Abstract
With an increasing incidence of prostate cancer, identification of new tumor drivers or modulators is crucial. Genetically engineered mouse models (GEMM) for prostate cancer are hampered by tumor heterogeneity and its complex microevolution dynamics. Traditional prostate cancer mouse models include, amongst others, germline and conditional knockouts, transgenic expression of oncogenes, and xenograft models. Generation of de novo mutations in these models is complex, time-consuming, and costly. In addition, most of traditional models target the majority of the prostate epithelium, whereas human prostate cancer is well known to evolve as an isolated event in only a small subset of cells. Valuable models need to simulate not only prostate cancer initiation, but also progression to advanced disease.
Here we describe a method to target a few cells in the prostate epithelium by transducing cells by viral particles. The delivery of an engineered virus to the murine prostate allows alteration of gene expression in the prostate epithelia. Virus type and quantity will hereby define the number of targeted cells for gene alteration by transducing a few cells for cancer initiation and many cells for gene therapy. Through surgery-based injection in the anterior lobe, distal from the urinary track, the tumor in this model can expand without impairing the urinary function of the animal. Furthermore, by targeting only a subset of prostate epithelial cells the technique enables clonal expansion of the tumor, and therefore mimics human tumor initiation, progression, as well as invasion through the basal membrane.
This novel technique provides a powerful prostate cancer model with improved physiological relevance. Animal suffering is limited, and since no additional breeding is required, overall animal count is reduced. At the same time, analysis of new candidate genes and pathways is accelerated, which in turn is more cost efficient.
Keywords: Cancer Research, Issue 134, Prostate cancer, genome editing, gene therapy, gene alteration, virus delivery, virus injection, CRISPR/Cas9, adeno-associated virus, in vivo tumor model, tumor initiation
Introduction
Detection and treatment of prostate cancer have significantly improved over the last decade. Still, the incidence of prostate cancer is increasing, following life expectancy. With an estimated 1.1 million new cases worldwide, it is among the most common causes of cancer-related death in men 1. Prostate cancer is slow in its development, but when the cancer has progressed to an advanced metastatic state, prognosis is poor due to limited treatment options. So far, only a few genes have been identified as common drivers in this cancer, and its heterogeneity and multifocality impedes detection of biomarkers and targetable disease drivers2,3.
Classical techniques of generating GEMMs are often impaired by their complexity, timely expenses, and costs. Conditional knockout models have been widely used to study prostate cancer candidate genes, that result in embryonic lethality when inactivated in the germline4. Most common models involve a prostate-specific Cre recombinase driven by either a modified Probasin5 or a PSA6 promoter integrated in the GEMM by additional cross-breeding. In these models, the gene of interest will be targeted in the majority of prostate epithelial cells, generating hyperplasia in the entire organ, which may impair the animal's urinary tract function7.
Viral delivery of the Cre protein by injection into the anterior lobe of the murine prostate can resolve this problem by only targeting a few cells8. Taking laboratories technical prerequisites, expertise, and objectives into account, the method benefits from a broad range of possible variations. Successful approaches utilizing Adenovirus targeting JunB and Pten9 or Lentivirus targeting Pten and Trp5310 have been shown amongst others. Adding transgenes, such as luciferase, to the viral construct or to the GEMM will furthermore enable non-invasive monitoring of disease progression via bioluminescence imaging11.
Genome editing based on the CRISPR/Cas9 technology reveals a new and rapid opportunity to study cancer through rapid generation of somatic knockouts12. Viral delivery of single guide RNAs (sgRNAs) directed to the anterior lobe of the murine prostate establishes a physiologically more relevant model of prostate cancer. By this means, single cells carrying chosen mutations can form clones that are capable of expansion and invasion. Furthermore, use of guide RNAs for multiple target genes will generate cell clones with alterations in different genes. This will allow tumor heterogeneity and a natural selection pressure on cancer progression, which can reveal the importance of each gene alteration or epistatic mechanisms.
Here we present a method to deliver viral particles to the murine prostate for alteration of gene expression. By a small abdominal incision, the murine anterior prostate lobe is exposed and viral particles are injected into the lobe. Five days post-surgery, surgical clips can be removed from the skin and the prostatic cancer can be analyzed from 8 weeks after. Overall, this is a rapid and cost-efficient procedure, which has little impact on the mouse and allows larger tumor to develop without compromising the mouse.
Protocol
This protocol involves a surgical procedure in laboratory mice. All animal experiments must be individually reviewed and approved by an Institutional Animal Care and Use Committee (IACUC). As the approach is based on animal recovery and survival, ensure appropriate anesthesia, pain management, and an aseptic surgical environment at all time. Use a heating pad to prevent hypothermia during surgery and until recovery from anesthesia.
1. Starting Considerations
Depending on the virus used, different titer requirements may apply; dilute the virus accordingly. NOTE: In this example, an Adeno-associated virus diluted in PBS is injected at a titer of 1 x 1012 IU/mL. The virus has serotype 9 and contains guide RNA to Pten and expression of Cre protein under a ubiquitin promotor (Figure 1). Loss of Pten increased pAKT and proliferation of the prostatic epithelium13.
Use male mice at an age of 8 weeks at the time of surgery to ensure adequate prostate development. NOTE: Mice shown in this experiment are Rosa26-LSL-Cas9-EGFP knockin mice on C57BL/6J background12 (Figure 1).
- Freshly prepare a general anesthesia mix and if possible keep it sterile. NOTE: For better animal recovery after surgery, an anesthesia antidote is highly recommended.
- To prepare a total volume of 5 mL anesthetic, mix 0.15 mL medetomidine hydrochloride (1 mg/mL), 0.4 mL midazolam (5 mg/mL), and 0.25 mL butorphanol (10 mg/mL) with 4.2 mL sterile saline water. NOTE: Alternative anesthesia methods, such as inhalable isoflurane, can be used according to the institutional animal care guidelines.
- To reverse the effect of medetomidine hydrochloride post-surgery, mix 0.1 mL atipamezole (5 mg/mL) with 4.9 mL sterile saline water.
Prepare an aseptic surgical environment by proper disinfection and sterilization. Autoclave surgical instruments before use.
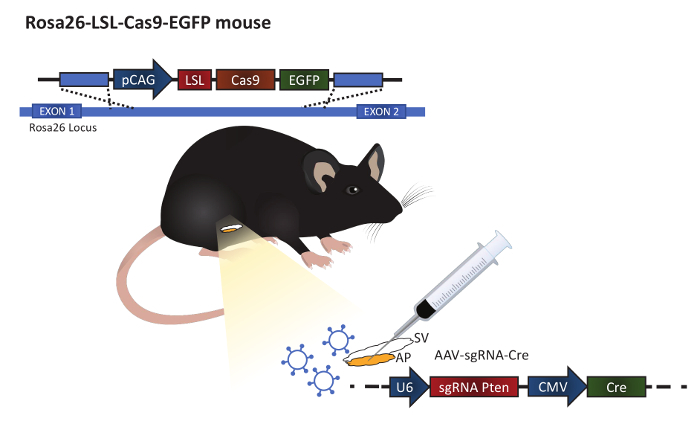
2. Virus-delivery to the Murine Prostate
Anesthetize the animal by intraperitoneal injection with a sterile 1 mL syringe and a 27G x ½" needle. Use an anesthesia dose of 0.01 mL/g body weight. If the procedure lasts longer than 30 min, maintain anesthesia by injecting 1/3 of the starting dose every 30 min.
Examine the anesthetic depth by assessing muscle relaxation, pedal withdrawal, and palpebral reflexes. When loss of reflexes is observed, shave the lower abdomen of the animal.
Carefully cover the animal's eyes with veterinary ophthalmic ointment using a sterile cotton swab to prevent blindness due to lack of corneal reflex.
Wipe the shaved abdomen with 70% ethanol and 10% povidone iodine to disinfect the surgical area. Scrub the surgical area from the center of the surgical area toward the periphery.
Perform a vertical skin incision at the low-abdominal midline of approximately 1 cm using a sterile surgical scissor.
Lift the peritoneum using a fine point forceps to prevent damaging the organs that are laying underneath. Carefully make an < 8 mm incision using surgical scissors through the peritoneum. NOTE: In contrast to human, a rodent's prostate gland comprises four separate lobes. The anterior lobe is attached to the seminal vesicle.
Gently move the fat tissue aside to uncover the seminal vesicle. Using a ring forceps, carefully lift up the seminal vesicle until the anterior prostate can be identified (Figure 1).
Inject a total volume of 30 µL virus (3 x 1010 viral particles) solution in the anterior prostate epithelium using a 0.5 mL insulin syringe with a 30G x 8 mm needle. Minimize leakage and ensure the fluid is absorbed within the tissue, forming a small bubble. Place the seminal vesicle back in the abdominal cavity.
Suture the peritoneum with 2-3 simple interrupted stitches using 6-0 absorbable sutures with a 13 mm, 3/8 circle, and a taper point needle.
Staple the skin with 3 sterile 4.8 x 6.5 mm clips lifting the skin with forceps to avoid damaging the peritoneum.
For better recovery, apply the anesthesia antidote in a dose of 0.01 mL/g body weight by intraperitoneal injection using a sterile 1 mL syringe and a 27G x ½" needle. Carefully place the animal back in its cage.
3. Post-surgery Procedures
Keep the cage with mice that underwent surgery on a heating pad for 1 h after the procedure, to prevent hypothermia until fully recovered. If the antidote is applied, the animal should be awake a few minutes after the injection.
Monitor the animals daily for appropriate wound healing and pain indications. If necessary, administer additional pain killers according to institutional animal care guidelines.
Remove skin clips after 4-5 days, once the wound is closed.
Representative Results
To assess virus delivery to the murine prostate, samples were analyzed three months after the surgery. The Rosa26-LSL-Cas9-EGFP mice12 express GFP in cells that have been exposed to Cre protein expressed by the virus. The prostate samples were examined with a fluorescence microscopy to identify areas with GFP signal (Figure 2A). The GFP signal indicates Cre activity in the prostate epithelium but not whether gene editing has been induced by the CRISPR guide. Immunohistochemical sections showed focal areas of cells with high expression of pAKT (Figure 2B), indicating loss of Pten13. An immunofluorescence co-staining of pAKT and GFP identified double positive cells (Figure 2C). This confirmed the transformation of prostatic cells by the Adeno-associated virus. Overall, these results show that in vivo CRISPR/Cas9 gene editing can be performed in the prostate epithelium by using the Adeno-associated virus and Rosa26-LSL-Cas9-EGFP mouse.
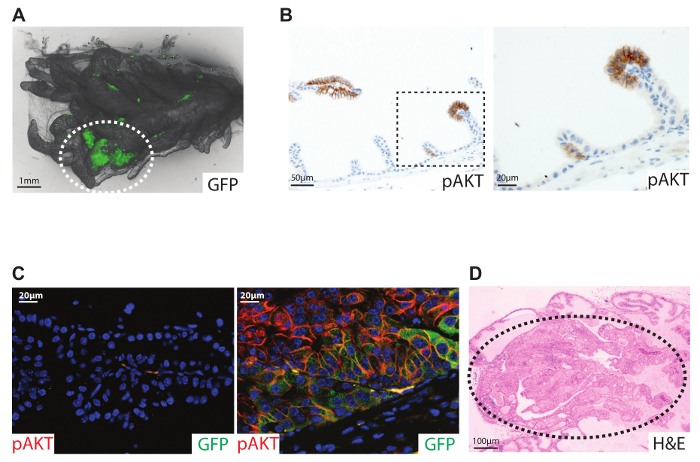
Figure 1: Illustration of the procedure. The procedure is carried out in the Rosa26-LSL-Cas9-EGFP mouse generated by Platt et al.12 The anterior prostate (AP) is attached to the seminal vesicle (SV). Virus particles expressing guide RNA and a Cre protein are injected into the anterior prostate to alter gene expression. Please click here to view a larger version of this figure.
Figure 2: Gene alteration in the murine prostate through orthotopic virus delivery. 7-week-old male Rosa26-LSL-Cas9-EGFP mice were injected with Adeno-associated virus containing a guide RNA for Pten and coding for a Cre protein. Samples were analyzed 3 months after virus injection. (A) GFP fluorescence imaging of the anterior prostate. (B) Histological section (4 µm) stained for pAKT (brown) marks the clonal area with loss of Pten expression. The dashed box marks the high magnification filed. (C) Immunofluorescence staining for GFP (green) and pAKT (red). Nuclear acids were stained with DAPI. Left: staining of the non-injected anterior lobe showing no signal for GFP or pAKT. Right: staining of the injected lobe showing co-localization of GFP (cytoplasmic staining) and pAKT (cell membrane localization). (D) Ptenflox/flox mice injected with Cre-expressing Adenovirus. H&E staining of a histological section (4 µm) of the anterior lobe 6 months after virus delivery. Dotted ellipse marks the area of high grade prostatic intraepithelial neoplasia. For details see reference 9. Please click here to view a larger version of this figure.
Discussion
In this protocol, we describe a method to alter gene expression in the anterior lobe of the murine prostate by virus injection, creating a powerful new mouse model for prostate cancer (Figure 2). The successful administration of an Adenovirus was first described by Leow et al. in 20058. We have previously shown how an Adenovirus coding for a Cre recombinase protein can replace time-consuming cross-breeding of a Cre allele for tissue-specific deletion 9 (Figure 2D). Since the virus infects a few cells9, this model mimics the human scenario of clonal expansion14,15 and is optimal for cancer studies (Figure 2B).
The discovery of CRISPR/Cas9 technology has opened new opportunities for in vivo gene editing. It is now possible to alter multiple genes simultaneously, providing a highly valuable technique for this heterogenic cancer in a both cost-saving and time-efficient manner. Research using animal models benefits from the advantage of generating gene alterations not only in the germline, but also in adult tissues. Furthermore, the development of Cas9 expressing mice allows viral delivery of both the Cre and sgRNAs (Figure 1). As the guides have no impact on the genome without Cas9 expression, the work with this virus is non-hazardous.
Adeno-associated viruses induce very low immune responses, and even though present for up to a year, the virus does not integrate into the host genome, which makes it preferable for in vivo knockout studies16. In this context, it must be noted that different serotypes may impact transduction efficiency in distinct tissue types. Therefore, optimization for the targeted tissue is crucial to achieve high efficiency. Using genome-integrating Lentivirus can on the other hand allow long-term oncogene expression10.
The human prostate is not separated in distinct lobes and it has been discussed which of the murine prostate lobes best represents the human prostate. While the lateral lobe was proposed, no significant difference has been shown between the different lobes with respect to tumor initiation9,17,18,19. Another critical aspect of the virus delivery is the possible infection of other cells in the body. We have observed prostate stroma cells that have been transduced. The risk can be minimized during the orthotopic delivery procedure, avoiding blood vessels and preventing leakage while injecting. Further virus design including a prostate specific promoter, such as the Probasin promoter and serotype, could increase cell-specificity.
Disclosures
The authors have nothing to disclose.
Acknowledgments
MR was funded by a fellowship from the Danish cancer society (R146-A9394-16-S2). MFB and MKT were funded by AUFF NOVA (AUFF-E-2015-FLS-9-8). MR and MFB were co-funded by Graduate School, HEALTH, AU. The E.F.W. laboratory is supported by grants from the Spanish Ministry of Economy (SAF2015-70857, co-funded by the ERDF-EU) and an ERC advanced grant (741888 - CSI-Fun).
We want to thank Liliana Fajardo Mellor (Genes, Development and Disease; National Cancer Research Center) for critical reading of the manuscript.
References
- Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell. 2015;162:454. doi: 10.1016/j.cell.2015.06.053. [DOI] [PubMed] [Google Scholar]
- Gama Sosa MA, De Gasperi R, Elder GA. Animal transgenesis: an overview. Brain Struct Funct. 2010;214:91–109. doi: 10.1007/s00429-009-0230-8. [DOI] [PubMed] [Google Scholar]
- Wu X, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- Ma X, et al. Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res. 2005;65:5730–5739. doi: 10.1158/0008-5472.CAN-04-4519. [DOI] [PubMed] [Google Scholar]
- Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow CC, Wang XD, Gao WQ. Novel method of generating prostate-specific Cre-LoxP gene switching via intraductal delivery of adenovirus. Prostate. 2005;65:1–9. doi: 10.1002/pros.20244. [DOI] [PubMed] [Google Scholar]
- Thomsen MK, et al. Loss of JUNB/AP-1 promotes invasive prostate cancer. Cell Death Differ. 2015;22:574–582. doi: 10.1038/cdd.2014.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, et al. a novel GEM model for metastatic prostate cancer analysis and therapy, reveals myc as a driver of Pten-mutant metastasis. Cancer Discov. 2014;4:318–333. doi: 10.1158/2159-8290.CD-13-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, et al. Rapid in vivo validation of candidate drivers derived from the PTEN-mutant prostate metastasis genome. Methods. 2015;77-78:197–204. doi: 10.1016/j.ymeth.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt RJ, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159:440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Liu W, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner MC, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123:4918–4922. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. Comparative Aspects of Development and Structure in the Prostate. Natl Cancer Inst Monogr. 1963;12:1–27. [PubMed] [Google Scholar]
- McNeal JE. Anatomy of the prostate: an historical survey of divergent views. Prostate. 1980;1:3–13. doi: 10.1002/pros.2990010103. [DOI] [PubMed] [Google Scholar]
- Thomsen MK, et al. SOX9 elevation in the prostate promotes proliferation and cooperates with PTEN loss to drive tumor formation. Cancer Res. 2010;70:979–987. doi: 10.1158/0008-5472.CAN-09-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]


