Abstract
The blood-brain barrier (BBB) is a dynamic barrier tissue that responds to various pathophysiological and pharmacological stimuli. Such changes resulting from these stimuli can greatly modulate drug delivery to the brain and, by extension, cause considerable challenges in the treatment of central nervous system (CNS) diseases. Many BBB changes that affect pharmacotherapy, involve proteins that are localized and expressed at the level of endothelial cells. Indeed, such knowledge on BBB physiology in health and disease has sparked considerable interest in the study of these membrane proteins. From a basic science research standpoint, this implies a requirement for a simple but robust and reproducible method for isolation of microvessels from brain tissue harvested from experimental animals. In order to prepare membrane samples from freshly isolated microvessels, it is essential that sample preparations be enriched in endothelial cells but limited in the presence of other cell types of the neurovascular unit (i.e., astrocytes, microglia, neurons, pericytes). An added benefit is the ability to prepare samples from individual animals in order to capture the true variability of protein expression in an experimental population. In this manuscript, details regarding a method that is utilized for isolation of rat brain microvessels and preparation of membrane samples are provided. Microvessel enrichment, from samples derived, is achieved by using four centrifugation steps where dextran is included in the sample buffer. This protocol can easily be adapted by other laboratories for their own specific applications. Samples generated from this protocol have been shown to yield robust experimental data from protein analysis experiments that can greatly aid the understanding of BBB responses to physiological, pathophysiological, and pharmacological stimuli.
Keywords: Neuroscience, Issue 135, Blood-brain barrier, Brain Microvessels, Dextran Separation, Differential Centrifugation, Endothelial Cell, Membrane Proteins, Molecular Pharmacology, Transporters, Tight Junctions, Western Blotting
Introduction
The blood-brain barrier (BBB) exists at the interface between the central nervous system (CNS) and the systemic circulation and plays an essential role in the maintenance of brain homeostasis. Specifically, the BBB functions to precisely control solute concentrations in brain extracellular fluid and to efficiently supply those nutrients that are required by brain tissue to fulfill the considerable metabolic demands of the CNS1. These roles imply that the BBB, which exists primarily at the level of the microvascular endothelial cell, must possess discrete mechanisms that enable some substances to access brain parenchyma while ensuring that potentially harmful xenobiotics cannot accumulate. Indeed, brain microvascular endothelial cells are not fenestrated and exhibit limited pinocytosis, which ensures a lack of non-selective permeability2. Additionally, brain microvessel endothelial cells express tight junction and adherens junction proteins that act to form a physical "seal" between adjacent endothelial cells and greatly restrict paracellular diffusion of blood-borne substances into brain parenchyma. Indeed, selective permeability of endogenous and exogenous substances requires functional expression of uptake and efflux transporters3. Overall, tight junctions, adherens junctions, and transporters work in concert to maintain the unique barrier properties of the BBB.
The BBB is a dynamic barrier that responds to physiological, pathophysiological, and pharmacological stimuli. For example, hypoxia/reoxygenation stress has been shown to modulate expression of critical tight junction proteins (i.e., occludin, zonulae occluden-1 (ZO-1)), which is associated with increased paracellular permeability to vascular markers such as sucrose4,5,6. Similar observations have been made at the BBB in the setting of traumatic brain injury7 and peripheral inflammatory pain8,9. These same diseases can also modulate transport mechanisms at the BBB10,11,12,13,14. Indeed, hypoxia/reoxygenation injury enhances functional expression of organic anion transporting polypeptide 1a4 (Oatp1a4) at the BBB, which can lead to significant increases in the blood-to-brain transport of specific Oatp transport substrates such as taurocholate and atorvastatin13. BBB properties can also be altered by pharmacotherapy itself, a mechanism that can form a basis for both profound changes in the drug effectiveness in the brain and for drug-drug interactions. For example, acetaminophen targets nuclear receptor signaling mechanisms in the brain microvascular endothelial cells, increases functional expression of the critical efflux transporter P-glycoprotein (P-gp), and modifies time-dependent analgesia conferred by morphine, an opioid analgesic drug and established P-gp transport substrate15. A thorough understanding of BBB changes, that can be induced by diseases or by drugs, also requires identification and characterization of specific regulatory mechanisms that control these modifications. Indeed, discrete signaling pathways have been identified in brain microvascular endothelial cells that control the molecular expression of tight junction proteins16,17 and transporters15,18,19. Taken together, these observations indicate that complex molecular pathways are involved in the regulation of BBB tight junctions and transporters in both health and disease.
A significant challenge in the study of the BBB is the absolute requirement of a simple and effective method for isolation of microvessels from brain tissue derived from experimental animals and subsequent preparation of membrane samples. These samples must be prepared so that they are both enriched in brain microvascular endothelial cells and limited in presence of other cell types. Over the past several years, multiple methodologies for isolation of microvasculature from rodent brain have been reported in the scientific literature13,20,21,22. This article describes a simple, robust, and reproducible method for isolation of microvessels from rat brain and for preparation of endothelial membrane-enriched samples that can be used for the analysis of protein expression. An advantage of this microvessel isolation protocol is the ability to obtain sample preparations of high quality and with sufficient protein yield from an individual experimental animal. This enables the consideration of inter-animal variability in protein expression. Such an advance in this protocol has greatly improved the robustness of BBB studies because over-estimation (or under-estimation) of the true magnitude of protein changes at the BBB can now be avoided. Additionally, the inclusion of multiple centrifugation steps with dextran enables improved enrichment of microvessels in experimental samples while facilitating removal of unwanted cellular constituents such as neurons.
Protocol
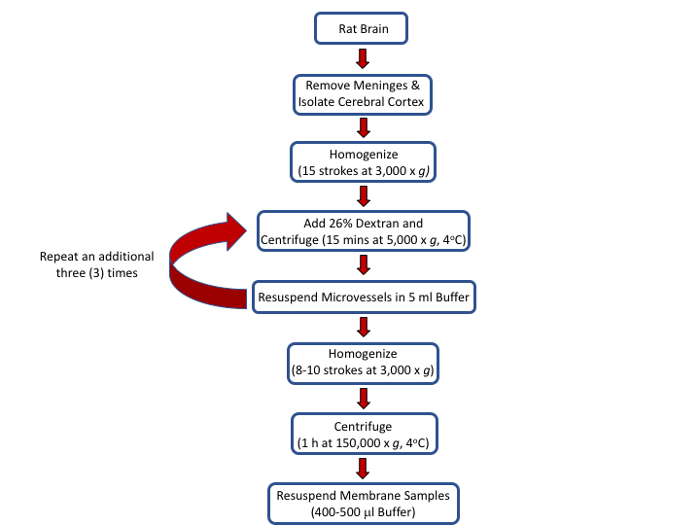
All procedures outlined below have been approved by an Institutional Animal Care and Use Committee (IACUC) and conform to National Institutes of Health (NIH) and Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines. The procedural flow for the protocol is depicted in Figure 1.
1. Set-up for the Procedure
Prepare the brain microvessel buffer (BMB). Start by weighing 54.66 g D-mannitol, 1.90 g EGTA, and 1.46 g 2-amino-2-(hydroxymethyl)-1,3-propanediol (i.e., Tris) base into a clean beaker. Add 1.0 L of deionized water. Mix the components of the BMB buffer using a magnetic stirrer. Once the solution has been thoroughly mixed, adjust the pH to 7.4 using 1.0 M HCl.
- Prepare 26% dextran (MW 75,000) solution (w/v) prior to anesthetizing animals. Prepare the dextran solution as described in the following steps. NOTE: It is critically important to prepare this solution ahead of time because dextran can take approximately 1.5-2 h to dissolve in aqueous buffer.
- Weigh out the powdered dextran (26 g per 100 mL buffer) into a clean beaker.
- Measure out the appropriate volume of BMB and dispense into a separate, clean beaker.
- Slowly pour BMB into the beaker containing powdered dextran. Manually mix the powdered dextran during pouring of BMB using a glass stir rod.
- Adjust the pH to 7.4 with 1.0 M HCl immediately prior to use. NOTE: A typical isolation of brain microvessels from 12 individual Sprague-Dawley rats (male or female; 3 months of age; 200-250 g each) requires 200 mL of dextran solution (i.e., 52 g dextran in 200 mL of BMB).
2. Extraction of Brain Tissue from Sprague-Dawley Rats
Following the desired experimental treatment, anesthetize Sprague-Dawley rats using ketamine (50 mg/kg; 2.5 mL/kg i.p.) and xylazine (10 mg/mL; 2.5 mL/kg I,p.). Dilute ketamine and xylazine in 0.9% saline to final concentrations of 20 mg/mL and 4.0 mg/mL respectively. Euthanize animals by decapitation using a sharpened guillotine in accordance with IACUC guidelines. Use the same procedure for both male and female Sprague-Dawley rats.
Resect the skin from the rat skull by making a single transverse cut using surgical scissors.
Using rongeurs, carefully remove the skull plate and expose the brain.
Remove the brain using a spatula. Detach the cerebrum and place the isolated brain tissue into a 50 mL conical tube containing 5 mL of BMB. Add 1.0 μL of protease inhibitor cocktail per 1.0 mL of BMB buffer immediately prior to use.
3. Brain Processing
Transfer the brain tissue from the 50 mL conical tube to a clean petri dish.
Using forceps, gently roll the brain on 12.5 cm diameter filter paper to remove outer meninges, which are loosely adhered to the cerebral cortex. Gently press the brain tissue against filter paper and roll the tissue again. Turn the filter paper frequently during this step.
Separate the choroid plexus from the cerebral hemispheres using forceps. NOTE: The choroid plexus appears as a clear membranous tissue localized to the surface of the cerebral ventricles.
Gently flatten the brain tissue and remove remaining meninges and olfactory bulbs using forceps.
Place the cortical brain tissue into a chilled glass mortar. Add 5.0 mL of BMB containing protease inhibitor cocktail to the mortar.
Using an overhead power homogenizer, homogenize the brain tissue using 15 up and down strokes at 3,700 rpm. Perform homogenization using a 10 mL mortar and pestle tissue grinder. Between homogenization of each individual sample, clean the pestle using 70% ethanol. NOTE: Homogenization strokes should be consistent in terms of pace and magnitude.
Pour the homogenate into labeled centrifuge tubes.
4. Centrifugation Steps
Add 8.0 mL of 26% dextran solution to each labeled centrifuge tube containing brain homogenate.
Invert the tube twice and then thoroughly vortex the sample. Conduct the vortexing of each sample using multiple angles to ensure a thorough mixing of brain homogenate solution with 26% dextran solution.
Centrifuge samples at 5,000 x g for 15 min at 4 °C. NOTE: For some analyses, it is useful to compare brain microvessels with brain parenchyma. Should the assessment of protein expression in brain parenchyma be required, the supernatant from this centrifugation step (i.e., brain parenchymal fraction) can be collected and stored at -80 °C for future use.
Remove the supernatant using a vacuum aspirator and glass Pasteur pipette. NOTE: Caution must be taken to not disturb the pellet. Otherwise, quantity of the brain microvessels collected will be reduced, which will significantly decrease the protein yield from this procedure.
Resuspend the pellet in 5.0 mL of BMB containing protease inhibitor cocktail (i.e., 1.0 μL protease inhibitor cocktail per 1.0 mL of BMB buffer). Vortex the pellet to ensure thorough mixing.
Add 8.0 mL of 26% dextran to each centrifuge tube and vortex as described in steps 4.1-4.2 of this protocol.
Centrifuge samples at 5,000 x g for 15 min at 4 °C.
Using a vacuum flask and glass pipette, aspirate supernatant and ensure that pellet containing brain microvessel is not disrupted. NOTE: Excess material that has adhered to the tube wall does not contain microvessels and should be carefully cleaned and removed.
Repeat steps 4.5 through 4.8 an additional two times.
Once 4 dextran centrifugation steps have been completed, add 5.0 mL of BMB to each pellet and vortex to resuspend the sample. NOTE: Following completion of step 4.10, whole microvessels can be collected for analysis of protein localization. This can be accomplished by taking a 50 μL aliquot of re-suspended microvessel pellet and smearing on a glass microscope slide. Microvessels are then heat-fixed at 95 °C for 10 min on a heating block followed by fixation in ice-cold ethanol for an additional 10 min. Slides can then be stored at 4 °C until required for imaging studies.
5. Ultracentrifugation to Prepare Samples of Total Brain Microvascular Membranes
Transfer samples to the chilled glass homogenizer.
Using an overhead power homogenizer, homogenize brain tissue using 8-10 up and down strokes at 3,000 rpm. Homogenization is conducted using a 10 mL mortar and pestle tissue grinder. Clean the pestle using 70% ethanol following homogenization of each individual sample.
Transfer samples to clean ultracentrifuge tubes. Number and weigh each individual tube. Balance and pair the tubes prior to loading into the ultracentrifuge rotor. NOTE: All weighing and pairing of ultracentrifuge tubes must be conducted with the caps on to ensure accurate measurement of tube weights.
Centrifuge samples at 150,000 x g for 1 h at 4 °C.
Using a vacuum flask and glass Pasteur pipette, aspirate supernatant using care not to disrupt the capillary membrane pellet.
Based on the pellet size, add an appropriate volume of storage buffer, which is comprised of deionized water and BMB in a 1:1 (v/v) ratio. Typically, pellets are resuspended in 400-500 μL of storage buffer. Add protease inhibitor cocktail to the storage buffer in a ratio of 1.0 μL per 1.0 mL of storage buffer.
Vortex samples to resuspend pellet in storage buffer.
Using a glass Pasteur pipette, transfer capillary membrane samples to a labeled 1.5 mL microcentrifuge tube.
Pass sample through a needle syringe 5x to ensure the sample is thoroughly mixed and that no cellular aggregates exist.
Store samples at -80 °C until required for analysis. NOTE: Protein content of each microvessel membrane sample is measured using the Bradford method.
Representative Results
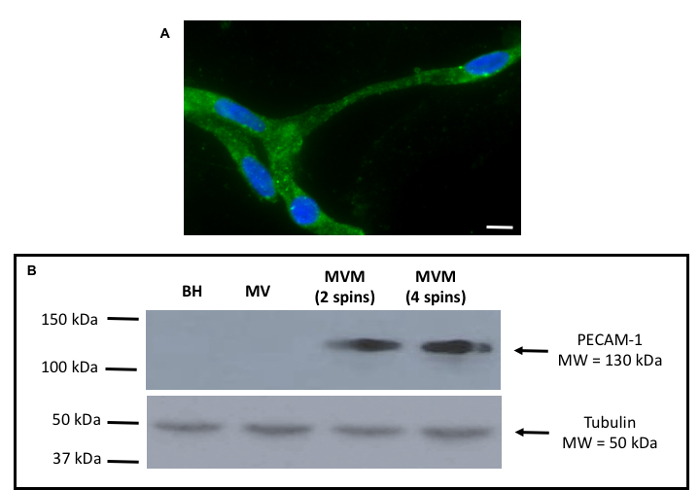
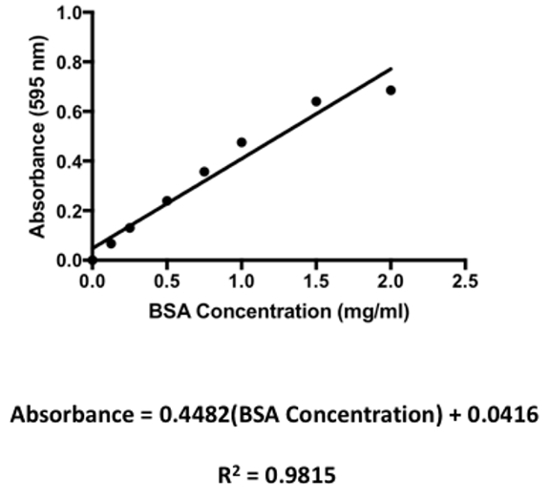
The experimental flow for isolation of rat brain microvessels and for the preparation of microvessel membrane samples is shown in Figure 1. Using the procedure presented here, successful isolation of intact microvessels from rat brain is demonstrated (Figure 2A). These vessels were obtained following completion of centrifugation with dextran and immediately prior to commencing ultracentrifugation to prepare membrane samples (i.e., following completion of step 4.10). In this image, the microvessel is stained using an antibody against Oatp1a4, a transporter that has been shown to be well expressed at the plasma membrane of brain microvascular endothelial cells2,11,13,18. Using western blot analysis (Figure 2B), membrane preparations from microvessels harvested from female Sprague-Dawley rats were shown to be enriched in platelet endothelial cell adhesion molecule-1 (PECAM-1; also known as CD31), a marker protein for brain microvasculature. PECAM-1 is an inhibitory co-receptor involved in T-cell and B-cell signaling that is highly expressed on the plasma membrane of vascular endothelial cells. During optimization of this protocol, PECAM enrichment was observed in membrane samples prepared using two and four dextran centrifugation steps. As shown in Figure 2B, there was no difference in PECAM expression between samples prepared using these different centrifugation steps; however, four dextran spins resulted in improved expression of endothelial transport proteins, which indicates improved microvessel enrichment in the samples. Additionally, the use of four dextrans spins improved removal of unwanted cellular constituents as indicated by reduced expression of neuronal marker proteins such as synaptophysin18. Therefore, all subsequent preparations for brain microvessel membranes were performed using four dextran centrifugation steps. In our western blot experiments, the microtubule constituent protein tubulin was used as a loading control. As determined by the Bradford protein assay, membrane preparations typically yield protein concentrations ranging between 5.0 mg/mL and 10.0 mg/mL. Representative protein quantification results are depicted in Table 1. These protein values were determined based on a standard curve that was generated using bovine serum albumin (BSA; 2.0 mg/mL) as the standard (Figure 3). Protein samples were diluted 10-fold in the Bradford assay.
Following protein quantification, microvessel membrane samples can be utilized for biochemical methodologies for protein analysis such as western blot analysis, dot blots, or co-immunoprecipitation. Figure 4A depicts a representative western blot for the BBB transport protein glucose transporter-1 (Glut-1) where samples from Table 1 were analyzed. Clear single bands at the appropriate molecular weight (i.e., predicted to be 54 kDa) for this highly-expressed BBB protein were detected in each membrane sample. Each individual lane in this western blot represents microvessel membrane sample prepared from a single experimental animal. Equal protein in each lane was determined using sodium-potassium ATPase (i.e., Na+/K+ ATPase; predicted molecular weight = 113 kDa) as a loading control. As shown in Figure 4B, higher expression of Glut-1 at the BBB was observed in male rats as compared to their female counterparts. No difference in expression of Na+/K+ ATPase was observed between male and female animals. For this densitometric analysis, bands were quantitated using ImageJ software.
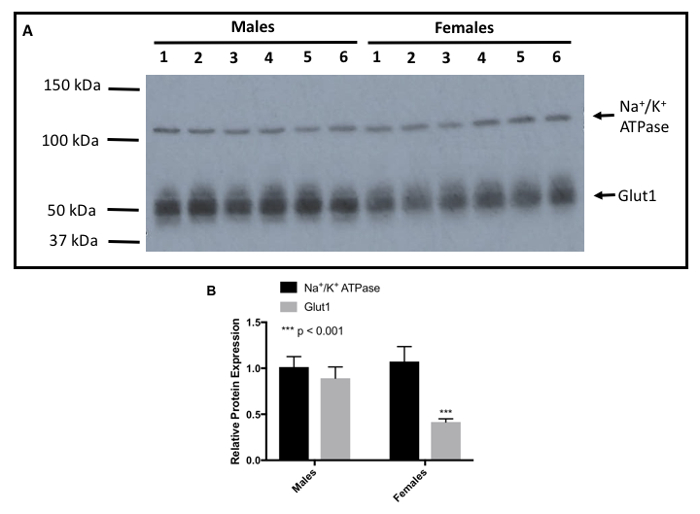
Figure 1: Outline of procedures and protocols to isolate rat brain microvessels and to prepare membrane samples. Please click here to view a larger version of this figure.
Figure 2: Representative data showing intact microvessel and expression of a vascular endothelial cell marker protein.A: Immunofluorescence staining showed localization of Oatp1a4, a transporter that has been shown to be well expressed at the BBB, in an intact brain microvessel isolated as described in this protocol. Oatp1a4 localization was detected using a specific rabbit polyclonal antibody against Oatp1a4 at a dilution of 1:10. The secondary antibody was a fluorescent conjugated anti-rabbit IgG that was used at a dilution of 1:300. Scale bar = 7.5 μm. B: Western blot analysis demonstrated the purity of microvessel samples by showing enrichment of the specific endothelial cell marker protein PECAM-1, which was detected using a mouse monoclonal antibody at a 1:100 dilution. The secondary antibody was an anti-mouse IgG at a 1:50,000 dilution. BH = brain homogenate, MV = crude microvessel fraction, MVM = brain microvessel membranes. Please click here to view a larger version of this figure.
Figure 3: A Representative standard curve for the Bradford Protein Assay. Bovine serum albumin (BSA) was used as a standard and was diluted to the following concentrations: 0, 0.125, 0.25, 0.50, 0.75, 1.0, 1.5, and 2.0 mg/mL. Absorbance was measured at = 595 nm. Each data point represents an average of three absorbance readings per BSA concentration. Please click here to view a larger version of this figure.
Figure 4: Representative western blot showing Sex Differences in Glut-1 Expression in Membrane Samples Prepared from Isolated Rat Brain Microvessels. A: Western blot analysis shows expression of Glut-1 in membrane samples prepared from microvessel isolated from male and female Sprague-Dawley rats. Glut-1 was detected using a rabbit monoclonal antibody at a 1:500 dilution. The secondary antibody was a monoclonal anti-rabbit IgG at a 1:40,000 dilution. Na=/K+ ATPase, a plasma membrane marker, and the loading control, was detected using a polyclonal anti-rabbit IgG at a 1: 40,000 dilutions. B: Densitometric analysis of Glut-1 expression in membrane samples isolated from male and female Sprague-Dawley rats. Results are expressed as mean SEM from 6 experimental animals per treatment group. Asterisks indicate data points that are statistically significant from control. A value of p < 0.05 was considered to be statistically significant. Please click here to view a larger version of this figure.
| Animal | Average Absorbance | Adjusted Absorbance | Calculated Concentration | Actual Concentration |
| (λ = 595 nm) | (mg/ml) | (mg/ml) | ||
| Male 1 | 0.7967 | 0.3859 | 0.768 | 7.68 |
| Male 2 | 0.7849 | 0.3741 | 0.7417 | 7.42 |
| Male 3 | 0.8187 | 0.4079 | 0.8173 | 8.17 |
| Male 4 | 0.7985 | 0.3877 | 0.7721 | 7.72 |
| Male 5 | 0.7943 | 0.3835 | 0.7628 | 7.63 |
| Male 6 | 0.7251 | 0.3143 | 0.6084 | 6.08 |
| Female 1 | 0.7114 | 0.3006 | 0.578 | 5.78 |
| Female 2 | 0.7993 | 0.3885 | 0.7738 | 7.74 |
| Female 3 | 0.8201 | 0.4093 | 0.8203 | 8.2 |
| Female 4 | 0.7526 | 0.3418 | 0.6698 | 6.7 |
| Female 5 | 0.8008 | 0.39 | 0.7773 | 7.77 |
| Female 6 | 0.7975 | 0.3867 | 0.77 | 7.7 |
Table 1: Representative Protein Concentrations from Brain Microvessel Preparations Derived from Male and Female Sprague-Dawley Rats. Adjusted absorbance values are determined by subtracting background absorbance at l = 595 nm from average absorbance values for each experimental animal. In this assay, background absorbance was determined to be 0.4108.
Discussion
In this article, a simple and effective method of preparing membrane protein samples from microvessels freshly isolated from rat brain tissue is described. Several approaches for isolation of rat brain microvessels and/or generation of membrane preparations from isolated microvasculature have been reported in the literature13,20,21,22,24. Although the microvessel isolation protocol described above is similar in principle, this approach has been optimized to enable preparation of membrane samples with excellent protein yield from a single experimental animal. This advance has eliminated the need to pool microvessels from at least three Sprague-Dawley rats in order to obtain samples with satisfactory protein enrichment for use in various approaches for protein analysis. Such approaches include, but are not limited to, western blot analysis, dot blots, and co-immunoprecipitation. It should be noted that the practice of pooling microvessels from multiple experimental animals to generate a single sample greatly reduces inter-group variability. To this extent, microvessel pooling can result in data with the reduced spread that does not represent true variability in protein expression observed in a population of experimental animals. Since samples can be prepared from single experimental animals, the true variability of a study population is captured, which allows for experimental data to be obtained that is more representative of the pathophysiological or pharmacological process under examination. An additional benefit is that microvessel membrane samples can be prepared using a reduced number of experimental animals.
Perhaps the most critical consideration in the execution of this protocol involves the centrifugation steps. Indeed, centrifuges can differ between laboratories due to their age, manufacturer, and/or overall condition and integrity. This can lead to significant variations in the actual g level (or rpm level) from spin to spin. The role of this variation in the sample integrity can be minimized by centrifugation of all samples in a single comparative analysis together in the same rotor at the same time. For example, if a rotor has only twelve available slots for sample tubes, then the isolation and preparation of microvessel samples in an individual experiment will be limited to twelve. This consideration ensures that prepared samples are of comparable quality and that any variability in mean protein expression between experimental groups can be assigned to the treatment condition itself.
An additional consideration involves the preparation of the 26% dextran solution, which is required for separation of brain microvessels and brain parenchymal tissue via differential centrifugation. Dextran is a glucose polymer with a prevalence of α-1,6-linked units and typically possesses a linear structure25. It also has a high water-binding capacity. For example, 1 g of dextran 75 (i.e., dextran with a mean molecular weight of 75,000 Da as is used in this protocol) retains approximately 20-25 mL of water. This property of dextran can lead to profound challenges in dissolving the powder into the aqueous buffer. Aqueous solubility can be improved by heating the dextran solution to a maximum temperature of 40 °C. Upon heating, the polymer interacts less with water molecules, thereby causing dextran to adopt a less expanded conformation (i.e., lower degree of water retention) in solution. Heating to higher temperatures will cause the dextran polymer to break down and, therefore, it will be ineffective as a separating reagent. Additionally, it is essential that the pH of the dextran solution be adjusted to 7.4 immediately before use. Solutions of 26% dextran can experience significant pH shifts, even over 1-2 hours following preparation. Due to these considerations, the 26% dextran solution must be prepared on the day of the experiment but with sufficient time prior to isolation of brain tissue to allow for dissolution and appropriate adjustment of pH.
A limitation of this protocol involves the presence of other cell types of the neurovascular unit (NVU) in microvessel preparations. In addition to endothelial cells, the NVU is comprised of various cellular components including glial cells (i.e., astrocytes, microglia), pericytes, and neurons 3,26,27. Isolation of microvessels from rodent brain typically shows enrichment with vascular endothelial cells; however, expression of neuronal marker proteins as well as expression of the glial fibrillary acidic protein (GFAP), an established astrocyte marker, is also observed. Additionally, pericytes are present in sample preparations due to their intimate association with the vascular endothelium. At present, it is virtually impossible to isolate brain microvessels from experimental animals and eliminate all pericytes, neurons, and astrocytes. However, this protocol has been advanced via multiple centrifugation steps so that the samples are enriched in endothelial cells (i.e., as indicated by improved expression of PECAM-1 following each centrifugation step) with limited presence of other cell types (i.e., as indicated by reduced expression of the neuronal marker protein synaptophysin-1 following each centrifugation step).
Overall, this approach for isolation of rat brain microvessels and for the preparation of membrane samples can be adapted in any laboratory with an interest in studying BBB proteins under pathophysiological or pharmacological conditions. For example, this protocol provides microvessel samples that can be utilized for the study of drug transport proteins that are endogenously expressed at the BBB2,18. The robustness of this approach has enabled the observation of discrete changes in Oatp1a4 in response to pharmacological stimuli, experimental data that has informed design of rigorous studies to assess transporter function and regulation at the BBB. The protocol described above can also be applied to protein analyses of tight junction proteins and adherens junction proteins in an effort to understand the BBB under physiological conditions and to study changes in BBB integrity in response to pathophysiological and pharmacological stressors. Clearly, this protocol can be adapted for multiple applications in the BBB field and, therefore, represents a simple yet robust and reproducible method that can be incorporated into BBB studies requiring protein analysis.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-NS084941) and the Arizona Biomedical Research Commission (ADHS16-162406) to PTR. WA has received past support from a pre-doctoral appointment to a National Institutes of Health Training Grant (T32-HL007249).
References
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77(3):731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Brzica H, Abdullahi W, Ibbotson K, Ronaldson PT. Role of Transporters in Central Nervous System Drug Delivery and Blood-Brain Barrier Protection: Relevance to Treatment of Stroke. J Cent Nerv Syst Dis. 2017;9:1179573517693802. doi: 10.1177/1179573517693802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. Targeting transporters: promoting blood-brain barrier repair in response to oxidative stress injury. Brain Res. 2015;1623:39–52. doi: 10.1016/j.brainres.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285(6):H2820–H2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, et al. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem. 2009;110(1):58–71. doi: 10.1111/j.1471-4159.2009.06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30(9):1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke-Wold BP, et al. Bryostatin-1 Restores Blood Brain Barrier Integrity following Blast-Induced Traumatic Brain Injury. Mol Neurobiol. 2015;52(3):1119–1134. doi: 10.1007/s12035-014-8902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CR, Ocheltree SM, Hom S, Egleton RD, Davis TP. Nociceptive inhibition prevents inflammatory pain induced changes in the blood-brain barrier. Brain Res. 2008. pp. 6–13. [DOI] [PMC free article] [PubMed]
- Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab. 2009;29(6):1084–1098. doi: 10.1038/jcbfm.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102(5):1677–1690. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Finch JD, Demarco KM, Quigley CE, Davis TP. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharmacol Exp Ther. 2011;336(3):827–839. doi: 10.1124/jpet.110.174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop V, et al. Early brain injury alters the blood-brain barrier phenotype in parallel with beta-amyloid and cognitive changes in adulthood. J Cereb Blood Flow Metab. 2013;33(2):205–214. doi: 10.1038/jcbfm.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, et al. Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood-brain barrier: relevance to CNS drug delivery. J Cereb Blood Flow Metab. 2014;34(4):699–707. doi: 10.1038/jcbfm.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome ME, et al. P-glycoprotein traffics from the nucleus to the plasma membrane in rat brain endothelium during inflammatory pain. J Cereb Blood Flow Metab. 2016;36(11):1913–1928. doi: 10.1177/0271678X16661728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosky LM, et al. Acetaminophen modulates P-glycoprotein functional expression at the blood-brain barrier by a constitutive androstane receptor-dependent mechanism. Mol Pharmacol. 2013;84(5):774–786. doi: 10.1124/mol.113.086298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artus C, et al. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab. 2014;34(3):433–440. doi: 10.1038/jcbfm.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, et al. Long-term exposure to ethanol downregulates tight junction proteins through the protein kinase Calpha signaling pathway in human cerebral microvascular endothelial cells. Exp Ther Med. 2017;14(5):4789–4796. doi: 10.3892/etm.2017.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullahi W, Brzica H, Ibbotson K, Davis TP, Ronaldson PT. Bone morphogenetic protein-9 increases the functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier via the activin receptor-like kinase-1 receptor. J Cereb Blood Flow Metab. 2017;37(7):2340–2345. doi: 10.1177/0271678X17702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesev EV, Miller DS, Cannon RE. Ceramide 1-Phosphate Increases P-Glycoprotein Transport Activity at the Blood-Brain Barrier via Prostaglandin E2 Signaling. Mol Pharmacol. 2017;91(4):373–382. doi: 10.1124/mol.116.107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz AL, Csejtey J, Goldstein GW. Hexose transport and phosphorylation by capillaries isolated from rat brain. Am J Physiol. 1979;236(1):C96–C102. doi: 10.1152/ajpcell.1979.236.1.C96. [DOI] [PubMed] [Google Scholar]
- Yousif S, Marie-Claire C, Roux F, Scherrmann JM, Decleves X. Expression of drug transporters at the blood-brain barrier using an optimized isolated rat brain microvessel strategy. Brain Res. 2007;1134(1):1–11. doi: 10.1016/j.brainres.2006.11.089. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, et al. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem. 2007;103(6):2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- Brzica H, et al. The liver and kidney expression of sulfate anion transporter sat-1 in rats exhibits male-dominant gender differences. Pflugers Arch. 2009;457(6):1381–1392. doi: 10.1007/s00424-008-0611-5. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J Neurochem. 2008;106(3):1298–1313. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- Pustylnikov S, Sagar D, Jain P, Khan ZK. Targeting the C-type lectins-mediated host-pathogen interactions with dextran. J Pharm Pharm Sci. 2014;17(3):371–392. doi: 10.18433/j3n590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullahi W, Davis TP, Ronaldson PT. Functional Expression of P-glycoprotein and Organic Anion Transporting Polypeptides at the Blood-Brain Barrier: Understanding Transport Mechanisms for Improved CNS Drug Delivery? AAPS J. 2017;19(4):931–939. doi: 10.1208/s12248-017-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


