Abstract
The detection of insulin secretion is critical for elucidating mechanisms of regulated secretion as well as in studies of metabolism. Though numerous insulin assays have existed for decades, the recent advent of homogeneous time-resolved Förster Resonance Energy Transfer (HTRF) technology has significantly simplified these measurements. This is a rapid, cost-effective, reproducible, and robust optical assay reliant upon antibodies conjugated to bright fluorophores with long lasting emission which facilitates time-resolved Förster Resonance Energy Transfer. Moreover, HTRF insulin detection is amenable for the development of high-throughput screening assays. Here we use HTRF to detect insulin secretion in INS-1E cells, a rat insulinoma-derived cell line. This allows us to estimate basal levels of insulin and their changes in response to glucose stimulation. In addition, we use this insulin detection system to confirm the role of dopamine as a negative regulator of glucose-stimulated insulin secretion (GSIS). In a similar manner, other dopamine D2-like receptor agonists, quinpirole, and bromocriptine, reduce GSIS in a concentration-dependent manner. Our results highlight the utility of the HTRF insulin assay format in determining the role of numerous drugs in GSIS and their pharmacological profiles.
Keywords: Medicine, Issue 135, Endocrine, beta cell, Homogeneous time resolved FRET (HTRF), antibodies, insulin, glucose stimulated insulin secretion, dopamine, quinpirole, bromocriptine
Introduction
The regulation of energy metabolism is fine-tuned by a major anabolic hormone, insulin. Insulin is synthesized and released by pancreatic beta cells in response to increased extracellular glucose levels. The released insulin triggers the uptake of glucose by insulin-sensitive tissues1,2. Physiologically, this is linked to the elevation of glucose concentration after a meal, followed by secretion of insulin to regulate glucose uptake. Disturbances in glucose homeostasis lead to metabolic impairments culminating in insulin resistance and ultimately in the onset of type 2 diabetes2,3,4.
Although insulin secretion has been extensively studied, its regulatory mechanisms remain poorly understood. A critical area of investigation has been identification of novel modulators of insulin secretion by beta cells5,6,7,8. These studies require a better understanding of the coupling relationship between glucose stimulation and insulin secretion. Therefore, the ability to accurately monitor and quantify the levels of glucose-stimulated insulin secretion (GSIS) has been essential. To date, however, only a limited number of methods were available to allow quantification of GSIS using insulin-secreting cell lines and/or pancreatic islets. One is radioimmunoassay (RIA), which utilizes radioisotope-tagged insulin and antibodies. The main limitations of this approach include safety issues due to the handling and disposal of radioactive materials. Additionally, this method is labor-intensive, involving multiple long washing and incubation steps. Enzyme-linked immunosorbent assay (ELISA) is another costly and labor-intensive approach that utilizes antibodies for insulin detection. Variation in antibody affinities and in the efficiency of recognizing insulin are limiting factors of this method and can affect the reproducibility of the results. Neither ELISA nor RIA was designed for high-throughput experiments. AlphaScreen is a homogeneous assay used for detecting and measuring levels of insulin secretion. AlphaScreen technology is based on the conversion of ambient oxygen into an excited oxygen singlet state that can react with chemiluminescent species, resulting in the generation of chemiluminescence. Because the assay is homogenous, many of the washing steps associated with RIA and ELISA are eliminated. However, the instability of the signal due to the nature of the reaction is a limiting factor that may affect the readout of the assay. (TR-PINCER, developed by Heyduk and colleagues9, is another homogenous approach to insulin measurement based on the binding of two separate antibodies to different epitopes on the insulin molecule. The antibodies are each chemically linked to double stranded DNA with short complementary single stranded DNA overhangs. Binding of the antibodies to insulin brings them together and leads to a double stranded DNA duplex. Each antibody is also associated with a respective donor or acceptor fluorophore, and the association of the DNA duplex brings together these fluorophores to generate Förster resonance energy transfer (FRET). One potential limitation of TR-PINCER, however, rests with the FRET itself. The inability to rapidly dissipate background fluorescence during the FRET reaction may lead to relatively high levels of background fluorescence and a low signal to noise ratio within the assay. Therefore, a need still exists for a reliable, robust, and cost-effective assay for quantifying GSIS in a high-throughput manner.
Recent advances in biophysics have culminated in the development of a homogeneous time-resolved fluorescence energy resonance transfer (HTRF) based assay. Specifically, while the energy transfer within the assay may be described as FRET-based, more accurately, HTRF relies on luminescence energy resonance transfer (LRET)10 which is the non-radiative transfer of energy between the donor and acceptor species11,12,13. This distinction is important, since the timing of a fluorescence or quenching based FRET interaction is much different than it is for LRET, though the same types of gating can be used for FRET and LRET. Moreover, the use of rare earth lanthanide cryptate compounds such as europium or terbium cryptate in HTRF produces long fluorescence half-lives12,14. This offers the unique advantage of the introduction of a time delay (µsec) between donor excitation and the measurement of emission from the acceptor (i.e., time-resolved assay). This time delay allows for sufficient time for the background fluorescence to dissipate prior to measurement of acceptor emission fluorescence. Consequently, the readout is free of non-specific fluorescence and thus, a high signal-to-noise ratio is achieved (Figure 1). Furthermore, the homogenous nature of HTRF eliminates the need for washing steps to wash off the unbound species, making the assay much more rapid than ELISA or RIA-based methods.
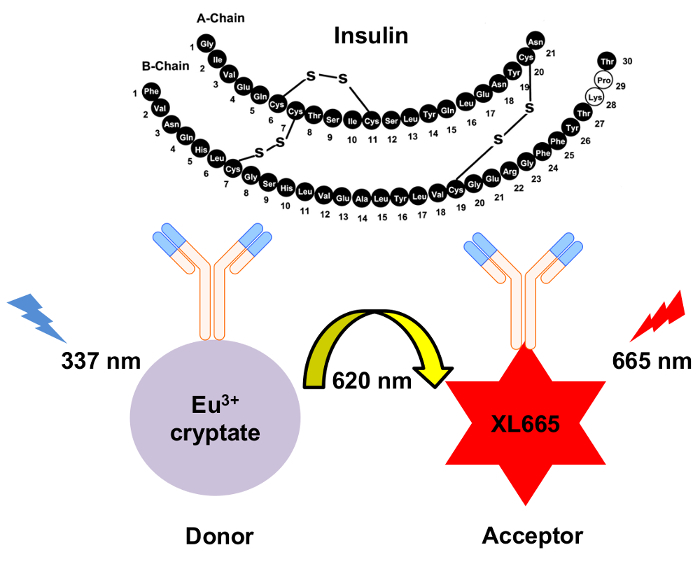
Figure 1: Schematic of the mechanism for HTRF insulin detection. Two independently generated monoclonal antibodies specifically recognize and bind to insulin at separate sites. These antibodies are conjugated to either the europium cryptate donor or the XL665 acceptor. Excitation of the donor at 337 nm results in emission at 620 nm. The resulting energy transfer causes XL665 to emit at a longer wavelength, 665 nm. Please click here to view a larger version of this figure.
Here, we provide a detailed protocol for using an HTRF-based approach to determine the levels of GSIS from INS-1E cells, a well-established insulin-secreting beta cell-derived rat insulinoma cell line15. In addition, this assay may be used for identifying the pharmacological profile of molecular regulators of insulin secretion. We apply this HTRF-based insulin assay to examine dopamine D2-like receptor regulation of GSIS. Increasing studies have revealed that the neurotransmitter dopamine is an important regulator of GSIS8,16,17,18,19,20,21,22. Dopamine affects GSIS in a negative autocrine/paracrine manner via actions on the dopamine D2-like receptors (D2, D3, D4 receptors) expressed at the surface of beta pancreatic cells8,16,19. Using this assay, we confirm dopamine's role as a negative regulator of GSIS and demonstrate that the dopamine D2-like receptor agonists bromocriptine and quinpirole also reduce GSIS.
Protocol
1. INS-1E Cells: Maintenance and Plating
Maintain INS-1E cells in a humidified 37 ˚C/5% CO2 incubator and cultured with RPMI 1640 medium supplemented with 5% (v/v) heat-inactivated fetal bovine serum, 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 100 U/mL Penicillin/Streptomycin solution, 50 µM β-mercaptoethanol. Culture cells in 10 mL complete RPMI 1640 medium (per plate), until they reach 80 - 90% confluence, when they can be trypsinized and passaged or used for the insulin secretion assay.
Day 1: Aspirate media and wash cells once with 5 mL of pre-warmed PBS. Add 0.5 mL of trypsin (0.025%) diluted 1:1 in 0.5 mL PBS to trypsinize the cells and incubate for 3 - 4 min at 37 ˚C. Deactivate trypsin by adding 9 mL complete media and transfer cells to a 15 mL centrifuge tube by pipetting.
Pellet cells by centrifugation and re-suspend the cell pellet in approximately 5 mL fresh media.
Take 10 µL of the re-suspended cells and mix with 10 µL Trypan Blue vital dye to check for cell viability. Count live and dead cells in 10 µL of this mixture using a hemocytometer. Viability level should be above 90%. Dilute the re-suspended cells in fresh media to 1 million cells per mL.
Seed 0.5 mL INS-1E cells per well in a poly-L-Lysine pre-coated 24-well plate, at a density of 500,000 cells/well.
Day 2: Remove media 18 - 24 h after plating and add 500 µL/well of fresh RPMI 1640 media. Incubate the cells for another 24 h to allow cells to fully recover from the prior passaging step. This additional time permits the cells to spread on the tissue culture plate.
2. Insulin Secretion Assay (Day 3)
Prepare KRB buffer: 132.2 mM NaCl, 3.6 mM KCl, 5 mM NaHCO3, 0.5 mM NaH2PO4, 0.5 mM MgCl2, 1.5 mM CaCl2, and 0.001 g/mL bovine serum albumin (BSA), pH 7.4.
Aspirate media from cells and wash twice with pre-warmed PBS.
For the glucose starvation step, add 450 µL/well KRB (containing BSA) without glucose for 1 h at 37 ˚C/5% CO2.
- During the glucose starvation step, prepare serial dilutions of the drug(s) in KRB containing 200 mM Glucose (10x concentration).
- Prepare drug at 10x the final concentration in KRB supplemented with 200 mM glucose (also 10x the final assay glucose concentration). If the drug stock (plus the added 10x glucose) is in DMSO, make sure DMSO percentage is kept consistent throughout the assay (ideally final percentage of less than 0.1% DMSO).
- For dopamine and quinpirole treatments, use a final assay drug concentration range of 100 µM to 100 pM (from highest to lowest concentration), with the last point of the dose response containing no drug. For bromocriptine, use a final assay concentration range of 10 µM to 10 pM, with the last point of the dose response being the drug-free control.
After glucose starvation, add drug serial dilutions to the assay to produce a dose response.
Add 50 µL/well of each serial dilution to the corresponding wells (total assay volume 500 µL).
For the glucose stimulation step, incubate cells with the respective drug serial dilutions (in the presence of 20 mM glucose) for 90 min at 37 ˚C/5% CO2. Include a set of control wells: (1) stimulation with 20 mM glucose alone in the absence of any additional drug and (2) cells that are neither stimulated with drug nor glucose (which provides a basal rate of secretion).
After the stimulation step, carefully remove the supernatants (use directly or store at 4 ˚C). NOTE: An additional 5 min gentle centrifugation step (600 x g, 1 min) may be introduced at this point to remove any remaining cells in the assay supernatants.
3. HTRF to Measure Insulin Secretion
Dilute assay supernatants 1:10 in KRB (without BSA), preferably in clear 96-well plates.
Prepare the insulin standard curve for the HTRF insulin assay (Table 1).
| Standard stock solution 500 ng/ml | Serial dilutions | Working [insulin] ng/ml |
| STD 7 | 30 µl Stock + 140 µl KRB | 150 |
| STD 6 | 30 µl STD 7 + 45 µl KRB | 60 |
| STD 5 | 30 µl STD 6 + 45 µl KRB | 24 |
| STD 4 | 30 µl STD 5 + 45 µl KRB | 9.6 |
| STD 3 | 30 µl STD 4 + 45 µl KRB | 3.84 |
| STD 2 | 30 µl STD 3 + 45 µl KRB | 1.54 |
| STD 1 | 30 µl STD 2 + 45 µl KRB | 0.61 |
| STD 0 | 45 µl KRB | 0 |
| Note: STD Stock is 500 ng/ml |
Table 1. Serial dilutions to make the insulin standard curve.
Add the standard curve samples and the diluted assay supernatants to the HTRF plate. The measurement of secreted insulin by HTRF can be carried out in either a 96-well or a 384-well plate format, keeping in mind that the assay volume has to be re-adjusted. Use 10 µL/well sample in 96-well white half-area plates or 5 µL/well in a 384-well white low-volume, round-bottom plate (see Table of Materials).
Prepare antibody mix in detection buffer (see Table of Materials) in a 1:2 donor (cryptate)/acceptor (XL-665) ratio. NOTE: Further specific details about the HTRF assay are available from the manufacturer.
Add antibody mix to the assay at 30 µL/well (for a 96 well-plate assay format) or 15 µL/well (for a 384-well plate assay format).
Seal the plate and incubate at room temperature.
Read plate after 2 h, 4 h, and/or overnight incubation with antibodies using the plate reader and the appropriate HTRF optic module (337 665 620 nm) (see Table of Materials and manufacturer's instructions). Set the integration start at 60 µs and the integration time at 400 µs. Use 200 flashes per well. NOTE: These parameters were based on the use of our particular reader. The readout at 620 nm and 665 nm may vary between different instruments. This is one of the reasons we suggest using the 665/620 ratio. In calculating this ratio, any potential differences from reader to reader will be normalized and provide consistent values irrespective of the instrument used to measure HTRF.
4. Data Analysis and Normalization
Calculate the insulin concentrations of the assay wells via extrapolation of ratiometric fluorescence readings (665 nm/620 nm) to a second order quadratic polynomial curve (Figure 2).
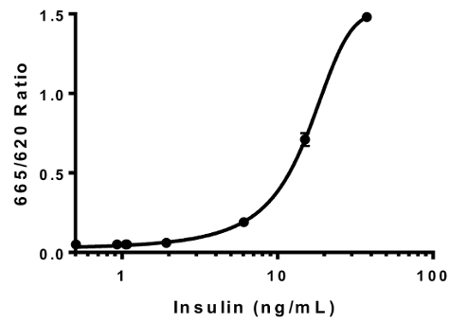
Figure 2: Insulin standard curve. Human insulin stock of known concentrations was used to generate the insulin standard curve. The resulting HTRF ratios (665 nm/ 620 nm) were plotted against the insulin concentrations. The data was best fit to a second order quadratic polynomial curve (R2 = 0.99996). This is a representative standard curve. Error bars = SEM. Please click here to view a larger version of this figure.
With the extrapolated data as ng/mL of insulin secreted, normalize (insulin secreted in response to increasing ligand concentrations) to the average value of the % maximum insulin secretion assay wells (20 mM glucose alone condition).
Use the curve fit (R2) from a single experiment to calculate the intraplate variation. Within the experiment, derived the individual R2 values from the intra-experimental duplicates, allowing calculation the standard error of the mean for the curve fit.
For determining the interplate variation, use the data from at least three individual experiments to calculate the standard error of the mean for the R2 value of the collective curve.
Representative Results
We validated our insulin HTRF assay by generating an insulin standard curve using purified human insulin standards of predefined concentrations (Figure 2). Generation of the standard curve permitted us to extrapolate the ratiometric fluorescence readings and thus to determine the secreted insulin levels in response to the drug treatments (Figure 2). Intraplate variation for curve fitting was minimal (R2 = 0.99996 ± 5.0 x 10-5) as was the interplate variation (R2 = 0.947 ± 5.5 x 10-5; n = 3 plates).
We next determined the basal levels of insulin secretion from INS-1E cells. Using a seeding density of 5 x 105 cells per well in a 24-well plate format, we found that cells secreted 54.53 ± 2.2 ng/mL of insulin in the absence of glucose over a duration of 90 min incubation in a static bath. Addition of 20 mM glucose to stimulate GSIS resulted in a significant enhancement in GSIS (112.4 ± 5.2 ng/mL insulin; p <0.0001) (Figure 3).
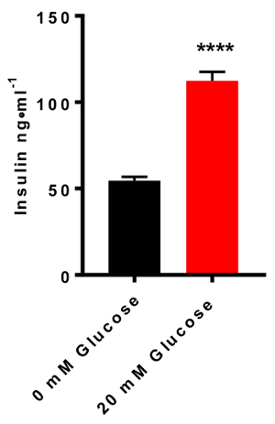
Figure 3: Estimation of basal versus stimulated insulin secretion. INS-1E cells were treated in the absence or the presence of high glucose (20 mM) stimulation for 90 min. A doubling in insulin secretion levels was detected in response to high glucose stimulation relative to the unstimulated cells (basal secretion condition; **** p <0 .0001, two-tailed unpaired t-test). N = 5; all assays were carried out in triplicates. Error bars = SEM. Please click here to view a larger version of this figure.
To investigate the role of dopaminergic D2-like receptor stimulation in GSIS within our INS-1E cell system, we incubated cells in the presence of D2-like receptor agonist drugs (dopamine, quinpirole, and bromocriptine) during the 90-min stimulation period with 20 mM glucose (Figure 4).
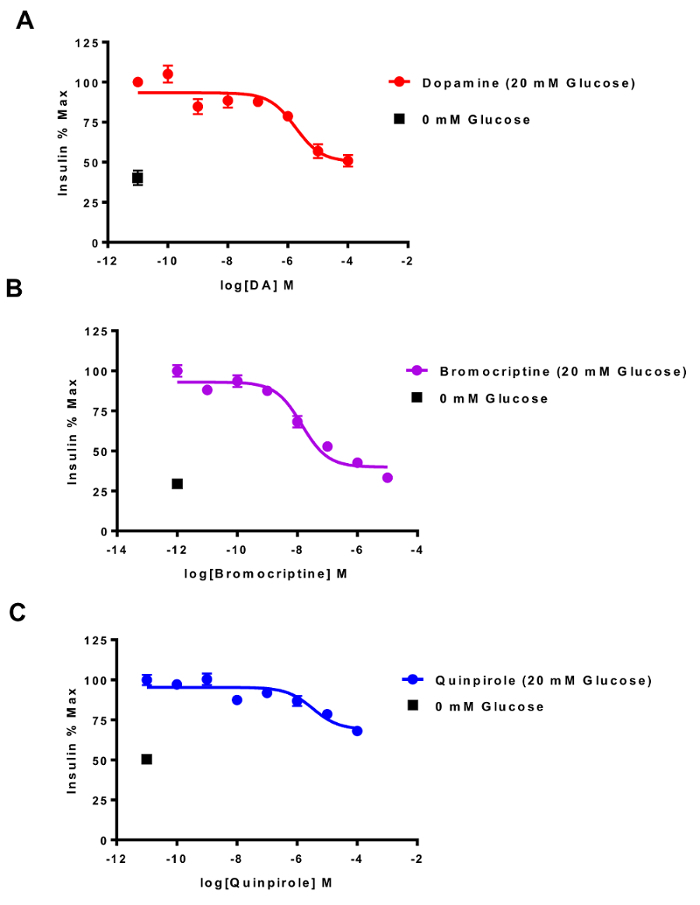
Figure 4: HTRF detection of dopamine D2-like receptor agonist inhibition of GSIS. INS-1E cells were treated with increasing concentrations of dopamine D2-like receptor agonists for 90 min in the presence of high glucose (20 mM; 37 ˚C). (A) Dopamine inhibited GSIS with an IC50= 1.78 ± 3.9 µM (R2 = 0.4355). (B) Bromocriptine was more potent than dopamine in reducing GSIS (IC50 = 13.9 ± 2.4 nM, R2 = 0.7501). (C) Quinpirole's potency was similar to dopamine (IC50 = 3.3 ± 3 µM, R2 = 0.3617). All data were best fit to a three-parameter fit sigmoidal curve. The '0 mM Glucose' point indicates basal insulin secretion by the INS-1E cells in the absence of high glucose (20 mM) stimulation. N >3; all assays were carried out in triplicates on separate experimental days. Error bars = SEM. Please click here to view a larger version of this figure.
The efficacy and potency of these agonist drugs was determined by graphing the insulin secretion curves. We found that in the absence of stimulation with high glucose, the INS-1E cells continued to secrete insulin, albeit to a markedly lower degree (indicated by the 0 mM condition), consistent with earlier reports15. Our results demonstrated that dopamine, the endogenous agonist of D2-like receptors, inhibited GSIS in a concertation-dependent manner (DA IC50 = 1.78 ± 3.9 µM; Figure 4A). In contrast, bromocriptine, a drug approved to treat type 2 diabetes23,24, was markedly more potent than dopamine in producing GSIS inhibition (IC50 = 13.9 ± 2.4 nM; Figure 4B). Lastly, though less efficacious than bromocriptine, quinpirole exhibited similar potency to dopamine in GSIS inhibition (IC50 = 3.3 ± 3 µM; Figure 4C).
Discussion
The HTRF insulin assay described here offers a rapid, efficient system to measure insulin secretion from a cultured cell-based system. Among its most important advantages, this assay offers a low background signal due to the high signal-to-noise ratio. Additionally, we have confirmed that the HTRF signal is stable for extended periods of time (>24 h)7. Nevertheless, since the insulin-binding monoclonal antibodies quickly reach binding saturation after addition to the assay, kinetics of antibody binding may be affected by ambient temperature or variations in secreted levels of insulin. Therefore, we read the assay plate at multiple time points (2 h, 4 h, and overnight) to precisely identify when the antibodies have reached full saturation and HTRF signal is most robust7. We have also recently shown the insulin antibodies do not significantly cross-react with proinsulin or c-peptide, suggesting that the assay is quite specific for mature insulin7.
An important step in the optimization of the assay involved establishing a broad dynamic range of insulin secretion. This dynamic range is defined as the difference in detected insulin during basal (unstimulated) versus stimulated insulin secretion. We find that an initial brief glucose starvation step (0 mM glucose, 1 h) preceding high glucose stimulation expands the dynamic range by priming the INS-1E cells to secrete more insulin during GSIS. In earlier work, we showed the assay's dynamic range to be from 0.17 ng/mL (limit of detection) to as high as ~110 ng/mL insulin with a signal to noise ratio of 10.027. Additionally, the amount of secreted insulin is dependent on initial cell number plated per well. We further optimized the assay by standardizing the passaging and handling of the INS-1E cells. Maintaining consistency in passaging schedule and plating in a specific plate type (e.g., 10 cm dishes) is essential for obtaining consistent secreted insulin levels.
Among potential sources of assay variability is bubbling in the assay solutions which interferes with the HTRF readout. An important source of these bubbles is the presence of bovine serum albumin (BSA) in the various KRB-based assay solutions. To troubleshoot this potential problem, reducing or even eliminating BSA significantly minimizes bubbles in the solution and therefore improves HTRF signal reliability. This is particularly important when generating the insulin standard curve and drug dilutions. Cell passage number-dependent differences in basal insulin secretion are another source of potential assay variability (data not shown). This is consistent with earlier work by Merglen and colleagues who also suggested that optimal insulin secretion is found between INS-1E cell passage numbers ~60 - 10015. Therefore, to minimize possible variability in insulin secretion related to cell passage number, we recommend limiting secretion assays to this well-defined cell passage range.
The primary limitation of the cell-based insulin secretion assay is the variability of the basal secretion intrinsic to the INS-1E cells. This may be related to the growing insulin-secreting cells outside of the physiological context of the pancreatic islet. Indeed, the other cell types within the islet including alpha and delta cells secrete numerous factors that may modulate basal and stimulated insulin secretion from beta cells in a local, paracrine manner25. The absence of these other islet cell types may disturb the tight regulation of insulin secretion, leading to greater variability than otherwise observed physiologically. Another potential limitation is the relatively small linear range of the assay, since insulin secreted in response to 20 mM glucose was almost saturating in some cases. As a result, in the undiluted cell supernatants, effects of insulin secretagogues on stimulated insulin secretion may be out of the linear portion of the calibration curve. This is, however, obviated by diluting the insulin-containing supernatant sufficiently to allow the insulin to fall into the linear range of the calibration curve.
We validated our assay by showing that agonism of dopamine D2-like receptors via dopamine, bromocriptine and quinpirole treatments inhibited GSIS in a concentration-dependent manner. These data allowed us to determine pharmacological properties of these drugs (i.e., IC50 and efficacy) in terms of their effects on GSIS. Our results are consistent with a growing body of evidence suggesting an important role for dopamine and dopaminergic signaling outside of the central nervous system20,21,22,26,27. Notably, our findings showing that the dopamine D2-receptor agonist bromocriptine potently inhibits GSIS, yet was recently approved by the FDA to treat type 2 diabetes24, raises the important question: how can this drug treat type 2 diabetes (T2D) if it lowers insulin secretion, at least acutely? One potential explanation may be related to bromocriptine's capacity to incur "beta cell rest." According to this hypothesis, since one of the cardinal features of T2D is an overall oversecretion of insulin, that ultimately may contribute to beta cell failure28. Moreover, we suggest that insulin oversecretion during T2D may also exacerbate insulin resistance by diminishing insulin sensitivity of organs including skeletal muscle, liver and adipose tissue. Thus, blocking or diminishing GSIS may result in gradual resensitization of these target tissues, making them more responsive to insulin and thus improving insulin sensitivity. Having an assay system amenable to high-throughput screening opens the door to future work to both dissect the intracellular signaling pathways mediated by dopamine D2-like receptors in pancreatic beta cells that are responsible for paracrine/autocrine GSIS inhibition as well as to further elucidate mechanisms by which drugs like bromocriptine may improve symptoms of diabetes.
The development of a cell-based insulin secretion assay that takes advantage of HTRF technology for insulin secretion and represents a significant advance in rapid, and efficient detection of insulin at significantly diminished cost relative and/or greater safety compared to older insulin detection assays relying on RIA and ELISA-based approaches. Indeed, though ELISA and RIA insulin assays are comparably sensitive and specific to the HTRF insulin assay, the homogenous nature of the detection, eliminates numerous additional washing steps present in these other assay systems7. Furthermore, the relative stability of the HTRF signal provides another key advantage for reliable insulin measurement.
Taken together, the HTRF insulin secretion assay has the capability of rapidly discriminating between the pharmacological properties of multiple drugs in the context of cellular insulin secretion. Moreover, this assay's relatively ease of use and robust signal make it amenable for high-throughput screening.
Disclosures
We thank Nicolas Pierre (Cisbio Bioassays) for helpful advice and Dr. Pierre Maechler (University of Geneva) for generously providing INS-1E cells. This work was supported by funding from the Department of Defense (grant PR141292 to Z.F.), and the John F. and Nancy A. Emmerling Fund of The Pittsburgh Foundation (to Z.F.).
Acknowledgments
The authors have nothing to disclose.
References
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: Insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Vetere A, Choudhary A, Burns SM, Wagner BK. Targeting the pancreatic beta-cell to treat diabetes. Nat Rev Drug Discov. 2014;13(4):278–289. doi: 10.1038/nrd4231. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Barg S. Mechanisms of exocytosis in insulin-secreting B-cells and glucagon-secreting A-cells. Pharmacol Toxicol. 2003;92(1):3–13. doi: 10.1034/j.1600-0773.2003.920102.x. [DOI] [PubMed] [Google Scholar]
- Braun M, Ramracheya R, Rorsman P. Autocrine regulation of insulin secretion. Diabetes Obes Metab. 2012;14 Suppl 3:143–151. doi: 10.1111/j.1463-1326.2012.01642.x. [DOI] [PubMed] [Google Scholar]
- Farino ZJ, et al. Development of a rapid insulin assay by homogenous time-resolved fluorescence. PLoS One. 2016;11(2):e0148684. doi: 10.1371/journal.pone.0148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson N, et al. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol Endocrinol. 2012;26(10):1757–1772. doi: 10.1210/me.2012-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk E, Moxley MM, Salvatori A, Corbett JA, Heyduk T. Homogeneous insulin and C-Peptide sensors for rapid assessment of insulin and C-peptide secretion by the islets. Diabetes. 2010;59(10):2360–2365. doi: 10.2337/db10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk T. Measuring protein conformational changes by FRET/LRET. Curr Opin Biotechnol. 2002;13(4):292–296. doi: 10.1016/s0958-1669(02)00332-4. [DOI] [PubMed] [Google Scholar]
- Degorce F. HTRF((R)): Pioneering technology for high-throughput screening. Expert Opin Drug Discov. 2006;1(7):753–764. doi: 10.1517/17460441.1.7.753. [DOI] [PubMed] [Google Scholar]
- Degorce F, et al. HTRF: A technology tailored for drug discovery - a review of theoretical aspects and recent applications. Curr Chem Genomics. 2009;3:22–32. doi: 10.2174/1875397300903010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis G. HTRF(R) Technology. J Biomol Screen. 1999;4(6):309–314. doi: 10.1177/108705719900400605. [DOI] [PubMed] [Google Scholar]
- Daijo JE, Sportsman JR. A time-resolved fluorescence immunoassay for insulin in rodent plasma. J Pharm Biomed Anal. 1999;19(3-4):335–342. doi: 10.1016/s0731-7085(98)00126-5. [DOI] [PubMed] [Google Scholar]
- Merglen A, et al. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145(2):667–678. doi: 10.1210/en.2003-1099. [DOI] [PubMed] [Google Scholar]
- Ustione A, Piston DW. Dopamine synthesis and D3 receptor activation in pancreatic beta-cells regulates insulin secretion and intracellular [Ca(2+)] oscillations. Mol Endocrinol. 2012;26(11):1928–1940. doi: 10.1210/me.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustione A, Piston DW. A simple introduction to multiphoton microscopy. J Microsc. 2011;243(3):221–226. doi: 10.1111/j.1365-2818.2011.03532.x. [DOI] [PubMed] [Google Scholar]
- Ustione A, Piston DW, Harris PE. Minireview: Dopaminergic regulation of insulin secretion from the pancreatic islet. Mol Endocrinol. 2013;27(8):1198–1207. doi: 10.1210/me.2013-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubi B, et al. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280(44):36824–36832. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- Garcia-Tornadu I, et al. Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology. 2010;151(4):1441–1450. doi: 10.1210/en.2009-0996. [DOI] [PubMed] [Google Scholar]
- Garcia-Tornadu I, et al. New insights into the endocrine and metabolic roles of dopamine D2 receptors gained from the Drd2 mouse. Neuroendocrinology. 2010;92(4):207–214. doi: 10.1159/000321395. [DOI] [PubMed] [Google Scholar]
- Lopez Vicchi F, et al. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol Res. 2016;109:74–80. doi: 10.1016/j.phrs.2015.12.029. [DOI] [PubMed] [Google Scholar]
- Kalra S, Kalra B, Agrawal N, Kumar S. Dopamine: The forgotten felon in type 2 diabetes. Recent Pat Endocr Metab Immune Drug Discov. 2011;5(1):61–65. doi: 10.2174/187221411794351842. [DOI] [PubMed] [Google Scholar]
- Mahajan R. Bromocriptine mesylate: FDA-approved novel treatment for type-2 diabetes. Indian J Pharmacol. 2009;41(4):197–198. doi: 10.4103/0253-7613.56070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol. 2013;24(1):11–21. doi: 10.1016/j.semcdb.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon JS, Pajvani U, Freyberg Z, Leibel RL, Lieberman JA. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol Metab. 2014. [DOI] [PubMed]
- Freyberg Z, Aslanoglou D, Shah R, Ballon JS. Intrinsic and antipsychotic drug-induced metabolic dysfunction in schizophrenia. Front Neurosci. 2017;11:432. doi: 10.3389/fnins.2017.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw van Weenen EJ, et al. The dopamine receptor D2 agonist bromocriptine inhibits glucose-stimulated insulin secretion by direct activation of the alpha2-adrenergic receptors in beta cells. Biochem Pharmacol. 2010;79(12):1827–1836. doi: 10.1016/j.bcp.2010.01.029. [DOI] [PubMed] [Google Scholar]


