–500 Hz and digitized at a rate of 1000 Hz. Finally, the shock was administered on the left wrist.
Data analysis
Performance
Correct go responses were go trials followed by button press. Correct nogo responses were nogo trials followed by no button press. Performance was determined for each condition (threat, safe) and trial type (go, nogo) by dividing the number of correct response by the total number of each trial type. The trial following a shock was excluded from analyses. Mean RT was calculated for correct-go to evaluate speed–accuracy trade-off (Peebles & Bothell, 2004). In addition, go RT variability (standard error) and RT coefficient of variation (standard deviation/mean RT) were also calculated, as these measures reflect the ability to sustain attention across trials (Stuss et al. 1995).
Signal-detection sensitivity (dL) and response bias (CL) scores were calculated for each participant as done by others (McVay & Kane, 2009) using the formulas for logistic distributions (Snodgrass & Corwin, 1988) (see details in the online Supplementary material).
The skill index was calculated as: skill index = 1000 × (mean nogo accuracy ratio/mean go-trial RT) (Saucedo Marquez et al. 2013; Seli, 2016; Seli et al. 2016). (The index is multiplied by 1000 to reduce the number of decimals; Seli, 2016.)
Startle reflex
After full-wave rectification and smoothing the EMG signal, peak startle/eyeblink magnitude was determined in the 20–100 ms time-frame following stimulus onset relative to a 50-ms pre-stimulus baseline. The startle responses from each participant were converted to t scores.
Data were analysed with mixed-model repeated-measures analyses of variance (ANOVAs) and t tests. Cohen’s d and partial eta squared () are reported for effect sizes. Preliminary analyses showed no significant order effects of the sequence of stimulus presentation on the key variables.
Ethical statement
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
Demographics
The demographic data are presented in Table 1. The two groups did not differ in age (t80 = 1.2, N.S.). As expected, the patients had higher scores of state anxiety (t80 = 8.3, p < 0.0009, d = 1.8), trait anxiety (t80 =12.6, p < 0.0009, d = 2.6) and on the Beck Depression Inventory (BDI) (t79 = 5.9, p < 0.0009, d = 1.3) compared with the healthy participants. Note that the BDI score of one subject was missing in the patient group.
Performance
Each performance score (CL, dL, nogo accuracy, go accuracy, go RT, go RT coefficient of variation, and skill index) was analysed using ANOVAs with group (healthy participants, anxiety patients) as a between-subject factor and condition (safe, threat) as a within-subject factor.
As expected, given the nature of the task, the response bias index (CL) was negative (Fig. 2, left), reflecting a ‘go’ bias (McVay et al. 2013). Consistent with our hypothesis, the go bias was reduced (i.e. increased nogo bias) in the threat compared with the safe condition (F1,80 = 6.5, p = 0.01, ), and in the patients compared with the healthy participants (F1,80 = 4.4, p = 0.04, ). However, these effects did not interact with one another (condition × group: F1,80 = 1.4, N.S., ). The group difference remained unchanged when the BDI score was used as a covariate (group main effect: F1,78 = 5.8, p = 0.02, ). Thus, both heightened state anxiety and clinical anxiety independently resulted in greater nogo biases, leading the nogo response bias to be lowest in the controls/safe condition and greatest in the patients/threat condition (Fig. 2, left).
Fig. 2.
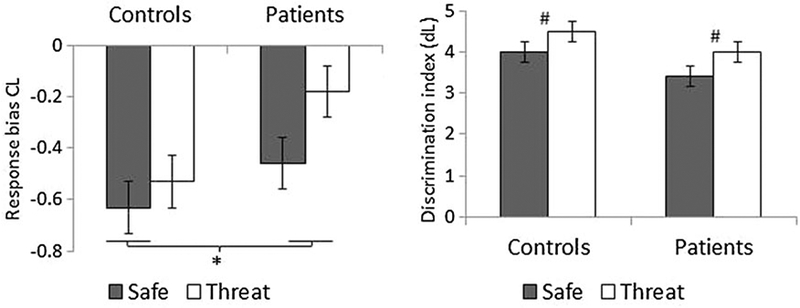
Response bias (CL) and signal-detection sensitivity (dL) scores in the two groups in the safe and threat conditions. Values are means, with standard errors represented by vertical bars. * Significant overall group difference (p < 0.05).# Significant difference between the safe and threat conditions across groups (p = 0.001).
The signal-detection sensitivity index (dL) was higher in the threat compared with the safe condition (condition: F1,80 = 14.1, p < 0.001, ), reflecting better perceptual discrimination during heightened state anxiety (Fig. 2, right). There was also a trend for larger dL in the healthy participants compared with the patients (group: F1,80 = 3.5, p = 0.066, ), but this trend disappeared when the BDI score was used as a covariate (F1,78 = 1.9, N.S., ). The condition × group effect was not significant (p > 0.1).
Nogo accuracy, go accuracy, skill index, go RT and go RT-variability are presented in Table 2. Nogo accuracy did not differ significantly among the healthy participants and patients (F1,80 = 0.04, N.S., ), but it was higher (fewer commission errors) in the threat compared with the safe condition, with a similar threat effect in both groups (condition: F1,80 = 12.5, p = 0.001, ; condition × group: F1,80 = 0.03, N.S., ). Go accuracy was lower (i.e. increased omission errors) in the patients compared with the healthy participants (F1,80 = 4.5, p = 0.04, ), but it was not affected by threat (condition: F1,80 = 0.05, N.S., ; condition × group: F1,80 = 0.4, N.S., ). The go accuracy group difference remained significant after controlling for depression symptoms (F1,78 = 5.4, p = 0.02, ).
Table 2.
Performance scores
| Healthy participants | Anxiety patients | |||
|---|---|---|---|---|
| Safe | Threat | Safe | Threat | |
| Nogo correct omissiona | 0.76 (0.02) | 0.80 (0.02) | 0.76 (0.01) | 0.81 (0.02) |
| Go correct hitb | 0.91 (0.01) | 0.90 (0.01) | 0.87 (0.01) | 0.87 (0.02) |
| Go RT, msc | 387.8 (16.2) | 377.4 (14.7) | 337.3 (10.7) | 332.2 (10.9) |
| Skill indexd | 2.1 (0.07) | 2.3 (0.08) | 2.3 (0.07) | 2.5 (0.08) |
| Go RT variabilitye | 148.4 (10.0) | 133.4 (9.8) | 124.9 (8.8) | 105.1 (7.3) |
| Go RT coefficient of variationf | 0.38 (0.02) | 0.35 (0.02) | 0.36 (0.02) | 0.31 (0.02) |
Data are given as mean (standard error).
RT, Reaction time.
Nogo trials followed by no button press.
Go trials followed by button press.
RT to correct button press to go trials.
1000 × (mean nogo accuracy ratio/mean go-trial RT) (Saucedo Marquez et al. 2013; Seli, 2016; Seli et al. 2016).
Go RT standard error.
Standard deviation/mean RT to go trials.
Go RT was faster in the patients compared with the healthy participants (F1,80 = 6.0, p = 0.02, ). No other go RT effects were significant (all p > 0.05). Go RT variability was smaller in the patients compared with the healthy participants (F1,80 = 4.9, p = 0.03, ), and in the threat compared with the safe condition across both groups (F1,80 = 10.3, p = 0.002, ). The difference between the threat and safe conditions remained significant after controlling for individual differences in RT (coefficient of variation) (F1,80 = 8.7, p = 0.004, ), but the group difference did not (group: F1,80 = 1.8, N.S., ; group × condition: F1,80 = 0.6, N.S., ). The group differences in go RT and go RT variability remained significant when the BDI score was used as a covariate (F1,78 = 6.4, p = 0.01, and F1,78 = 6.8, p = 0.01, , respectively).
The skill index was larger in the patient group compared with the control group (F1,80 = 5.9, p = 0.017, ) and in the threat compared with the safe condition (F1,80 = 16.5, p < 0.0009, ) without significant group x condition interaction (F1,80 = 0.1, N.S., ). The group difference in skill index remained significant when the BDI score was used as a covariate (F1,78 = 9.3, p = 0.003, ).
Startle reflex and subjective anxiety
Analysis of the startle data with raw scores and t score led to similar results. The raw scores are presented here (Fig. 3, top) and the t scores in the online (Supplementary material Fig. S1). These data were analysed with a group (healthy participants, anxiety patients) × task (no task, GNG) x condition (safe, threat) ANOVA. Startle magnitude was larger in the threat compared with the safe condition (i.e. fear-potentiated startle: F1,80 = 77.1, p < 0.0009, ) and in the no-task compared with the GNG task condition (F1,80 = 19.9, p < 0.0009, ), but these effects were qualified by (1) a group × condition interaction (F1,80 = 4.6, p = 0.03, ), reflecting greater fear-potentiated startle in the patients compared with the healthy participants, and (2) a task x condition interaction (F1,80 = 4.2, p = 0.04, ), due to reduced fear-potentiated startle in the GNG task compared with the no-task condition. The group x condition interaction remained significant when the BDI score was used as a covariate (F1,78 = 12.2, p = 0.001, ).
Fig. 3.
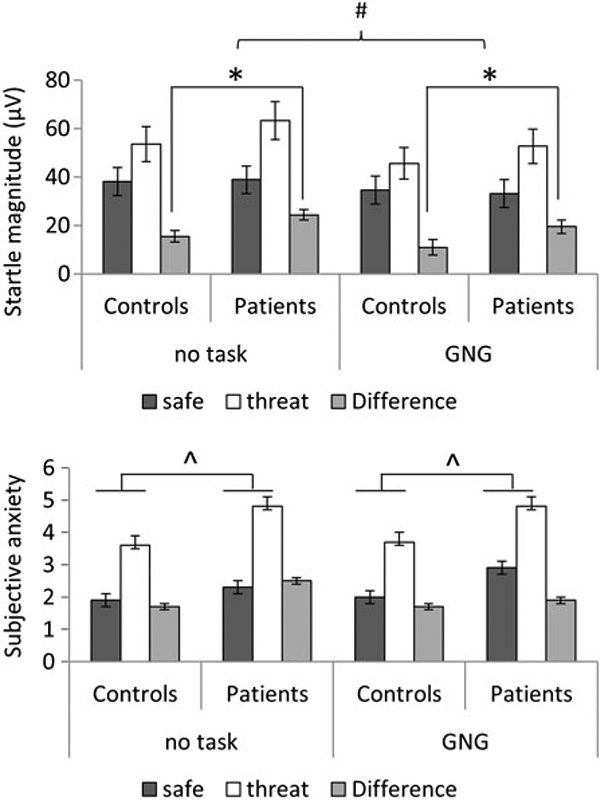
Startle magnitude (top) and subjective anxiety (bottom) in each condition in the two groups. Difference is difference scores of threat minus safe (fear-potentiated startle). Values are means, with standard errors represented by vertical bars. * Significantly greater fear-potentiated startle in the patients compared with the control group (p < 0.05). # Significantly greater fear-potentiated startle in no task compared with Go/nogo (GNG) task (p < 0.05). ^ Significantly greater overall subjective anxiety in the patients compared with the control group (p < 0.05).
Subjective anxiety (Fig. 3, bottom) was higher overall in the patients compared with the healthy participants (F1,80 = 9.0, p = 0.003, ) (including after controlling for BDI, F1,78 = 4.7, p = 0.03, ), in the threat compared with the safe condition (F1,80 = 76.2, p < 0.0009, ), and (at trend) in the control task compared with the GNG task (F1,80 = 3.7, p = 0.057, ). No interaction was significant (all p > 0.1).
Discussion
This study examined the interacting effects of state and clinical anxiety on response inhibition. As predicted, clinical anxiety was associated with excessive response inhibition, as measured by the signal-detection response bias index (CL), leading to decreased go accuracy. This effect was independent of the threat manipulation. These findings suggest that clinical anxiety is associated with overactivation of an adaptive defense mechanism (i.e. the behavioral inhibition system) that promotes an excessive response inhibition tendency, leading to a maladaptive behavioral effect (i.e. impaired go performance). This maladaptive behavior is a marker of the disease rather than the result of excessive state anxiety arising from an actual threat.
The finding that threat of shock in the healthy participants improved nogo accuracy without affecting go accuracy or go RT replicates and extends our original study (Robinson et al. 2013a). The signal-detection analysis suggests that two factors contributed to this improvement. First, the response bias index CL indicated that heightened state anxiety decreased go tendency (i.e. increased nogo tendency), aligning behavior preferentially with the task of stopping the motor response on nogo trials. Second, heightened state anxiety improved signal-detection sensitivity (dL), probably leading to better target discrimination. This latter effect is consistent with findings that heightened state anxiety facilitates early perceptual and sensory processing (Vuilleumier, 2005; Baas et al. 2006; Cornwell et al. 2007; Pessoa et al. 2012). These sensory effects may have facilitated stimulus detection, also contributing to improved nogo accuracy (Smallwood, 2013).
Successful performance during GNG tasks did not depend solely on response inhibition. For example, it also involves changes in response strategy. However, the result that RT was not affected by heightened state anxiety indicates two points: participants (including the anxiety patients) did not adopt a more deliberate, cautious and controlled response strategy (Gold & Shadlen, 2007) and improved nogo performance did not come at the expense of efficiency (Eysenck et al. 2007), which would be reflected in a speed–accuracy trade-off (i.e. slower RT) (Peebles & Bothell, 2004). Rather, the skill index indicated superior nogo performance efficiency during the threat condition when RT was taken into account to evaluate nogo performance.
Clinical anxiety, like heightened state anxiety, increased response inhibition based on the response bias index (Fig. 2, left). In healthy participants, the GNG task promoted a strong go pre-potency in an innocuous context (safe condition), which resulted in a low response inhibition tendency that increased the likelihood of nogo commission errors. As state anxiety was heightened in the healthy participants (threat condition) and in the anxiety patients (safe and threat conditions), response inhibition increased. This facilitated stopping on nogo trials in both groups, but increased go omission errors in the patients. Thus, consistent with Gray’s models (Quay, 1997; Gray & McNaughton, 2000; Epstein et al. 2001; Sylwan, 2004), a transient increase in response inhibition under threat is normative, but excessive response inhibition characterizes clinical anxiety. This excessive behavioral inhibition in anxiety patients has beneficial effects such as superior nogo performance (based on the skill index scores), but it is also detrimental for go accuracy, compared with the healthy participants. The exact implication of these findings remains to be determined. A key question is how increased response inhibition relates to maladaptive behaviors, such as avoidance and fearful inhibited temperament, which is characterized by a behavioral inhibition phenotype (Kagan et al. 1988; Buss et al. 2004). Indeed, it is probably context dependent, increased inhibition could be adaptive in the face of threat, but it could be maladaptive in the face of positive outcomes. Future work should explore the impact of inhibitory processes across valenced outcomes.
During go trials, a balance is established between pre-potent go responses and the need to adjust motor readiness in anticipation of stopping the response on nogo trials (i.e. proactive response inhibition) (Aron, 2011). The go results point to excessive proactive response inhibition in clinical anxiety. However, one could alternatively argue that the anxiety patients failed to develop pre-potent go responses. This explanation is unlikely given that (1) RT was faster in the patients compared with the healthy participants and (2) it is inconsistent with the literature that shows no detrimental effect of anxiety on pre-potent responses. For example, eye movement studies show no impairment in pro-saccade, a pre-potent response, in trait anxious individuals (Ansari et al. 2008; Ansari & Derakshan, 2011). In addition, acute stress and chronic stress promote rather than impair the development of habit behaviors and pre-potent responses in humans and in animals (Kim et al. 2001; Schwabe et al. 2008; Schwabe & Wolf, 2009).
The present findings have several implications. First, organisms need to react adaptively to threat with a set of behavioral adjustments including attentional bias towards threat, increased arousal and negative affect, and behavioral inhibition. Clinical anxiety has long been associated with attentional bias for threat and excessive arousal (Robinson et al. 2013b). We now show that it is also marked by excessive behavioral inhibition, which has previously been suggested as an underlying cause of anxiety disorders (Quay, 1988). Second, one of the objectives of RDoC is to identify neurobiological dimensions of dysfunction that characterize psychiatric disorders. We propose that response inhibition is an important behavioral component that can inform our understanding of clinical anxiety. Future studies should examine whether response inhibition is an enduring feature of clinical anxiety or a pre-existing vulnerability and whether strong response inhibition tendencies are associated with passive or avoidant behaviors as it has been proposed (Kooijmans et al. 2000).
The present results seem to contradict the prominent view that anxiety impairs a host of cognitive functions, including cognitive control and inhibition (Eysenck et al. 2007; Ansari et al. 2008; Derakshan et al. 2009). However, this view needs to be revisited. First, inhibition reflects an umbrella of concepts (Bari & Robbins, 2013). Inhibiting a premotor response and inhibiting intrusion from task-irrelevant thought or stimuli (e.g. Stroop effect) relies on distinct mechanisms. There is no reason to assume that anxiety would affect these two types of inhibition similarly. In addition, in most inhibition studies, anxiety refers to trait anxiety, but trait anxiety is different from state anxiety or clinical anxiety. It is important to distinguish among the expression of these different forms of anxiety as they may present commonalities but also important differences (Bijsterbosch et al. 2015). In fact, most studies do not show a detrimental effect of trait anxiety in GNG tasks (Righi et al. 2009; Sehlmeyer et al. 2010).
This study had strengths and limitations. Among the strengths, the study relied on a within-subject design with well-established methods of fear induction and measurement (Grillon & Baas, 2003; Kaye et al. 2016). In addition, the study was based on strong a priori hypotheses from prior studies, and replicated the effect of heightened state anxiety on response inhibition. Finally, the anxiety patients were off medication. A limitation was that the safe condition is not fully affectively neutral. Participation in a study where shocks are administered raises the level of state anxiety even when shocks are not imminent. It is therefore possible that anxiety caused by the threatening context was responsible for the group difference in performance observed in this study. However, one could argue that if this contextual anxiety was responsible for the performance difference between the controls and the patients in the safe condition, the patients should have been even more affected by the much greater anxiety evoked by the actual shock threat in the threat condition. We could have then expected the two groups to show differential performance over the safe and threat conditions, a result which was not obtained. Nevertheless, future studies should examine response inhibition in anxiety patients in a non-threatening context. Another limitation was that the anxiety patients were not homogeneous with regard to diagnosis. However, our approach was consistent with current conceptualization of mental disorders (Insel et al. 2010). In addition, there was no performance difference among the anxiety groups (online Supplementary material, Table S1). Finally, the small number of males, reflective of the clinical prevalence, prevented us from testing for sex differences.
To conclude, heightened state anxiety shifts motor action tendencies towards increased response inhibition, improving individuals’ ability to stop motor responses. Clinical anxiety is characterized by excessive response inhibition tendencies, which lead to inappropriate stopping. These results have clinical implications. Discovery of clinical biological pheno-types is a key step in improving clinical classification and identifying pathophysiological mechanisms that could be targeted for treatment development. This study suggests that increased response inhibition may be a clinical phenotype of anxiety disorders. The study of response inhibition in anxiety as a state (Hagenaars et al. 2014; Wright et al. 2014) or a disorder is in its infancy. However, there is substantial knowledge of the underlying mechanisms of response inhibition in healthy individuals. This knowledge provides a benchmark to identify underlying dysfunction associated with pathological anxiety.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Mental Health (ZIAMH002798) (protocol 03-M-0093; NCT00055224).
Footnotes
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291716002555
Declaration of Interest
The authors declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association: Washington, DC. [Google Scholar]
- Ansari TL, Derakshan N (2011). The neural correlates of impaired inhibitory control in anxiety. Neuropsychologia 49, 1146–1153. [DOI] [PubMed] [Google Scholar]
- Ansari TL, Derakshan N, Richards A (2008). Effects of anxiety on task switching: evidence from the mixed antisaccade task. Cognitive, Affective, and Behavioral Neuroscience 8, 229–238. [DOI] [PubMed] [Google Scholar]
- Aron A (2011). From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological Psychiatry 69, 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas JMP, Milstein J, Donlevy M, Grillon C (2006). Brainstem correlates of defensive states in humans. Biological Psychiatry 59, 588–593. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology 108, 44–79. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA (1987). BDI: Beck Depression Inventory. The Psychological Corporation, Harcourt Brace Jovanovich, Inc.: New York. [Google Scholar]
- Beutel ME, Stark R, Pan H, Silbersweig D, Dietrich S (2010). Changes of brain activation pre-post short-term psychodynamic inpatient psychotherapy: an fMRI study of panic disorder patients. Psychiatry Research: Neuroimaging 184, 96–104. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch J, Smith S, Bishop SJ (2015). Functional connectivity under anticipation of shock: correlates of trait anxious affect versus induced anxiety. Journal of Cognitive Neuroscience 27, 1840–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Davidson RJ, Kalin NH, Goldsmith HH (2004). Context-specific freezing and associated physiological reactivity as a dysregulated fear response. Developmental Psychology 40, 583–594. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Baas JMP, Johnson L, Holroyd T, Carver FW, Lissek S, Grillon C (2007). Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. NeuroImage 37, 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty TK, Quay HC, Ramos L (1993). Response perseveration, inhibitory control, and central dopaminergic activity in childhood behavior disorders. Journal of Genetic Psychology 154, 177–188. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Ansari TL, Hansard M, Shoker L, Eysenck MW (2009). Anxiety, inhibition, efficiency, and effectiveness. Experimental Psychology (formerly Zeitschrift für Experimentelle Psychologie) 56, 48–55. [DOI] [PubMed] [Google Scholar]
- Epstein J, Johnson D, Varia I, Conners CK (2001). Neuropsychological assessment of response inhibition in adults with ADHD. Journal of Clinical and Experimental Neuropsychology 23, 362–371. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG (2007). Anxiety and cognitive performance: attentional control theory. Emotion 7, 336–353. [DOI] [PubMed] [Google Scholar]
- Forster S, Lavie N (2014). Distracted by your mind? Individual differences in distractibility predict mind wandering. Journal of Experimental Psychology – Learning Memory and Cognition 40, 251–260. [DOI] [PubMed] [Google Scholar]
- Geen RG (1985). Test anxiety and visual vigilance. Journal of Personality and Social Psychology 49, 963–970. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN (2007). The neural basis of decision making. Annual Review of Neuroscience 30, 535–574. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N (2000). The Neuropsychology of Anxiety: An Inquiry into the Function of the Septo-Hippocampal System. Oxford University Press: Oxford. [Google Scholar]
- Grillon C, Baas JM (2003). A review of the modulation of the startle reflex by affective states and its application to psychiatry. Clinical Neurophysiology 114, 1557–1579. [DOI] [PubMed] [Google Scholar]
- Grillon C, Robinson O, Krimsky M, O’Connell K, Alvarez G, Ernst M (2016). Anxiety-mediated facilitation of behavioral inhibition: threat processing and defensive reactivity during a go/nogo task. Emotion. Published online 19 September 2016. doi: 10.1037/emo0000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars MA, Oitzl M, Roelofs K (2014). Updating freeze: aligning animal and human research. Neuroscience and Biobehavioral Reviews 47, 165–176. [DOI] [PubMed] [Google Scholar]
- Hagopian LP, Ollendick TH (1994). Behavioral inhibition and test anxiety: an empirical investigation of Gray’s theory. Personality and Individual Differences 16, 597–604. [Google Scholar]
- Helton WS (2009). Impulsive responding and the sustained attention to response task. Journal of Clinical and Experimental Neuropsychology 31, 39–47. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N (1988). Biological basis of childhood shyness. Science 240, 167–171. [DOI] [PubMed] [Google Scholar]
- Karch S, Jäger L, Karamatskos E, Graz C, Stammel A, Flatz W, Lutz J, Holtschmidt-Täschner B, Genius J, Leicht G, Pogarell O, Born C, Möller H-J, Hegerl U, Reiser M, Soyka M, Mulert C (2008). Influence of trait anxiety on inhibitory control in alcohol-dependent patients: simultaneous acquisition of ERPs and BOLD responses. Journal of Psychiatric Research 42, 734–745. [DOI] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ (2016). Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology 53, 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG (2001). Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. Journal of Neuroscience 21, 5222–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijmans R, Scheres A, Oosterlaan J (2000). Response inhibition and measures of psychopathology: a dimensional analysis. Child Neuropsychology 6, 175–184. [DOI] [PubMed] [Google Scholar]
- Li CS, Chao HH, Lee TW (2009). Neural correlates of speeded as compared with delayed responses in a stop signal task: an indirect analog of risk taking and association with an anxiety trait. Cerebral Cortex 19, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maack DJ, Tull MT, Gratz KL (2012). Examining the incremental contribution of behavioral inhibition to generalized anxiety disorder relative to other Axis I disorders and cognitive–emotional vulnerabilities. Journal of Anxiety Disorders 26, 689–695. [DOI] [PubMed] [Google Scholar]
- McVay JC, Kane MJ (2009). Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology. Learning, Memory, and Cognition 35, 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Meier ME, Touron DR, Kane MJ (2013). Aging ebbs the flow of thought: adult age differences in mind wandering, executive control, and self-evaluation. Acta Psychologica 142, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BE, van Honk J, Hermans EJ, Scholten MRM, Stein DJ, Kahn RS (2009). Gray’s BIS/BAS dimensions in non-comorbid, non-medicated social anxiety disorder. World Journal of Biological Psychiatry 10, 925–928. [DOI] [PubMed] [Google Scholar]
- Neo PSH, Thurlow J, McNaughton N (2011). Stopping, goal-conflict, trait anxiety and frontal rhythmic power in the stop-signal task. Cognitive, Affective and Behavioral Neuroscience 11, 485–493. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Sergeant JA (1996). Inhibition in ADHD, aggressive, and anxious children: a biologically based model of child psychopathology. Journal of Abnormal Child Psychology 24, 19–36. [DOI] [PubMed] [Google Scholar]
- Peebles D, Bothell D (editors) (2004). Modelling Performance in the Sustained Attention to Response Task. Carnegie Mellon University/University of Pittsburgh: Pittsburgh, PA. [Google Scholar]
- Pessoa L, Padmala S, Kenzer A, Bauer A (2012). Interactions between cognition and emotion during response inhibition. Emotion 12, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay H (1988). The behavioral reward and inhibition system in childhood behavior disorder In Attention Deficit Disorder (ed. Bloomingdale LM), pp. 176–186. Pergamon Press: Oxford, UK. [Google Scholar]
- Quay HC (1997). Inhibition and attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology 25, 7–13. [DOI] [PubMed] [Google Scholar]
- Righi S, Mecacci L, Viggiano M (2009). Anxiety, cognitive self-evaluation and performance: ERP correlates. Journal of Anxiety Disorders 23, 1132–1138. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, Grillon C (2013a). The impact of induced anxiety on response inhibition. Frontiers in Human Neuroscience 7, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Lieberman L, Allen P, Vytal K, Grillon C (2015). The dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit in unmedicated generalized and social anxiety disorders. Lancet Psychiatry 1, 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Vytal K, Cornwell BR, Grillon C (2013b). The impact of anxiety upon cognition: perspectives from human threat of shock studies. Frontiers in Human Neuroscience 7, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K, van Peer J, Berretty E, Jong Pd, Spinhoven P, Elzinga BM (2009). Hypothalamus–pituitary–adrenal axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. Biological Psychiatry 65, 336–343. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Hirshfeld DR, Bolduc EA, Faraone SV, Kagan J, Snidman N, Reznick JS (1991). Further evidence of an association between behavioral inhibition and anxiety disorders – results from a family study of children from a non-clinical sample. Journal of Psychiatry Research 25, 49–65. [DOI] [PubMed] [Google Scholar]
- Saucedo Marquez CM, Zhang X, Swinnen SP, Meesen R, Wenderoth N (2013). Task-specific effect of transcranial direct current stimulation on motor learning. Frontiers in Human Neuroscience 7, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Dalm S, Schächinger H, Oitzl MS (2008). Chronic stress modulates the use of spatial and stimulus-response learning strategies in mice and man. Neurobiology of Learning and Memory 90, 495–503. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT (2009). Stress prompts habit behavior in humans. Journal of Neuroscience 29, 7191–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Konrad C, Zwitserlood P, Arolt V, Falkenstein M, Beste C (2010). ERP indices for response inhibition are related to anxiety-related personality traits. Neuropsychologia 48, 2488–2495. [DOI] [PubMed] [Google Scholar]
- Seli P (2016). The attention-lapse and motor decoupling accounts of SART performance are not mutually exclusive. Consciousness and Cognition 41, 189–198. [DOI] [PubMed] [Google Scholar]
- Seli P, Risko EF, Smilek D (2016). On the necessity of distinguishing between unintentional and intentional mind wandering. Psychological Science 27, 685–691. [DOI] [PubMed] [Google Scholar]
- Smallwood J (2013). Penetrating the fog of the decoupled mind: the effects of visual salience in the sustained attention to response task. Canadian Journal of Experimental Psychology 67, 32–40. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J (1988). Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology: General 117, 34–50. [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press: Palo Alto, CA. [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, Picton TW (1995). A multidisciplinary approach to anterior attentional functions. Annals of the New York Academy of Sciences 769, 191–212. [DOI] [PubMed] [Google Scholar]
- Sylwan RP (2004). The control of deliberate waiting strategies in a stop-signal task. Brazilian Journal of Medical and Biological Research 37, 853–862. [DOI] [PubMed] [Google Scholar]
- Thomas SJ, Gonsalvez CJ, Johnstone SJ (2016). Electrophysiology of facilitation priming in obsessive–compulsive and panic disorders. Clinical Neurophysiology 127, 464–478. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Science 9, 585–594. [DOI] [PubMed] [Google Scholar]
- Wright L, Lipszyc J, Dupuis A, Thayapararajah S, Schachar R (2014). Response inhibition and psychopathology: a meta-analysis of go/no-go task performance. Journal of Abnormal Psychology 123, 429–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


