Abstract
Objective
The roles of nonsteroidal anti-inflammatory drugs (NSAIDs) in the occurrence and prognosis of hepatocellular carcinoma (HCC) remain controversial. This analysis aimed to summarize the relationships between NSAIDs and HCC development.
Methods
Studies published prior to October 1, 2017, in the PubMed, Embase, Ovid, Web of Science, and Cochrane Library databases were systematically searched and analyzed.
Results
Eleven studies were included in this analysis. A meta-analysis of five studies revealed that aspirin use could significantly decrease the risk of HCC occurrence (hazards ratio [HR] = 0.64, 95% confidence interval [CI] = 0.45–0.91, P = 0.014). No significant difference was found for the use of NSAIDs (six studies) and non-aspirin NSAIDs (three studies) in HCC occurrence (HR = 0.74, 95%CI = 0.53–1.02, P = 0.064 and HR = 0.98, 95%CI = 0.87–1.12, P = 0.81, respectively). However, subgroup analysis of cohort studies demonstrated that NSAIDs significantly decreased the risk of HCC occurrence (HR = 0.58, 95%CI = 0.43–0.78, P < 0.001). HCC patients who received NSAIDs achieved better disease-free survival and overall survival compared with the non-NSAID users (HR = 0.79, 95%CI = 0.74–0.84, P<0.001 and HR = 0.60, 95%CI = 0.50–0.72, P<0.001, respectively). Additionally, a meta-analysis of two studies showed that aspirin treatment in HCC patients could significantly decrease the 2-year and 4-year mortalities (rate ratio [RR] = 0.50, 95%CI = 0.36–0.69, P < 0.001 and RR = 0.67, 95%CI = 0.45–0.998, P = 0.049, respectively). A meta-analysis of two studies showed that aspirin use was not associated with a higher risk of bleeding in HCC patients (HR = 0.71, 95%CI = 0.41–1.23, P = 0.223).
Conclusion
The use of NSAIDs, especially aspirin, is linked to a lower risk of HCC development and better survival in HCC populations. High-quality, well-designed trials should be conducted to reevaluate the relationships between NSAIDs and HCC.
Keywords: NSAID, hepatocellular carcinoma, aspirin, overall survival, recurrence
Introduction
It has been reported that worldwide 70%–90% of diagnosed primary liver cancers are hepatocellular carcinoma (HCC), which is the fastest growing cause of cancer-related death.1–3 There has been a marked increase in HCC-related annual death rates in the past two decades.2,4 The incidence and prevalence of HCC are increasing in Western countries, where HCC is now the leading cause of death in patients with liver cirrhosis. In addition, a recent study using the SEER registry projects has shown that the incidence of HCC will continue to rise until 2030;1,5 that is, HCC imposes an enormous health burden worldwide.3,6
Nonsteroidal anti-inflammatory drug (NSAID) use, especially of aspirin, has been linked to reduced chronic inflammation and a risk of several cancers. The effects of aspirin and non-aspirin NSAIDs rely on several possible mechanisms, partly related to the inhibition of cyclooxygenase (COX) enzymes and prostaglandins, such as a decrease in angiogenesis and cancer cell proliferation, an increase in apoptosis, and a reduction in proinflammatory cytokine production.7–9 Clinically, several studies have revealed that the long-term use of NSAIDs, including aspirin, is associated with a reduced risk of cancers and better survival.10–13 However, for some cancers, no association was found.14 Additionally, the effects of NSAIDs including aspirin were inconsistent in HCC. For example, Sitia et al15 found that the effects of aspirin were present in hepatitis B virus-related carcinogenesis but not in chemical liver carcinogenesis. Sahasrabuddhe et al’s study showed that the use of aspirin alone was associated with a 41% reduced risk of developing HCC, but the risk of developing HCC in non-aspirin NSAID users remained unchanged.16
The purpose of this study was to systematically review and analyze the published studies regarding the epidemiology and outcomes of HCC patients who received NSAIDs, via meta-analysis.
Materials and methods
Search strategy
We searched the PubMed, Ovid, Embase, Web of Science, and Cochrane Library databases for studies published prior to October 1, 2017. The following medical subject terms were used: “hepatocellular carcinoma”, “liver cancer”, “liver neoplasms”, “hepatoma”, “aspirin”, “NSAIDs”, and “non-steroidal anti-inflammatory drug”. Electronic searches were supplemented with manual searches of the reference lists of all retrieved review articles, primary studies, and abstracts from meetings to identify additional studies not found electronically. The literature was searched by two authors (YT and YL) independently.
Eligibility criteria
Two authors (YT and YL) independently selected the studies and discussed them with each other when inconsistencies were found. The articles that met the following criteria were included: (1) case–control, cohort studies or randomized controlled trials (RCTs), (2) subjects 18 years of age or older, and (3) availability of the hazard ratio (HR), rate ratio (RR), or odds ratio (OR) estimates, clinicopathological features or survival data of patients with HCC who received NSAIDs or aspirin. If the duration and sources of the recruited study populations overlapped by more than 30% in two or more studies by the same authors, we only included the most recent study or the study with the larger number of HCC patients.
Data extraction
Two researchers independently read the full texts and extracted the following information: publication data, study design, sample size, population, patients’ ages, controlled variables, and follow-up period.
Methodological quality assessment
The Newcastle–Ottawa Scale (NOS) was used to assess the methodological quality of all cohort or case–control publications selected in the final analysis.17 The scales allocated a maximum of nine stars for the quality of selection, comparability, exposure, and outcome of the study participants.18 Two authors (YT and YL) independently assessed the study quality, and any inconsistency was discussed with the review team.
Statistical analysis
Stata software version 14.0 (Stata Corporation, College Station, TX, USA) was used for data analysis. The effect measures of interest were the HR, RR, and the corresponding 95% confidence intervals (CIs). RRs were directly considered as HR. Odds ratios (ORs) were transformed into RRs according to the formula RR = OR/[(1−P0)+(P0×OR)], where P0 stands for HCC incidence in the nonexposed group.19 Heterogeneity across studies was assessed using the Cochran Q test. Higgins I2 statistics were used to determine the degree of between-study heterogeneity.20 Publication bias was examined by Begg’s funnel plots and Egger’s regression tests.21 Sensitivity analyses were conducted to investigate the robustness of our results and to assess whether any of the included studies had a considerable influence on the results. A fixed-effects model was initially used for our meta-analyses. Since sensitivity analysis could not determine the source of heterogeneity, a random-effects model was subsequently used to accommodate the anticipated heterogeneity across studies. If a direct report of patient survival was not available,22 an estimated value was derived indirectly from the Kaplan–Meier curves using Engauge Digitizer software (http://markummitchell.github.io/engauge-digitizer). A descriptive analysis was performed when the quantitative data could not be pooled. All statistical tests were two-tailed, and differences with P < 0.05 were considered statistically significant.
Results
Study and patient characteristics
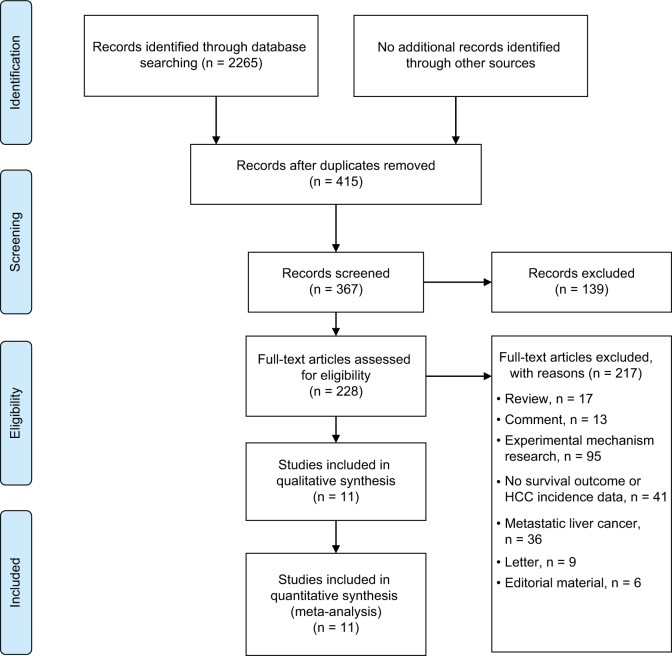
As shown in Figure 1, 2265 records were identified through database searches. Overall, 228 full-text articles were assessed for eligibility after removing duplicates and screening records. Eleven studies16,22–31 were included in our analysis. Six studies16,23–27 reported the epidemiology data of NSAID use related to HCC occurrence, and five22,28–31 presented survival data. The baseline characteristics of the included studies are summarized in Tables 1 and 2.
Figure 1.
Study selection process
Table 1.
Characteristics of studies with HCC occurrence risk data
| Studies | Study design | Country | NSAIDs | NSAID duration | RR (95%CI) | Controlled variables | Hepatitis virus infection |
|---|---|---|---|---|---|---|---|
| Coogan et al (2000)23 | Case–control | USA | Salicylates, indoles, propionic acids, fenamates, and/or oxicams | At least 4 days/week for at least 3 months, at least 1 year before admission | Regular use initiated >1 year; 0.9 (0.3–2.9); Nonregular use; 1.6 (0.8–3.1) | Age, sex | NA |
| Lee et al (2017)24 | Cohort, retrospective | Korea | Aspirin | 38.5 months (IQR, 10.4–68.7 months) | 0.26 (0.09–0.74) | Cirrhosis | HBV |
| Oh et al (2017)25 | Retrospective | Korea | Aspirin | NA | 0.27 (0.09–0.76) in propensity score-matched pairs; 0.27 (0.10–0.74) in time-varying Cox proportional analyses | Time-varying Cox proportional hazards model; propensity score-matching | NA |
| Petrick et al (2015)26 | Cohort, prospective | USA | Aspirin, ibuprofen | NA | 0.68 (0.57–0.81) | NA | 42 (26.6%) were anti-HCV positive and five (3.2%) were HBsAg positive in HCC cases; 10 (2.5%) were anti-HCV positive and three (0.8%) were HBsAg positive in controls |
| Sahasrabuddhe et al (2012)16 | Cohort, prospective | USA | Aspirin, ibuprofen, naproxen, ketoprofen, piroxicam, sulindac, indomethacin, nambumetone | NA | 0.59 (0.45–0.77) for aspirin use; 1.08 (0.84–1.39) for non-aspirin NSAID use | NA | NA |
| Yang et al (2016)27 | Nested case–control | UK | Aspirin, COX-2 inhibitors, propionic acid derivatives, fenamic acid derivatives, acetic acid derivatives, and enolic acid derivatives | Two or more NSAID prescriptions | 1.11 (0.86–1.44) for aspirin use; 1.05 (0.88–1.24) for NSAIDs | Age, sex, general practice, and number of years in the CPRD prior to the case’s index date | HBV and/or HCV infection, 170 (14.2%) in HCC cases and 23 (0.5%) in controls |
Abbreviations: CPRD, Clinical Practice Research Datalink; NSAID, nonsteroidal anti-inflammatory drug; COX-2, cyclooxygenase-2; NA, not available; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
Table 2.
Baseline characteristics of studies with HCC survival outcomes
| Studies | Study design | Country/area | HCC cases, n | NSAIDs | NSAID duration | Aspirin administration | Treatment regimen | Hepatitis virus infection | Controlled variables | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al (2016)22 | Cohort | Taiwan | 9461 | Aspirin | ≥90 days | 100 mg/day | Liver resection | HBV | Propensity score-matching | ≥90 days |
| Li et al (2016)28 | Case–control, retrospective | China | 120 | Aspirin | ≥90 days | 100 mg/day | TACE | HBV, n = 110; HCV, n = 5 | Age, sex, date of HCC diagnosis, Child-Pugh score | 12.0 (1.0–48.0) months |
| Takami et al (2016)29 | RCT | Japan | 232 | Meloxicam | NA | None | Hepatic resection and/or microwave ablation | HBV, n = 24; HCV, n = 152 | Age, sex, etiology | NA |
| Wu et al (2012)30 | Cohort | Taiwan | 4569 | NA | At least 1 day per month on average | NA | Liver resection | HBV | NA | ≥90 days |
| Yeh et al (2015)31 | Cohort, retrospective | Taiwan | 15,574 | Conventional NSAIDs, aspirin, and COX-2 inhibitors | NA | NA | Liver resection | HBV, n = 8002; HCV, n = 4575 | Age, sex, extent of liver resection, comorbidities and drug usage | 1.8 (0.8–3.9) years |
Abbreviations: HBV, hepatitis B virus; TACE, transarterial chemoembolization; NA, not available; RCT, randomized controlled trial.
Quality assessment of included studies
The NOS quality assessment scale was used to examine potential bias risks of case control studies and cohort studies. The detailed NOS items and scores of each study are presented in Table 3. Cohort studies by Sahasrabuddhe et al16 and Wu et al30 did not present adjusted variables in exposed and non-exposed individuals, leading to a one-star score for comparability. We did not conduct a methodological quality assessment for the study reported in the abstract.25 The study by Takami et al29 was randomized and non-blinded with no patients lost to follow-up, which we considered to have a low risk of selection bias, attrition bias, and reporting bias.
Table 3.
NOS quality assessment of included studies (case control studies and cohort studies)
| Case–control studies | Coogan et al (2000)23 | Yang et al (2016)27 | Li et al (2016)28 | |
|---|---|---|---|---|
| Selection | Is the case definition adequate? | * | * | * |
| Representativeness of the cases | * | * | * | |
| Selection of controls | * | * | * | |
| Definition of controls | * | * | * | |
| Comparability | Comparability of cases and controls on the basis of the design or analysis | ** | ** | ** |
| Exposure | Ascertainment of exposure | * | * | * |
| Same method of ascertainment for cases and controls | * | * | * | |
| Non-response rate | ||||
| NOS scores | 8 | 8 | 8 |
| Cohort studies | Sahasrabuddhe et al (2012)16 | Lee et al (2017)24 | Petrick et al (2015)26 | Lee et al (2016)22 | Wu et al (2012)30 | Yeh et al (2015)31 | |
|---|---|---|---|---|---|---|---|
| Selection | Representativeness of the exposed cohort | ||||||
| Selection of the non-exposed cohort | * | * | * | * | * | * | |
| Ascertainment of exposure | * | * | * | * | * | * | |
| Demonstration that outcome of interest was not present at start of study | * | * | * | * | * | * | |
| Comparability | Comparability of cohorts on the basis of the design or analysis | * | ** | ** | ** | * | ** |
| Exposure | Assessment of outcome | * | * | * | * | * | * |
| Was follow-up long enough for outcomes to occur | * | * | * | * | * | * | |
| Adequacy of follow-up of cohorts | * | * | * | * | * | * | |
| NOS scores | 7 | 8 | 8 | 8 | 7 | 8 |
Notes: A study can be awarded a maximum of one asterisk (*) for each numbered item within the Selection and Exposure categories. A maximum of two asterisks (**) can be given for Comparability.
Abbreviation: NOS, Newcastle–Ottawa scale.
NSAIDs and HCC risk
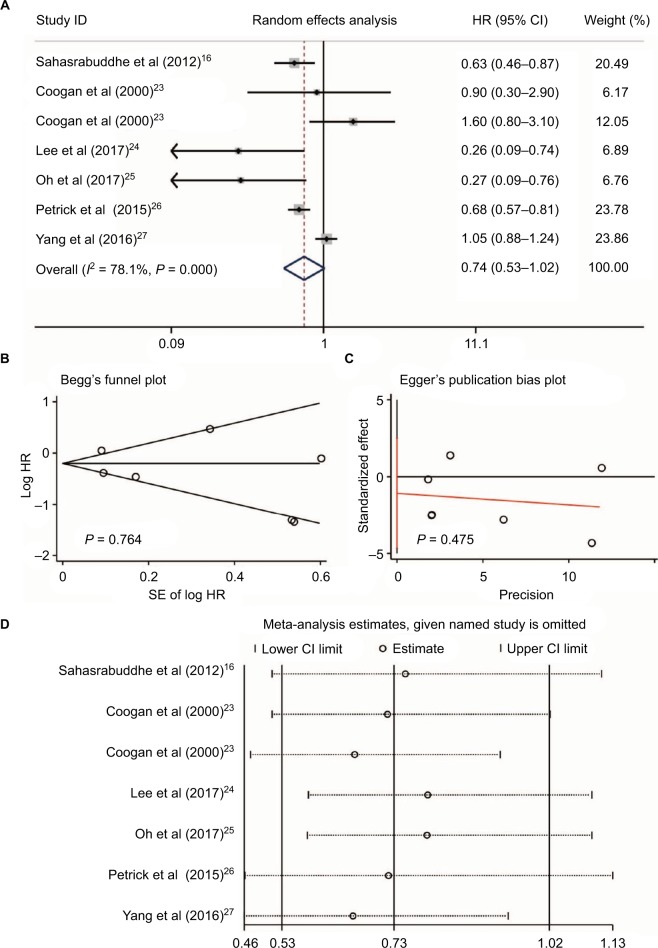
Heterogeneity was found when evaluating the association between NSAID use and HCC risk (I2 = 78.1%, P < 0.001, Figure 2A). We performed a publication bias analysis and sensitivity analysis of these six studies.16,23–27 No publication bias was found by Begg’s and Egger’s tests (P = 0.764 and P = 0.475, respectively, Figure 2B and 2C). When each named study was omitted, no large influence was found in our sensitivity analysis (Figure 2D). Thus, a meta-analysis of six studies16,23–27 with the random-effect model demonstrated that patients who received NSAIDs had a similar risk of HCC occurrence compared with those without NSAIDs (HR = 0.74, 95%CI = 0.53–1.02, P = 0.064, Figure 2A).
Figure 2.
NSAIDs, including aspirin use and HCC risk. The use of NSAIDs, including aspirin and HCC risk (A); publication bias of included studies by Begg’s (B) and Egger’s tests (C); and sensitivity analysis of included studies (D).
Abbreviations: NSAIDs, nonsteroidal anti-inflammatory drugs; HCC, hepatocellular carcinoma.
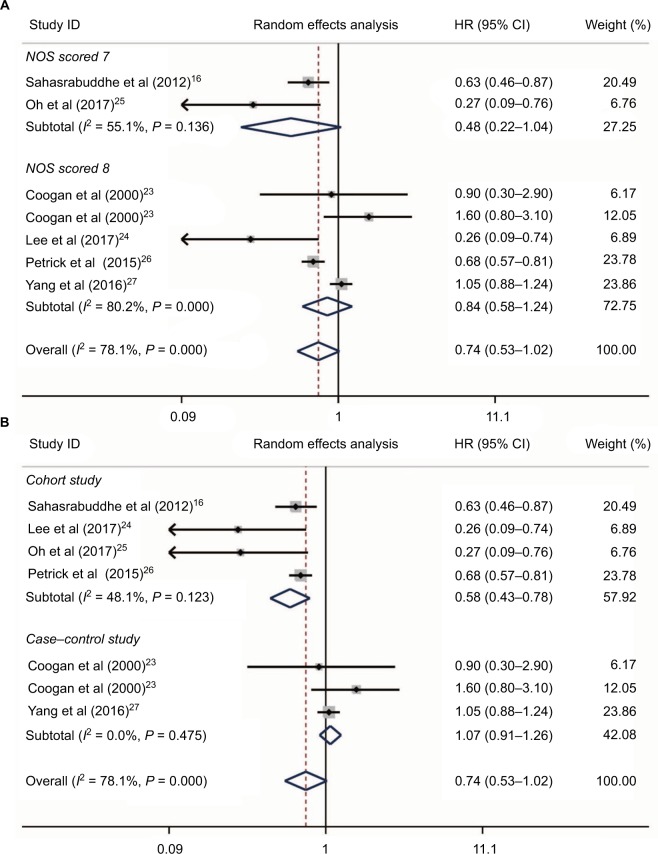
No large influence was detected in NSAID use and HCC occurrence and, thus, we conducted further subgroup analysis based on NOS scores and study design (Figure 3). As shown in Figure 3A, no heterogeneity was found in studies with lower NOS scores (I2 = 55.1%, P = 0.136, Figure 3A), while heterogeneity was still detected in studies with high NOS scores (I2 = 80.2%, P < 0.001, Figure 3A). Additionally, no significant associations were found between NSAIDs and the risk of HCC occurrence in both the groups with lower and higher NOS scores (HR = 0.48, 95%CI = 0.22–1.04, P = 0.064 and HR = 0.84, 95%CI = 0.58–1.24, P = 0.386, respectively, Figure 3A). Interestingly, when we performed subgroup analysis based on study design, no heterogeneity was found in either subgroup (I2 = 48.1%, P = 0.123 and I2 = 0.0%, P = 0.475, respectively, Figure 3B). Meta-analysis revealed that NSAID use could significantly decrease the HCC occurrence risk in four cohort studies16,24–26 (HR = 0.58, 95%CI = 0.43–0.78, P < 0.001, Figure 3B), while no association between NSAIDs and HCC risk was found in case–control studies23,27 (HR = 1.07, 95%CI = 0.91–1.26, P = 0.402, Figure 3B). Therefore, we assumed that the study design contributed to heterogeneity, and well-designed trials should be considered to evaluate the relationships between NSAIDs and HCC risk.
Figure 3.
Subgroup analysis of links between NSAIDs and HCC risk based on NOS scores (A) and study design (B).
Abbreviations: NSAIDs, nonsteroidal anti-inflammatory drugs; HCC, hepatocellular carcinoma.
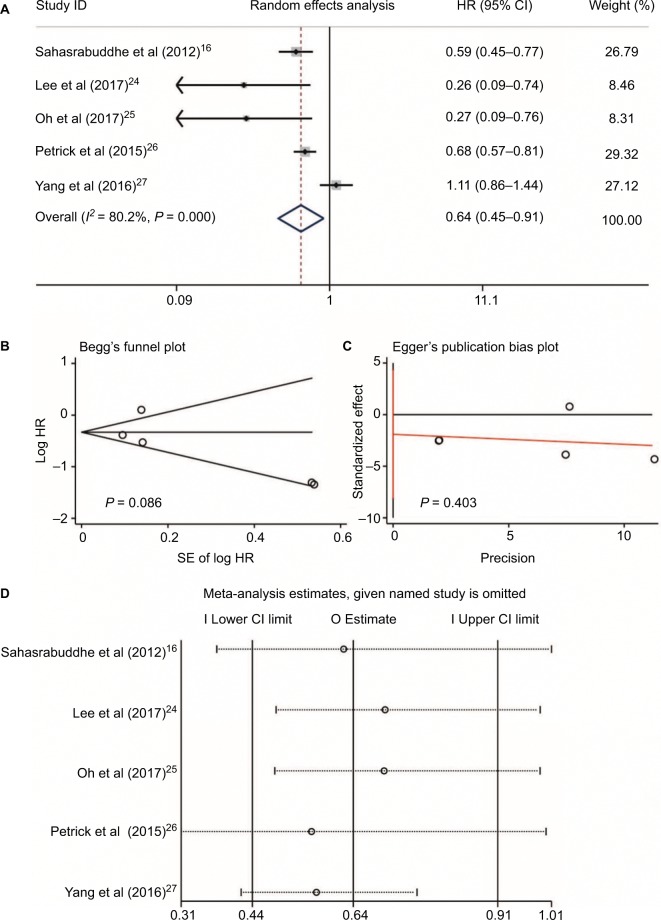
Five studies16,24–27 reported aspirin use and risk of HCC occurrence. Heterogeneity was found (I2 = 80.2%, P < 0.001, Figure 4A); no publication bias was found by Begg’s and Egger’s tests (P = 0.086 and P = 0.403, respectively, Figure 4B and 4C); and no large influence was found after each named study was omitted by sensitivity analysis (Figure 4D). Accordingly, a meta-analysis of five studies with a random-effects model16,24–27 with data on aspirin use and HCC risk revealed that aspirin use could significantly decrease the risk of HCC occurrence (HR = 0.64, 95%CI = 0.45–0.91, P = 0.014; Figure 4A). In two studies reported by Yang et al27 and Oh et al,25 the dose and daily frequency of aspirin were not available. However, in the studies of Sahasrabuddhe et al16 and Petrick et al,26 patients with any frequency of aspirin use were included. In the study by Lee et al,24 100 mg aspirin was used daily.
Figure 4.
Aspirin use and HCC risk. Relationship between the use of aspirin and HCC risk (A); publication bias of included studies by Begg’s (B) and Egger’s tests (C); and sensitivity analysis of included studies (D).
Abbreviation: HCC, hepatocellular carcinoma.
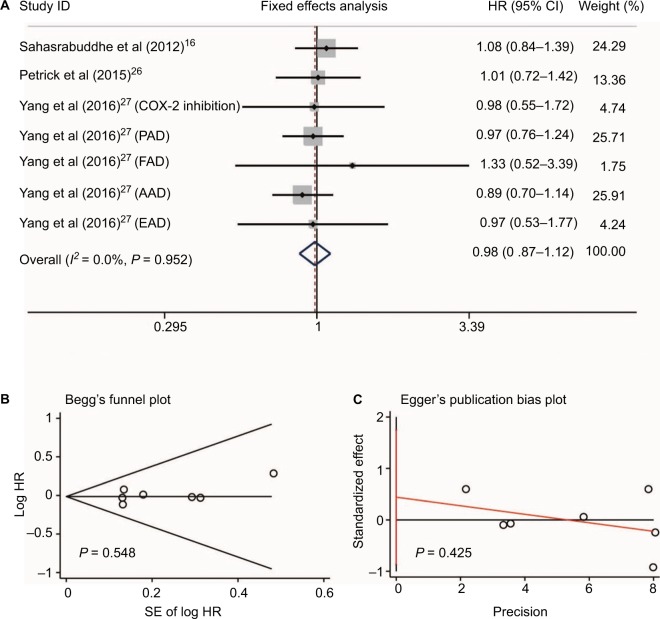
Three studies16,26,27 reported data on non-aspirin NSAID use and HCC risk. The non-aspirin NSAIDs in these three studies included ibuprofen,26 COX-2 inhibitors (rofecoxib, celecoxib, and etoricoxib), propionic acid derivatives (ibuprofen, naproxen, ketoprofen, and tiaprofenic acid), fenamic acid derivatives (mefenamic acid), acetic acid derivatives (diclofenac, indomethacin, etodolac, and nabumetone), and enolic acid derivatives (piroxicam and meloxicam).27 Since no heterogeneity was found between these three studies (I2 = 0.0%, P = 0.952, Figure 5A), sensitivity analysis was not conducted. Meta-analysis with a fixed-effect model showed that no significant difference was found for the use of non-aspirin NSAIDs in HCC occurrence (RR = 0.98, 95%CI = 0.87–1.12, P = 0.81; Figure 5A). Additionally, no publication bias was detected by Begg’s and Egger’s tests (P = 0.548 and P = 0.425, respectively, Figure 5B and 5C).
Figure 5.
Non-aspirin NSAIDs use and HCC risk. Relationship between the use of non-aspirin NSAIDs and HCC risk (A); publication bias of included studies by Begg’s (B) and Egger’s tests (C).
Abbreviations: NSAIDs, nonsteroidal anti-inflammatory drugs; HCC, hepatocellular carcinoma; COX-2, cyclooxygenase-2; PAD, propionic acid derivatives; FAD, fenamic acid derivatives; AAD, acetic acid derivatives; EAD, enolic acid (oxicam) derivatives
NSAIDs and HCC survival
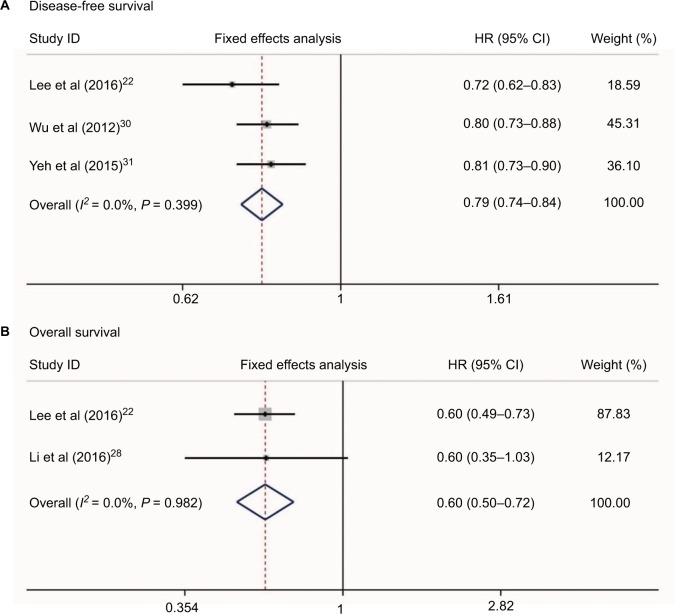
No heterogeneity was found when evaluating disease-free survival (DFS) and overall survival (OS) of HCC patients who received NSAIDs (I2 = 0.0%, P = 0.399 and I2 = 0.0%, P = 0.982, respectively, Figure 6). Three studies22,30,31 reported the DFS risk of HCC patients treated with NSAIDs. Two studies22,28 presented OS data. Meta-analysis with a fixed-effect model showed that HCC patients who received NSAIDs achieved better DFS and OS compared with non-NSAID users (HR = 0.79, 95%CI = 0.74–0.84, P<0.001 and HR = 0.60, 95%CI = 0.50–0.72, P<0.001, respectively, Figure 6A and 6B).
Figure 6.
Relationship between NSAIDs use and disease-free survival (A) and overall survival (B) in HCC patients.
Abbreviation: HCC, hepatocellular carcinoma.
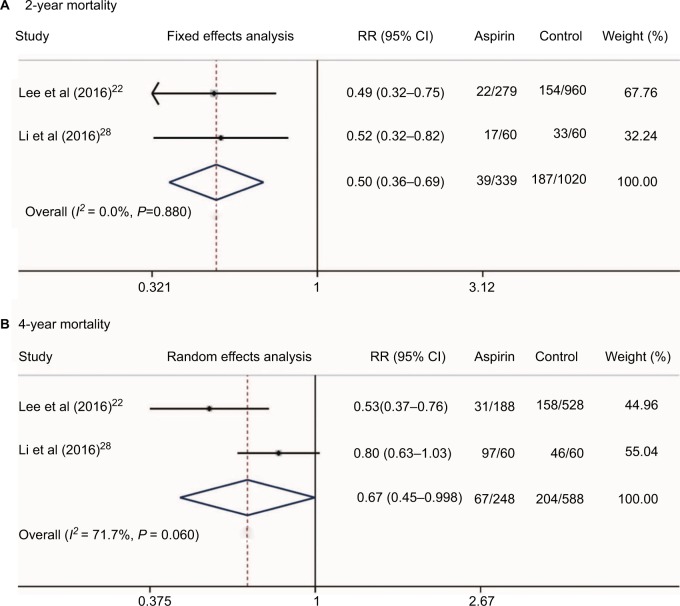
Additionally, we conducted a meta-analysis of the mortality rate of HCC patients who received aspirin treatment in two studies.22,28 Our results showed that aspirin treatment in HCC patients could significantly decrease the 2-year and 4-year mortalities (RR = 0.50, 95%CI = 0.36–0.69, P < 0.001 and RR = 0.67, 95%CI = 0.45–0.998, P = 0.049, respectively, Figure 7). Since only three studies22,30,31 presented data on NSAID use and HCC survival and two studies22,28 reported data on aspirin use and HCC survival, the relationships between NSAID use and HCC clinical outcomes should be reevaluated by well-designed, large sample, multiple-centered RCTs in future.
Figure 7.
Two-year (A) and 4-year mortalities (B) of HCC patients who received aspirin treatment.
Abbreviation: HCC, hepatocellular carcinoma.
Adverse events
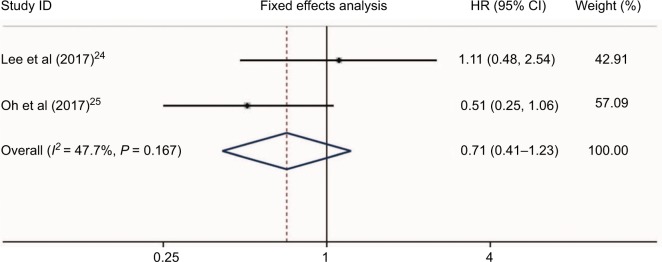
Two studies24,28 presented the hazard risk of bleeding in HCC patients who received aspirin treatment. No heterogeneity was found (I2 = 47.7%, P = 0.167, Figure 8). Meta-analysis showed that aspirin use was not associated with a higher risk of bleeding in HCC patients (HR = 0.71, 95%CI = 0.41–1.23, P = 0.223, Figure 8). Additionally, a study by Li et al28 also found no differences of bleeding risk between the aspirin group (6/60, 10%) and non-aspirin group (7/60, 11.7%) in unresectable HCC. Another study by Yeh et al31 evaluated the bleeding risk of HCC in patients who received NSAIDs after curative liver resection. They found that the incidence of de novo upper gastrointestinal bleeding in NSAID users was significantly higher than that of nonusers (25.3% vs 18.7%; P < 0.001). Therefore, the bleeding risk of NSAIDs including aspirin in HCC patients still needs further evaluation.
Figure 8.
Bleeding risk of HCC patients who received aspirin treatment.
Abbreviation: HCC, hepatocellular carcinoma.
Discussion
HCC is a type of inflammation-associated cancer;32 an environment of chronic inflammation results in continuous rounds of cell injury, necrosis, and regeneration that leaves the liver prone to the development of activating mutations in oncogenes and the inactivating genetic and epigenetic suppression of tumor suppressor genes.33–35 Currently, anti-inflammatory therapy is effective at preventing early neoplastic progression and malignant conversion.35–37
NSAIDs are potential agents for chemoprevention against liver cancer based on their anti-inflammatory properties. Previous experimental studies have revealed that NSAIDs may inhibit liver cancer cellular growth and induce cell apoptosis by modifying COX enzymatic pathways, which mediate inflammation.38,39 Although many epidemiological studies demonstrated that treatment with NSAIDs reduces the incidence and mortality of certain malignancies, including HCC, no consistent conclusion was found.23 In the studies included in our analysis, Sahasrabuddhe et al16 and Petrick et al26 conducted two large sample cohort studies and showed that NSAIDs were associated with an approximately 30%–40% reduced risk of developing HCC. Consistent results were also observed in patients who used aspirin. However, the risk of developing HCC remained unchanged in the users of non-aspirin NSAIDs.16,26 The evidence presented in these two studies is not robust enough to recommend the use of NSAIDs or aspirin in the prevention of HCC.7 The case–control studies by Yang et al27 and Coogan et al23 showed that NSAIDs, including aspirin monotherapy and non-aspirin NSAIDs, were not associated with HCC occurrence. Oh et al’s study25 was reported as conference abstract and Lee et al’s study24 was performed in chronic hepatitis B patients on antiviral treatment, leading to the conclusion that the aspirin group showed a significantly lower risk of HCC.
This meta-analysis of cohort studies showed that NSAIDs could significantly decrease the risk of HCC occurrence. Our results showed that aspirin use could significantly reduce the risk of developing HCC. HCC patients who received NSAIDs or aspirin treatment achieved better survival compared with non-users. In vitro studies have shown that NSAIDs can potentiate apoptotic pathways by increasing death signal-associated receptors and decreasing proinflammatory signals.40,41 Considering the anti-inflammatory mechanisms of NSAIDs, the anti-platelet effects of aspirin and our results, we cautiously drew the conclusion that NSAIDs, especially aspirin, might be a therapeutic strategy for HCC. However, as reviewed by Sahin et al,42 the pattern of COX-2 expression in HCC is different from that of other cancers. A low expression of this critical enzyme is associated with poor differentiation, whereas higher expression levels of COX-2 have been observed in well-differentiated HCC samples.43,44 Therefore, the exact roles of non-aspirin NSAIDs in HCC aggressiveness need to be studied further.
Previous research has demonstrated that platelets are key facilitators of this inflammation-mediated injury. The elevation of the circulating platelet count is frequently found in patients with cancer45,46 and is associated with an increased risk of venous thromboembolism and poor prognosis.46,47 Moreover, platelet depletion or the inhibition of platelet function has been shown to reduce or prevent cancer growth and distant metastasis in animal models.15,48–50 Unlike other NSAIDs, aspirin has an antiplatelet effect. In an animal study, aspirin prevented HCC formation and prolonged the survival of mice with a chronic immune-mediated inflammatory liver disease by attenuating platelet-mediated inflammation.15 Platelets are involved in thrombosis, inflammatory responses, liver regeneration,51,52 and the regulation of angiogenesis.53,54 In several tumor cell lines, platelets affected the epithelial-mesenchymal transition, invasion, and metastasis.55 Clinical studies have shown that high platelets are involved in the extrahepatic metastasis and recurrence of HCC.56–58 Buergy et al59 demonstrated that an elevated platelet level at the time of diagnosis was associated with a shorter survival in many solid tumors, including pancreatic adenocarcinoma, gastric cancer, and HCC. Nouso et al60 recruited 157 HCC patients with Child-Pugh class C cirrhosis and found that a high platelet level was an independent predictor of poor OS. Considering previously published reports and our results, we assumed that aspirin could decrease HCC risk and improve survival. Unfortunately, all the included studies that reported the survival data of NSAIDs were small sample, non-RCT studies. Further trials are needed in the future.
This meta-analysis has some limitations. First, most of the studies included in this analysis were not RCTs, leading to potential biases. Second, some studies included in this analysis did not report HCC risk factors, including hepatitis B virus/hepatitis C virus infection status. Third, the included studies that reported the survival outcomes of HCC patients who received NSAIDs had small sample sizes, and most of them included patients who received hepatic resection and might have had small HCC tumors and compensatory liver function, which poses a high risk of selection bias.
Conclusion
In summary, our results indicate that the use of NSAIDs, especially aspirin, could reduce HCC risk and improve survival in this population. Some NSAIDs have moderate selectivity for COX-1, others inhibit both COX isoforms including aspirin, indomethacin, naproxen and ibuprofen, other NSAIDs favor COX-2 inhibition, and the other ones including celecoxib, rofecoxib, lumiracoxib, valdecoxib and etoricoxib are highly selective for COX-2. Different types of NSAIDs and drug doses had different roles in the inhibition of COX-1 and COX-2.61 Therefore, further studies to understand the impact of various doses and durations in the use of NSAIDs, especially aspirin intake, on the development and progression of HCC, are needed. The causal relationship between NSAIDs and HCC risk cannot be determined and further well-designed trials are required.
Acknowledgments
This work was mainly sponsored by Shanghai Sailing Program (17YF1416000), Shanghai Youth Physician Training Grant Program 2015 (to ZY), National Natural Science Foundation of China (81502059 and 81472582), and Shanghai Rising-Star Program (16QB1402900).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 2.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34(15):1787–1794. doi: 10.1200/JCO.2015.64.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 7.Kim AK, Dziura J, Strazzabosco M. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma: the egg of columbus or another illusion? Hepatology. 2013;58(2):819–821. doi: 10.1002/hep.26498. [DOI] [PubMed] [Google Scholar]
- 8.Hossain MA, Kim DH, Jang JY, et al. Aspirin enhances doxorubicin-induced apoptosis and reduces tumor growth in human hepatocellular carcinoma cells in vitro and in vivo. Int J Oncol. 2012;40(5):1636–1642. doi: 10.3892/ijo.2012.1359. [DOI] [PubMed] [Google Scholar]
- 9.Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. Am J Physiol Gastrointest Liver Physiol. 2015;309(2):G59–G70. doi: 10.1152/ajpgi.00101.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua X, Phipps AI, Burnett-Hartman AN, et al. Timing of aspirin and other nonsteroidal anti-inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival. J Clin Oncol. 2017;35(24):2806–2813. doi: 10.1200/JCO.2017.72.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Staalduinen J, Frouws M, Reimers M, et al. The effect of aspirin and nonsteroidal anti-inflammatory drug use after diagnosis on survival of oesophageal cancer patients. Br J Cancer. 2016;114(9):1053–1059. doi: 10.1038/bjc.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel D, Kitahara CM, Park Y, et al. Thyroid cancer and nonsteroidal anti-inflammatory drug use: a pooled analysis of patients older than 40 years of age. Thyroid. 2015;25(12):1355–1362. doi: 10.1089/thy.2015.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skriver C, Dehlendorff C, Borre M, et al. Low-dose aspirin or other nonsteroidal anti-inflammatory drug use and prostate cancer risk: a nationwide study. Cancer Causes Control. 2016;27(9):1067–1079. doi: 10.1007/s10552-016-0785-7. [DOI] [PubMed] [Google Scholar]
- 14.Lad AA, Ma Y, Lee JA, et al. No association between nonsteroidal anti-inflammatory drug use and pancreatic cancer incidence and survival. Pancreas. 2017;46(5):e43–e45. doi: 10.1097/MPA.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA. 2012;109(32):E2165–E2172. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed August 1, 2018]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PC, Yeh CM, Hu YW, et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann Surg Oncol. 2016;23(Suppl 5):874–883. doi: 10.1245/s10434-016-5520-9. [DOI] [PubMed] [Google Scholar]
- 23.Coogan PF, Rosenberg L, Palmer JR, et al. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev. 2000;9(1):119–123. [PubMed] [Google Scholar]
- 24.Lee M, Chung GE, Lee JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66(5):1556–1569. doi: 10.1002/hep.29318. [DOI] [PubMed] [Google Scholar]
- 25.Oh S, Shin S, Lee SH, et al. Aspirin and the risk of hepatocellular carcinoma development in patients with compensated alcoholic cirrhosis. J Hepatol. 2017;66(1):S629–S630. [Google Scholar]
- 26.Petrick JL, Sahasrabuddhe VV, Chan AT, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the liver cancer pooling project. Cancer Prev Res (Phila) 2015;8(12):1156–1162. doi: 10.1158/1940-6207.CAPR-15-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang B, Petrick JL, Chen J, et al. Associations of NSAID and paracetamol use with risk of primary liver cancer in the Clinical Practice Research Datalink. Cancer Epidemiol. 2016;43:105–111. doi: 10.1016/j.canep.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li JH, Wang Y, Xie XY, et al. Aspirin in combination with TACE in treatment of unresectable HCC: a matched-pairs analysis. Am J Cancer Res. 2016;6(9):2109–2116. [PMC free article] [PubMed] [Google Scholar]
- 29.Takami Y, Eguchi S, Tateishi M, et al. A randomised controlled trial of meloxicam, a Cox-2 inhibitor, to prevent hepatocellular carcinoma recurrence after initial curative treatment. Hepatol Int. 2016;10(5):799–806. doi: 10.1007/s12072-016-9704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 31.Yeh CC, Lin JT, Jeng LB, et al. Nonsteroidal anti-inflammatory drugs are associated with reduced risk of early hepatocellular carcinoma recurrence after curative liver resection: a nationwide cohort study. Ann Surg. 2015;261(3):521–526. doi: 10.1097/SLA.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 32.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 33.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48(6):2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 34.Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25(2):212–225. doi: 10.1055/s-2005-871200. [DOI] [PubMed] [Google Scholar]
- 35.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coffelt SB, de Visser KE. Cancer: inflammation lights the way to metastasis. Nature. 2014;507(7490):48–49. doi: 10.1038/nature13062. [DOI] [PubMed] [Google Scholar]
- 37.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4(12):2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38(3):756–768. doi: 10.1053/jhep.2003.50380. [DOI] [PubMed] [Google Scholar]
- 39.Fodera D, D’Alessandro N, Cusimano A, et al. Induction of apoptosis and inhibition of cell growth in human hepatocellular carcinoma cells by COX-2 inhibitors. Ann N Y Acad Sci. 2004;1028:440–449. doi: 10.1196/annals.1322.052. [DOI] [PubMed] [Google Scholar]
- 40.Fredriksson L, Herpers B, Benedetti G, et al. Diclofenac inhibits tumor necrosis factor-alpha-induced nuclear factor-kappaB activation causing synergistic hepatocyte apoptosis. Hepatology. 2011;53(6):2027–2041. doi: 10.1002/hep.24314. [DOI] [PubMed] [Google Scholar]
- 41.Abiru S, Nakao K, Ichikawa T, et al. Aspirin and NS-398 inhibit hepatocyte growth factor-induced invasiveness of human hepatoma cells. Hepatology. 2002;35(5):1117–1124. doi: 10.1053/jhep.2002.32676. [DOI] [PubMed] [Google Scholar]
- 42.Sahin IH, Hassan MM, Garrett CR. Impact of non-steroidal anti-inflammatory drugs on gastrointestinal cancers: current state-of-the science. Cancer Lett. 2014;345(2):249–257. doi: 10.1016/j.canlet.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Koga H, Sakisaka S, Ohishi M, et al. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29(3):688–696. doi: 10.1002/hep.510290355. [DOI] [PubMed] [Google Scholar]
- 44.Shiota G, Okubo M, Noumi T, et al. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46(25):407–412. [PubMed] [Google Scholar]
- 45.Hwang SJ, Luo JC, Li CP, et al. Thrombocytosis: a paraneoplastic syndrome in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10(17):2472–2477. doi: 10.3748/wjg.v10.i17.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simanek R, Vormittag R, Ay C, et al. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) J Thromb Haemost. 2010;8(1):114–120. doi: 10.1111/j.1538-7836.2009.03680.x. [DOI] [PubMed] [Google Scholar]
- 48.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho-Tin-Noe B, Goerge T, Wagner DD. Platelets: guardians of tumor vasculature. Cancer Res. 2009;69(14):5623–5626. doi: 10.1158/0008-5472.CAN-09-1370. [DOI] [PubMed] [Google Scholar]
- 50.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9(5):259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 51.Kondo R, Yano H, Nakashima O, Tanikawa K, Nomura Y, Kage M. Accumulation of platelets in the liver may be an important contributory factor to thrombocytopenia and liver fibrosis in chronic hepatitis C. J Gastroenterol. 2013;48(4):526–534. doi: 10.1007/s00535-012-0656-2. [DOI] [PubMed] [Google Scholar]
- 52.Starlinger P, Assinger A, Haegele S, et al. Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology. 2014;60(1):257–266. doi: 10.1002/hep.26950. [DOI] [PubMed] [Google Scholar]
- 53.Walsh TG, Metharom P, Berndt MC. The functional role of platelets in the regulation of angiogenesis. Platelets. 2015;26(3):199–211. doi: 10.3109/09537104.2014.909022. [DOI] [PubMed] [Google Scholar]
- 54.Dineen SP, Roland CL, Toombs JE, et al. The acellular fraction of stored platelets promotes tumor cell invasion. J Surg Res. 2009;153(1):132–137. doi: 10.1016/j.jss.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 55.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xue TC, Ge NL, Xu X, Le F, Zhang BH, Wang YH. High platelet counts increase metastatic risk in huge hepatocellular carcinoma undergoing transarterial chemoembolization. Hepatol Res. 2016;46(10):1028–1036. doi: 10.1111/hepr.12651. [DOI] [PubMed] [Google Scholar]
- 57.Lee CH, Lin YJ, Lin CC, et al. Pretreatment platelet count early predicts extra-hepatic metastasis of human hepatoma. Liver Int. 2015;35(10):2327–2336. doi: 10.1111/liv.12817. [DOI] [PubMed] [Google Scholar]
- 58.Pang Q, Zhang JY, Xu XS, et al. Significance of platelet count and platelet-based models for hepatocellular carcinoma recurrence. World J Gastroenterol. 2015;21(18):5607–5621. doi: 10.3748/wjg.v21.i18.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130(12):2747–2760. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 60.Nouso K, Ito Y, Kuwaki K, et al. Prognostic factors and treatment effects for hepatocellular carcinoma in Child C cirrhosis. Br J Cancer. 2008;98(7):1161–1165. doi: 10.1038/sj.bjc.6604282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cervello M, Montalto G. Cyclooxygenases in hepatocellular carcinoma. World J Gastroenterol. 2006;12(32):5113–5121. doi: 10.3748/wjg.v12.i32.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]