Abstract
Background
Pars plana vitrectomy (PPV) combined with phacoemulsification and primary intraocular lens implantation can be performed for the repair of primary rhegmatogenous retinal detachment (RRD; PHACOVIT group). The safety and efficacy of this combined ophthalmic procedure on RRD surgery outcomes remain unclear compared with more conventional PPV technique alone (VITRET group). We explored the need for reoperation after primary surgical procedure in these two groups.
Methods
Retrospective, longitudinal, register-based cohort of RRD patients was operated in University Eye Clinic, Helsinki, Finland, during 2008–2014. The main outcome measure was reoperation rate during a postoperative follow-up period of 1 year due to retinal re-detachment, vitreous rehemorrhage, postoperative endophthalmitis, secondary pucker, macular hole or other reasons.
Results
We analyzed 1,690 consecutive RRD cases, out of which 1,564 patients were treated in the PPV VITRET group and 126 patients in the PHACOVIT-operated group. Risk for reoperation was 2.67 times higher in the PHACOVIT group compared to the PPV VITRET group (95% CI 1.85–3.85).
Conclusion
The reoperation rate was higher in RRD eyes operated with combined cataract surgery plus PPV, suggesting that RRD eyes should not primarily undergo combined PHACOVIT surgery.
Keywords: cataract surgery, rhegmatogenous retinal detachment, proliferative vitreoretinopathy, cohort study, epidemiology, vitreoretinal surgery, statin therapy
Introduction
Cataract surgery is the most common surgical procedure in the world, with few serious sight-threatening adverse events (rhegmatogenous retinal detachment [RRD], endophthalmitis, suprachoroidal hemorrhage).1,2 Of note, vitreoretinal (VR) surgery is the third most common intraocular surgery performed after refractive and cataract surgeries in the US.3 Great technological advances in both cataract and VR surgery have been achieved, attributed to technological innovations in the machinery (such as the availability of microincisional cataract and vitrectomy technology) and instrumentation (pupil stretchers, capsular tension ring, microforceps, wide-angle microscope and even 3D heads-up viewing systems, perioperative optical coherence tomography imaging, pharmacologic agents [staining of the anterior lens capsule and chromovitrectomy] and therapeutic antiangiogenic agents).4–6
Currently, surgeon’s preferences to treat different VR pathologies are known to differ between US and European VR surgeons.7 Both medical and nonmedical factors including economic factors are known to affect the decision-making of VR surgeons.7 Interestingly, phacoemulsification with intraocular lens (IOL) implantation at the time of vitrectomy is much more commonly performed in Europe than in the US. Today, most RRD-related studies compare different VR techniques such as pars plana vitrectomy (PPV), PPV plus scleral buckle (SB), SB alone or pneumatic retinopexy, showing no differences in surgical success rates between different RRD groups.8–10 Historically, phacoemulsification at the time of RRD vitrectomy was avoided due to the increased complication rate such as possibility of optic capture, corneal edema, secondary glaucoma, fibrinous uveitis, development of posterior synechiae, capsular phimosis, decentered IOL and secondary pupillary block. If needed, phacofragmentation through the pars plana was performed when needed, although this method was mainly used for advanced proliferative vitreoretinopathy (PVR) eyes with an anterior PVR component to remove more efficiently the potential scaffold for reproliferation.11
Thanks to improvement of surgical technology, VR surgeons have started to treat combined posterior segment pathology with combined surgeries despite some increased risks such as potential postoperative refractive surprises, especially toward myopia in RRD eyes and other complication risks.12–14 Of note, Savastano et al15 showed no increase in postoperative complications in 565 eyes operated with combined cataract surgery with 25-gauge PPV for vitreomacular diseases ie epiretinal membrane, vitreomacular traction or macular hole (MH). In addition, in a small study by Moon et al,16 combined small-gauge sutureless vitrectomy and clear corneal phacoemulsification with IOL implantation for RRD repair was proven safe and effective.16
Owing to scarcity of the existing literature related to combined cataract and vitrectomy surgery, our study aimed to find out whether multiple interventions, ie, combined cataract plus vitrectomy surgery (PHACOVIT), are as safe and effective as phakic vitrectomy (PPV alone) as a primary surgical strategy in RRD eyes.
Methods
Study design and cohort
The current register-based study was based on adult RRD patients operated in a tertiary academic referral VR surgical unit, Helsinki University Hospital, Finland.17 The patients underwent retinal repair surgery due to RRD from May 12, 2008, to December 31, 2014, covering altogether 6.5 years. After exclusions, our final sub-study population comprised 1,690 RRD patients.
Register data sources and collection
Our register-based data were collected from the following sources: treating institution Helsinki University Hospital, Social Insurance Institution and Statistics Finland (SF), linked by means of the unique personal identification number assigned to all people living in Finland, as described previously.17
Briefly, the baseline data collected from the university hospital operating room management system (OPERA® GE Healthcare, Helsinki, Finland) database consisted of the surgical procedure and patient-related variables, the mode of admission (day surgery/inpatient stay/emergency) and the main indications for surgery (International Classification of Diseases [ICD]-10 code [http://urn.fi/URN:NBN:fi fe201205085423]).
The eyes operated due to RRD were coded as H33.0. The primary surgical procedure for RRD repair was recorded as follows (http://urn.fi/URN:ISBN:978-952-245-858-2 [in Finnish and Swedish]): combined vitrectomy and retinal procedure (VITRET, CKD92) and combined phacoemulsification and IOL implantation together with pars plana vitrectomy (PPV; PHACOVIT, CKD94). The IOL power was calculated preoperatively using IOLMaster (Carl Zeiss Meditec Optical Biometer; Carl Zeiss Meditec AG, Jena, Germany). Exclusion criterion was vitrectomy with combined SB and/or encircling band (SBVIT, CKD93). Encirclement procedures (cerclage, SB) make the eye surgery more complicated, and therefore, these procedures were not included or analyzed in our study. Postoperatively, operated patients received dexamethasone (1 mg/mL)/Dexa-Chlora® (chloramphenicol; 2 mg/mL) eye drops four times a day for 4 weeks or alternatively, levofloxacin 5 mg/mL (Oftaquix®) for 2 weeks and Pred Forte® (prednisolone acetate) 10 mg/mL (1%) for 3–4 weeks. Nonsteroidal anti-inflammatory drugs (NSAIDs) were prescribed according to surgeons’ preference.
Accordingly, ophthalmic surgery and patient-related characteristics (age, sex, height, weight and body mass index [BMI] and the American Society of Anesthesiologists [ASA] Physical Status classification) were recorded as shown in our previous paper.17 Only minority of our RRD VR cases were operated under general anesthesia. Most of our patients (>95%) were operated under local peribulbar anesthesia.
Systemic diseases and concomitant medication of the study patients were obtained from the Social Insurance Institution database. Information on the systemic and ocular diseases was collected as described earlier.17
All purchased and reimbursed medications were recorded nationwide in the Finnish Prescription Register (FPR) and Finnish Registry for Reimbursed Medication (FRM), with generic name and World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) code (http://www.whocc.no/atc_ddd_index/). For each drug, reimbursement-related factors, including the dispensing date (date of purchase), the WHO ATC code and the quantity dispensed (amount in defined daily doses), were recorded as classified by WHO (WHOCC 2012; WHOCC - ATC/defined daily dose Index).18
Details were obtained regarding the drugs prescribed in the year before and following the first operation, including statins, insulins, other diabetes drugs, beta-blocking agents and antithrombotic drugs. We assumed that the drug was used at the time of the first surgery if the purchase before surgery would cover the operation date based on the information of the DDD purchased. We used only baseline usage of drugs as covariates.
Mortality data were retrieved from SF, where the vital status was collected for all Finnish citizens into the Finnish Causes of Death Register.19
RRD surgery
The surgical technique was the standard three-port 20-, 23-, or 25-gauge transconjunctival PPV (Constellation Vision System; Alcon Laboratories, Inc., Fort Worth, TX, USA).17
Our sub-study population included consecutive RRD cases with first-performed operations coded with vitrectomy with retinal procedure (including removal of membranes, use of perfluorocarbon liquid if needed, endolaser, fluid-air exchange, posterior drainage retinotomy with air, short- or long-acting gas [SF6, C2F6, C3F8] or silicone oil tamponade; VITRET, CKD92) and standard sutureless clear corneal phacoemulsification and foldable IOL implantation combined with vitrectomy and retinal procedure called phacovitrectomy (PHACOVIT, CKD94). In combined surgical cataract plus RRD cases, altogether five incisions were made to the operated eye.
Follow-up time and end points
The follow-up period started after the first operation. The primary end point event was the reoperation during the postoperative follow-up period due to retinal re-detachment (H33.0), vitreous hemorrhage (H43.1), endophthalmitis (H44.0), secondary pucker, MH (H35.38/H35.37/H35.39) or other reason.
Revitrectomy was defined as described earlier.17 Only the first revitrectomy of the operated eye was taken into account. If there was no revitrectomy in 1 year after the start of follow-up, censoring took place.17 Death before 1 year after the start of follow-up was accounted as a censoring event.
Ethics
Our study protocol was approved by the ethics committees of the Hjelt Institute, University of Helsinki, and the Hospital District of Helsinki and Uusimaa, Helsinki, Finland.
Statistical analysis
Statistical analyses were performed as described earlier.17 Incidence of resurgery was modeled using Poisson regression models. Incidence rate ratio (IRR) was calculated, and cumulated follow-up time was taken into account.
We controlled for confounding using background variables such as age, sex, BMI, year of surgery, type of surgery, duration of operation, baseline usage of insulin, other antidiabetic drugs (OADs), antithrombotic drugs and beta-blockers. However, the precise ophthalmic surgical procedure-related variables such as removal of membranes, use of perfluorocarbon liquid if needed, endolaser, posterior drainage retinotomy or used tamponade could not be taken into account in our analysis.
All data analysis was performed using R language (R Core Team, 2016; available at https://www.R-project.org/).20
Results
Baseline patient and eye-related characteristics of our RRD study cohort (N = 1,690) are given in Table 1. Of note, 87% of VITRET-operated patients and 89.7% of PHACOVIT-operated RRD patients were presbyopic (>50 years of age). Duration of operation was >1 h 13 min in 84.9% of PHACOVIT-operated patients compared to 62.6% of VITRET-operated patients. There were no differences between the groups regarding systemic medical treatment. In our study, the most common systemic diseases were hypertension, diabetes mellitus and coronary artery disease that did not differ between the two study groups.
Table 1.
Basic characteristics of study population
| Patient and ophthalmic surgery related characteristics | VITRET, CKD92 group (n = 1,564), n (%) | PHACOVIT, CKD94 group (n = 126), n (%) | P-value |
|---|---|---|---|
| Sex | 0.16 | ||
| Male | 1,034 (66.1) | 75 (59.5) | |
| Female | 530 (33.9) | 51 (40.5) | |
| Age of the patient (years) | 0.004 | ||
| <40 | 56 (3.6) | 6 (4.8) | |
| 40–<50 | 147 (9.4) | 7 (5.5) | |
| 50–<60 | 482 (30.8) | 23 (18.3) | |
| ≥60 | 879 (56.2) | 90 (71.4) | |
| Year of surgery | 0.07 | ||
| Before 2011 | 696 (44.5) | 45 (35.7) | |
| 2011 or later | 868 (55.5) | 81 (64.3) | |
| Statin therapy | 0.66 | ||
| No | 1,273 (81.4) | 100 (79.4) | |
| Yes | 291 (18.6) | 26 (20.6) | |
| Insulin use | 0.99 | ||
| No | 1,520 (97.2) | 122 (96.8) | |
| Yes | 44 (2.8) | 4 (3.2) | |
| OAD use | 0.99 | ||
| No | 1,451 (92.8) | 117 (92.9) | |
| Yes | 113 (7.2) | 9 (7.1) | |
| Antithrombotics | 0.10 | ||
| No | 1,478 (94.5) | 114 (90.5) | |
| Yes | 86 (5.5) | 12 (9.5) | |
| Beta-blockers | 0.82 | ||
| No | 1,334 (85.3) | 106 (84.1) | |
| Yes | 230 (14.7) | 20 (15.9) | |
| Duration of operation (h) | <0.0001 | ||
| (0.3, <1.13) | 584 (37.3) | 19 (15.1) | |
| (1.13, <1.5) | 573 (36.6) | 44 (34.9) | |
| (1.5, 6.38) | 407 (26.0) | 63 (50.0) |
Notes: P-value based on the chi-square test for independence. P ≤ 0.05 was considered as statistically significant. VITRET, CKD92, combined vitrectomy and retinal procedure; PHACOVIT, CKD94, combined phacoemulsification and IOL implantation together with PPV.
Abbreviations: OAD, oral antidiabetic drugs; IOL, intraocular lens; PPV, pars plana vitrectomy.
Our RRD surgeries were performed by ten VR surgeons. Since personal preferences and decision-making of each surgeon are by nature subjective, the rate of type of operation between different surgeons differed in our study (χ2 = 62.01, df = 9, P-value < 0.0001).
Primary RRD surgical procedure (VITRET) compared with combined phacovitrectomy (PHACOVIT)
Interestingly, vitrectomy with retinal procedure (VITRET, CKD92) was performed in 1,564 (92.5%) patients and combined phacovitrectomy (PHACOVIT, CKD94) in 126 (7.5%) cases.
Of the VITRET-operated patients, 291 (18.6%) used statin compared with 26 (20.6%) of patients who were phacovitrectomy operated (Table 1).
Reoperation rate in the operated RRD eyes
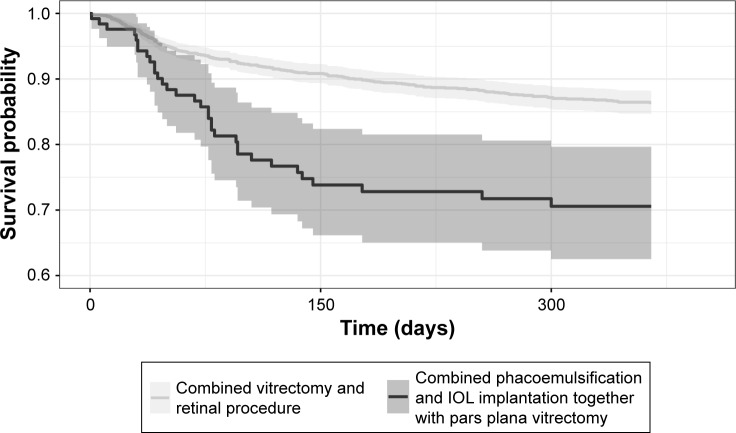
Altogether, reoperation was performed in 232 (13.7%) of our subcohort consisting of RRD eyes (N = 1,690). Reoperation rate was lower in VITRET (CKD92)-treated RRD eyes (n = 199) compared with PHACOVIT (CKD94)-treated (n = 33) RRD eyes, with a rate of 15.6 per 100 person-years (95% CI 13.5–18) as compared to a rate of 41.7 per 100 person-years (95% CI 28.7–58.6). Taken together, reoperations were performed in 12.7% of VITRET-operated eyes and 26.2% of PHACOVIT-operated eyes (IRR 2.67, 95% CI 1.85–3.85) (Table 2 and Figure 1).
Table 2.
Reoperation rate (per 100 person-years) with 95% CI
| Operation type | Person-years (100) | Reoperations (n) | Rate + CI | IRR |
|---|---|---|---|---|
| CKD92 | 12.718 | 199 | 15.65 (13.55–17.98) | Reference |
| CKD94 | 0.791 | 33 | 41.73 (28.72–58.60) | 2.67 (1.85–3.85) |
Notes: VITRET, CKD92, combined vitrectomy and retinal procedure; PHACOVIT, CKD94, combined phacoemulsification and IOL implantation together with PPV. IRR from univariate Poisson model.
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; IOL, intraocular lens; PPV, pars plana vitrectomy.
Figure 1.
Survival from reoperation (N = 1,690) differed according to the surgical procedure (VITRET, CKD92 versus PHACOVIT, CKD94).
Notes: Kaplan-Meier curves with 95% CI. VITRET, CKD92, combined vitrectomy and retinal procedure; PHACOVIT, CKD94, combined phacoemulsification and IOL implantation together with PPV.
Abbreviations: CI, confidence interval; IOL, intraocular lens; PPV, pars plana vitrectomy.
Out of the all the reoperated RRD patients, 173 developed redetachment, 30 secondary epiretinal fibrosis, six MHs, three vitreous hemorrhage, six IOL complications and 14 other reasons for reoperation (Table 3).
Table 3.
Diagnose codes for reoperations
| Diagnosis for reoperation | VITRET, CKD92 group (n = 199) | PHACOVIT, CKD94 group (n = 33) |
|---|---|---|
| Retinal redetachment (H33.0) | 149 | 24 |
| Secondary pucker (H35.38) | 28 | 2 |
| Secondary MH (H35.37) | 4 | 2 |
| Exudative AMD (H33.1) | 1 | 0 |
| Vitreous hemorrhage (H43.1) | 1 | 2 |
| Mechanical complication of IOL (T85.2) | 6 | 1 |
| Secondary glaucoma (H40) | 0 | 1 |
| Complicated cataract (H26.2) | 6 | 0 |
| Suspected endophthalmitis (H44) | 0 | 1 |
| Secondary malignancy (C79.41) | 1 | 0 |
| No code | 3 | 0 |
Notes: VITRET, CKD92, combined vitrectomy and retinal procedure; PHACOVIT, CKD94, combined phacoemulsification and IOL implantation together with PPV.
Abbreviations: ICD, International Classification of Diseases; MH, macular hole; AMD, age-related retinal degeneration; IOL, intraocular lens; PPV, pars plana vitrectomy.
In Poisson regression analysis, usage of statin therapy at the time of operation was associated with a lower reoperation risk (IRR 0.62, 95% CI 0.42–0.92).
Generally, survival from reoperation was significantly better in eyes operated with the VITRET procedure as compared with phacovitrectomized RRD eyes.
Bayesian prediction model
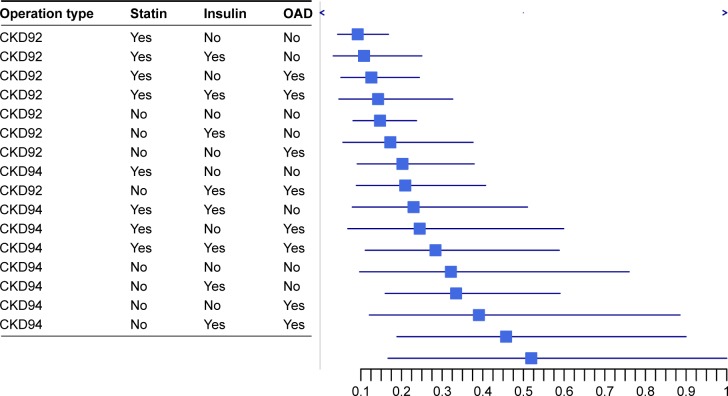
The risk for reoperation was lowest in VITRET (CKD92)- operated RRD eyes (n = 1,564) if the patient had statin treatment and no diabetes. Noteworthy, the risk for reoperation was highest in the PHACOVIT (CKD94)-operated RRD group (n = 126) if the patient had no statin treatment but was diabetic using both oral antidiabetic medication and insulin (Figure 2).
Figure 2.
Bayesian prediction of reoperation of RRD eyes according to eye procedure (VITRET, CKD92 versus PHACOVIT, CKD94), use of statin, use of insulin and use of oral diabetic treatment.
Note: VITRET, CKD92, combined vitrectomy and retinal procedure; PHACOVIT, CKD94, combined phacoemulsification and IOL implantation together with PPV.
Abbreviations: RRD, rhegmatogenous retinal detachment; IOL, intraocular lens; PPV, pars plana vitrectomy; GAD, oral antidiabetic drugs.
Discussion
Herein, we analyzed all consecutive RRD eyes that underwent either primary standard three port pars plana microincision vitrectomy with retinal procedure (VITRET) alone or combined PHACOVIT with IOL implantation in our hospital during 2008–2014. Our historical retrospective register based cohort study demonstrated that the reoperation rate was 2.67 times higher in RRD eyes operated with combined PHACOVIT surgery. Of note, systemic statin medication seemed to lower the risk of reoperation in our RRD eyes according to Bayesian prediction.
Repair of tissue following any surgical procedure (injury) involves dynamic interactions between multiple cell types, growth factors, inflammatory mediators and components of the extracellular matrix. Indeed, wound healing is one of the most complex biological processes to occur in tissues.21 Presently, multiple ophthalmic procedures are considered more inflammatory than single procedures.22 Surgical techniques including tissue manipulation, instrumentation and drugs may also modulate the postoperative inflammatory process, making evaluation of performed surgical procedures and their success rates challenging but important.
Previously, it has been shown that intact natural crystalline lens could provide a physical barrier for transmission of inflammatory cytokines and/or growth factors from the anterior chamber to the vitreous cavity.23 Interestingly, after cataract surgery, increased levels of various cytokines and growth factors (such as TGF-beta, FGF and VEGF) may be involved in postoperative inflammatory processes, leading to several ocular complications.24–27 Additional complication such as iris damage at the time of cataract surgery has been associated with elevation in the levels of aqueous protein and cytokines (IL-1-alpha, IL-1-beta, IL-6, IL-8, IL-10, TNF- alpha), which have also been implicated with PVR formation in the posterior segment.28,29 Vice versa, a long time after vitrectomy, the levels of inflammatory cytokines (such as IL-6, IL-8 and ICAM-1) can still be increased in the anterior chamber of vitrectomized eyes.25 Taken together, both aqueous and/or vitreous cytokines and growth factors are known to promote cell migration, proliferation and tissue emodeling (epithelial-mesenchymal transition of both lens epithelial and retinal pigment epithelial cells), resulting in various manifestations of intraocular fibrosis such as posterior capsular opacification after cataract surgery and PVR after vitrectomy, making combined surgery potentially more risky than single ophthalmic procedures.21,30–36
According to our data, we could speculate that both the breakdown of blood-aqueous-barrier due to cataract surgery and the breakdown of blood-retinal barrier due to vitrectomy together could cause stronger ocular inflammatory condition, leading to a higher reoperation rate after combined phacovitrectomy than after single vitrectomy procedure in our RRD eyes.37 Retinal detachment per se is known to trigger the release of large numbers of intravitreal proinflammatory and profibrotic cytokines and growth factors. Therefore, postoperative inflammatory mechanisms could be more active in eyes operated with combined cataract plus RRD than in eyes operated with combined cataract plus neurodegenerative posterior segment pathology (such as epiretinal membrane, vitreomacular traction or MH).15,17,38–40 Supporting these findings, the risk for reoperation indeed was highest in our PHACOVIT-operated RRD eyes if the patient had diabetes with oral antidiabetic medication and insulin but no anti-inflammatory and anti-fibroproliferative statin treatment (Bayesian model).30,41 Accordingly, on the other hand, our findings of the lowest reoperation rate in VITRET-operated RRD patients with statin use and no systemic disease supported the same hypotheses. Of note, as shown by our previous studies, intravitreal levels of angiopoietin-2 and VEGF, two key factors involved in vascular permeability and inflammation, together with the activity of matrix metalloproteinase-2, the major factor connected with the breakdown of basement membrane and fibroproliferation, have been found to be lower in RRD eyes of patients with statin treatment.42,43 Pharmacological modulation of these ocular processes with statin might also explain our novel epidemiological findings in RRD eyes.
The strength of our study was that we included a relatively large sample size and assessed reoperation rates in a real-world clinical setting by analyzing all consecutive multiple surgeon-operated RRD cases in our institution. We do acknowledge the following limitations such as the unbalanced patient groups that can also occur in multicenter studies including multiple surgeons. Noteworthy, we had no access to patient archives to check the precise ophthalmic4 status of the operated RRD eyes.17 Therefore, it is unclear why and when a surgeon decided for a combined PHACOVIT procedure instead of VITRET procedure. Of note, we could not report on the functional visual outcomes in our RRD eyes.44 Preoperative eye-related characteristics (such as refraction, axial length, extent of RRD, number of holes/breaks, PVR grade C or worse, macula on/off, vitreous hemorrhage, localized/extensive laser retinopexy) or intraoperative surgical management-related characteristics (such as phacoemulsification or posterior segment surgical complications) could not be analyzed in our epidemiological study setting without patient contact.17,44 Thus, more thorough prospective clinical surgical studies and randomized clinical trials are warranted to address these unanswered but important eye and surgery-related features in the future.
In observational studies, it is always possible that there are remaining confounding factors connected to data analysis. We controlled for this confounding using background variables as model covariates and also applied the inverse probability of treatment weighting method. Despite these attempts, it is possible that some bias remains.
Conclusion
Registry databases in Finland allow a powerful tool for understanding the best clinical practices in surgical procedures that have not been globally established and out of which there is limited literature published. Indeed, combined cataract surgery together with RRD-vitrectomy are two separate ophthalmic surgical procedures without recommendations from the current ophthalmic literature.22 Our study evaluated surgical outcome and adverse events associated with combined phacovitrectomy and vitrectomy alone in RRD eyes in the biggest tertiary ophthalmic referral center in Finland. According to our preliminary results, we cannot recommend the combined PHACOVIT procedure as a standard routine surgery in the RRD eyes. Even though cataract surgery is the most often performed surgical procedure in the whole world,45 its removal can still be more challenging in patients suffering from RRD than in patients with normal age-related VR interface diseases, and/or combined PHACOVIT surgery per se could cause more intraocular inflammation, leading to an increased risk of reoperations in RRD eyes. Interestingly, combined phacovitrectomy surgery was very recently rated as more inflammatory condition than single cataract surgery.22
Interestingly, combined cataract-vitrectomy surgery in eyes with uveitis has also been associated with increased inflammation more frequently than single vitrectomy surgery.46 In addition, the incidence of postoperative complications has shown to be higher in the eyes that require tamponade, corresponding to the severity of each RRD case and the complexity of the RRD surgical procedure.47 Our study suggested that PHACOVIT surgery could be regarded as a more inflammatory procedure, and the source of the “additional” inflammation is located in the anterior chamber. During the study period of 2008–2014, the PHACOVIT patients were not systematically subjected to a more aggressive or prolonged anti-inflammatory topical treatment after surgery (eg, by increasing the dosage or including NSAIDs nepafenac, ketorolac or bromfenac). NSAID treatment could result in lower concentrations of aqueous cytokines and growth factors and a lower rate of complications after combined PHACOVIT surgery. Pre- and postoperative anterior chamber laser flare meter measurements obtained from RRD eyes (operated either with vitrectomy or phacovitrectomy) would offer valuable information regarding this important clinical aspect in the future.48 As a whole, more thorough clinical studies are needed to answer important open questions related to the best topical therapy after single and phaco-combined RRD surgery.
In our study, we observed a difference between the two operated RRD groups. This difference may be due to patient selection for different operations because of the ophthalmic characteristics (opacity of crystalline lens, larger area of RRD, multiple holes) or because of the surgeon’s preference. Thus, based on this observational study, no causal relation between the operation type and the outcome may be determined. Our current study should strongly be regarded as a trigger for further in-depth investigation to find out the best possible surgical approach to treat RRD eyes.
Acknowledgments
This work was supported by TYH2016230 grant (SL) and the University of Helsinki and the National Institute for Health and Welfare, Finland (JH).
Footnotes
Author contributions
JH and SL designed the sub-study. JH performed statistical analyses. SL and JH drafted the manuscript. SL and JH contributed to the conception and design of the study, acquisition and interpretation of the data, and have approved the final version of the work. All authors agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology. 2011;1128(9):1716–1723. doi: 10.1016/j.ophtha.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein JD. Serious adverse events after cataract surgery. Curr Opin Ophthalmol. 2012;23(3):219–225. doi: 10.1097/ICU.0b013e3283524068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Amir AN, Keenan TD, Abu-Bakra M, Tanner V, Yeates D, Goldacre MJ. Trends in rates of retinal surgery in England from 1968 to 2004: studies of hospital statistics. Br J Ophthalmol. 2009;93(12):1585–1590. doi: 10.1136/bjo.2009.159939. [DOI] [PubMed] [Google Scholar]
- 4.Sharma T, Fong A, Lai TY, Lee V, Das S, Lam D. Surgical treatment for diabetic vitreoretinal diseases: a review. Clin Exp Ophthalmol. 2016;44(4):340–354. doi: 10.1111/ceo.12752. [DOI] [PubMed] [Google Scholar]
- 5.Khan MA, Shahlaee A, Toussaint B, et al. Outcomes of 27 gauge microincision vitrectomy surgery for posterior segment disease. Am J Ophthalmol. 2016;161:36–43. e1–2. doi: 10.1016/j.ajo.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Eckardt C, Paulo EB. Heads-up surgery for vitreoretinal procedures: an experimental and clinical study. Retina. 2016;36(1):137–147. doi: 10.1097/IAE.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 7.D’Amico DJ. Different preferences between United States and European vitreoretinal surgeons: personal observations. Curr Opin Ophthalmol. 2016;27(3):196–200. doi: 10.1097/ICU.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, Sun T, Xu Y, et al. Primary vitrectomy versus scleral buckling for the treatment of rhegmatogenous retinal detachment: a meta-analysis of randomized controlled clinical trials. Curr Eye Res. 2012;37:492–499. doi: 10.3109/02713683.2012.663854. [DOI] [PubMed] [Google Scholar]
- 9.Soni C, Hainsworth DP, Almony A. Surgical management of rhegmatogenous retinal detachment: a meta-analysis of randomized controlled trials. Ophthalmology. 2013;120:1440–1447. doi: 10.1016/j.ophtha.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Haugstad M, Moosmayer S, Bragadóttir R. Primary rhegmatogenous retinal detachment - surgical methods and anatomical outcome. Acta Ophthalmol. 2017;95(3):247–251. doi: 10.1111/aos.13295. [DOI] [PubMed] [Google Scholar]
- 11.Benson WE, Blankenship GW, Machemer R. Pars plana lens removal with vitrectomy. Am J Ophthalmol. 1977;84(2):150–152. doi: 10.1016/0002-9394(77)90846-7. [DOI] [PubMed] [Google Scholar]
- 12.Cho KH, Park IW, Kwon SI. Changes in postoperative refractive outcomes following combined phacoemulsification and pars plana vitrectomy for rhegmatogenous retinal detachment. Am J Ophthalmol. 2014;158(2):251.e2–256.e2. doi: 10.1016/j.ajo.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Rahman R, Bong CX, Stephenson J. Accuracy of intraocular lens power estimation in eyes having phacovitrectomy for rhegmatogenous retinal detachment. Retina. 2014;34(7):1415–1420. doi: 10.1097/IAE.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 14.Pinarci EY, Bayar SA, Sizmaz S, Yesilirmak N, Akkoyun I, Yilmaz G. Anterior segment complications after phacovitrectomy in diabetic and nondiabetic patients. Eur J Ophthalmol. 2013;23(2):223–229. doi: 10.5301/ejo.5000203. [DOI] [PubMed] [Google Scholar]
- 15.Savastano A, Savastano MC, Barca F, Petrarchini F, Mariotti C, Rizzo S. Combining cataract surgery with 25-gauge high-speed pars plana vitrectomy: results from a retrospective study. Ophthalmology. 2014;121(1):299–304. doi: 10.1016/j.ophtha.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Moon H, Sohn HJ, Lee DY, Lee JY, Nam DH. Combined 23-gauge sutureless vitrectomy and clear corneal phacoemulsification for rhegmatogenous retinal detachment repair. Int J Ophthalmol. 2015;8(1):122–127. doi: 10.3980/J.ISSN.2222-3959.2015.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loukovaara S, Sahanne S, Takala A, Haukka J. Statin use, and vitreoretinal surgery: findings from a Finnish population-based cohort study. Acta Ophthalmol. 2018 Jan 16; doi: 10.1111/aos.13641.. Epub. [DOI] [PubMed] [Google Scholar]
- 18.WHOCC ATC/DDD Index. [Accessed September 11, 2012]. Available from: http://www.whocc.no/atc_ddd_index/
- 19.Gissler M, Haukka J. Finnish health and social welfare registers in epidemiological research. Norsk Epidemiologi. 2004;14:113–120. [Google Scholar]
- 20.Core Team R [home page on the Internet] R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Accessed June 30, 2018]. Available from: https://www.R-project.org/ [Google Scholar]
- 21.Shu DY, Lovicu FJ. Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog Retin Eye Res. 2017;60:44–65. doi: 10.1016/j.preteyeres.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aptel F, Colin C, Kaderli S, et al. Management of postoperative inflammation after cataract and complex ocular surgeries: a systematic review and Delphi survey. Br J Ophthalmol. 2017;101(11):1–10. doi: 10.1136/bjophthalmol-2017-310324. [DOI] [PubMed] [Google Scholar]
- 23.Kon CH, Asaria RH, Occleston NL, Khaw PT, Aylward GW. Risk factors for proliferative vitreoretinopathy after primary vitrectomy: a prospective study. Br J Ophthalmol. 2000;84(5):506–511. doi: 10.1136/bjo.84.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakobsson G, Sundelin K, Zetterberg H, Zetterberg M. Increased levels of inflammatory immune mediators in vitreous from pseudophakic eyes. Invest Ophthalmol Vis Sci. 2015;56(5):3407–3414. doi: 10.1167/iovs.15-16837. [DOI] [PubMed] [Google Scholar]
- 25.Gu R, Zhou M, Jiang C, Yu J, Xu G. Elevated concentration of cytokines in aqueous in post-vitrectomy eyes. Clin Exp Ophthalmol. 2016;44(2):128–134. doi: 10.1111/ceo.12638. [DOI] [PubMed] [Google Scholar]
- 26.Iyengar L, Patkunanathan B, McAvoy JW, Lovicu FJ. Growth factors involved in aqueous humour-induced lens cell proliferation. Growth Factors. 2009;27(1):50–62. doi: 10.1080/08977190802610916. [DOI] [PubMed] [Google Scholar]
- 27.Pennock S, Haddock LJ, Mukai S, Kazlauskas A. Vascular endothelial growth factor acts primarily via platelet-derived growth factor receptor α to promote proliferative vitreoretinopathy. Am J Pathol. 2014;184(11):3052–3068. doi: 10.1016/j.ajpath.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kita T, Hata Y, Arita R, et al. Role of TGF-beta in proliferative vitreo-retinal diseases and ROCK as a therapeutic target. Proc Natl Acad Sci USA. 2008;105(45):1750–17509. doi: 10.1073/pnas.0804054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennock S, Haddock LJ, Eliott D, Mukai S, Kazlauskas A. Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog Retin Eye Res. 2014;40:16–34. doi: 10.1016/j.preteyeres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara S, Hata Y, Kita T, et al. Potent inhibition of cicatricial contraction in proliferative vitreoretinal diseases by statins. Diabetes. 2008;57(10):2784–2793. doi: 10.2337/db08-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garweg JG, Tappeiner C, Halberstadt M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv Ophthalmol. 2013;58(4):321–329. doi: 10.1016/j.survophthal.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Pennock S, Kim D, Mukai S, et al. Ranibizumab is a potential prophylaxis for proliferative vitreoretinopathy, a nonangiogenic blinding disease. Am J Pathol. 2013;182(5):1659–1670. doi: 10.1016/j.ajpath.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moysidis SN, Thanos A, Vavvas DG. Mechanisms of inflammation in proliferative vitreoretinopathy: from bench to bedside. Mediators Inflamm. 2012;2012:815937. doi: 10.1155/2012/815937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Li H, Jiang P, Liu X, Xu D, Wang F. Investigating the pathological processes of rhegmatogenous retinal detachment and proliferative vitreoretinopathy with metabolomics analysis. Mol Biosyst. 2014;10:1055–1062. doi: 10.1039/c3mb70386j. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhary R, Dretzke J, Scott R, Logan A, Blanch R. Clinical and surgical risk factors in the development of proliferative vitreoretinopathy following retinal detachment surgery: a systematic review protocol. Syst Rev. 2016;5(1):107. doi: 10.1186/s13643-016-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastor JC, Rojas J, Pastor-Idoate S, Di Lauro S, Gonzalez-Buendia L, Delgado-Tirado S. Proliferative vitreoretinopathy: a new concept of disease pathogenesis and practical consequences. Prog Retin Eye Res. 2016;51:125–155. doi: 10.1016/j.preteyeres.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Coca-Prados M. The blood-aqueous barrier in health and disease. J Glaucoma. 2014;23(8 Suppl 1):S36–S38. doi: 10.1097/IJG.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 38.Symeonidis C, Diza E, Papakonstantinou E, Souliou E, Dimitrakos SA, Karakiulakis G. Correlation of the extent and duration of rhegmatogenous retinal detachment with the expression of matrix metalloproteinases in the vitreous. Retina. 2007;27(9):1279–1285. doi: 10.1097/IAE.0b013e3180592c00. [DOI] [PubMed] [Google Scholar]
- 39.Hoerster R, Hermann MM, Rosentreter A, Muether PS, Kirchhof B, Fauser S. Profibrotic cytokines in aqueous humour correlate with aqueous flare in patients with rhegmatogenous retinal detachment. Br J Ophthalmol. 2013;97(4):450–453. doi: 10.1136/bjophthalmol-2012-302636. [DOI] [PubMed] [Google Scholar]
- 40.Loukovaara S, Lehti K, Robciuc A, et al. Increased intravitreal angiopoietin-2 levels associated with rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2014;252(6):881–888. doi: 10.1007/s00417-013-2508-z. [DOI] [PubMed] [Google Scholar]
- 41.Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000;47(4):648–657. doi: 10.1016/s0008-6363(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 42.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27(12):552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Tuuminen R, Haukka J, Loukovaara S. Statins in rhegmatogenous retinal detachment are associated with low intravitreal angiopoietin-2, VEGF and MMP-2 levels, and improved visual acuity gain in vitrectomized patients. Graefes Arch Clin Exp Ophthalmol. 2015;253(10):1685–1693. doi: 10.1007/s00417-014-2873-2. [DOI] [PubMed] [Google Scholar]
- 44.Sahanne S, Tuuminen R, Haukka J, Loukovaara S. A retrospective study comparing outcomes of primary rhegmatogenous retinal detachment repair by scleral buckling and pars plana vitrectomy in Finland. Clin Ophthalmol. 2017;11:503–509. doi: 10.2147/OPTH.S128746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.eurostat [webpage on the Internet] Surgical Operations and Procedures Statistics - Statistics Explained. [Accessed November 30, 2017]. Available from: http://ec.europa.eu/eurostat/statistics-explained/index.php?title=Surgical_operations_and_procedures_statistics&oldid=270601.
- 46.Senn P, Schipper I, Perren B. Combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation in the capsular bag: a comparison to vitrectomy and subsequent cataract surgery as a two-step procedure. Ophthalmic Surg Lasers. 1995;26(5):420–428. [PubMed] [Google Scholar]
- 47.Honjo M, Ogura Y. Surgical results of pars plana vitrectomy combined with phacoemulsification and intraocular lens implantation for complications of proliferative diabetic retinopathy. Ophthalmic Surg Lasers. 1998;29(2):99–105. [PubMed] [Google Scholar]
- 48.Schroder S, Muether PS, Caramoy A, et al. Anterior chamber aqueous flare is a strong predictor for proliferative vitreoretinopathy in patients with rhegmatogenous retinal detachment. Retina. 2012;32(1):38–42. doi: 10.1097/IAE.0b013e3182173753. [DOI] [PubMed] [Google Scholar]