Summary
Perceptual decisions require both analysis of sensory information and selective routing of relevant information to decision networks. This study explores the contribution of a midbrain network to visual perception in chickens. Analysis of visual orientation information in birds takes place in the forebrain sensory area called the Wulst, as it does in the primary visual cortex (V1) of mammals. In contrast, the midbrain, which receives parallel retinal input, encodes orientation poorly, if at all. We discovered, however, that small electrolytic lesions in the midbrain severely impair a chicken’s ability to discriminate orientations. Focal lesions were placed in the optic tectum (OT) and in the nucleus isthmi pars parvocellularis (Ipc) – key nodes in the midbrain stimulus selection network – in chickens trained to perform an orientation discrimination task. A lesion in the OT caused a severe impairment in orientation discrimination specifically for targets at the location in space represented by the lesioned location. Distracting stimuli increased the deficit. A lesion in the Ipc produced similar but more transient effects. We discuss the possibilities that performance deficits were caused by interference with orientation information processing (sensory deficit) versus with the routing of information in the forebrain (agnosia). The data support the proposal that the OT transmits a space specific signal that is required to gate orientation information from the Wulst into networks that mediate behavioral decisions, analogous to the role of ascending signals from the SC in monkeys. Furthermore, our results indicate a critical role for the cholinergic Ipc in this gating process.
Keywords: Attention, Optic tectum, Superior colliculus, Bird, Perceptual decision, Cognition, Behavior, Vision, Chicken
Introduction
Perceptual decisions require both the analysis of sensory information and the selective routing of that information to networks in the brain that mediate cognitive decisions. Interruption of either process will result in an animal being unable to utilize the stimulus for perceptual decisions. In this study, we test the contributions of two midbrain structures, both implicated in the selective routing of visual information to the forebrain based on neurophysiological data [1, 2], to visual perceptual decisions by chickens.
The central visual system in vertebrates contains two major pathways: a retinogeniculate pathway, which projects to primary visual areas in the forebrain, and a retinotectal pathway, which projects to the optic tectum (OT; equivalent to the superior colliculus, SC, in mammals) in the midbrain. In primates, the majority of retinal ganglion cells travel in the retinogeniculate pathway, and this pathway analyzes independent features of the visual scene (for example, contour orientation, color, and motion direction) in specialized circuits in V1 [3]. By contrast in birds, the majority of retinal ganglion cells travel in the retinotectal pathway, and the OT conveys information about certain features, such as color, luminance and motion, in parallel pathways to the thalamic nucleus rotundus (Rt; equivalent to the pulvinar nucleus in mammals) [4, 5].
However, at least one visual feature – the orientation of local contours – is analyzed in the retinogeniculate pathway in both birds and mammals. Like in the mammalian V1, neurons in the Wulst are organized in a topographic map of space, the majority respond best to lines of particular orientations and, in owls, they cluster in orientation columns [6–10]. In contrast, neurons in the dorsal lateral geniculate nucleus (dLGN), which provides visual input to the Wulst, have small visual receptive fields and they are rarely sensitive to line orientation [10, 11]. Therefore, a key transformation in the neural coding for contour orientation occurs in the bird Wulst, as it does in the mammalian V1.
Conversely, there is no evidence that neurons in the OT, or in the forebrain areas that receive input from the OT, respond differentially to the orientation of local contours [4, 12–14]. Neurophysiological studies of the OT, Rt and entopallium (Ent, equivalent to high-order cortex in mammals) [1]) have demonstrated that neurons in these structures can be selective for stimulus features, such as color, luminance, figure versus ground motion, and motion in depth [4, 15–18]. None of these studies, however, reports tuning for line orientation, a tuning that is easy to detect and that is readily apparent in the Wulst. Based on these data, there is no reason to expect that lesions in the OT should impair the ability of birds to discriminate orientations.
Behavioral studies have shown, however, that bilateral lesions of the OT render pigeons unable to discriminate among different shapes [19]. Why might this impairment occur if contour orientations are analyzed in the Wulst and not in the OT? One possibility is that the birds in the study discriminated shapes based largely on features that did not depend on contour orientations and that were processed in the OT. To resolve this possibility, the effects of OT lesions must be tested with stimuli that differ only in local orientation (as we do in this study). A second possibility is that the OT lesions interfered with the routing of orientation information from the Wulst to decision networks that guide behavioral choice. A recent modeling study indicates that, in monkeys, the signal that ascends from the SC to the thalamus exerts such a routing effect on visual information in the forebrain [20].
A network of structures has been identified in the bird midbrain that generates a signal that could act as a gate for routing sensory information in the forebrain [21]. The network comprises structures located in both the tectum and tegmentum of the midbrain, structures that are conserved across vertebrate species [22, 23]. The OT is the hub of this network. It combines spatial information from a variety of sensory modalities with information from the forebrain that signals the behavioral relevance of stimuli, and it represents the highest priority stimulus (combination of its salience and relevance) as the site of greatest activity in a topographic map of space [2, 24]. This network has been shown to generate signals that are appropriate for selectively routing sensory information in the forebrain [1]. However, its contribution to information routing has never been tested behaviorally.
A key circuit in the midbrain network is formed by the nucleus isthmi pars parvocellularis (Ipc; equivalent to a portion of the nucleus parabigeminus in mammals), a cholinergic nucleus that connects reciprocally and topographically with the ipsilateral OT [25]. Ipc spike bursts, which occur rhythmically at gamma frequencies (25–60 Hz), encode the highest priority stimulus and are not selective for the orientation of local contours [26]. These bursts amplify and synchronize sensory responses in both the OT and in a parallel cholinergic circuit formed by the nucleus isthmi pars semilunaris (SLu) [1, 27–29]. The ascending drive from the OT and SLu synchronizes sensory responses in the Rt as well as in the entopallium (Ent, equivalent to high-order cortex in mammals)[1]. Thus, the Ipc circuit plays a central role in generating the signal that encodes the highest priority stimulus.
In this study, we demonstrate behaviorally that signals from the midbrain stimulus selection network are necessary for chickens to discriminate orientations at specific locations in the visual field. The results support the hypothesis that signals from the midbrain network route high priority sensory information to decision making networks that control behavior.
Results
A novel protocol tor testing the causal role of the midbrain network
We trained chickens to report the horizontal or vertical orientation of small (3º diameter) Gabor gratings (Figure 1A) (Methods; Movie S1), a stimulus property that is analyzed by neurons in the Wulst [7–10], but not by neurons in the Ipc or OT [12–14, 30]. The responses of the birds were quantified based on the location and timing of pecks registered by a touch-sensitive computer screen. To receive a reward, the bird had to peck within 15º of a response box following a horizontal grating or within 15º of the center cross following a vertical grating (Figure 1A, red and blue arrows). The cross and box were presented within easy reach, and were separated from each other sufficiently that responses to the cross and box were distinct from each other (Figure 1B, dashed lines). By separating the location of the target stimulus from the locations of the cross and box, and by placing the cross and box at locations that were unaffected by the lesion, we could distinguish the effects of brain lesions on the planning and execution of movements from their effects on perceptual decisions.
Figure 1. Orientation discrimination task and spatial patterns of peck responses.
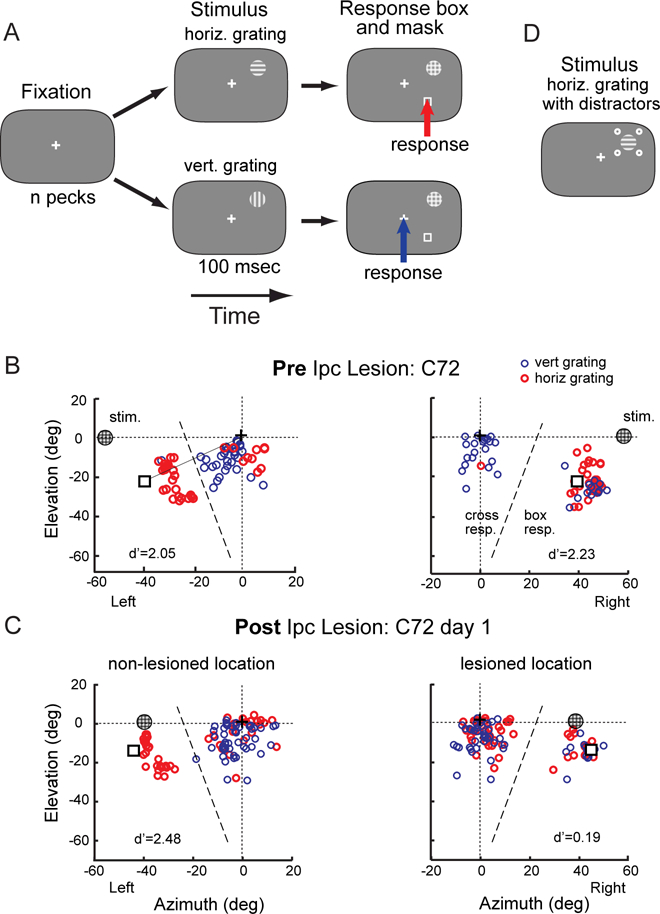
(A) The sequence of stimuli for the single-location and mirror-locations protocols are shown as a time series from left to right. The chicken was rewarded with brief access to food for pecking within 15º of the box following the horizontal grid (red arrow) or of the cross following the vertical grating (blue arrow). The visual stimuli and locations are not drawn to scale. (B) The spatial pattern of peck responses on the touch-sensitive computer screen for a single test session from C72 before an Ipc lesion (baseline). Responses to the horizontal grating are shown in red, and responses to the vertical grating in blue. The locations of the grating, response box and cross on the screen are indicated. This bird was tested with the mirror-locations protocol (Methods), in which two grating locations at mirror symmetrical positions (circled plaids at left and right 58º, 0º elevation) were randomly interleaved. The dashed line indicating the boundary used to define box responses and cross responses was drawn perpendicular to the line connecting the cross to the box (solid line). The d’ values for this particular test session are indicated. (C) Peck responses recorded on day 1 after the Ipc lesion. The lesioned (right) and non-lesioned (left) locations (at left and right 39º, 0º elevation) were tested on randomly interleaved trials. (D) Grating surrounded by distractors, used to test birds in group 2. The contrast of the four distractor dots was varied randomly across trials. See also Figure S1.
We tested four protocols of successively increasing levels of difficulty that enabled us to explore progressively more complex manifestations of the lesion deficit. The target was always a Gabor grating, 1 cycle per degree, at moderate contrast, subtending 3º, and lasting 100 ms followed by a high contrast plaid mask. The short target duration was chosen to make the task difficult (hold d’ at <2), thereby increasing the demand on orientation information from the Wulst. Distractors also served to increase the difficulty of the task. For distracting stimuli, 4 dots, each subtending 1º, were presented 5º from the center of the target grating at the corners of a square (Figures 1D, S1), modeled after [31]. We analyzed performance in terms of percent correct and we applied signal detection theory to compute discrimination accuracy (d’) and criterion (c), in order to separate effects on discrimination accuracy from effects on response bias (Methods). We also analyzed response latency. However, because latencies were found to be variable and idiosyncratic, we report those data only in Methods.
Each bird received two, unilateral, electrolytic lesions: one in the Ipc (Figure 2) and a second in the OT on the same side of the brain, but at different locations in the space maps. After making each Ipc lesion, we verified that rhythmic bursting responses (a conspicuous signature of Ipc activity in the OT [32]) at the corresponding location in the OT space map was eliminated, demonstrating that the Ipc lesion was effective. The OT lesion was made 8–16 weeks after the Ipc lesion. Before each lesion was made in the Ipc or OT, we measured the location of the visual receptive field for the neural activity at the lesion site. Because both the brain lesions and the target stimulus size were small, the target had to be positioned accurately at the “lesioned location” (Methods) in order to observe a behavioral effect.
Figure 2. Histological reconstructions of Ipc lesion sites.
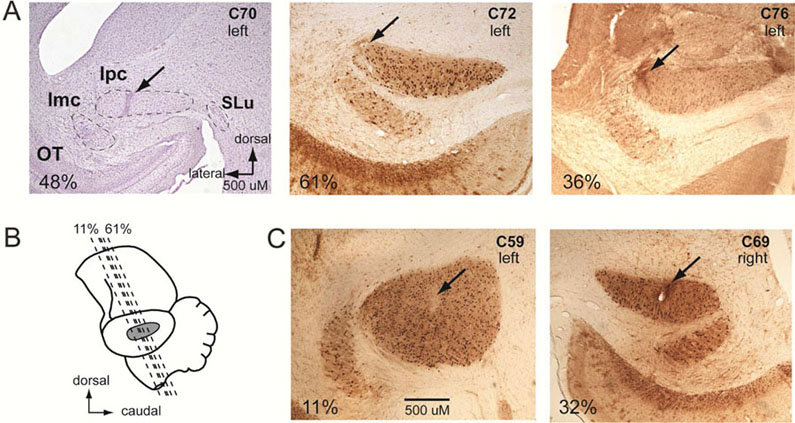
(A) Lesion sites in group 1 birds. Transverse sections through the Ipc stained for cell bodies with cresyl violet (left) or for choline acetyl transferase (ChaT), the synthetic enzyme for the neurotransmitter acetylcholine. Note the intense somatic ChaT staining in the Ipc. Arrows indicate lesion sites. Important nuclei in the midbrain selection network are labeled in the left panel: OT, optic tectum; Ipc, nucleus isthmi pars parvocellularis; Imc, nucleus isthmi pars magnocellularis; SLu, nucleus isthmi pars semilunaris. (B) Lateral view of the chicken brain. Dashed lines: the % rostrocaudal locations of the transverse sections shown in A and C. (C) Lesion sites (arrows) in group 2 birds. Scale bar is the same for all sections.
Effects of focal Ipc lesions
The effects of focal Ipc lesions on orientation discrimination were tested in two groups of birds. In the first group, the target stimulus was presented without distracting stimuli (Figure 1A). In the second group, the same target stimulus was presented simultaneously with distracting stimuli in order to test for deficits in attention control (Figure 1D; Methods). In both groups, orientation discrimination performance with the target positioned at the lesioned location was compared with performance measured both prior to the lesion (baseline), and on the same day but at a non-lesioned location (Figure 1B,C).
Ipc group 1; baseline performance.
Prior to the lesion, orientation discrimination without distractors was consistent across birds (Figures 1B, 3A-C). Birds C70 and C76 were tested with the single-location protocol, and C72 with the mirror-locations protocol (Figure 1B; Methods). During the week prior to the Ipc lesion, average % correct was approximately 70% for all birds (Figure 3A; chance performance = 50%), with best performance reaching over 80% in individual sessions. Average values for d’ ranged from 0.94 to 1.32 (Figure 3B,C). The birds exhibited little bias for either response (Methods): values for c ranged from −0.14 (Figure S2; negative value indicates a bias to peck on the response box) to 0.52 (positive value indicates a bias to peck on the cross).
Figure 3. Orientation discrimination performance before and after an Ipc lesion.
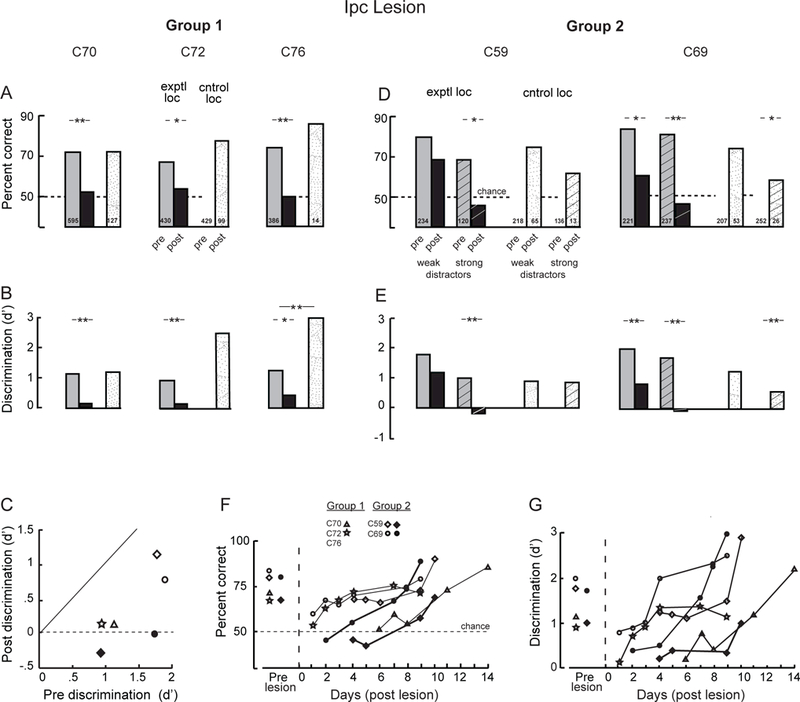
(A,B) Data for group 1 birds. Performance measured at baseline (before the Ipc lesion; gray and white bars), at the Ipc-lesioned location (exptl loc; black bar), and at a non-lesioned location (cntrol loc; stippled bar). C70 and C76 were tested with the single-location protocol, and C72 with the mirror-locations protocol (Methods). The post-lesion data for the lesioned and non-lesioned locations were collected on the same day. The post-lesion data for C72 and C76 were collected on day 1, and for C70 on day 6, following the lesion. Number inside each bar: sample size. Single asterisk: p<0.05, double asterisk: p<0.01, randomization test comparing pre-lesion with post lesion responses. (A) Percent correct performance. (B) Discrimination accuracy, d’ (Methods). (C) Comparison of d’ measured before (pre) and immediately after (post) Ipc lesions for all birds (symbol key). (D,E) Data for group 2 birds. The birds were tested with the distractors protocol (Methods). Gray and black bars: lesioned location (exptl loc); white and stippled bars: non-lesioned location (cntrol loc). Bars without hatching: performance with weak distractors; bars with hatching: performance with strong distractors (defined in Figure S1). Post lesion data were collected from C59 on day 4 and from C69 on day 1 for weak distractors and day 2 for strong distractors. (F,G) Recovery of orientation discrimination following the Ipc lesion for all birds. (F) Percent correct performance. (G) Discrimination accuracy (d’). The data represent responses to target gratings presented at the lesioned location plotted as a function of days following the Ipc lesion in each bird. The data from each bird are plotted with a distinct symbol (symbol key) and connected by a line. Open symbols: tested with weak or no distractors; filled symbols: strong distractors. See also Figure S2.
Ipc group 1; effects of an Ipc lesion.
After baseline data were collected, an electrolytic lesion was made in the left Ipc of each bird (Figure 2A). The lesion in C70 was centered in the Ipc. The lesions in C72 and C76 were placed at the lateral edge of the nucleus where Ipc axons that project to the OT fasciculate. Following these lesions, observation of the birds outside the test chamber revealed no apparent behavioral abnormalities. However, testing the birds in the chamber exposed a profound, but temporary deficit in orientation discrimination when targets were positioned at the lesioned location (Methods).
For C72 and C76, the lesioned location was found during the first day after making the Ipc lesion; for C70 it was found on day 6 post-lesion. When the target stimulus was presented at the lesioned location, discrimination performance dropped to chance (binomial test: p>0.2; performance not significantly different from chance; Figure 3A), and discrimination accuracy (d’) was no longer significantly different from zero (p>0.05; randomization test) for all of the birds (Figure 3B,C). In contrast, when the target was presented at a non-lesioned location, performance was either unimpaired (C70 and C72) or improved relative to baseline (C76; p<0.01, randomization test). In addition, all birds exhibited a bias for selecting the cross when the target was at the lesioned location (Figure S2A).
Discrimination performance at the lesioned location was followed across days post-lesion for two birds, C70 and C72 (Figure 3F,G). For both birds, discrimination performance improved rapidly and returned to baseline values across days of testing (p>0.05; randomization tests).
Ipc group 2; baseline performance.
In a different group of animals (birds C59 and C69), orientation discrimination was measured with the target grating presented both with and without task-irrelevant distractors, and with the target at mirror symmetric locations in the right and left hemifields on randomly interleaved trials (distractors protocol; Methods). Distractor contrasts <5.5% are referred to as “weak”; contrasts ≥5.5% are referred to as “strong.” The effect of distractor contrast on baseline performance is shown in Figure S1. Prior to the Ipc lesion, both birds discriminated target orientation reliably on both sides (Figure 3 D,E) and criteria were near zero (Figure S2).
Ipc group 2; effects of an Ipc lesion.
We made a lesion in the left Ipc of C59 and in the right Ipc in C69 (Figure 2C). Apparent damage from these lesions was contained within the nucleus. Bursting neural activity was eliminated from corresponding locations in the OT space maps, verifying that the lesions were effective [32] (Methods). Observation of the birds outside the test chamber revealed no apparent behavioral abnormalities following the Ipc lesions.
The lesioned location was identified on day 4 after the lesion in C59 and on day 1 in C69 (Methods). For both birds, performance decreased, but it did not drop to chance levels, when distractors were weak (Figure 3D,E). However, it did drop to chance levels (binomial test: C59, p=0.580, n=22; C69, p=0.432, n=26) when distractors were strong (Figure 3D).
At the non-lesioned location, performance remained unchanged for most conditions (p>0.05, randomization test). The one exception was a decrease in the performance of C69 when tested with strong distractors (Figure 3 D,E right side, hatched stippled bars). This unexpected decrease may have been caused by a loss of motivation resulting from poor performance at the lesioned location, tested on interleaved trials, and the difficulty of performing the task with strong distractors. When tested on day 1 at a pair of locations that did not include the lesioned location (at left and right 58º, −39º instead of at left and right 57º, −20º), C69 performed well on both sides (lesioned side: 83%; d’=2.05 c=−.0.41, n=30; non-lesioned side: 93%, d’= 2.87, c= −.03, n=30).
Orientation discrimination recovered across days following the lesion (Figure 3F,G).
In summary, a small lesion placed within the Ipc or Ipc output fascicle impaired orientation discrimination performance at the lesioned location, but not at a non-lesioned location in the same (C70 and C76) or opposite (C72, C59, C69) visual hemifield. Strong distracting stimuli increased the deficit, indicating an impairment in attention control [33]. The deficits were less severe for the group 2 birds than for the group 1 birds, perhaps due to the superior pre-lesion performance of the group 2 birds (Figure 3C) or due to the target being positioned closer to the periphery of the lesioned zone (Methods). Along with the loss of target orientation discrimination, the birds exhibited a bias to select the cross as their response (Figure S2). This bias probably resulted from the birds being unable to perform the perceptual discrimination and, therefore, adopting an idiosynchratic response strategy. Once the lesioned location was found and testing at the lesioned location began, the rate of recovery was similar across all birds (Figure 3F,G), suggesting a similar location-specific, experience-dependent, compensatory mechanism.
Effects of focal OT lesions
The effect of a focal OT lesion on orientation discrimination was tested in two groups of animals. For all birds, the OT lesion (Figure 4) was placed on the same side of the brain as the previous Ipc lesion, but at a different location in the space map. In the first group of birds (C70, C72 and C76), the target stimulus was presented as described for Ipc group 1, except that for C72 and C76, the target was presented with distractors in some blocks of trials. In the second group of birds (C59 and C69), the birds were tested with more complex sequences of stimuli (Methods; Movie S1).
Figure 4. Histological reconstructions of OT lesion sites.
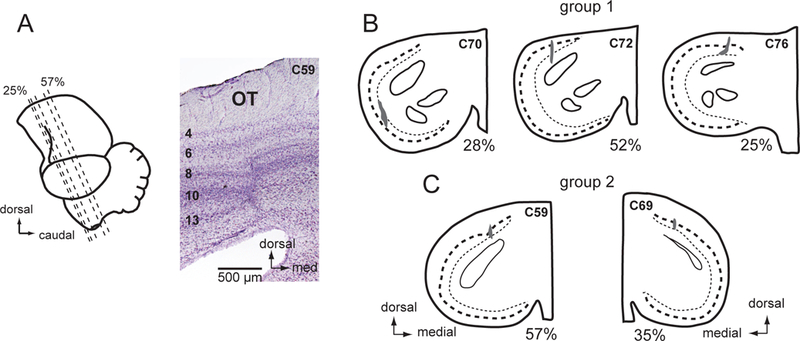
(A) Lateral view of the chicken brain showing the plane of section and a cresyl violet stained transverse section through the center of the OT lesion in C59. Numbers on the left identify the OT layers. (B) OT lesions in the group 1 birds, C70, C72, and C76. Camera lucida drawings of cresyl violet stained transverse sections. The dark shading indicates the extent of overt scaring. Dashed line: layer 10; dotted line: layer 13. The % rostrocaudal location of each section is defined in (A).(C) OT lesions in the group 2 birds, C59 and C69.
OT group 1; baseline performance.
Pre-lesion baseline performance measured with weak or no distractors was consistent across birds (Figures 5A and 6 A-C).
Figure 5. Spatial patterns of peck responses before and after an OT lesion.
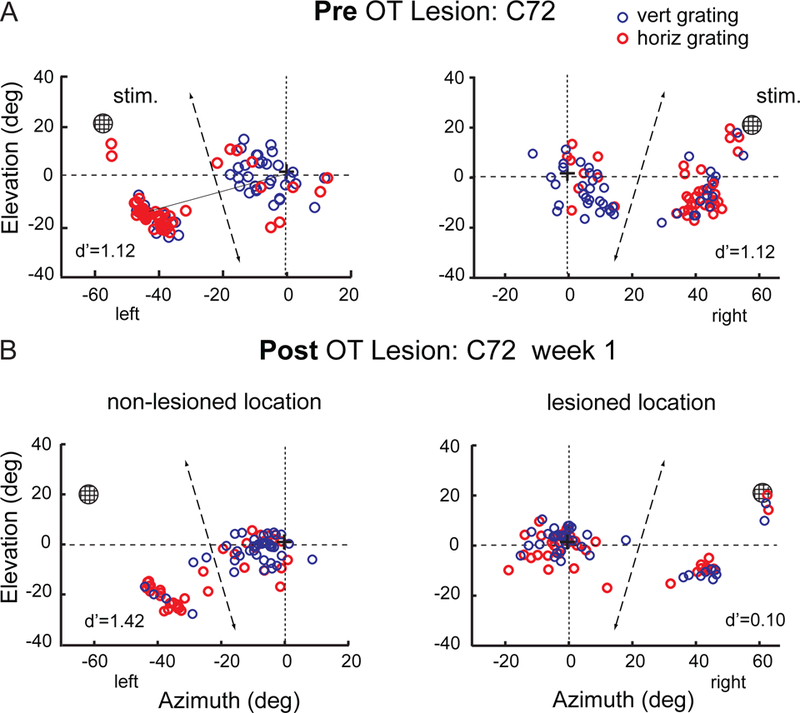
Peck responses of C72 from single sessions, before and on day 1 following the OT lesion. The data are plotted as described in the Figure 1B caption. Responses to the horizontal grating are shown in red, and responses to the vertical grating in blue. The d’ values for these particular test sessions are indicated. (A) Baseline session before the OT lesion. Responses to the targets at left and right 63º, up 22º are shown on the left and right, respectively. (B) Peck responses recorded on day 1 after the OT lesion. The lesioned (right) and non-lesioned (left) locations were tested on randomly interleaved trials.
Figure 6. Orientation discrimination performance before and after an OT lesion.
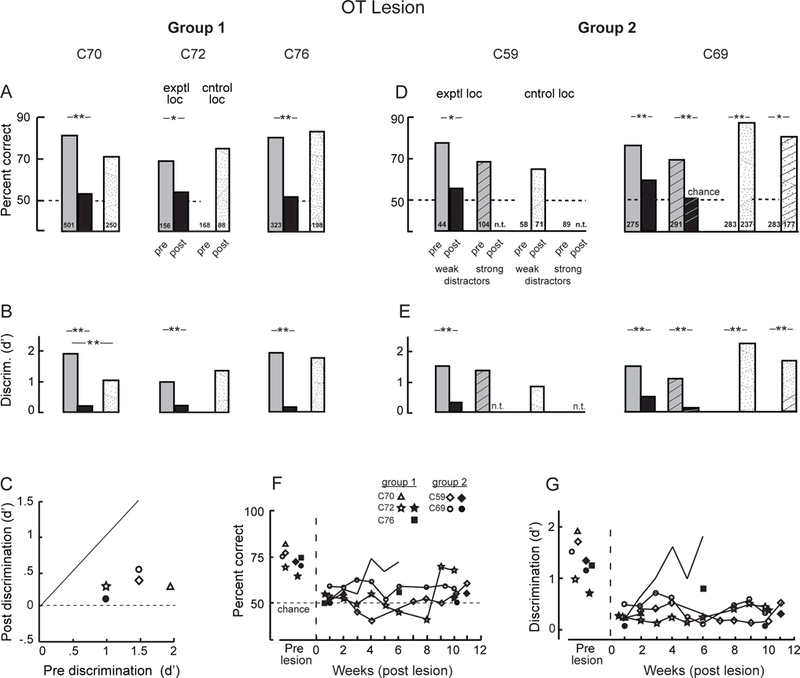
(A-C) Data for group 1 birds are presented as described in the Figure 3 caption. The data represent performance only on trials with weak or no distractors. C70 and C76 were tested with the single-location protocol, and C72 was tested with the distractors protocol (Methods). (D,E) Data for the group 2 birds measured during week 1 (C69) or week 2 (C59) following the OT lesion are presented as described in the Figure 3 caption. The birds were tested with the cued-location protocol (Methods), but the data were combined across cueing conditions. n.t.: not tested. (F,G) Time-course of orientation discrimination performance following the OT lesion. The data were combined across days of testing according to the week post-lesion when they were collected. (F) Percent correct performance. (G) Discrimination accuracy (d’). The data represent responses to target gratings presented at the lesioned location plotted as a function of weeks (data combined across days of testing) following the OT lesion for each bird. The data from each bird are plotted with a distinct symbol (symbol key) and are connected by a line. Open symbols: tested with weak or no distractors; filled symbols: tested with strong distractors. See also Figures S1 and S3.
OT group 1; effects of an OT lesion.
The lesions were centered in layer 13 for C70 and C72 and in layer 10 for C76 (Figure 4B). However, in all of these birds, histological evidence of degeneration (gliosis and somatic atrophy) extended into surrounding layers 6 through 13. Following the OT lesions, observation of the birds outside the test chamber revealed conspicuous behavioral abnormalities. They ignored salient stimuli (a small food dish) brought toward them from certain directions in the lesioned portion of the visual field, they tended to turn and move toward the non-lesioned (left) side, and they tended to hold the head turned slightly toward the lesioned (right) side relative to the body.
When tested in the chamber, all three birds (C70, C72 and C76) exhibited a complete loss of orientation discrimination at the lesioned location (p>0.07; binomial test), even with weak or no distractors (Figures 5B right side, 6A,B). Criterion values increased, the bias favoring responses to the cross in every case (Figure S3A). In contrast, discrimination performance tested at non-lesioned locations on the same day was accurate and responses were made with little bias (Figures 5B left side, 6A,B,S3A).
Long-term effects.
The long-term effects of an OT lesion were assessed in two of the three birds: C72 and C76. C72 exhibited no orientation discrimination for 8 weeks post-lesion (Figure 6F,G). Performance without distractors improved to 60% correct (p=0.016, n=105; binomial test, performance significantly different from chance) during week 9. However, d’ remained low (Figure 6G). Performance with strong distractors remained at chance (54%; p=0.180, n=96; binomial test, performance not significantly different from chance) with a d’ =0.39 through week 10 (Figure 6F,G, black star) and a strong bias to choose the cross (c =1.14).
For C76, the effect of the OT lesion was equally profound initially, but performance recovered over a period of weeks (Figure 6F,G). By week 4 following the lesion, performance with weak distractors had improved to 74% correct, with d’ =1.62 and c =0.71. Performance with strong distractors remained poor through week 6 (57% correct, p=0.014, n=176, binomial test, performance significantly different from chance; d’ =0.78, decreased from pre-lesion, p=0.020, randomization test).
OT group 2; baseline performance
The effect of distractor strength on discrimination performance increased following the Ipc lesions in the group 2 birds (Figure 3C-G), indicating a lesioned-induced deficit in attention control. To test specifically for lesion effects on top-down control of attention, we trained the group 2 birds (C59 and C69) on a cued-location protocol (Figure 7A; Methods; Movie S1). The results were analyzed first with data from validly cued, neutrally cued, and non-cued trials combined to assess the main effects of the lesion. Then, the results from validly and neutrally cued trials were compared to determine the effects of the lesion on cueing.
Figure 7. Spatially cued orientation discrimination task.
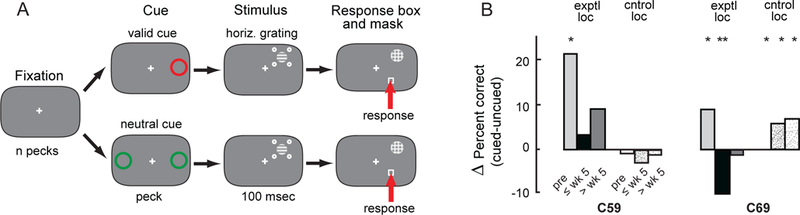
(A) The sequence of stimuli for the cued-location protocol (Methods; Movie S1) is shown as a time series from left to right; only one of the multiple, interleaved target conditions is shown. Either a valid spatial cue (red circle; top) or a neutral spatial cue (two green circles; bottom) preceded the stimulus sequence: left-right mirror symmetric locations tested on interleaved trials, with the contrast of the four distractor dots varied randomly across trials. For the illustrated condition (horizontal grating), the correct response is to the box in both cases (red arrows). The visual stimuli and locations are not drawn to scale. (B) Data for the group 2 birds (C59 and C69) tested with the cued-location protocol before the OT lesion, ≤5 weeks post-lesion, and >5 weeks post-lesion. The bars indicate the difference in percent correct measured for valid versus neutrally cued trials at the lesioned location (exptl loc) and non-lesioned location (cntrol loc). Data were combined across distractor strengths. Single asterisk: p<0.05, double asterisk: p<0.01; Fisher’s exact test. Pre-lesion, C59 exhibited a cueing benefit only for targets on the right side (exptl loc: p=0.019, n= 195 trials). Following the OT lesion, the cueing benefit was reduced dramatically ≤5 weeks post-lesion (p>0.9, n=64 trials). A benefit from cueing was marginal, but not significant, at the lesioned location (exptl loc) >5 weeks post-lesion (p>0.1, n=294 trials; weeks 6–11 post-lesion). No benefit appeared at the non-lesioned location (cntrol loc) during this period (p>0.7, n=294 trials; weeks 6–11 post-lesion). Pre-lesion, C69 exhibited improved discrimination performance on both sides with cueing (exptl loc (left): p=0.037, n= 712 trials; cntrol loc (right): p=0.028, n= 712 trials). Cueing had a detrimental effect on performance at the lesioned (exptl) location (p=0.003, n=825 trials; weeks 1–5 post-lesion), while continuing to benefit performance at the cntrol location (p=0.015, n=825 trials; weeks 1–5 post-lesion). No benefit from cueing appeared at the lesioned (exptl) location up to 12 weeks post-lesion (p>0.5, n=679 trials; weeks 6–12 post-lesion), while the benefit from cueing continued at the non-lesioned (cntrol) location throughout this period (p= 0.021, n=688 trials; weeks 6–12 post-lesion).
At baseline with weak distractors, C59 performed at 77% correct (d’=1.66) and C69 at 76% (d’=1.45) (Figure 6D,E). With 100% predictive spatial cueing, C59 exhibited a cueing benefit only for targets on the right side (Figure 7B, “exptl loc”), indicating that she had learned to use the cue only on this side. C69 exhibited improved discrimination performance on both sides with cueing (Figure 7B).
OT group 2; effects of an OT lesion.
A focal electrolytic lesion was made in the left OT of C59 (affecting the side on which she used the cue; Figure 7B) and in the right OT of C69 (Figure 4C), on the same side as the previous Ipc lesion. The lesions were placed in the dorsal OT, centered in layer 12 for C59 and in layer 10 for C69, but both lesions caused apparent damage across layers 6–13.
Observation of the birds outside the test chamber immediately following the lesion revealed similar behavioral abnormalities as reported above for the group 1 OT birds. When tested inside the chamber, they exhibited severely impaired orientation discrimination at the lesioned location, even with weak or no distractors (Figure 6C-E). The first test session in which C59 provided a sample size adequate to evaluate performance was during week 2: At the lesioned location with weak distractors, performance was at chance (56%, p=0.14, binomial test, n=70; d’ =0.32 and c =−0.48). At the non-lesioned location, performance was 65% correct (p=0.004, binomial test, n=71), not different from pre-lesion baseline (67%) for this location (Figure 6D). In addition, cueing no longer provided a significant benefit at the lesioned location, when tested on week 4 post-lesion (Figure 7B).
In contrast, C69 performed the task beginning day 1 following the OT lesion. At the lesioned location, performance was poor with weak distractors (59% correct; p=0.004, binomial test, n=227) and at chance with strong distractors (51% correct; p= 0.41, binomial test, n=186) (Figure 6D,E). At the same time, performance with targets at the non-lesioned location was improved relative to baseline (p<0.05; randomization test). Cueing now had a detrimental effect on performance at the lesioned location, while continuing to benefit performance at the non-lesioned location (Figure 7B).
Long-term effects.
Neither of the group 2 birds showed convincing evidence of improving orientation discrimination at the lesioned location over a period of almost 3 months (Figure 6F,G). At the non-lesioned location, discrimination performance continued to be superior (p<0.001, randomization test) to pre-lesion baseline with strong distractors (weak distractors: 79% correct, d’=1.43, c =−0.11; strong distractors: 77% correct, d’ =1.35, c =−0.18). For C59 after >5 weeks post-lesion, the benefit from cueing at the lesioned location was marginal, but still not significant (Figure 7B). For C69, no benefit from cueing appeared at the lesioned location up to 12 weeks post-lesion, while the benefit from cueing continued at the non-lesioned location throughout this period (Figure 7B).
To summarize, a focal lesion in the OT resulted in a severe impairment in orientation discrimination at the lesioned location with weak distractors, and a complete loss of discrimination in the presence of strong distractors. For most birds, there was little recovery of discrimination performance over a period of more than 2 months. However, one bird (C76) recovered discrimination over a period of weeks. The more persistent lesion effects observed in the group 2 birds versus the group 1 birds may reflect better positioning of the target grating in the region of space affected by the lesions (Methods). The lesions also produced a persistent increase in median response latency at the lesioned location for 3 of the 5 birds (Figure S3B; Methods). Although the group 2 birds were able to exploit spatial cueing to improve discrimination at baseline, even 100% valid spatial cueing did not ameliorate the discrimination deficit caused by the OT lesion.
Discussion
Seminal behavioral studies, reported several decades ago, demonstrated that pigeons with large bilateral OT lesions were unable to discriminate different visual patterns (horizontal versus vertical bars; apex up versus down triangles); the birds behaved as though blind [19, 34]. At that time, little was known about how visual information was processed in birds, and the studies did not consider the possible causes of the blindness: an interruption of visual information (sensory deficit) versus a disruption of the ability to access visual information for behavioral choices (agnosia). Instead, researchers assumed that the deficit was due to an interruption of visual signals, because of the massive direct anatomical projection from the retina to the OT in birds [35].
Since that time, much has been learned about visual information processing in birds. Neurophysiological studies in the Wulst have shown that the retinogeniculate pathway conveys information that is used to construct neurons that are tuned for the orientation of local contours, both in birds with eyes oriented laterally (pigeons, chickens and finches [7–9]) or frontally (owls [6, 10]). Also during this time, the OT has been shown to be the hub of a sophisticated midbrain network that selects and transmits the highest priority visual information for further analysis in the forebrain [2, 21]. Moreover, unlike in the Wulst, no evidence has been found that neurons in the OT or in its target structures are tuned for the orientation of local contours. With our current knowledge, the persistent perceptual deficits reported in the early, seminal OT lesion studies can be explained, in part, by the inability of the birds to access the pattern information provided by the Wulst to make behavioral choices, consistent with the conclusion of this study.
Additional behavioral studies employed the same target stimuli described above to show that bilateral lesions in the thalamus of either the dLGN (retinogeniculate pathway) or the Rt (retinotectal pathway) alone had only modest or temporary effects on target discrimination [34, 36]. In contrast, lesions of both structures eliminated stimulus discrimination, an effect that persisted over time. These data demonstrated that the birds were able to use visual pattern information provided by the dLGN to discriminate the stimuli. The features analyzed by the retinogeniculate and retinotectal pathways are likely to be complementary, consistent with the known functional properties of these pathways. The results imply that the birds could discriminate among the target stimuli that were used in these early studies on the basis of multiple distinguishing stimulus properties.
In order to distinguish the roles of the retinotectal and retinogeniculate pathways following OT lesions, birds must be trained to discriminate stimuli based on a property that is analyzed specifically by the retinogeniculuate pathway. To this end, we employed small horizontal and vertical gratings that could be discriminated easily by neurons in the Wulst, but not by neurons in the OT. Our strategy was to force the birds to use local contour orientation information to solve the task. We found that a lesion placed in either the OT or Ipc rendered chickens unable to discriminate grating orientation at the lesioned location, at least under certain conditions. We can rule out sensorimotor impairments, because the birds reported their decisions with a motor response directed toward a location different from the location of the target stimulus. Therefore, the results demonstrate a perceptual deficit. The two remaining possibilities are that 1) the lesions disrupted the orientation information itself or 2) they disrupted the routing of orientation information into networks that drive behavioral choice. In the discussion that follows we consider the likelihood of each of these possibilities, and we discuss the implications of these results for how the bird visual system processes information for making perceptual decisions.
The possibility of a deficit in orientation information processing
When the effects of lesioning a structure render an animal unable to discriminate a stimulus, as is the case with OT lesions, then behavioral data cannot distinguish between an interruption in sensory information (sensory deficit) and an inability to access sensory information for guiding behavior (agnosia). In this case, the interpretation depends on the information that is processed by the structure. As discussed above, there are no physiological data to support the possibility that the OT lesions interfered with the processing of orientation information. The visual Wulst and the OT receive parallel projections from the retinae [35, 37]. Many studies document that local contour orientation is analyzed in the visual Wulst. Conversely, no studies indicate that the OT or its target structures analyze orientation information.
Nevertheless, the absence of evidence does not demonstrate that the lesions did not interfere with orientation information processing. It is possible that an as yet undiscovered structure receives topographic, high spatial resolution information from the OT to compute contour orientation (in parallel with the Wulst), and that birds depend specifically on information from this structure (and not from the Wulst) to make behavioral choices. This scenario raises the question: What then is the function of the orientation information that is computed in the Wulst? Moreover, all current data indicate that thalamic nuclei that receive input from the OT contain neurons with large visual receptive fields, which cannot support the analysis of local orientations [37]. One possible exception is the dLGN itself, which receives a projection from the superficial layers of the OT [38]. Although the effect of this input from the OT on dLGN responses has not been studied, it may contribute to orientation processing in the Wulst.
All neurophysiological data are consistent with the hypothesis that the information used for target discrimination in our experiments was analyzed in the Wulst, but the information could not be accessed to guide behavioral choice. A test of this hypothesis would be to repeat the same behavioral experiments with lesions in the Wulst.
The possibility of a deficit in routing orientation information
For the birds to perform the task, information about the target’s orientation had to be routed to the networks that mediate decisions for guiding behavior. The forebrain mediates complex behaviors, such as courtship, navigation and hunting, which require that stimuli be identified [2, 39]. The main projections of the Wulst are to a number of high-order areas in the forebrain, including the frontal, intermediate and caudal nidopallium, the intermediate arcopallium, and the lateral striatum [40]. These same high-order areas also receive input from the parallel, retinotectal (OT-Rt-Ent) pathway [41].
The routing of information according to task demands is a fundamental mechanism of selective attention. A modeling study of behavioral data from monkeys engaged in attention demanding tasks indicated that the effects of SC inactivations are accounted for best by a disruption in the routing of visual information in the forebrain [20]. The results presented in this study are consistent with OT lesions having the same effect in birds.
The midbrain stimulus selection network has been shown to compute a signal that acts as a space specific gate for transmitting the highest priority visual information from the OT to the Rt [1]. We hypothesize that this signal also acts as a space specific gate for orientation information from the Wulst to decision making networks within the forebrain. One pathway that could act as a space specific gate for visual information is the projection from the superficial OT to the dLGN [38]. This input to the dLGN is likely to provide information with high spatial resolution that is modulated by the midbrain stimulus selection network, which regulates the efficacy of retinal afferents in the superficial OT [21]. A second pathway that could act as a space specific gate is the cholinergic projection from the SLu to the thalamus. SLu neurons have restricted spatial receptive fields and they project to both visual and multimodal nuclei in the thalamus [1, 17, 42]. SLu neurons receive gamma-periodic drive from a special class of neurons in the OT [25, 29]. Normally, SLu activity depends on Ipc activity, signaling the highest priority location [1]. (Following the Ipc lesions, recovery of discrimination performance may have resulted from SLu neurons increasing their sensitivity to OT input, thereby becoming independent of Ipc activation and restoring the effectiveness of the midbrain selection signal.) The ascending signal from the SLu could gate presynaptically visual inputs to the thalamus from the selected location, as well as space-specific inputs from other sensory modalities (for example, auditory and somatosensory) that provide the forebrain with information about stimulus identity [43].
If this hypothesis is true, then the midbrain selection signal exerts more powerful control of information routing in birds than in monkeys. Inactivation of the OT causes birds to be unable to make decisions about target orientation without any distractors present, in a highly impoverished visual environment, even when the upcoming location of the target is cued or completely predictable (single-location task, C70 and C76). In contrast, although monkeys are perceptually impaired during SC inactivation, they still are able to make decisions about the visual features of target stimuli that are presented alone [44–46].
The ability of monkeys to make perceptual decisions about stimuli without a signal from the SC at least under impoverished sensory conditions is likely due to the action of a top-down selection signal from the frontoparietal attention network, which is uniquely well developed in primates [47]. However even in primates, when the top-down and midbrain selection signals conflict (for example, when SC inactivation causes the SC signal to select the location of a non-cued competing stimulus), the SC signal determines the information that is used for behavioral choice [44]. The dominance of the midbrain selection signal over the top-down signal, even in monkeys, makes sense given that the midbrain signal represents external stimuli that may be of immediate importance to survival, whereas the top-down signal represents the animal’s internal goals [2]. Given that the basic anatomy of the midbrain network is conserved across vertebrate phylogeny, we propose that it serves this critical function in all vertebrate species.
STAR Methods
CONTACT FOR RESOURCE SHARING
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Eric I Knudsen (eknudsen@stanford.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Experiments were conducted on five adult female white leghorn chickens (Gallus domesticus, Hyline strain). Training began at 10–12 months of age, and measurements finished before 4 years of age. All procedures were in compliance with the guidelines of the National Institutes of Health for the care and use of laboratory animals and were approved by the Institute Animal Care and Use Committee of Stanford University (Protocol ID: 20287). The birds were kept on a food-restricted schedule that maintained 70% of their free feeding weight. Water was available ad-libitum in the home cage.
METHOD DETAILS
Experimental apparatus
Training and testing were conducted with custom-made equipment, housed in a large sound isolation chamber (3×3×2.5 m). The chamber provided light, temperature and ventilation control. During the experiments, the chamber was dark, and the experimenter viewed the bird from outside the chamber on a TV screen with infrared (IR) video cameras (Sony, NightVision) mounted above the bird.
A black plexiglass, test booth (.5×.5×1 m), containing a touch-sensitive computer screen (Acoustic Pulse Recognition Technology, Elo Touch Systems, 37.5× 30 cm), was located at the center of the sound chamber. A feeder box with a computer-controlled access door was positioned at the base of the computer screen. The body of the bird was placed inside a box with the head protruding, and the box was secured in front of the computer screen so that the front of the eyes were 5 cm from the screen and level with the center of the screen when the bird was in resting position. From this position, the bird could easily peck all response targets displayed on the screen and the feeder box at the base of the screen.
The position and orientation of the head were monitored continuously with an IR based tracking system (Natural Point; OptiTrack Systems)[48]. Head position measurements were based on signals from three IR-reflecting markers embedded in a cap and attached to the head during testing. These signals were monitored with 2 overhead cameras. The system tracked 3 dimensions of head position relative to a zero-point with a precision of <0.2 mm as well as 3 dimensions of angles with a precision of <1º. The data were sampled at 120 Hz and stored for post-hoc analysis.
Responses were quantified based on the timing and location of pecks registered by the touch-sensitive computer screen. The timing and location of each peck and details of the stimulus configuration were stored for post-hoc analysis.
Behavioral Conditions
The birds performed two-alternative forced choice (2-AFC), orientation discrimination tasks. Stimulus presentation and data acquisition were controlled by custom Matlab scripts running Psychophysics Toolbox extensions. To initiate a trial, the bird had to peck accurately, 2–5 times, on a zeroing cross that appeared at the center of the computer screen. Pecking engages a stereotyped action pattern of binocular frontal fixation, during which the eyes assume standard converged positions in the head for a period of >150 ms following the peck [48]. Pecking on the cross therefore forced the head and eyes into standard positions relative to the screen. The stability and stereotypy of the head and eye positions during this period enabled us to present visual stimuli at known locations in the visual field [49].
Locations on the screen were calibrated in degrees of visual angle measured 5 cm from the screen, the average distance (standard deviation < ± 0.5 mm in the z dimension) from the screen to the midpoint between the eyes immediately following pecks on the cross. Azimuth refers to degrees left or right from the vertical plane that contained the average center point between the eyes and the cross on the screen. Elevation refers to degrees up or down from the plane that contained the average optical axes of the eyes and the cross.
Immediately following accurate zeroing pecks on the cross, a target stimulus appeared on the screen for 100 ms (Figure 1A). The target stimulus was a Gabor grating, 1 cycle per degree, subtending 3º, on a dark background. The grating was oriented either horizontally or vertically. After 100 ms, the grating was replaced with a full contrast, plaid mask that had the same size and spatial frequency as the stimulus grating; the mask prevented afterimages of the grating from being used for discrimination and increased the difficulty of the task. Synchronous with the plaid mask, a response box appeared on the same side as the target stimulus but located lower in the visual field and at a different azimuth. The mask, box and cross remained on the screen for up to 2 s. To receive a reward, the bird had to peck within 15º of the box following a horizontal grating or within 15º of the cross following a vertical grating (Figure 1A, red and blue arrows). When the bird responded correctly, the stimulus array was extinguished, a green reward light appeared, and the bird was given access to the feeder for 2 s. After a variable interval (2–5 s) following the extinguishing of the array, the zeroing cross re-appeared, and the bird could initiate another trial.
The cross and box were presented within easy reach, and were separated from each other sufficiently that responses to the cross and box were distinct from each other (Figure 1B, dashed lines). By separating the location of the target stimulus from the locations of the cross and box, and by placing the cross and box at locations that were unaffected by the lesion, we could distinguish the effects of brain lesions on sensory perception from their effects on orienting movements.
The contrast of the target grating (calibrated with an OceanOptics spectrometer) was held constant at an intermediate value of 25%. At this contrast, performance was consistent and it allowed distracting stimuli to be presented at contrasts that were either higher or lower than that of the target (Figure S1).
Task Protocols
We tested four protocols of successively increasing levels of difficulty.
Single-location protocol:
The simplest protocol required the bird to report the orientation of the target grating when it appeared alone, without distracting stimuli, at a single location in the visual field (Figure 1A); all of the birds were trained on this protocol. The target location remained constant throughout a single block of trials. In subsequent blocks in the same session, the target (but not the cross or box) was moved to test discrimination at another location in the same visual hemifield.
Mirror-locations protocol:
Same as the single-location protocol, except that two different target locations were sampled on randomly interleaved trials during the block. The sampled locations were mirror symmetrical across the vertical meridian (Figure 1B, plaids). The response box always appeared at mirror symmetrical locations in the visual field, on the same side as the target stimulus.
Distractor protocol:
Same as the mirror-locations protocol, except that task-irrelevant distracting stimuli were presented simultaneously with the target grating (Figure 1D). The distracting stimuli were 4 dots, each subtending 1º and centered 5º from the center of the target grating at the vertices of a square, modeled after [31]. This distractor configuration was chosen to promote strong suppression of the target’s representation in the OT [50]. The contrast of the distractors, calibrated with the spectrometer, varied across trials, the contrast being sampled randomly from one of eight levels (0.0033 – 50%) uniformly spaced on a logarithmic scale.
Cued-locations protocol:
The most elaborate protocol tested the effects of spatial cueing on orientation discrimination, with and without the distractors (Figure 7A; Movie S1). This protocol was the same as the distractor protocol, except that cueing stimuli preceded the presentation of the target stimulus. The cued-locations protocol was modeled on a protocol published previously [49]. In 50% of the trials, a 100% valid spatial cue (10º radius, red annulus) was presented on the side of the target stimulus, at the horizon, and centered 15º beyond where the target stimulus would appear. Trials with a valid spatial cue were pseudorandomly interleaved with trials in which a neutral spatial cue was presented. The neutral cue consisted of two, 10º radius, green annuli, one located at the identical location as the valid cue and the other at the mirror symmetrical location in the opposite visual hemifield. The neutral cue provided no spatial information, but did provide the same temporal information as the valid cue. The cue stimuli were triggered by the peck that initiated a trial. The cue was extinguished by a subsequent peck (median cue duration = 400 ms; 350–550 ms 68% confidence interval, CI). Another peck on the cross immediately triggered the presentation of the target grating.
QUANTIFICATION AND STATISTICAL ANALYSIS
Percent Correct Performance
The results report the performance of 5 adult female chickens. Percent correct performance was computed as the number of correct responses (either a peck on the response box following a horizontal grating or a peck on the cross following a vertical grating) relative to the total number of trials of each type. Although only accurate peck responses (<15º from the box or cross) were rewarded, all peck responses were scored as either pecks on the cross or on the box, respectively, by partitioning the data with a line that was perpendicular to, and bisected, the line connecting the locations of the cross and box (Figure 1B, dashed lines). Percent correct data were compared against chance performance using binomial statistics. Percent correct data were also compared pre- versus post lesion with a randomization test by permuting trials in a contingency table with unfixed marginals, and calculating the chi-squared statistics. Specifically, we created a 2×2 contingency table with counts of correct trials in the first column and incorrect trials in the second column, and pre-lesion in the first row and post-lesion in the second row. To generate a null distribution under the hypothesis that performance was independent of lesion condition, we generated random draws based on the expected proportions and matched the number of random draws to the number of observed trials in the dataset. We calculated a chi-squared statistic, comparing the randomly observed response counts with the expected counts, and compared this chi-statistic with the observed statistic. This randomization was performed 1000 times. The proportion of iterations in which the randomized chi-squared statistic exceeded the observed statistic is reported as a p-value. p-values were Bonferroni corrected for multiple comparisons, when appropriate. Effects of the lesion on accuracies with and without cueing were measured with the Fisher’s exact test, pooling trials across experimental sessions. Differences in accuracies were quantified by averaging over distractor values, and by counting all sessions equally (regardless of slight differences in numbers of trials across sessions).
We applied signal detection theory (SDT) to the response choices to compute discrimination accuracy (d’) and criterion (c). Hit rates (h) were arbitrarily assigned as the proportion of correct responses to the horizontal grating (that resulted in pecks on the response box). False-alarm rates (f) were assigned as the proportion of incorrect responses to the vertical grating (that resulted in pecks on the response box). d’ and c were computed as follows:
where Φ−1 denotes the probit function [51, 52]. In lesion/inactivation experiments, it is essential to distinguish whether a change in performance (percent correct) results from a change in discrimination accuracy, a change in response criterion, or both. SDT analysis enabled us to demonstrate that changes in performance could not be explained solely by changes in response bias.
Values of d’ and c were compared against chance performance using a randomization test. Contingency tables were constructed from multiple (10000) random draws from a binomial distribution with identical hit and false alarm rates (h=f=0.5) that preserved the total number of trials of each type of stimulus (horizontal and vertical), and null distributions of d’ and c were computed based on each contingency table. p-values denote the proportion of d’ or c values in the respective null distributions that exceeded the experimentally observed d’ or c value. Values of d’ and c were compared pre- versus post lesion by a similar randomization test. In this case, the null distribution was constructed from multiple (10000) random distributions based on the hit and false alarm probabilities corresponding to the pre-lesion baseline. p-values denote the proportion of d’ or c values in the pre-lesion null distribution that exceeded (or fell below) the corresponding d’ or c value measured in the post-lesion condition.
The specific conditions for measurements are given in the Figure captions. Sample sizes (number inside the bars of histograms) refer to number of trials for that condition. To assess baseline performance and the effects of OT lesions, data gathered from multiple test sessions across multiple days were combined.
Response Latency
Response latency was measured as the time from the onset of a horizontal target grating until the peck on the response box. Only these correct trials were included in computing median latencies. Latencies were compared with Mann-Whitney U tests.
Prior to Ipc lesions, baseline response latencies were idiosyncratic and variable: median response latency (68% confidence interval, CI) for each bird across all sessions was: C70 = 528 ms (503–576 ms; n=199), C72 = 744 ms (655–819 ms; n=147), C76 = 482 ms (323–550 ms; n=137), C59 = 277 ms (240–408 ms; n=104), and C69 = 600 ms (528–631 ms; n=82). Following Ipc lesions, there was no systematic change in response latency for any of the birds (p>0.05, Mann-Whitney U tests).
Prior to OT lesions, for birds in group 1, baseline median response latencies (68% CI) with weak or no distractors were idiosynchratic: C70 =599 ms (552–625 ms; n=181), C72 =984 ms (922–1033 ms; n=59), C76 =242 ms (240–282 ms; n=111). After the OT lesions, median response latencies for targets presented at the lesioned location remained unchanged for C70, shortened (p<0.01; Mann-Whitney U test) for C72 (note that this bird had an exceptionally long baseline median latency of 984 ms), and lengthened for C76 (Figure S3B). Median response latencies at the non-lesioned location changed (p<0.01; Mann-Whitney U test) in the same direction as at the lesioned location, but the magnitude of change was less (Figure S3B). Median response latency at the lesioned location returned to baseline (p>0.05; Mann-Whitney U test) by week 6 for both C72 and C76.
For birds in group 2, prior to OT lesions, median response latencies with weak distractors were C59 =386 ms (336–432) and C69 = 552 ms (528–600). After the OT lesions, median latency for C59 with weak distractors was 131 ms longer than baseline at the lesioned location and 108 ms longer at the non-lesioned location (p<0.001, Mann-Whitney U test) (Figure S3). For C69, median response latency with weak distractors was 168 ms longer than baseline (p<0.001, Mann-Whitney U test) with targets at the lesioned location, but was unchanged (p=0.390, Mann-Whitney U test) at the non-lesioned location (Figure S3B). After a period of 3 months post-lesion, median response latency for C59 remained 336 ms longer than baseline at the lesioned-location, but only 86 ms longer at the non-lesioned location; for C69, median response latency remained 119 ms longer than baseline at the lesioned location, but 24 ms shorter at the non-lesioned location.
Brain Lesions
Targeting of lesions
Each bird received two, unilateral, electrolytic lesions: one in the Ipc and a second in the OT on the same side of the brain, but at different locations in the space maps. The OT lesion was made 8–16 weeks after the Ipc lesion. The order of the Ipc and OT lesions was determined by anatomical considerations: the OT is the hub of the network and is the source of the major pathway that connects the network to the forebrain, whereas the Ipc connects only locally within the midbrain network [2, 25]. Consequently, an Ipc lesion that is made after an OT lesion at corresponding locations in the space maps would probably have no effect. Therefore, the Ipc lesion was always made first, followed by the OT lesion at a different location in the space map.
Before each lesion was made, we measured the location of the visual receptive field for the neural activity at the lesion site. In preparation for making the lesions, each bird was anesthetized with 1.5% isoflurane and nitrous oxide/oxygen (45:55 by volume), a craniotomy was made over the midbrain, and a recording chamber was surgically implanted in the skull. The chamber included a plate for mounting the IR reflectors used for head tracking during behavioral testing. This procedure was completed before behavioral training began.
On the day of a lesion, the bird was anesthetized, wrapped in a jacket and suspended in front of the computer screen. The head was mounted in a holder, aligned with the screen so that the midsagittal and horizontal planes aligned with the cross, and secured in place. Anesthesia was then discontinued.
A custom microdrive was locked to the implanted recording chamber. Single and multi-unit responses were recorded with an epoxy-coated tungsten microelectrode (impedance=1–5 MΩ at 1 kHz). Visual stimuli consisting of moving bright dots, which drove Ipc and OT units effectively, were displayed under computer control. Stimulus location and spike times were analyzed by custom Matlab (Mathworks) software to generate a map of the receptive field location. Under these conditions of head restraint, the birds tended to hold the eyes in a consistent resting position [48], yielding a consistent visual receptive field location. The electrode was moved in the Ipc or OT space map until the visual receptive field was at a location that would be located on the computer screen when the bird was engaged in the behavioral task (see below). Then, a lesion was made by passing direct anodal current for 30 s through the electrode: Ipc, 20 µA; OT, 50 µA.
Identification of the “lesioned location”
Because both the brain lesions and the target stimulus size were small, the target had to be positioned accurately at the lesioned location in order to observe a behavioral effect. Positioning of the target was guided by the visual receptive field location measured at the lesion site prior to making the lesion. However, the positions of the eyes in the head differ between a bird that is head-fixed and resting (visual receptive field measurements) and a bird that has just pecked at a stimulus (behavioral measurements). This difference in eye positions was estimated based on results from a prior study [48]. Beginning with this estimated location, we searched in both azimuth and elevation, for a target location that resulted in an apparent change in discrimination performance (the locations of the response box and cross remained constant). We sampled each location with 10–40 trials using the single-location protocol. Because the birds performed this simple task with high reliability (70–85% correct), an impairment in orientation discrimination was readily apparent from such a small sample. When a candidate location was discovered, we ran a larger block of trials with the target stimulus at that location to determine whether performance was indeed abnormal. If so, this location was designated the “lesioned location” for that bird, more thorough testing with targets at that location commenced, and we made no further attempt to adjust target location to increase the behavioral effect.
This search strategy for finding the lesioned location introduced two potential sources of error. One is that the loss of orientation discrimination at a particular target location may have been unrelated to the lesion. Arguing against this possibility is the observation that the behaviorally determined lesion locations correlated with the anatomical locations of the lesions in the respective nuclei. Behaviorally determined lesioned locations ranged from contralateral 39º to 58º in azimuth and 0º to −29º in elevation for the Ipc lesions, and from contralateral 50º to 63º in azimuth and +22º to −22º in elevation for the OT lesions. Linear regressions (minus data from C72, see below) of behaviorally measured, lesioned location azimuths on rostrocaudal anatomical locations yielded: Ipc R2=0.53 and OT R2=0.82. Linear regressions of lesioned location elevations on mediolateral anatomical locations in the Ipc yielded R2=0.82, and on percent around from the dorsomedial to ventromedial border of the OT yielded R2=0.95. Only one lesioned location deviated substantially from these regressions: the Ipc lesion in C72 was located further rostral and lateral than expected either from the visual receptive field location measured at the time the lesion was made or from the behaviorally measured lesioned location. However, the Ipc lesion in this bird was placed in the Ipc output fiber fascicle and not in the nucleus itself.
A second potential source of error that resulted from this search strategy was that a lesioned location may have been at the edge of the affected zone and not at its center. In birds that were tested at the edge of the lesioned area, the magnitude of the impairment could have been underestimated because of trial-to-trial variability in the position of the target inside versus outside of the affected area, and improvement in performance may have resulted from plasticity in the OT [53] near the edge of the lesion that was not available near the center of the lesion. This source of error could have contributed to variations across individuals, both in terms of the magnitude of the impairment (which may have been greater had it always been tested at the center of the lesioned zone) and the amount of recovery over time post lesion (which may have been less had it always been tested at the center of the lesioned zone).
Histology
After completing behavioral measurements of the effects of the Ipc and OT lesions, we anesthetized the bird deeply with isoflurane, opened the thoracic cavity, and injected Beuthanasia D (0.5 cc) into the heart. The bird was perfused transcardially with 0.1 M phosphate buffer, followed by 4% paraformaldehyde in phosphate buffer. The brain was removed and sunk in 30% sucrose in fixative for 2 d before sectioning. The brain was sliced in 30 µm frozen sections, mounted on slides, and either stained with cresyl violet or reacted with an antibody to choline acetyl-transferase (ChAT; the synthetic enzyme for acetylcholine, ACh). An avidin-biotin-DAB reaction was used to visualize the antibody labeling [54].
Supplementary Material
Acknowledgements
We are grateful to S. van Winden for training and testing birds. We thank S. Evans for coding support and L. Giocomo and T. Moore for reviewing the manuscript. This work was supported by funding from the NIH (1R01 EY024243, EIK) and a Wellcome Trust DBT-India Alliance Fellowship (DS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marin GJ, Duran E, Morales C, Gonzalez-Cabrera C, Sentis E, Mpodozis J, and Letelier JC (2012). Attentional capture? Synchronized feedback signals from the isthmi boost retinal signals to higher visual areas. J Neurosci 32, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen EI, and Schwarz JS (2017). The optic tectum: a structure evolved for stimulus selection. In Evolution of the Nervous System 2nd edition, Kaas JH, ed. (Elsevier; ). [Google Scholar]
- 3.Livingstone M, and Hubel D (1988). Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science 240, 740–749. [DOI] [PubMed] [Google Scholar]
- 4.Wang YC, Jiang S, and Frost BJ (1993). Visual processing in pigeon nucleus rotundus: luminance, color, motion, and looming subdivisions. Vis Neurosci 10, 21–30. [DOI] [PubMed] [Google Scholar]
- 5.Wylie DRW, Gutierrez-Ibanez C, Pakan JMP, and Iwaniuk AN (2009). The Optic Tectum of Birds: Mapping Our Way to Understanding Visual Processing. Canadian Journal of Experimental Psychology-Revue Canadienne De Psychologie Experimentale 63, 328–338. [DOI] [PubMed] [Google Scholar]
- 6.Nieder A, and Wagner H (1999). Perception and neuronal coding of subjective contours in the owl. Nat Neurosci 2, 660–663. [DOI] [PubMed] [Google Scholar]
- 7.Wilson P (1980). The organization of the visual hyperstriatum in the domestic chick. II. Receptive field properties of single units. Brain Res 188, 333–345. [DOI] [PubMed] [Google Scholar]
- 8.Bischof HJ, Eckmeier D, Keary N, Lowel S, Mayer U, and Michael N (2016). Multiple Visual Field Representations in the Visual Wulst of a Laterally Eyed Bird, the Zebra Finch (Taeniopygia guttata). PLoS One 11, e0154927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng BS, Grabska-Barwinska A, Gunturkun O, and Jancke D (2010). Dominant vertical orientation processing without clustered maps: early visual brain dynamics imaged with voltage-sensitive dye in the pigeon visual Wulst. J Neurosci 30, 6713–6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettigrew JD, and Konishi M (1976). Neurons selective for orientation and binocular disparity in the visual Wulst of the barn owl (Tyto alba). Science 193, 675–678. [DOI] [PubMed] [Google Scholar]
- 11.De Britto LR, Brunelli M, Francesconi W, and Magni F (1975). Visual response pattern of thalamic neurons in the pigeon. Brain Res 97, 337–343. [DOI] [PubMed] [Google Scholar]
- 12.Jassik-Gerschenfeld D, and Guichard J (1972). Visual receptive fields of single cells in the pigeon’s optic tectum. Brain Res 40, 303–317. [DOI] [PubMed] [Google Scholar]
- 13.Hughes CP, and Pearlman AL (1974). Single unit receptive fields and the cellular layers of the pigeon optic tectum. Brain Res 80, 365–377. [DOI] [PubMed] [Google Scholar]
- 14.Verhaal J, and Luksch H (2013). Mapping of the receptive fields in the optic tectum of chicken (Gallus gallus) using sparse noise. PLoS One 8, e60782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost BJ, Scilley PL, and Wong SC (1981). Moving background patterns reveal double-opponency of directionally specific pigeon tectal neurons. Experimental brain research 43, 173–185. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Q, Li DP, and Wang SR (2006). Looming-sensitive responses and receptive field organization of telencephalic neurons in the pigeon. Brain Res Bull 68, 322–328. [DOI] [PubMed] [Google Scholar]
- 17.Reches A, and Gutfreund Y (2009). Auditory and multisensory responses in the tectofugal pathway of the barn owl. J Neurosci 29, 9602–9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun H, and Frost BJ (1998). Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat Neurosci 1, 296–303. [DOI] [PubMed] [Google Scholar]
- 19.Hodos W, and Karten HJ (1974). Visual intensity and pattern discrimination deficits after lesions of the optic lobe in pigeons. Brain Behav Evol 9, 165–194. [DOI] [PubMed] [Google Scholar]
- 20.Sridharan D, Steinmetz NA, Moore T, and Knudsen EI (2017). Does the superior colliculus control perceptual sensitivity or choice bias during attention? Evidence from a multialternative decision framework. J. Neurosci. 37, 480–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knudsen EI (2011). Control from below: the role of a midbrain network in spatial attention. Eur J Neurosci 33, 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruberg E, Dudkin E, Wang Y, Marin G, Salas C, Sentis E, Letelier J, Mpodozis J, Malpeli J, Cui H, et al. (2006). Influencing and interpreting visual input: the role of a visual feedback system. J Neurosci 26, 10368–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sridharan D, Schwarz JS, and Knudsen EI (2014). Selective attention in birds. Curr Biol 24, R510-513. [DOI] [PubMed] [Google Scholar]
- 24.Mysore SP, and Knudsen EI (2014). Descending control of neural bias and selectivity in a spatial attention network: rules and mechanisms. Neuron 84, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Luksch H, Brecha NC, and Karten HJ (2006). Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (Gallus gallus): a possible substrate for synchronizing tectal channels. J Comp Neurol 494, 7–35. [DOI] [PubMed] [Google Scholar]
- 26.Asadollahi A, Mysore SP, and Knudsen EI (2011). Rules of competitive stimulus selection in a cholinergic isthmic nucleus of the owl midbrain. J Neurosci 31, 6088–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sridharan D, Boahen K, and Knudsen EI (2011). Space coding by gamma oscillations in the barn owl optic tectum. J Neurophysiol 105, 2005–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asadollahi A, and Knudsen EI (2016). Spatially precise visual gain control mediated by a cholinergic circuit in the midbrain attention network. Nat Commun 7, 13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goddard CA, Huguenard J, and Knudsen E (2014). Parallel midbrain microcircuits perform independent temporal transformations. J Neurosci 34, 8130–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asadollahi A, Mysore SP, and Knudsen EI (2010). Stimulus-driven competition in a cholinergic midbrain nucleus. Nat Neurosci 13, 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Weerd P, Peralta MR 3rd, Desimone R, and Ungerleider LG (1999). Loss of attentional stimulus selection after extrastriate cortical lesions in macaques. Nat Neurosci 2, 753–758. [DOI] [PubMed] [Google Scholar]
- 32.Marin G, Mpodozis J, Sentis E, Ossandon T, and Letelier JC (2005). Oscillatory bursts in the optic tectum of birds represent re-entrant signals from the nucleus isthmi pars parvocellularis. J Neurosci 25, 7081–7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desimone R, and Duncan J (1995). Neural mechanisms of selective visual attention. Annu Rev Neurosci 18, 193–222. [DOI] [PubMed] [Google Scholar]
- 34.Jarvis CD (1974). Visual discrimination and spatial localization deficits after lesions of the tectofugal pathway in pigeons. Brain Behav Evol 9, 195–228. [DOI] [PubMed] [Google Scholar]
- 35.Remy M, and Gunturkun O (1991). Retinal afferents to the tectum opticum and the nucleus opticus principalis thalami in the pigeon. J Comp Neurol 305, 57–70. [DOI] [PubMed] [Google Scholar]
- 36.Hodos W, Karten HJ, and Bonbright JC Jr. (1973). Visual intensity and pattern discrimination after lesions of the thalamofugal visual pathway in pigeons. J Comp Neurol 148, 447–467. [DOI] [PubMed] [Google Scholar]
- 37.Wylie DR, Gutierrez-Ibanez C, Pakan JM, and Iwaniuk AN (2009). The optic tectum of birds: mapping our way to understanding visual processing. Can J Exp Psychol 63, 328–338. [DOI] [PubMed] [Google Scholar]
- 38.Wild JM (1989). Pretectal and tectal projections to the homologue of the dorsal lateral geniculate nucleus in the pigeon: an anterograde and retrograde tracing study with cholera toxin conjugated to horseradish peroxidase. Brain Res 479, 130–137. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu T, Cox K, and Karten HJ (1995). Intratelencephalic projections of the visual wulst in pigeons (Columba livia). J Comp Neurol 359, 551–572. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu T, Patton TB, and Husband SA (2010). Avian visual behavior and the organization of the telencephalon. Brain Behav Evol 75, 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Husband SA, and Shimizu T (1999). Efferent projections of the ectostriatum in the pigeon (Columba livia). J Comp Neurol 406, 329–345. [PubMed] [Google Scholar]
- 42.Hellmann B, Manns M, and Gunturkun O (2001). Nucleus isthmi, pars semilunaris as a key component of the tectofugal visual system in pigeons. J Comp Neurol 436, 153–166. [PubMed] [Google Scholar]
- 43.Sarter M, Hasselmo ME, Bruno JP, and Givens B (2005). Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev 48, 98–111. [DOI] [PubMed] [Google Scholar]
- 44.Zenon A, and Krauzlis RJ (2012). Attention deficits without cortical neuronal deficits. Nature 489, 434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovejoy LP, and Krauzlis RJ (2010). Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci 13, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McPeek RM, and Keller EL (2004). Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7, 757–763. [DOI] [PubMed] [Google Scholar]
- 47.Buschman TJ, and Kastner S (2015). From Behavior to Neural Dynamics: An Integrated Theory of Attention. Neuron 88, 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarz JS, Sridharan D, and Knudsen EI (2013). Magnetic tracking of eye position in freely behaving chickens. Front Syst Neurosci 7, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sridharan D, Ramamurthy DL, Schwarz JS, and Knudsen EI (2014). Visuospatial selective attention in chickens. Proc Natl Acad Sci U S A 111, E2056-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mysore SP, Asadollahi A, and Knudsen EI (2011). Signaling of the strongest stimulus in the owl optic tectum. J Neurosci 31, 5186–5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green DM, and Swets JA (1988). Signal detection theory and psychophysics, (Los Altos, California: Peninsula Publishing; ). [Google Scholar]
- 52.Macmillan NA, and Creelman DC (2005). Detection theory: A user’s guide, (Mahwah, New Jersey: Lawrence Erlbaum Associates Inc.). [Google Scholar]
- 53.DeBello WM, and Knudsen EI (2004). Multiple sites of adaptive plasticity in the owl’s auditory localization pathway. J Neurosci 24, 6853–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maczko KA, Knudsen PF, and Knudsen EI (2006). Auditory and visual space maps in the cholinergic nucleus isthmi pars parvocellularis of the barn owl. J Neurosci 26, 12799–12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


