Abstract
For the elderly, arterial stiffening is a good marker for aging evaluation and it is recommended that the arterial stiffness be determined noninvasively by the measurement of carotid to femoral pulse wave velocity (cf-PWV) (Class I; Level of Evidence A). In literature, numerous community-based or disease-specific studies have reported that higher cf-PWV is associated with increased cardiovascular risk. Here, we discuss strategies to evaluate arterial stiffness with cf-PWV. Following the well-defined steps detailed here, e.g., proper position operator, distance measurement, and tonometer position, we will obtain a standard cf-PWV value to evaluate arterial stiffness. In this paper, a detailed stepwise method to record a good quality PWV and pulse wave analysis (PWA) using a non-invasive tonometry-based device will be discussed.
Keywords: Medicine, Issue 135, arterial stiffness, carotid-femoral pulse-wave velocity
Introduction
Arterial stiffening is a good marker for vascular aging evaluation1,2. The measurement of arterial stiffening is traditionally conducted using a pulse wave velocity (PWV) methodology that is an important and reliable measure of arterial stiffness1,3,4,5. Specifically, PWV represents the stiffness of a specific arterial segment. The pulse wave is transmitted through the arterial vessels in a specific segment, and its speed is inversely related to the viscoelastic properties of the wall itself6. PWV value increases with arterial stiffening.
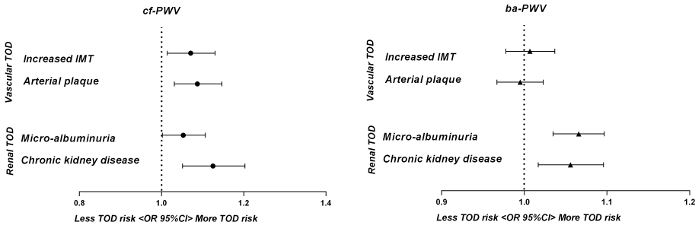
Carotid-femoral PWV (cf-PWV) and brachial-ankle PWV (ba-PWV) are the 2 most frequently applied PWV measurements. They are widely used in clinical practice, where cf-PWV is popular in western countries and ba-PWV is popular in Asian countries.7,8. In fact, cf-PWV has been considered as the 'gold-standard' measurement of arterial stiffness1. For cf-PWV, it is taken as representative of the PWV for the entire aorta. Besides, for ba- PWV, there is no true arterial pathway linking the measurement sites (brachial to ankle). The estimated ba-PWV represents the PWV for the entirety of the central and peripheral arterial system9. A previous study has reported that cf-PWV is superior to ba-PWV in associations with asymptomatic hypertensive target organ damage (TOD)10 (Figure 1).
Non-invasive devices for regional stiffness equipped with a specific tonometer are increasingly being used to measure the stiffness of the carotid to femoral segment1. In cf-PWV measurements, this device and a handheld tonometer create a steady waveform on the computer that can record high resolution digital waveform images and the specific PWV values (Figure 2). All these measurements need to be standardized. Here, we show how to record a good quality cf-PWV with this non-invasive tonometry-based device in a real-world setting.
Some established cardiovascular risk prediction models such as the Framingham risk score and the SCORE Risk Charts are mainly calculated and sorted by conventional risk factors11,12. However, some novel biomarkers should be added into the risk assessment model to improve the risk stratification13. In literature, arterial stiffening is considered as an intermediate state between conventional risk factors and clinical cardiovascular events14. Thus, adding cf-PWV into the risk assessment model may be a tool for risk stratification15,16.
Here, we generate a methodology plan to assess participants' cf-PWV, together with PWA, to establish a standard protocol for arterial stiffening assessment.
Protocol
This protocol was approved by the Ethics Committee of Shanghai Tenth People's Hospital.
1. Recruitment of Participants
Include Adults with a moderate heart rate (40 < HR < 160).
- Use the following exclusion criteria.
- Exclude those without internal arteriovenous fistula for renal dialysis or any other peripheral arteriovenous fistula like that.
- Exclude those without peripheral arterial spasm, for example the Raynaud disease.
- Exclude those without aortic valvular stenosis (transprosthetic pressure gradient > 60 mmHg).
- Exclude those without second or third-degree atrioventricular (AV) block.
- Exclude those without atrial fibrillation or atrial flutter.
- Exclude those without unstable carotid atherosclerotic plaques that may rupture after kneading. 9) Without cognitive impairment or unable to cooperate.
Ensure that the participants' bladder is empty.
Have participants refrain from smoking, tobacco use or caffeine use for at least 3 hours before the measurement. Abstain from alcohol for at least 12 hours before the measurement. Fast for at least 3 hours before the measurement.
2. Measurements of Vascular TOD
NOTE: cf-PWV and PWA measurements, like conventional four-limb blood pressure measurements, are performed through a non-invasive evaluating method. A standard protocol is crucial to obtain an accurate value, especially when there are multiple operators. Make sure to practice the method before the actual operation. The participant's cooperation is key for the measurement of cf-PWV.
Explain the procedure to the participant and obtain permission.
Measure the body height and weight before the start of the measurements.
- Explain that the process may take 5-10 min and involves letting the participant lie on the bed.
- Keep the patient's skin clean and dry when shifting supine positions. If the patient's skin is damp because of sweating, then wipe dry the skin.
- Perform the measurements in a quiet room with a stable room temperature.
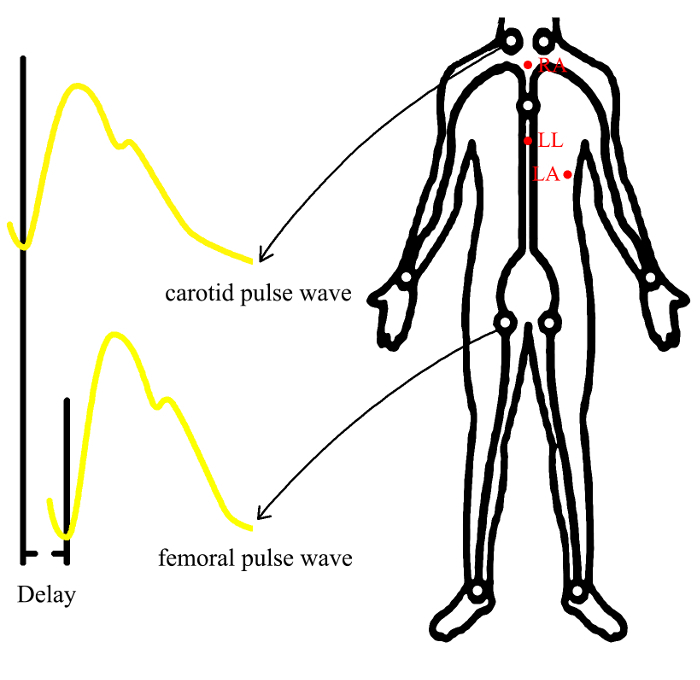
Put the participant's hands next to their body to make sure there is a smooth, undisturbed ECG signal. Connect up to 3-lead ECG monitors, positioning three electrodes (RA, LL, LA) according to the following diagram (Figure 2). NOTE: The use of disposable electrodes is recommended. The ECG leads and wires cannot be used in other ECG drawing equipment.
Have the patient rest for at least 5 minutes in the supine position. Then measure the patient's blood pressure with a semiautomatic oscillometric device. NOTE: The blood pressure is measured according to the recommendations of the European Society of Hypertension17.
Have the patient rest for at least 2 minutes. NOTE: The peripheral vascular dilatation caused by artery occlusion when measuring brachial artery blood pressure will change the brachial artery pulse transmission. Resting is essential to avoid excessive deviation.
While the participant rests, enter the participant's detailed information, including the supine blood pressure, body height, body weight and operator's name, into the measurement device (Figure 3).
After entering all the information, keep the participants in a supine position.
- Manually measure the following parameters (mm) (Figure 3).
- Measure the distance from the suprasternal notch (SSN) to the remote detection point (femoral artery).
- Measure the distance from the sternal notch to the proximal (carotid artery) detection point.
- Measure the distance from the remote detection point (femoral artery) to the proximal (carotid artery) detection point (cf-distance).
Choose the PWA mode. Enter '0' as SSN-carotid distance, and enter 0.8*cf-distance as SSN-femoral distance. If the device doesn't accept '0', put another value as the SSN-carotid distance and add that value to the 80% cf distance as SSN-femoral distance5. NOTE: The operator should sit in a most comfortable position.
- Press the button to capture data. Put the tonometer on the radial artery fluctuation point (1-2 cm above the radial styloid process at a point where the fluctuation is strong and stable) to measure the central blood pressure.
- Maintain the position of the tonometer to obtain a steady waveform on the computer. Once the quality and reproducibility of the tonometry measurements reach the standard, the signal recording is finished automatically by the computer.
- Keep the tonometer at 90°.
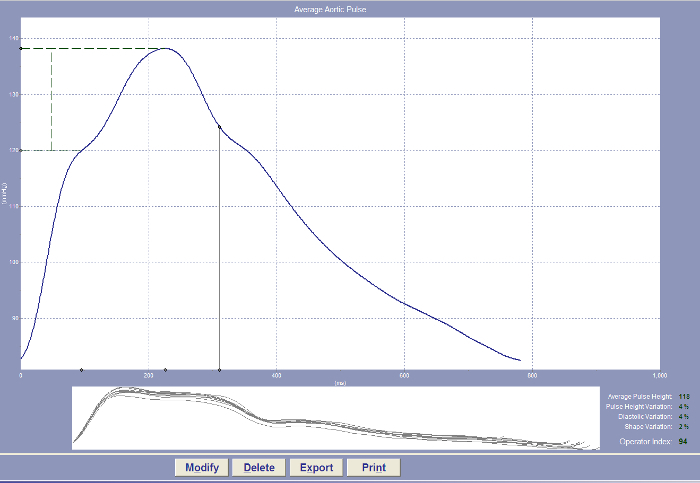
- To avoid parasite movement, immobilize the tonometer near the base with the fingers, firmly affix the tonometer to the skin. NOTE: The software automatically records the data as soon as the tonometer is placed in contact with the measurement site. The quality and reproducibility of the tonometry measurements are automatically tested. An operator index greater than 80% is considered as a reliable measurement. Pulse pressure amplification (PPA) is defined as the peripheral-to-central pulse pressure ratio and is calculated with the formula, PPA= (brachial SBP- brachial DBP)/ (central SBP- central DBP)-1. PWA can be observed on the device18 (Figure 4).
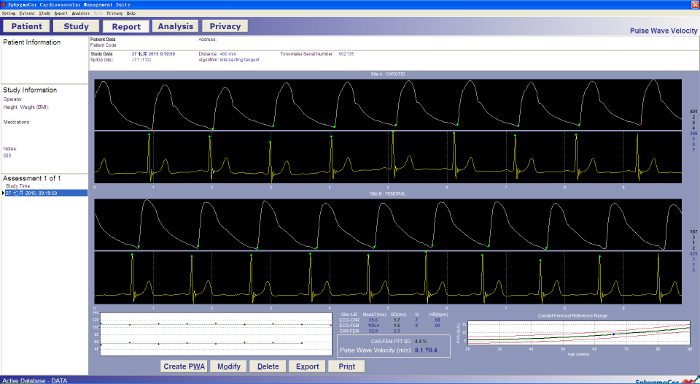
Choose the PWV mode. Put the tonometer on the carotid artery fluctuation point. Maintain the position of the tonometer to obtain a steady waveform on the computer. After recording a stable waveform for 15-20 seconds. Once the quality and reproducibility of the tonometry measurements reach the standard, finish recording manually (Figure 5).
Put the tonometer on the femoral artery fluctuation point (1-2 cm below the midpoint of the inguinal ligament at a point where the fluctuation is stable). Maintain the position of the tonometer to record a steady waveform. After recording a stable waveform for 15-20 seconds, finish recording manually once the quality and reproducibility of the tonometry measurements reach the standard. The PWV value can be observed on the device cf-PWV ≥ 10 m/s is considered as arterial stiffening (Figure 5)1.

Representative Results
Cf-PWV (with this method) and ba-PWV (with other method10) were conducted in all of the 2098 participants from Northern Shanghai Study19. Both cf-PWV and ba-PWV were used in the same logistic regression model. In this model, age and gender were adjusted. The results showed that only cf-PWV, but not ba-PWV, was significantly associated with increased IMT and arterial plaque, which indicates the superiority of cf-PWV over ba-PWV in the association with vascular abnormalities in the elderly (Figure 1).
Figure 1: The cf-PWV and ba-PWV in association with other hypertensive target organ damage in 2098 participants from Northern Shanghai Study. Odds ratios of cf-PWV and ba-PWV were presented using logistic regressions when cf-PWV and ba-PWV were both put into the same full-mode model. This data has been recalculated with the current 2098 participants. The figure was modified from a previous publication10. Abbreviations: IMT, carotid intima-media thickness; TOD, target organ damage. Please click here to view a larger version of this figure.
Figure 2: The positions of electrodes and the segments of measured arteries. By recording the ECG and tonometer signal, the device will measure transit time as the time delay between the arrival of the pulse wave at the common carotid artery and the common femoral artery. Carotid-femoral PWV is calculated by dividing traveled distance by transit time (PWV=distance/time) Abbreviations: RA: right arm; LL: left leg; LA: left arm Please click here to view a larger version of this figure.
Figure 3: Examiners and Patients Positioning for PWV measurement. Measurements should be performed in supine position after at least 5 min of rest. Measurements should preferentially be done at the right common carotid and common femoral arteries. The room should be quiet with stable room temperature.
Figure 4: A normal pulse wave analysis waveform (radial artery) imaged using a non-invasive tonometry-based device. The waveform will be recorded in the device and the operator index is provided directly. An operator index greater than 80% is considered as a reliable measurement. Please click here to view a larger version of this figure.
Figure 5: A normal pulse wave velocity waveform (carotid and femoral arteries) imaged using a non-invasive tonometry-based device. The waveform will be recorded in the device and the PWV value is provided directly. A cf-PWV over 10 m/s is considered as arterial stiffening. Please click here to view a larger version of this figure.
Discussion
Here, we demonstrate a widely accessible methodology to assess the participants' novel preclinical vascular TOD, arterial stiffness, evaluated by cf-PWV. In order to compare PWs with minimal hemodynamic differences between measurements done prior to devices, only accept data when the brachial systolic and diastolic BP varied by less than 3 mmHg. This reduces the deviation caused by human manipulation. In this protocol, the critical steps are to have standardized patient conditions using 80% of the direct straight distance between the carotid artery and femoral artery sites and using 10 m/s as standard cut-off value for carotid-femoral PWV as arterial stiffening.
With this tonometry-based device, we can non-invasively assess participants' PWV. In fact, among tonometry-based devices, they assess similar cardiovascular risk for individuals. However, they were not consistent in measurements of central BP and wave reflections in clinical practice, with considerable and significant differences among them20. This tonometry-based device is commonly used in the measurement of aortic PWV1.
We can also get the central BP measurements from this device. However, the accuracy of the central BP measurements from non-invasive devices is very much debated and should be improved. To achieve accurate non-invasive assessment of true central BP, more accurate non-invasive estimates of intra-arterial brachial BP are needed21. The data of central BP from this measurement can be further used to make comparisons with other non-invasive devices or invasive intra-arterial standards22.
Arterial stiffening can be considered as an intermediate state between risk factors and clinical CV events, which will affect medical decision making. For instance, long term hypertension may result in severe arterial stiffening. The anti-hypertensive treatment is important for hypertensive participants. However, we prefer to reverse, terminate or at least control the process of arterial stiffening with medications, instead of just focusing on hypertension. Arterial stiffening might better represent exposure to risk factors than hypertension itself. Thus, the arterial stiffening should also be considered into the risk prediction model. It is also important to consider the effect from TOD to make risk stratification strategies. In fact, arterial stiffening which is defined as increased PWV is a composite of aging and micro-changes. An increased PWV should be considered as the phenomenon of accelerated development of cardiovascular diseases in some patients. Researches focusing on PWV and its management may provide a much longer disease-free life span23. In this way, we can make a more accurate CV assessment so as to provide a more effective guidance for management and treatment.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work is under the financial support from National key research and development program of China (Grant No. 2017YFC0111800) and the Shanghai municipal government (Grant ID. 2013ZYJB0902 and 15GWZK1002). Dr. Yi Zhang was supported by the National Nature Science Foundation of China (Grant ID. 81300239 and 81670377).
References
- Laurent S, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Townsend RR, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66(3):698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiranen TJ, et al. Prevalence, Correlates, and Prognosis of Healthy Vascular Aging in a Western Community-Dwelling Cohort: The Framingham Heart Study. Hypertension. 2017;70(2):267–274. doi: 10.1161/HYPERTENSIONAHA.117.09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reference Values for Arterial Stiffness C, et al. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values. Eur Heart J. 2010;31(19):2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bortel LM, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- Salvi P. Pulse waves: how vascular hemodynamics affect blood pressure. Springer; 2011. [Google Scholar]
- Mancia G, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- Yamashina A, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25(3):359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- Tanaka H, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27(10):2022–2027. doi: 10.1097/HJH.0b013e32832e94e7. [DOI] [PubMed] [Google Scholar]
- Lu Y, et al. Comparison of Carotid-Femoral and Brachial-Ankle Pulse-Wave Velocity in Association With Target Organ Damage in the Community-Dwelling Elderly Chinese: The Northern Shanghai Study. J Am Heart Assoc. 2017;6(2) doi: 10.1161/JAHA.116.004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino RB, Sr, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Conroy RM, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- Zethelius B, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358(20):2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- Vernooij JW, et al. Hypertensive target organ damage and the risk for vascular events and all-cause mortality in patients with vascular disease. J Hypertens. 2013;31(3):492–499. doi: 10.1097/HJH.0b013e32835cd3cd. [DOI] [PubMed] [Google Scholar]
- van der Veen PH, et al. Hypertensive Target Organ Damage and Longitudinal Changes in Brain Structure and Function: The Second Manifestations of Arterial Disease-Magnetic Resonance Study. Hypertension. 2015;66(6):1152–1158. doi: 10.1161/HYPERTENSIONAHA.115.06268. [DOI] [PubMed] [Google Scholar]
- Ji H, et al. Shanghai Study: cardiovascular risk and its associated factors in the Chinese elderly-a study protocol of a prospective study design. BMJ Open. 2017;7(3) doi: 10.1136/bmjopen-2016-013880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23(4):697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- Agnoletti D, et al. Pulse wave analysis with two tonometric devices: a comparison study. Physiol Meas. 2014;35(9):1837–1848. doi: 10.1088/0967-3334/35/9/1837. [DOI] [PubMed] [Google Scholar]
- Ji H, et al. Shanghai Study: cardiovascular risk and its associated factors in the Chinese elderly-a study protocol of a prospective study design. BMJ Open. 2017;7(3):e013880. doi: 10.1136/bmjopen-2016-013880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. Comparison study of central blood pressure and wave reflection obtained from tonometry-based devices. Am J Hypertens. 2013;26(1):34–41. doi: 10.1093/ajh/hps031. [DOI] [PubMed] [Google Scholar]
- Sharman JE, et al. Validation of non-invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017;38(37):2805–2812. doi: 10.1093/eurheartj/ehw632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millasseau S, Agnoletti D. Non-invasive estimation of aortic blood pressures: a close look at current devices and methods. Curr Pharm Des. 2015;21(6):709–718. doi: 10.2174/1381612820666141023163748. [DOI] [PubMed] [Google Scholar]
- Olsen MH, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388(10060):2665–2712. doi: 10.1016/S0140-6736(16)31134-5. [DOI] [PubMed] [Google Scholar]


