Abstract
Vascular insufficiency and alterations in normal retinal perfusion are among the major factors for the pathogenesis of various sight-threatening ocular diseases, such as diabetic retinopathy, hypertensive retinopathy, and possibly glaucoma. Therefore, retinal microvascular preparations are pivotal tools for physiological and pharmacological studies to delineate the underlying pathophysiological mechanisms and to design therapies for the diseases. Despite the wide use of mouse models in ophthalmic research, studies on retinal vascular reactivity are scarce in this species. A major reason for this discrepancy is the challenging isolation procedures owing to the small size of these retinal blood vessels, which is ~ ≤ 30 µm in luminal diameter. To circumvent the problem of direct isolation of these retinal microvessels for functional studies, we established an isolation and preparation technique that enables ex vivo studies of mouse retinal vasoactivity under near-physiological conditions. Although the present experimental preparations will specifically refer to the mouse retinal arterioles, this methodology can readily be employed to microvessels from rats.
Keywords: Medicine, Issue 135, Retina, Mouse, Blood Vessel, Microvessel Preparation, Ex Vivo, Vasoreactivity, Videomicroscopy
Introduction
Disturbances in retinal perfusion have been implicated in the pathogenesis of various ocular diseases, such as diabetic retinopathy, hypertensive retinopathy, and glaucoma1,2,3. Thus, studies aimed at measuring vascular reactivity in the retina are important to understand the pathophysiology of these diseases and to develop new treatment approaches.
Due to the possibility of gene manipulation in the murine genome, the mouse has become a widely used animal model for studies of the cardiovascular system4. However, because of the small size of retinal blood vessels (≤ 30 µm), measurement of vascular reactivity in the mouse retina is challenging. For example, stereomicroscopic techniques for in vivo measurement are limited in their optical resolution and therefore only allow to exactly detect changes in diameter or blood flow in small blood of less than ≤ 30 µm diameter when equipped with additional sophisticated devices, such as a confocal microscope using fluorescent dyes or the Adaptive Optics Scanning Light Ophthalmoscope5,6. Moreover, the interpretation of in vivo measurements aimed at identifying local signaling mechanisms in retinal blood vessels can be confounded by anaesthetics, changes in systemic blood pressure and the influence of retrobulbar blood vessels.
Therefore, we developed a method to measure responses of mouse retinal blood vessels with high optic resolution ex vivo. The technique presented herein allows visualization of retinal arterioles via transmitted light microscopy. This method, which can also be used in rats, provides access to the advantages of gene targeting technology in ocular vascular research.
Protocol
The experimental procedures of this study were approved by the Animal Care Committee of Rhineland-Palatinate, Germany. Animal care conformed to the institutional guidelines and The Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research. Animals were treated according to the EU Directive 2010/63/EU for animal experiments. Male C57BL/6J mice (The Jackson Laboratory, Bar Harbour, ME, USA) aged 3-4 months were used for the experiments. Animals were housed under standard conditions (temperature 23 ± 2 °C, humidity range 55 ± 10% and 12 h light/dark cycles), and had access to standard mouse chow and water ad libitum.
1. Isolation of Mouse Retina
Place the dissection instruments on the preparation table and check if all instruments are complete to enable a quick preparation.
Sacrifice the mouse by CO2 inhalation.
Decapitate the mouse with steel scissors, cut the skull sagitally into two halves using the same pair of scissors and remove the skin and brain using eye scissors (Figure 1).
Cut off the skull bone to leave only the orbital bone by using eye scissors.
Transfer the orbit into a dissection dish containing ice-cold Krebs-Henseleit buffer.
Keep the dissection dish on ice or change the buffer every 10 min.
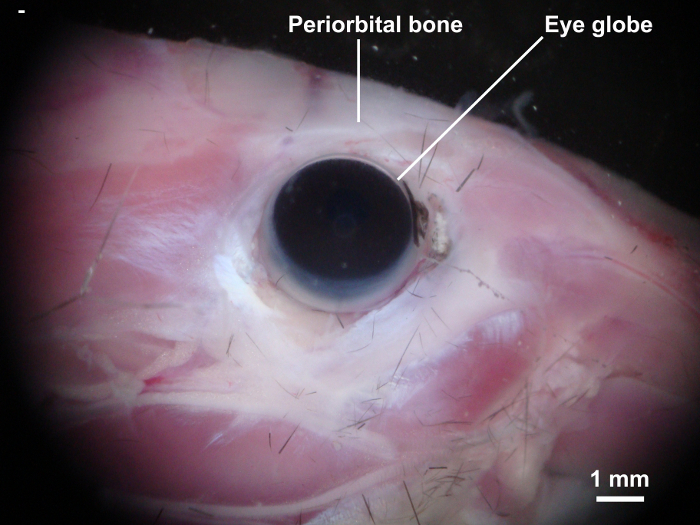
Cut the orbital bone using eye scissors and gently remove the eye globe together with the orbital tissue using type 4 precision tweezers and Student Vannas spring scissors (Figure 2).
Gently remove the Harderian gland, connective tissue, and the extraocular muscles using type 5 precision tweezers and Vannas capsulotomy scissors, taking care not to damage the main branch of the ophthalmic artery.
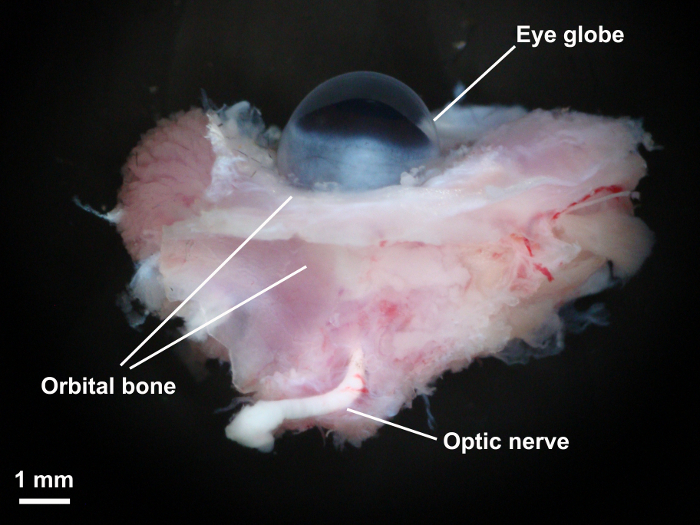
Ligate orbital branches of the ophthalmic artery and eventually the proximal end of the main branch of the ophthalmic artery using 10-0 Nylon sutures (Figure 3).
Transfer the eye globe into a Petri dish containing 70% ethanol for 10 s using type 4 precision tweezers. Place the eye globe back into the dissection dish and replace the buffer with fresh Krebs solution. The cornea will appear whitish after ethanol exposure .
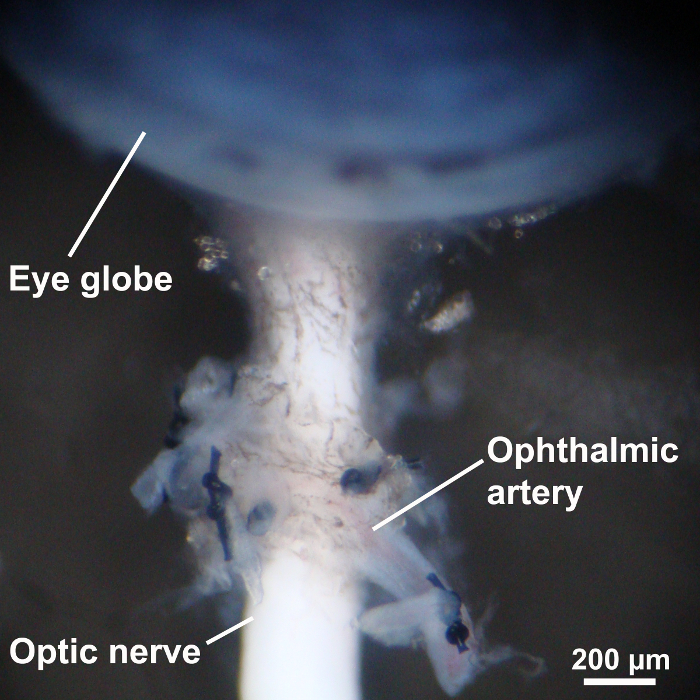
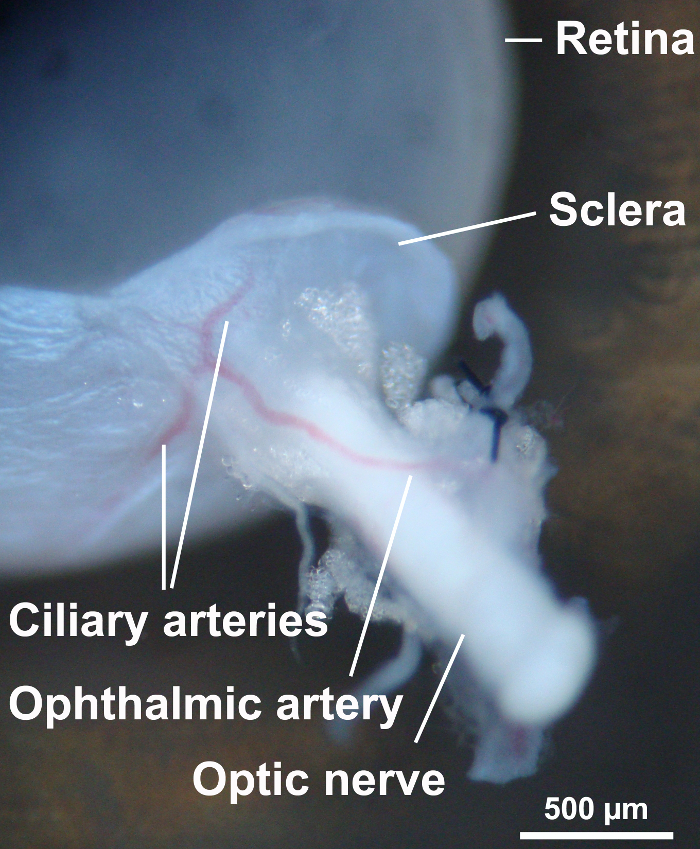
Puncture the peripheral cornea with a 30 gauge needle and dissect the cornea using Vannas capsulotomy scissors. Gently cut off the iris tissue, place one sharp point of the Vannas capsulotomy scissors between the choroid and retina, and cut off the choroid and sclera. Leave some scleral tissue around the optic nerve (Figure 4).
2. Mounting the Retina in the Perfusion Chamber
NOTE: The perfusion chamber used for retinal arteriole experiments is home-made. It consists of a transparent reservoir with an in- and outflow tube (Figure 5).One end of a silicone tube is glued to the bottom of the chamber with histoacryl adhesive and the other end attached to a three-way stopcock. Connect a syringe containing fresh Krebs buffer to the three-way stopcock and fill the tube with buffer.
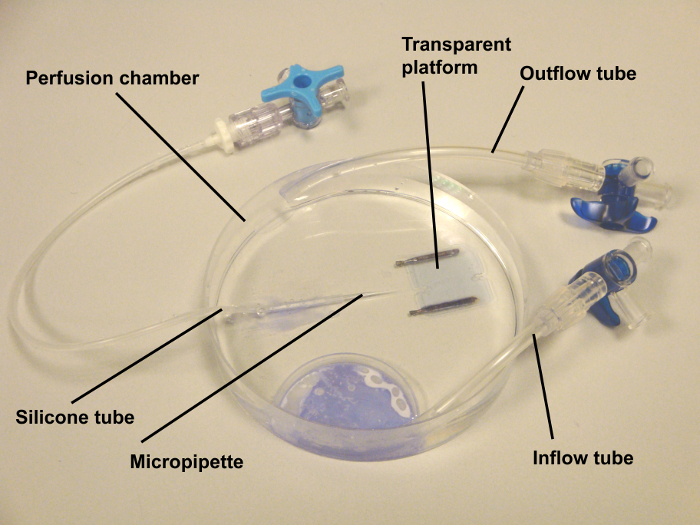
Take a glass micropipette with a needle holder and push it into the end of the tube at the bottom of the perfusion chamber. Then break the tip of the micropipette with type 4 scissors to obtain a tip of 100 µm diameter.
Focus on the tip of the micropipette and flush it via the syringe connected to the tube. Take care that the capillary is not occluded by debris. In case of occlusion, remove it, flush the tubing and insert another micropipette.
Fill the perfusion chamber with cold Krebs buffer and put the chamber under a stereo microscope.
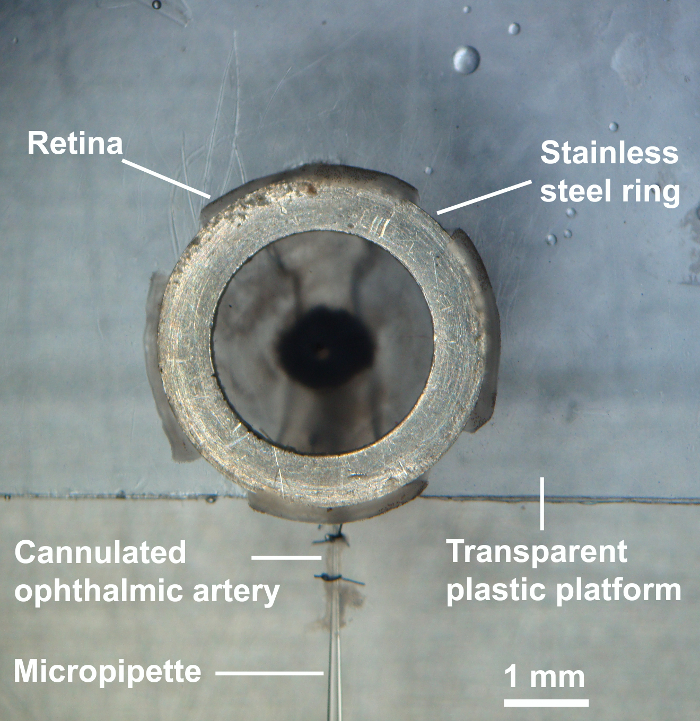
Place a transparent plastic platform into the perfusion chamber. This platform has an indentation of 1.8 mm width for the optic nerve and the ophthalmic artery and is placed on two steel wires of 1.6 mm diameter. Then, place a stainless-steel ring of 2.8 mm inner diameter and 4.0 mm outer diameter onto the platform.
Transfer the retina with the optic nerve and attached ophthalmic artery from the dissection chamber into the perfusion chamber with extreme care using a spoon. This step is important to prevent potential stretching of the tissues as it may be detrimental to the experiment.
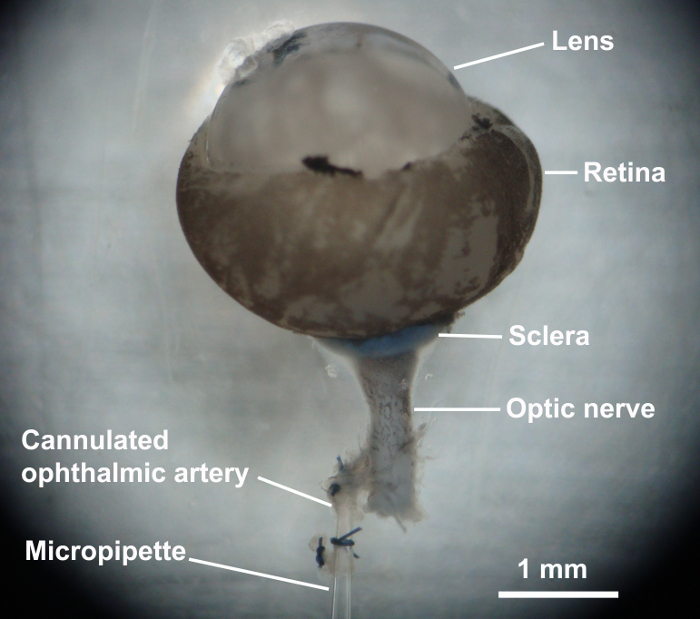
Cut off the sutured part of the proximal end of the ophthalmic artery, place two preformed loops of 10-0 nylon suture onto the micropipette, and cannulate the ophthalmic artery with the micropipette. Tie the artery to the micropipette (Figure 6) and place the retina onto the transparent platform using fine-point-tweezers.
Gently tear open the anterior lens capsula with two fine point tweezers, pull out the lens, and cut off the whole lens capsula using microscissors.
Subsequently, make four radial incisions into the retina half the distance from the pars plana to the optic nerve and place a stainless-steel ring onto the retina to fix it to the bottom. The retina is now ready for the experiment (Figure 7).
3. Preparation of Retinal Arterioles for the Experiment
Place the perfusion chamber under a light microscope. The objective is a 100X water-immersion objective lens.
Connect the two tubings for in- and out-flow to a pericyclic pump and circulate the chamber with Krebs buffer oxygenated with 95% O2 and carbonated with 5% CO2 at 37 °C. Use a flow rate between 100 and 120 mL/min.
Connect the silicone tube, which is connected to the micropipette, to a reservoir silicone hose via a three-way stopcock. Then, fill the reservoir with Krebs buffer to a height corresponding to 50 mm Hg but do not open the three-way stopcock yet.
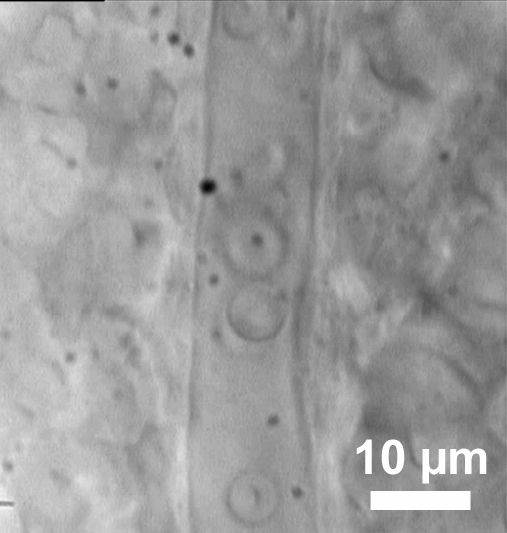
Look through the objectives of the microscope and focus on the retinal surface. Search for blood vessels or red blood cells. It is possible that the blood vessels are completely collapsed, so sometimes only some red blood cells are visible.
When a blood vessel is found, focus on it and open the three-way stopcock. The diameter of the vessel should increase immediately and the red blood cells move either centrifugally, if it is an arteriole, or centripetally in case of a venule. The arterioles are smaller in diameter compared to venules of the same order.
Once a blood vessel with a well visible wall is found, it can be employed for experiments following an equilibration period of 30 min (Figure 8). During the equilibration period, the arterioles develop only weak intrinsic tone resulting in less than 10% of luminal diameter reduction.
4. Performing the Experiment
Test the viability of vessels with the thromboxane mimetic 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U46619) by adding it to the bath solution. This agent constricts mouse retinal arterioles by about 50% from resting diameter at concentrations of ≥ 10-6 M.
Once the vessel is confirmed viable, wash the agonist out of the bath by circulating the pump and supply fresh Krebs buffer into the bath.
Representative Results
U-46619 produced concentration-dependent vasoconstrictor responses in retinal arterioles from wild-type mice of the C57Bl/6J background. At a concentration of 10-6 M, reduction in luminal diameter was ≈50% from resting diameter. Figure 9A shows a representative concentration-response curve of one retinal arteriole. In arterioles preconstricted with U46619, cumulative administration of acetylcholine evoked concentration-dependent increases in luminal diameter to ≈25% from the preconstricted diameter at 10-5 M, indicative of an intact vascular endothelium (Figure 9B).
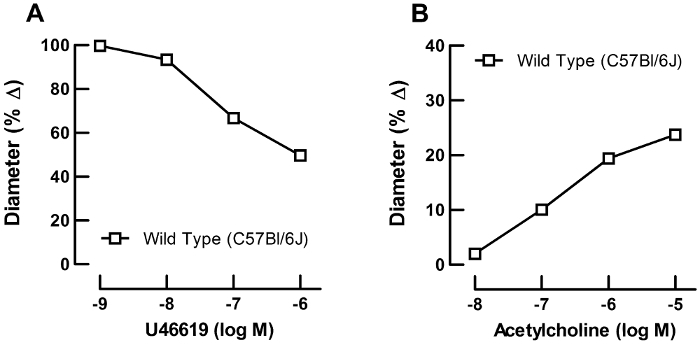
Figure 1: Dissection of the mouse skull. Skin on the decapitated mouse head is removed to expose the eyes and orbital cavity. Please click here to view a larger version of this figure.
Figure 2: Isolation of eye globe and orbital tissue. The orbital bone was cut with eye scissors and the eye globe together with the retrobulbar tissues and optic nerve were carefully isolated. Please click here to view a larger version of this figure.
Figure 3: The eye globe and preparation of the ophthalmic artery. Once the surrounding orbital tissues were dissected with fine microscissors, the ophthalmic artery was carefully exposed and their small branches ligated with 10-0 nylon monofilament sutures. Please click here to view a larger version of this figure.
Figure 4: Dissection of ocular structures. To visualize the retina, the sclera and uvea were dissected with Vannas capsulotomy scissors. Some scleral tissue around the optic nerve was left to avoid damage of the ophthalmic artery. Please click here to view a larger version of this figure.
Figure 5: Organ chamber for real-time video microscopy. The chamber was home made. It consisted of a Petri dish of 100 mm diameter with an in- and out-flow tube. The tubes were glued with histoacryl adhesive. Externally oxygenated and carbonated Krebs-Henseleit buffer was circulated by a peristaltic pump via these tubes. One end of a silicone tube was glued to the bottom of the chamber and the other end attached to a three-way stopcock. At the end of the tube, a glass micropipette with a tip of 100 µm was inserted, which served for cannulation of the ophthalmic artery. Via the silicone tube, the ophthalmic circulation was pressurized by filling a silicone tube with Krebs buffer to a level corresponding to 50 mm Hg. Please click here to view a larger version of this figure.
Figure 6: Cannulation of the ophthalmic artery. The ophthalmic artery was cannulated with glass micropipette and sutured with a 10-0 nylon suture. Please click here to view a larger version of this figure.
Figure 7: Cannulation of the ophthalmic artery. After placing the retina onto the transparent platform, the lens with the capsular bag was removed, four radial incisions were made into the retina, and a stainless steel of ring of 2.8 mm inner diameter and 4.0 mm outer diameter was placed onto the retina to fix it to the bottom. The retina was then ready for the experiment. Please click here to view a larger version of this figure.
Figure 8: Visualization of retinal blood vessels. An exemplary retinal arteriole with red blood cells inside.
Figure 9: Functional studies using video microscopy. Representative concentration response curves from a single retinal arteriole. (A) Concentration-response curve for the vasoconstrictor, U46619 (10-9 to 10-6 M), and (B) for the endothelium-dependent vasodilator, acetylcholine (10-8 to 10-5 M). Please click here to view a larger version of this figure.
Discussion
The measurement of vascular responses in the mouse retina is challenging due to the small size of retinal blood vessels. With the presented technique, retinal arterioles are visualized by transmitted light microscopy. This is possible, because the isolated retina is translucent. The advantage of the technique is the high optical resolution. The calculated spatial resolution is 11 px/µm. However, the real resolution for this optical system that uses white light is between 200 and 300 nm, which is explained by the Abbe diffraction limit. Since the first branch of mouse retinal arterioles has an internal diameter of 20 to 30 µm, diameter changes of approximately 1% are detectable with this system. There is no need of additional technical devices or fluorescent dyes as reported in other studies to visualize small retinal arterioles5,6,7. A disadvantage of the technique is the long preparation time, which is between 90 to 120 min by trained investigators. If the preparation time exceeds 180 min, the endothelial function starts to be attenuated.
There are several major critical steps in this technique. First, it is important to thoroughly ligate all retrobulbar arterial branches. If ligation is incomplete, retinal arterioles will not be pressurized due to leakage. Second, the immersion in ethanol for 10 s before opening the eye globe is important to deactivate the smooth muscle reactivity of the ophthalmic artery. We previously demonstrated that immersing for 10 s in 70% ethanol completely deactivates the ophthalmic artery smooth muscle8. In contrast, retinal arterioles are unlikely to be directly affected by ethanol, since they are protected by the bulbar wall. If this step is omitted, diameter changes in the ophthalmic artery may influence the perfusion of the retina. Of note, reactivity of the ophthalmic artery may markedly differ from that of retinal arterioles in response to the same agonist9,10. Third, avoid torsion of the ophthalmic artery. Especially while mounting the retinal preparation onto the platform, the ophthalmic artery may become twisted, resulting in occlusion of the lumen. Also, check carefully that the tip of the glass capillary is not occluded to enable pressurization of retinal arterioles. We suggest not to perform more than three consecutive concentration-response curves in the same retinal preparation, because we observed that responses to acetylcholine became weaker when repeating the concentration-response curves for more than three times. However, there may be differences regarding the repeatability of concentration-response curves depending on the agent applied and, thus, should be tested individually.
We applied pharmacological agents extraluminally in our studies, although intraluminal perfusion using a servo control pump is also possible. Since the technique allows to measure for local mechanisms of vascular reactivity in the retina of small laboratory animals including mice and rats, the advantages of gene-targeting technology by using genetically modified animals can be accessed with this method.
Disclosures
The authors have no competing financial interests.
Acknowledgments
This work was supported by grants from the Ernst und Berta Grimmke Stiftung and the Deutsche Ophthalmologische Gesellschaft (DOG).
References
- Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007;26(3):205–238. doi: 10.1016/j.preteyeres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Schuster AK, Fischer JE, Vossmerbaeumer C, Vossmerbaeumer U. Optical coherence tomography-based retinal vessel analysis for the evaluation of hypertensive vasculopathy. Acta Ophthalmol. 2015;93(2):e148–e153. doi: 10.1111/aos.12509. [DOI] [PubMed] [Google Scholar]
- Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol. 2013;13(1):36–42. doi: 10.1016/j.coph.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Sigmund CD. Vascular biology in genetically altered mice : smaller vessels, bigger insight. Circ Res. 1999;85(12):1214–1225. doi: 10.1161/01.res.85.12.1214. [DOI] [PubMed] [Google Scholar]
- Kornfield TE, Newman EA. Measurement of Retinal Blood Flow Using Fluorescently Labeled Red Blood Cells. eNeuro. 2015;2(2) doi: 10.1523/ENEURO.0005-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara-Torres A, Joseph A, Schallek JB. Label free measurement of retinal blood cell flux, velocity, hematocrit and capillary width in the living mouse eye. Biomed Opt Express. 2016;7(10):4229–4249. doi: 10.1364/BOE.7.004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallek J, Geng Y, Nguyen H, Williams DR. Morphology and topography of retinal pericytes in the living mouse retina using in vivo adaptive optics imaging and ex vivo characterization. Invest Ophthalmol Vis Sci. 2013;54(13):8237–8250. doi: 10.1167/iovs.13-12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke A, et al. Identification of the muscarinic acetylcholine receptor subtype mediating cholinergic vasodilation in murine retinal arterioles. Invest Ophthalmol Vis Sci. 2011;52(10):7479–7484. doi: 10.1167/iovs.11-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke A, et al. Functional role of alpha1-adrenoceptor subtypes in murine ophthalmic arteries. Invest Ophthalmol Vis Sci. 2011;52(7):4795–4799. doi: 10.1167/iovs.11-7516. [DOI] [PubMed] [Google Scholar]
- Bohmer T, et al. The alpha(1)B -adrenoceptor subtype mediates adrenergic vasoconstriction in mouse retinal arterioles with damaged endothelium. Br J Pharmacol. 2014;171(16):3858–3867. doi: 10.1111/bph.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]


