Abstract
Objective
Apoptotic destruction of insulin-producing pancreatic β-cells is involved in the aetiology of both type 1 and type 2 diabetes. AMP-activated protein kinase (AMPK) is a sensor of cellular energy charge whose sustained activation has recently been implicated in pancreatic β-cell apoptosis and in islet cell death post-transplantation. Here, we examine the importance of β-cell AMPK in cytokine-induced apoptosis and in the cytotoxic action of CD8+ T cells.
Research Design and Methods
Clonal MIN6 β-cells or CD1 mouse pancreatic islets were infected with recombinant adenoviruses encoding enhanced green fluorescent protein (eGFP/Null), constitutively-active AMPK (AMPK CA), or dominant-negative AMPK (AMPK DN) and exposed or not to tumour necrosis factor-α (TNF-α) interleukin-1β (IL-1β) and interferon-γ (IFN-γ). Apoptosis was detected by monitoring the cleavage of caspase-3 and DNA fragmentation. The cytotoxic effect of CD8+ purified T cells was examined against pancreatic islets from NOD mice infected with either Null or the AMPK DN-expressing adenoviruses.
Results
Exposure to cytokines, or expression of AMPK CA, induced apoptosis in clonal MIN6 β-cells and CD1 mouse pancreatic islets. By contrast, over-expression of AMPK DN protected against the proapoptotic effect of these agents, in part by preventing decreases in cellular ATP, and lowered the cytotoxic effect of CD8+ T cells towards NOD mouse islets.
Conclusions
Inhibition of AMPK activity enhances islet survival in the face of assault by either cytokines or T cells. AMPK may therefore represent an interesting therapeutic target to suppress immune mediated β-cell destruction, and may increase the efficacy of islet allografts in Type 1 diabetes.
Keywords: AMPK, type 1 diabetes, cytokines, pancreatic β-cells, islets, apoptosis, CD8+ T cells
Diabetes mellitus currently affects ~ 6 % of the population in westernised societies, an incidence expected to double by 2020 (1). Destruction of β-cells is now believed to be involved in the aetiology of both type 1 and type 2 diabetes (2). Strategies which delay or reverse the loss of β-cell mass in either case are therefore likely to be of significant therapeutic value. Furthermore, such strategies may enhance the survival of islet allograft transplanted into type 1 diabetic patients. Indeed, current and successful human transplantation protocols involve a substantial (60-80%) loss of functional islet mass after transplantation and means that several donors are still required for successful grafting (3). The development of strategies to enhance β-cell survival before and after transplantation ought therefore to provide clear therapeutic benefits.
In type 1 diabetes, autoreactive CD4+ and CD8+ T cells recognize their target autoantigens (such as insulin, GAD65) as peptide fragments presented by major histocompatibility complex molecules, become activated and travel to the pancreas, infiltrating (insulitis) and finally destroying the insulin producing β-cells (4). Both direct T cell-mediated cytotoxicity and indirect cytokine-, nitric oxide (NO)- or free radical-, and Fas ligand (FasL)-dependent mechanisms are likely to be responsible for β-cell apoptosis. These all lead to the activation of caspases by cleavage of the inactive zymogen counterpart. Caspase-3 is an effector caspase leading to the characteristic apoptotic morphological changes such as membrane blebbing, cytoplasmic and nuclear condensation, DNA fragmentation, and formation of apoptotic bodies (5;6).
AMP-activated protein kinase is a multisubstrate, trimeric serine/threonine kinase composed of one 63 KDa catalytic α-subunit, and two regulatory subunits, β and γ (7;8). AMPK activity is regulated allosterically by AMP (9) and through reversible phosphorylation at Thr-172 of the α-subunit by upstream kinases such as LKB1 (10), calmodulin kinase kinase (CaMKKβ) (11) or TGFβ-activated kinase (TAK1) (12). AMPK is thus a sensor of cellular energy charge that is activated by the fall in ATP/AMP ratios, an elevation in free Ca2+ concentration (13) or perhaps other mechanisms. In most cell types, activation of AMPK is associated with the phosphorylation of enzymes involved in ATP-consuming processes, such as fatty acid synthesis (acetyl-CoA carboxylase, ACC) and cholesterol biosynthesis (hydroxymethylglutaryl-CoA reductase, HMG-CoA reductase), and the consequent activation of mitochondrial fatty acid oxidation (7;14). In this way, regulation of AMPK ensures that cellular ATP is spared during times of nutrient deprivation. However, in the pancreatic islet β-cell, the role of AMPK may be more specialised and may represent a key part of the glucose-sensing machinery of these cells (7;14;15).
A good deal of data has emerged in the last 3-4 years showing that sustained AMPK activation can exert a proapoptotic effect in a variety of cell types (16;17). Thus, work in hepatocytes (18), gastric cancer (19), neuroblastoma (20), HT-29 colon cancer (21) and chronic lymphocytic leukaemia (22) cells, has implicated AMPK activation in cell death. Taken together, these data reinforce the view that whereas activation of AMPK is likely, in the short term, to reduce ATP consumption and thus to protect cells from transient metabolic stresses (23), sustained activation of the enzyme entrains a sequence of events ultimately leading to programmed cell death. Importantly, we have recently shown that adenovirus-mediated expression of an activated form of AMPK (AMPK CA) reduces the ability of syngeneic islet to reverse streptozotocin-induced diabetes, whilst a dominant-negative form of AMPK (AMPK DN) tended to enhance graft efficiency (24).
Various cellular and molecular mechanisms are involved in β-cell apoptosis. CD8+ T cells are increasingly recognized as key actors in the diabetes of the non-obese diabetic (NOD) mouse, which spontaneously develops diabetes remarkably similar to human type 1 diabetes (25) and constitute a useful model to study this disorder. CD8+ T cells are also likely to play a role in humans. Thus, in identical twins who had received a transplant from their non-diabetic co-twin, recurrent disease occurred within six weeks, with CD8+ T cells constituting a majority of the cells infiltrating the transplants (26). Biopsies performed in patients newly diagnosed with type 1 diabetes have also shown that CD8+ T cells make up a considerable proportion of the infiltrate (27). Finally, a number of recent studies have indicated that T cells recognizing proinsulin and IGRP may be detected with high sensitivity at onset of diabetes (28).
T-cells induce damage to islet β cells by a number of mechanisms including lysis by perforin/granzymes and induction of apoptosis by Fas/FasL interactions (29). In addition, in the insulitis lesion in type 1 diabetes, invading immune cells produce pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α), interferon-γ (INF-γ) and interleukin-1β (IL-1β) and constitute therefore also a key part of the type 1 diabetes mechanism (30).
Here, we demonstrate that AMPK is involved both (a) in regulating β-cell apoptosis induced by cytokines, and (b) in the cytotoxicity of CD8+ T cells towards islets from NOD mice.
Research Design and Methods
Materials
Collagenase, Histopaque (1077) and Hoechst (type V) were from Sigma (St Louis, MO). Mouse recombinant TNF-α, INF-γ and IL-1β were from PreproTech EC (London, UK). BM Chemiluminescence’s blotting substrate (ECL), anti-rabbit and anti-mouse Horseradish Peroxidase (HRP), as well as the [32P] γATP and the 51chromium sulphate, were from Amersham BioSciences (Buckinghamshire, UK). Firefly luciferase was from Promega (Madison, WI), the In Situ Cell Death Detection Kit, tetramethylrhodamine red (TMR Red) was from Roche (Basel, Switzerland). The CD8a+ T cell isolation kit was from MACS (Bergisch Gladbach, Germany). All the culture media and cell dissociation buffer were from Invitrogen (Paisley, UK). Scintillation fluid Ultima Gold LLT was from Perkin Elmer (Waltham, MA) and the scintillation 96 well plate 1450 micro β counter was from Wallac (Turku, Finland).
Animals
Wild-type CD-I mice (20-25 g) and 8-12 week old male NOD mice were used for islet isolation and killed by cervical dislocation immediately before the islet isolation procedure (see Cell culture and islet isolation). Insulin-specific TCR transgenic mice were generated from G9C8 cloned T cells (31) which had previously been shown to have specific reactivity to amino acids 15-23 of the insulin-β chain (32). TCR α and β founder lines were inter-crossed to produce αβ TCR transgenic mice (G9.NOD). G9Cα-/-.NOD mice expressing T cells monoclonal for the transgenic TCR were generated by crossing the αβ TCR transgenic mice to NOD.Cα-/- mice (>20 generations backcross to NOD mice). All animal procedures were in accordance with the British Home Office Animals (Scientific Procedures) Act, 1986.
Antibodies
Rabbit anti-phospho-AMPK (Thr-172) and anti-cleaved-caspase-3 (Asp-175) antibodies were purchased from Cell Signaling (Beverly, MA). Rabbit anti-ERK2 was from Santa Cruz (Santa Cruz, CA). Tetra methyl rhodamine isothiocyanate-conjugated (TRITC-conjugated) secondary antibody against rabbit IgG was purchased from Jackson (West Grove, PA).
Adenoviruses
Adenoviruses encoding enhanced green fluorescent protein (eGFP) only, hereafter named pAd-GFP (Null), or constitutively-active AMPK (AMPK CA), or a dominant-negative form of AMPKα1 (D157A) (AMPK DN), have been described by Woods and Carling et al. (33;34). AMPK CA comprises the amino terminal domain (amino acids 1-312) common to both AMPKα1 and α2, and is rendered constitutively-active by a T172D point mutation. Islets and MIN6 cells were infected at a multiplicity of infection of 100 viral particles / cell respectively overnight or 4 h and cultured for 48 h before experiments.
Cell culture and islet isolation
Clonal mouse pancreatic β-cells MIN6 were used between passages #18 and #30 and grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 25 mmol/l glucose and supplemented with 2 mmol/l L-glutamine, 15% heat-inactivated fetal calf serum (FCS), 50 µmol/l 2-mercaptoethanol, 100 U/ml penicillin and 100 g/ml streptomycin. Mouse mastocytoma P815 cells were grown in RPMI 1640 medium, 5% FCS, 2 mmol/l L-glutamine, 50 µmol/l 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin. Both were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
Mice were killed by cervical dislocation. Collagenase [1 mg/ml in Krebs Ringer bicarbonate (KRB) medium comprising (mmol/l): 120 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2, 24 NaHCO3, and 1 mg/ml bovine serum albumin, gassed with O2/CO2 (95% / 5%) to maintain a pH of 7.4] was then injected into the pancreatic duct (3 ml / mouse). The distended pancreas was then placed in a water bath at 37 °C for 11 min, and the islets were hand picked. Mouse islets were maintained in RPMI 1640 medium supplemented with 2 mmol/l L-glutamine, 10% FCS, 11 mmol/l glucose and antibiotics.
For immunocytochemistry, islets were dissociated with cell dissociation buffer before plating the liberated cells onto glass coverslips.
CD8+ T cell purification and activation
G9Cα-/-.NOD mice express monoclonal insulin reactive CD8 T cells. Cells were extracted from the spleen and activated overnight (ON) in presence of the Insulin B15-23 peptide, in RPMI 1640 medium containing 5% FCS, 2 mmol/l L-glutamine and 100 U/ml penicillin and 100 μg/ml streptomycin, at 37 °C in a humidified atmosphere containing 5% CO2. The day after, activated CD8+ T cells were purified from the splenocytes using a CD8+ T cell isolation kit (MACS) according to the manufacturer’s instructions. CD8+ T cells obtained by this method were 90-95% pure.
Immunocytochemistry and apoptosis detection
MIN6 cells or dispersed islets were washed three times with PBS before fixation with 4% (v/v) paraformaldehyde in 0.1 M NaH2PO4, pH 7.4. Cells were then permeabilized with 0.3% (vol/vol) Triton X-100 for 20 min and incubated for 1 h with primary rabbit polyclonal anti-cleaved caspase-3 antibody, at 1:200 dilution, at 4 °C. Primary antibody was revealed using TRITC-conjugated secondary antibody against rabbit IgG (1:500 dilution). Nuclear staining was achieved by incubating the cells in wash buffer containing Hoechst for 10 min at room temperature. Apoptotic cells were imaged either on Leica SP2 laser-scanning confocal or Leica SP5 MP/FLIM inverted optics confocal microscope, using a x40 or a x63 oil immersion objective with excitation at 488 nm (Ar) and 543 nm (He-Ne) and emission detected at >515 (green, eGFP) and >560 nm (red, TRITC). A Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) assay was also performed using the in Situ Cell Death Detection Kit, TMR red, to visualise the DNA strand breaks of apoptotic cells by fluorescence microscopy, according to the manufacturer’s recommendations. Briefly, the cells were treated as described above for fixation and permeabilization and the TUNEL reaction mixture was added for 1 h. Imaging was performed with an excitation wavelength range of 520-560 nm, detecting in the range 570-620 nm. Results were quantified by densitometry.
Western immunoblot Analysis
MIN6 cells or mouse islets were incubated and lysed as for measurements of caspase activity. Whole cellular extract (50 µg) was denatured for 5 min at 100°C in 2% (w/v) SDS, 5% mercaptoethanol, and resolved by 10% or 7.5% SDS-PAGE before transferring to PVDF membranes and immunoblotting. Secondary antibodies were revealed using BM Chemiluminescence’s blotting substrate. Intensities were measured by digital scanning of gels and quantified using Scion Image (www.scioncorp.com/).
ATP measurements
For total ATP assay, MIN6 cells were infected with adenoviruses and incubated with the indicated cytokines in culture medium and then at the given glucose concentrations in KRB medium for 30 min, before extraction into perchloric acid (10%, v/v). ATP was quantitated in extracts neutralized with Hepes-buffered KOH, using partially purifed firefly luciferase and photon counting, as described before (35).
Cytotoxicity assay
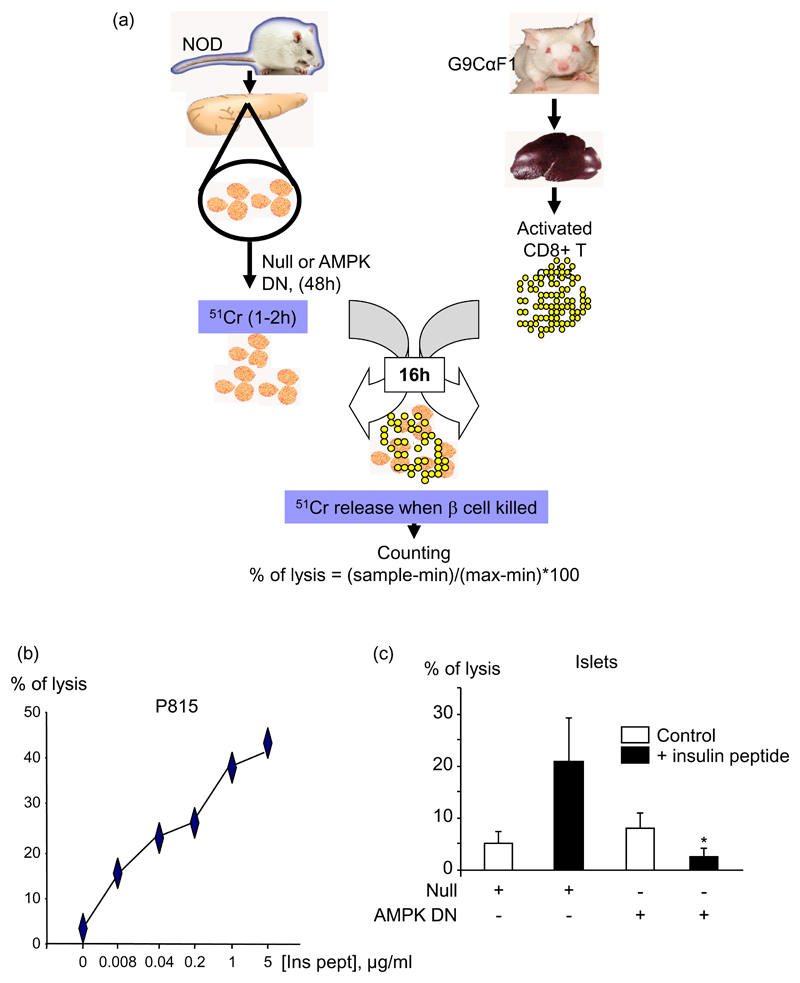
A chromium release assay was performed similar to that previously described (31). Islets from 8-12 week old NOD male mice were infected with either the Null adenovirus or the AMPK DN for 48 h. They were then labelled with 51chromium sulphate for 1 h 30, and washed (see Fig. 8 (a)). The purified activated CD8+ T cells were added for 16 h to the islets, with or without added insulin B15-23 peptide (1 μg/ml) at an effector:target ratio of 20:1 (assuming 1000 cells/islet). Cytotoxicity using P815 cells, labelled with 51chromium sulphate for 1 h and coated with insulin B15-23 peptide, at an effector:target ratio of 10:1, was used as a positive control. Results were expressed as % specific lysis determined as (((cytotoxic release-Min)/(Max-Min))x100) %, where the spontaneous lysis corresponds to the minimal release (Min), and the lysis provoked by addition of hydrochloric acid corresponds to the maximal lysis (Max).
Fig. 8. Dominant-negative AMPK decreases the cytotoxicity of insulin-reactive CD8+ T cells to NOD pancreatic islets.
(a) Scheme explaining the cytotoxic assay (see Research Design and Methods). (b) P185 control cells were labelled with 51chromium sulphate during 1 h, washed, and incubated for 16 h with insulin B15-23 reactive CD8+ T cells at a ratio of 1:10, in presence of increasing concentrations of insulin B15-23 peptide. The percentage of lysis was determined measuring the gamma radioactivity released in the supernatant by the lysed cells: % of lysis = ((sample release-min) / (max-min))*100. (c) The same procedure was used with islets from NOD mice which were infected with the AMPK DN adenovirus at 100 MOI for 48 h. 20 islets per condition were used in presence of 400 000 CD8+ T cells, estimating 1000 cells / islet, and insulin peptide was added or not at 1 μg/ml. *p<0.05 for the effect of AMPK DN virus in the presence of the peptide compared with Null virus. Data are given as means ± S.E.M. of triplicate analyses from three independent experiments.
Statistical Analysis
Results are expressed as means±S.E.M. of at least three independent experiments. Statistical significance was evaluated using the Student's t-test for unpaired comparison with Bonferroni correction as appropriate. A value of p<0.05 was considered statistically significant.
Results
Effect of cytokines on clonal MIN6 β-cell apoptosis
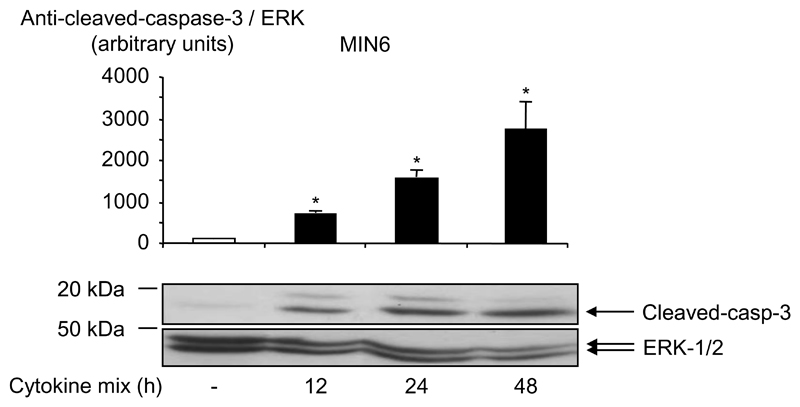
It has previously been reported that incubation of MIN6 cells (36) or islets (37;38) with different cytokine combinations can lead to the induction of apoptosis. Apoptosis can be prompted by several different mechanisms but the one usually implicated in islet cell death in type 1 diabetes and allograft transplantation involves cytotoxic cytokines secreted by macrophages and possibly also β-cells. Here, we observed that incubation of MIN6 cells for 12 h, 24 h or 48 h with a combination of the three cytokines, IL-1β, TNF-α and IFN-γ (Fig. 1) induced the cleavage of caspase-3, indicating the establishment of apoptosis in clonal MIN6 cells.
Fig. 1. Cytokines induce apoptosis in clonal MIN6 β-cells.
MIN6 cells were cultured with a cytokine mix containing IL-1β (50 U/ml), TNF-α (1000 U/ml) and IFN-γ (1000 U/ml), for the times indicated, in their culture medium (see Research Design and Methods). Induction of apoptosis was measured by western (immuno-) blotting for cleaved caspase-3 (Asp-175) (see Research Design and Methods) and total protein content was estimated using anti-ERK1/2. Data are given as means ± S.E.M. of three independent experiments: *p<0.05 for effect of cytokines compared with basal without cytokines.
AMPK activation induces MIN6 cell apoptosis
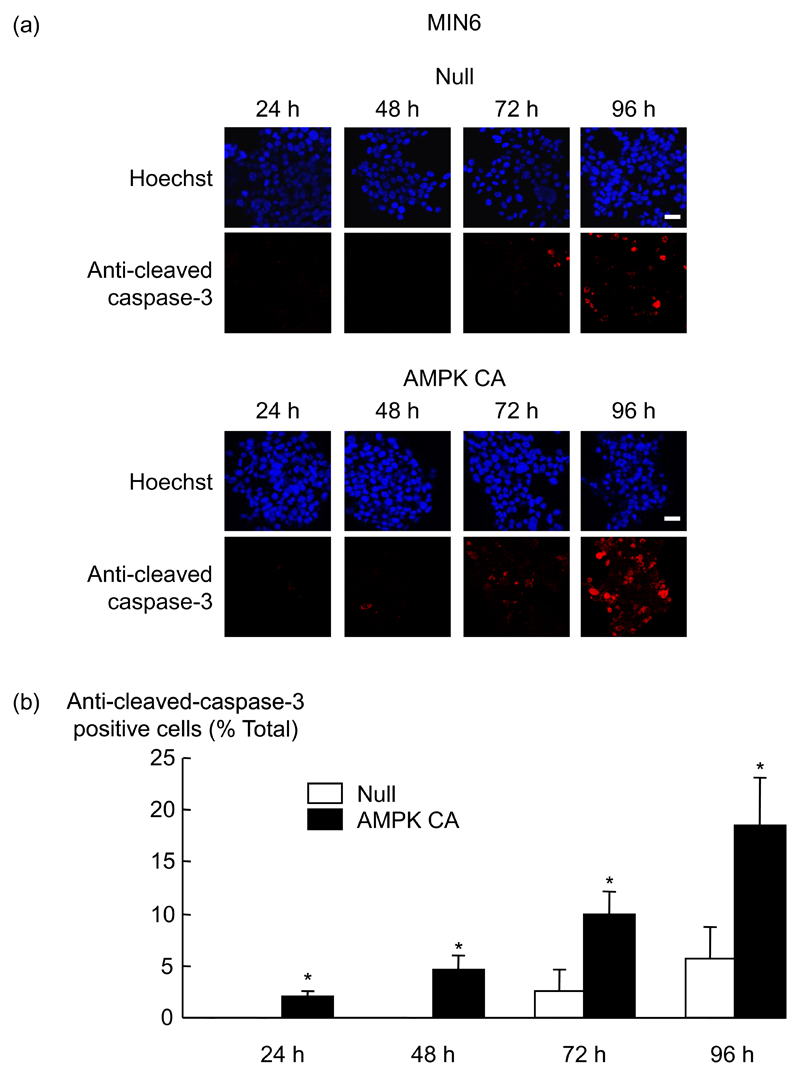
Previous studies have shown that AMPK activators such as 5-amino-4-imidazolecarboxamide riboside (AICAR) (17), or chronic incubation of MIN6 cells or islets with low concentrations (0-3 mmol/l) of glucose (39) or metformin (40) can lead to β-cell apoptosis. Consistent with these earlier findings, when MIN6 cells were infected with Null/GFP (Null) or the constitutively-active AMPK (AMPK CA) adenoviruses, and cultured for 24 h to 96 h, we observed a four-fold increase in the level of activated caspase-3 after 96 h of incubation with AMPK CA compared to Null virus-expressing cells (Fig. 2). Thus, AMPK activation alone is sufficient to induce apoptosis in MIN6 cells.
Fig. 2. Effect of AMPK CA adenovirus on caspase-3 activity in MIN6 β-cells.
(a) Cells were infected with the indicated adenoviruses at 100 MOI (Null/GFP, AMPK CA) for the times indicated. Induction of apoptosis was measured by immunocytochemistry with an antibody to cleaved-caspase-3. Primary antibody was revealed using TRITC-conjugated secondary antibody against rabbit IgG and a Hoechst coloration of the nucleus was performed. (b) The data from (a) are given as means ± SEM of triplicate analyses from three independent experiments. Results were analysed by densitometry and statistical differences were assessed by unpaired Students t-test (*p<0.05 for effect of AMPK CA virus compared with Null virus). Scale bar, 20 µm.
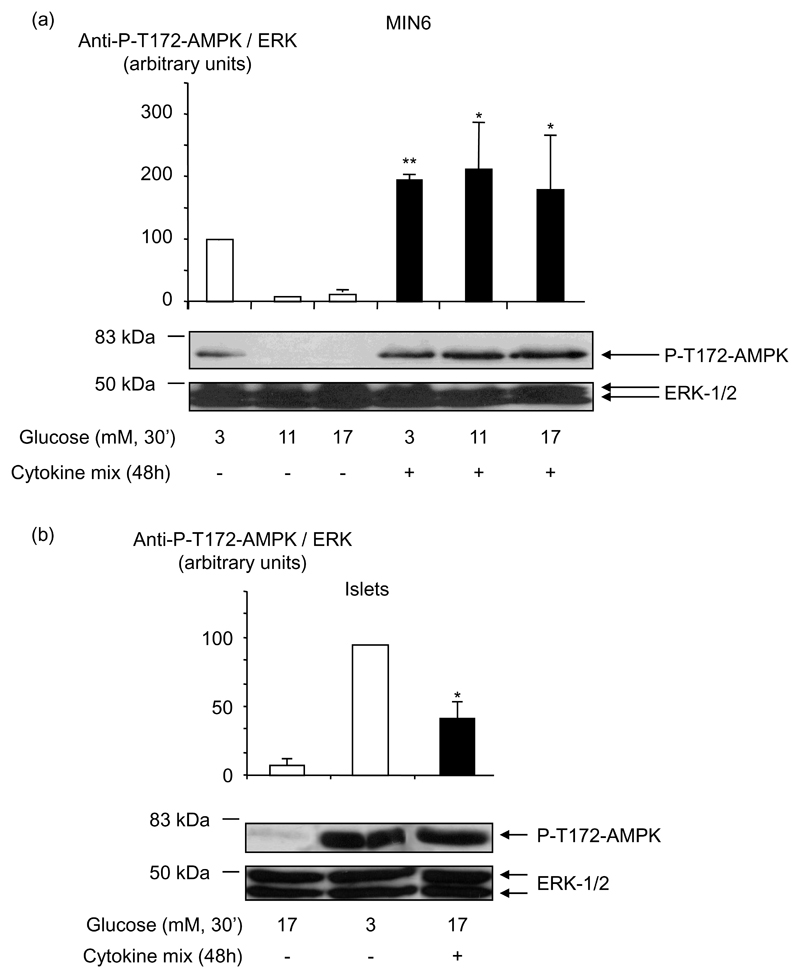
Cytokines induce an increase in MIN6 cells and mouse islets AMPK activity
Measured either in MIN6 cells (Fig. 3 (a)) or islets (Fig. 3 (b)), AMPKα phosphorylation at Thr-172 was markedly higher after incubation for 30 min in KRB containing low (0-3 mM) glucose concentrations, than at elevated (17 mmol/l) glucose, consistent with previous findings (15). Likewise, incubation for 48 h with a combination of IL-1β, TNF-α and IFN-γ increased by fifteen-fold and four-fold the level of phosphorylation on AMPK Thr-172 at 17 mmol/l glucose respectively in MIN6 cells and mouse islets.
Fig. 3. Effect of cytokines on AMPK activity in clonal MIN6 β-cells and mouse pancreatic islets.
(a) MIN6 cells or (b) mouse pancreatic islets were treated with cytokine mix for 48 h and incubated in KRB medium (see Research Design and Methods) at the indicated concentrations of glucose for 30 min. Phospho-Thr-172 AMPK phosphorylation was assessed using the appropriate phospho-specific antibody. Total protein was estimated using anti-ERK1/2 antibody. Results shown are from three independent experiments and statistical differences were assessed by unpaired Students t-test: *p<0.05 and **p<0.01 for the effect of cytokines compared without cytokines.
Cytokine-induced apoptosis requires AMPK activation in MIN6 cells
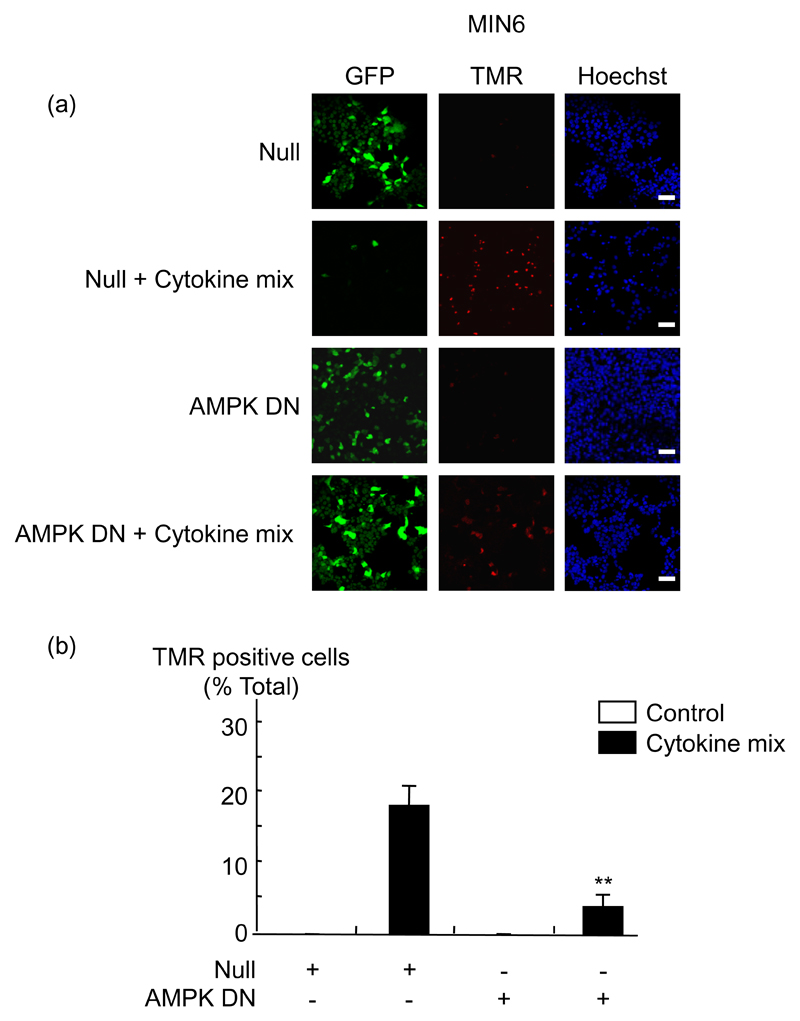
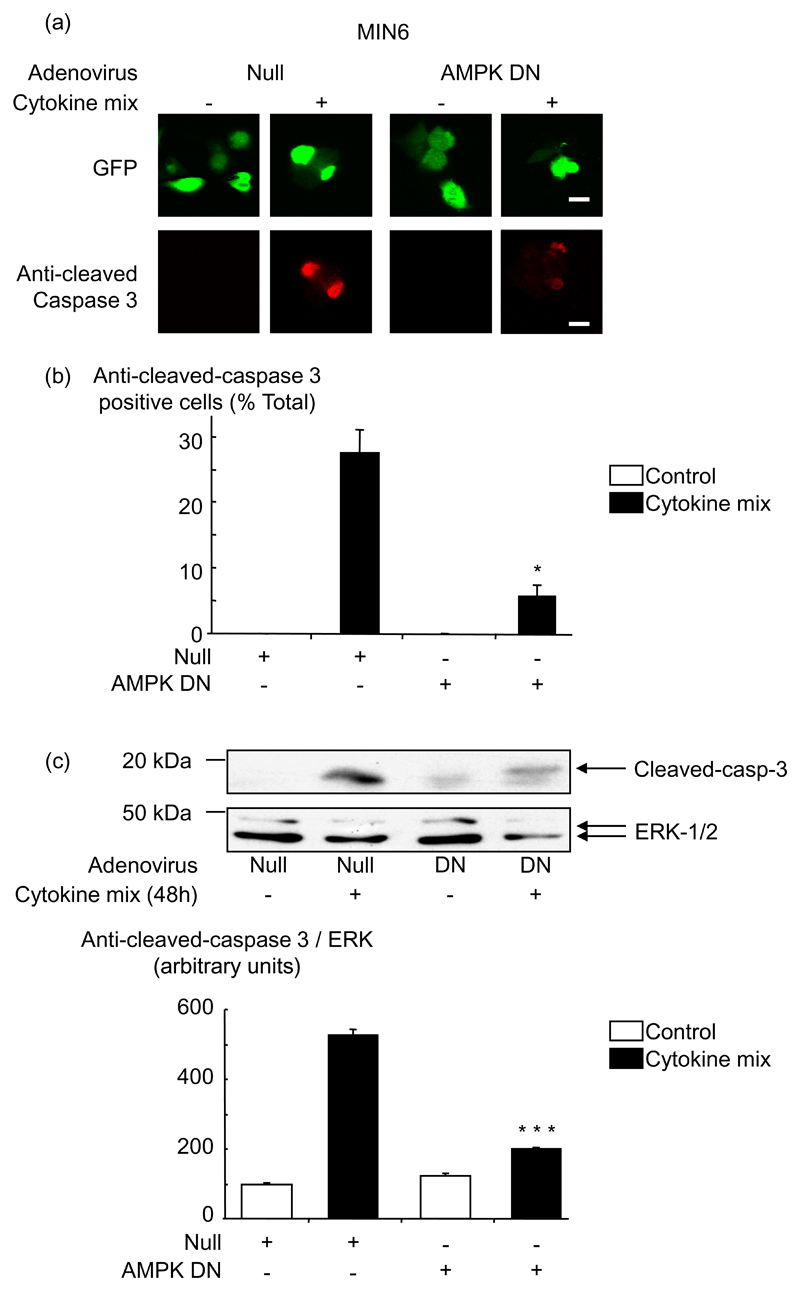
To determine whether cytokine-mediated apoptosis required AMPK activation, we used an adenovirus to express a dominant-negative form of AMPK (41). MIN6 cells were infected with the AMPK DN adenovirus for 48 h and then treated with cytokines for a further 48 h. As expected, the increased phosphorylation of AMPK induced by 3 mmol/l glucose or by cytokines was reduced in cells and islets infected with AMPK DN (data not shown). Inhibition of AMPK also reduced the pro-apoptotic effect of the cytokines. Thus, apoptosis was reduced about 4-5-fold in MIN6 cells infected with the AMPK DN adenovirus and treated 48 h with the three cytokines, as shown by TUNEL assay (Fig. 4) or measuring anti-cleaved caspase 3 either in single cells by immunochemistry (where infection with adenovirus could be confirmed on a cell basis by the presence of eGFP; Fig. 5 (a, b)), or in cell populations by Western (immuno-) blotting (Fig. 5 (c)).
Fig. 4. Dominant-negative AMPK blocks cytokine-mediated apoptosis in MIN6 β-cells.
(a) MIN6 cells were infected with the indicated adenoviruses at 100 MOI (Null/GFP, AMPK DN) for 48 h, before treatment with cytokines as in Fig. 1, for a further 48 h. Apoptosis was measured by TUNEL assay using the In Situ Cell Death Detection Kit, TMR Red, as described in Research Design and Methods, followed by nucleus coloration with Hoechst dye. Results were analysed by densitometry and statistical differences were assessed by unpaired Students t-test: **p<0.01 for the effects of AMPK DN virus in the presence of cytokines compared with Null virus. (b) The data from (a) are given as means ± SEM of triplicate analyses from three independent experiments involving 100 individual cells per condition. Scale bar, 20 µm.
Fig. 5. Dominant-negative AMPK blocks cytokine-activation of caspase 3 in MIN6 β-cells.
MIN6 cells were treated as in Fig. 4, and active caspase 3 was measured by (a, b) immunocytochemistry or (c) by western blot with anti cleaved-caspase-3 antibody. Results were analysed as in Fig. 4: *p<0.05 and ***p<0.001 for the effect of AMPK DN virus in the presence of cytokines compared with Null virus. (b) Data are given as means ± S.E.M. of triplicate analyses from three independent experiments involving 50 individual cells per condition for the immunocytochemistry and (c) as means ± S.E.M. of three independent experiments for the western blot. Scale bar, 10 µm.
Effect of cytokines on primary mouse islets apoptosis and implication of AMPK and caspase-3
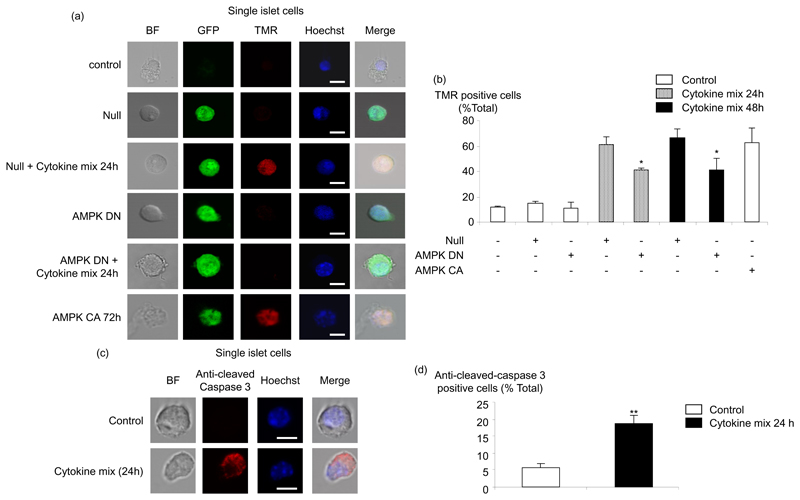
To examine the role of AMPK in primary β-cells, mouse islets were dispersed into single cells before infection with adenoviruses. This approach ensured high levels of infection such that >80% of cells were positive as judged by eGFP fluorescence. After treatment of the cells for a further 24 h and 48 h with cytokines, apoptosis was then assessed by TUNEL assay (Fig. 6 (a, b)). The level of apoptosis was between 60% and 70% after the incubation with cytokines and a significant decrease was apparent in cells expressing AMPK DN versus those infected with Null adenovirus. Moreover, activation of AMPK by the AMPK CA adenovirus during 72 h also led to an increase apoptosis in primary mouse islets, reaching 70% (Fig. 6 (a, b)). To determine if caspase-3 was also involved in the latter process, we measured caspase-3 cleavage (Fig. 6 (c, d)). Interestingly, the percentage of cells expressing cleaved-caspase-3 reached 20% after 24 h in presence of cytokines. These results confirm the involvement of caspase-3 in cytokine-induced apoptosis of primary β-cells. Nevertheless, this number is less than the percentage of apoptosis measured with the TUNEL assay (Fig. 6 (a, b)), even after 24 h of incubation with cytokines, and thus implies the involvement of other (non caspase 3-dependent) pathways.
Fig. 6. Effect of dominant-negative AMPK and AMPK CA on cytokine-mediated apoptosis in dispersed mouse pancreatic islets.
(a) Dispersed islets were infected or not with the indicated adenoviruses at 100 MOI (Null/GFP, AMPK DN, AMPK CA) for 48 h. Then, islets infected with Null/GFP or AMPK DN were treated or not with cytokines, as in Fig. 1, for a further 24 h or 48 h. Islets infected with AMPK CA were maintained for a further 24h. Apoptosis was measured by TUNEL assay as described in Fig. 4, followed by nucleus coloration with Hoechst dye (BF represents the bright field). (b) Data from (a) are given as means ± SEM of duplicate analyses from five experiments involving 50 individual cells per condition. Scale bar, 5 µm. *p<0.05 for the effect of AMPK DN with cytokines compared with Null virus in presence of cytokines. (c) Dispersed islets were treated with cytokines during 24 h and caspase-3 cleavage was measured by immunocytochemistry with the appropriate antibody, followed by nucleus coloration with Hoechst dye. (d) Data from (c) are given as means ± SEM of duplicate analyses from three experiments involving 50 individual cells per condition. Scale bar, 5 µm. **p<0.01 for the effect of the cytokines compared with the control without cytokines.
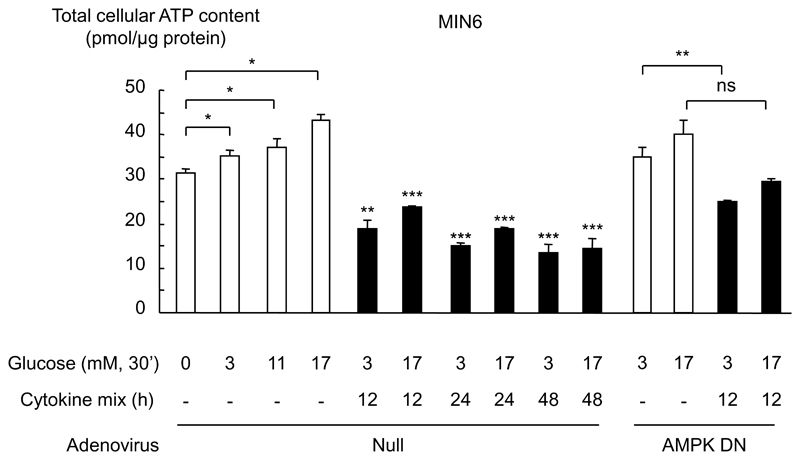
Cytokines induce a decrease in total cellular ATP content
AMPK is an AMP-sensitive enzyme whose activity is expected, at least in large part, to be regulated by changes in intracellular ATP/AMP ratio. Given that the adenylate kinase reaction is likely to be at near equilibrium in β-cells, we measured the total cellular ATP content as a guide to ATP/AMP ratio (Fig. 7). As anticipated, cellular ATP content was increased as glucose concentrations were elevated, consistent with a lowering of AMP levels and inhibition of AMPK (15;41). Interestingly, when cells were incubated for 12 h to 48 h with the cytokine combination, the cellular ATP content was decreased at both 3 and 17 mmol/l glucose. These results thus suggest that the action of the cytokines on AMPK is likely to be due to a fall in ATP/AMP ratio, and LKB1-mediated phosphorylation of AMPKα (13). By contrast, AMPK DN had no significant impact on the action of the cytokine combination to lower cellular ATP content (Fig. 7).
Fig. 7. Effect of cytokines on total cellular ATP content in MIN6 cells.
MIN6 cells were infected with the indicated adenoviruses at 100 MOI (Null/GFP, AMPK DN) for 48 h, before treatment with cytokines as in Fig. 1, for the indicated times. Prior to ATP assay, cells were incubated with the indicated concentrations of glucose for 30 min (see Fig. 3). Cells were then extracted in perchloric acid as described under “Research Design and Methods”, before assay of total ATP. *p<0.05, **p<0.01 and ***p<0.001 for effects of glucose or cytokines compared to the corresponding controls. Data are the means ± SEM of triplicates from observations on three separate cell cultures. Results shown are representative of three independent experiments.
Inhibition of AMPK decreases the cytotoxic effect of CD8+ T cells on NOD pancreatic islets
CD8+ T cell cytotoxicity towards islets probably occurs via a combination of effects including cytokine-mediated killing, Fas/FasL apoptosis and lysis by perforin/granzymes. To determine whether AMPK might be involved in the cytotoxicity of CD8+ T cells against islets during type 1 diabetes, we used a cytotoxic assay (Fig. 8 (a)) involving insulin B15-23-reactive CD8+ T cells as effectors, and islets isolated from NOD mice and infected either with the Null or the AMPK DN adenoviruses as targets. The insulin-specific T-cells used in this study are low avidity and require a relatively high peptide concentration to stimulate them (42). Although NOD β-cells do express endogenous peptide, the concentration is low. However, β-cells (both wild type and NOD) also express MHC class I molecules so they can present added peptide. In other words, β-cells have dual role in this assay, serving both as the targets of insulin-specific cytolytic T cells and as antigen-presenting cells for these T cells. Inhibition of AMPK led to a 50% decrease of CD8+ T cell cytotoxicity towards the islets (Fig. 8 (c)). Lysis of B15-23 coated P815 target cells was used as a positive control for CD8+ T cell cytotoxicity (Fig. 8 (b)).
Discussion
The main aim of the present study was to determine whether AMPK activation may be involved in β-cell death induced by cytokines or cytotoxic T cells, and might therefore represent an interesting therapeutic target to enhance the efficacy of transplantation protocols.
The nature of the immunological effectors that induce apoptosis in β-cells, and may thus lead to β-cell destruction and type 1 diabetes, is still debated. However, involvement of autoreactive T cells and an inflammatory response in which perforin, granzyme B, Fas ligand (FasL), TNF-α, IL-1β, INF-γ, NO, or a combination of all of the above, has been implicated in the destruction of pancreatic β-cells (2;5;6). These molecules are likely to act in synergy to induce apoptotic signalling cascades through a caspase-3-dependant cell death (6;30). Nevertheless, it is also now recognised that endoplasmic reticulum stress, possibly triggered by IL-1α and acting via NO formation and caspase 12 activation (43), as well as stress-activated kinases through reactive oxygen species (ROS) production and loss of mitochondrial membrane potential, possibly triggered by IFN-γ/TNF-α (36), are also key factors in primary β-cell apoptosis.
In our hands, 12 h - 48 h treatment of MIN6 β-cells with the cytokines TNF-α, IL-1β and IFN-γ led to increased apoptosis as demonstrated by enhanced caspase-3 cleavage (Fig. 1). These findings are in accordance with previous results in rat pancreatic islets (44) and clonal insulin-secreting cells (45). Although still clearly apparent (Fig. 6 (c,d)), cytokine-induced caspase-3 cleavage in primary mouse islet (expected to be largely β-) cells was less markedly increased than was TUNEL staining (Fig. 6 (a,b)), suggesting that different apoptotic mechanisms may be triggered in primary cells versus clonal MIN6 cells. Kefas et al. have previously shown that chronic treatment of MIN6 cells or rat islets with low glucose concentrations, or a sustained activation of AMPK with AICAR, also leads to programmed cell death (17). Correspondingly, our results show an increase in AMPK activity in response to cytokines (Fig. 3) and in apoptosis in MIN6 cells after an infection with a recombinant adenovirus expressing a constitutively-active form of AMPK (AMPK CA; Fig. 2).
These changes were matched by significant decreases in ATP content and hence probable increases in AMP level, which are likely to underlie the activation of AMPK (Fig. 7). Nevertheless, it is also conceivable that increases in intracellular free Ca2+ concentration, and hence activation of CaMKK1β (11;45), may also contribute.
We demonstrate for the first time that cytokine-mediated β-cell death is inhibited when the activation of AMPK is blocked by over-expression of a dominant-negative form of the enzyme (Figs 4,5,6). Interestingly, AMPK DN had no apparent effect on the cytokine-induced changes in intracellular ATP content, indicating that the pro-apoptotic pathways activated in response to AMPK act downstream of the decline in ATP. Several mechanisms could explain the link between AMPK and apoptosis. Firstly, activation of AMPK may cause cell cycle arrest, as observed in prostate cancer and smooth muscle cells (46). This observation is consistent with the fact that the upstream kinase LKB1 (47) is a tumour suppressor mutated in Peutz-Jeghers syndrome. Importantly, recent work demonstrates that AMPK activation induces phosphorylation of the tumour suppressor p53, an event required to initiate cell-cycle arrest in G1 phase (48). Whilst this arrest was reversible in the short term, persistent activation of AMPK led to cell death. Providing alternative mechanisms, Kefas et al. showed that AICAR treatment of β-cells lead to a c-jun N-terminal kinase (JNK) and caspase-3 dependant apoptosis whereas Jambal et al. and Inoki et al. showed an inhibition of protein kinase B and mTOR, respectively, by AMPK and thus an inhibition of the anti-apoptotic pathway and protein synthesis (16;49;50). AMPK activation is also associated with increased mitochondrial superoxide-derived radicals (ROS) production and decreased mitochondrial activity (51;52).
There has been much debate on the mode of cytotoxity towards islet β cells mediated by CD8+ T cells and it is likely that cytokines, Fas/FasL system, and granzyme/perforin all play a role in the attack (53). We demonstrate here that AMPK may be involved in cytokine-induced apoptosis and we also show that the inhibition of AMPK decreases the cytotoxic effect of CD8+ T cells (Fig. 8) though this finding alone is insufficient to pinpoint which aspect of T cell action was affected. This question will need further elucidation, for example by blocking each of these pathways using cells from mice expressing targeted mutations, or with blocking antibodies. It has recently been shown by Suzuki et al. that ARK5, a novel AMPK catalytic subunit family member of AMPK, whose activation is directly regulated by Akt, leads to the resistance of colorectal cancer cells to Fas-induced apoptosis by negatively regulating procaspase-6 (54). Taken with our own findings, the latter result suggests that activation of different AMPK family members may exert quite distinct effects on cell survival.
In summary, we demonstrate for the first time that AMPK is involved in regulating β-cell apoptosis induced by cytokines and that AMPK is involved in CD8+ T cell cytotoxicity towards NOD mouse islets. These data suggest that inhibition of AMPK activity may enhance the survival of islets in diabetes or after islet transplantation.
Supplementary Material
Acknowledgments
We thank Drs Gabriela da Silva Xavier, Isabelle Leclerc and Richard Smith for discussion and Dr Sarah Richards, Dr Laura Parton, Rebecca Rowe, Dr Magalie Ravier, Stephen Chapman, Alan Leard (Bristol MRC Imaging Facility) and Dr Martin Spitaler (Sir Alexander Fleming building, Imperial College London, Facility for Imaging by Light Microscopy) for their assistance. Supported by a Fellowship from ALFEDIAM (France; A, R-C) and grants to GAR from the Medical Research Council (G0401641) the Wellcome Trust (Programmes 067081/Z/02/Z and 081958/Z/07/Z) and Diabetes UK. G.A.R. was a Wellcome Trust Research Leave Fellow and F.S.W was a Wellcome Trust Senior Fellow in Clinical Science.
Abbreviations
- ACC
acetyl-CoA carboxylase
- AICAR
5-amino-4-imidazolecarboxamide riboside
- AMPK
AMP-activated protein kinase
- AMPK CA
constitutively-active AMPK
- AMPK DN
dominant-negative AMPK
- BF
Bright Field
- CaMKK
calmodulin kinase kinase
- DMEM
Dulbecco's modifed Eagle's medium
- eGFP
enhanced green fluorescent protein
- FasL
Fas ligand
- FCS
fetal calf serum
- HRP
Horseradish Peroxidase
- IFN
interferon
- IGRP
Islet specific glucose-6-phosphatase catalytic subunit related protein
- IL
interleukin
- KRB
Krebs Ringer bicarbonate medium
- NO
nitric oxide
- NOD
non-obese diabetic
- ON
overnight
- ROS
reactive oxygen species
- TMR
tetramethylrhodamine
- TNF
tumour necrosis factor
- TRITC
Tetramethyl rhodamine isothiocyanate
- TUNEL
Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433–1440. doi: 10.1016/s0006-2952(03)00494-5. [DOI] [PubMed] [Google Scholar]
- 3.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 4.Pearl-Yafe M, Kaminitz A, Yolcu ES, Yaniv I, Stein J, Askenasy N. Pancreatic islets under attack: cellular and molecular effectors. Curr Pharm Des. 2007;13:749–760. doi: 10.2174/138161207780249155. [DOI] [PubMed] [Google Scholar]
- 5.Lee SC, Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol. 2007;39:497–504. doi: 10.1016/j.biocel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Hui H, Dotta F, Di M U, Perfetti R. Role of caspases in the regulation of apoptotic pancreatic islet beta-cells death. J Cell Physiol. 2004;200:177–200. doi: 10.1002/jcp.20021. [DOI] [PubMed] [Google Scholar]
- 7.Rutter GA, da Silva Xavier GX, Leclerc I. Roles of 5'-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J. 2003;375:1–16. doi: 10.1042/BJ20030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leclerc I, Rutter GA. AMP-activated protein kinase: a new beta-cell glucose sensor?: Regulation by amino acids and calcium ions. Diabetes. 2004;53(Suppl 3):S67–74. doi: 10.2337/diabetes.53.suppl_3.s67. [DOI] [PubMed] [Google Scholar]
- 12.Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 13.Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 14.Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335(Pt 3):533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva Xavier G, Leclerc I, Salt IP, Doiron B, Hardie DG, Kahn A, Rutter GA. Role of AMP-activated protein kinase in the regulation by glucose of islet beta cell gene expression. Proc Natl Acad Sci U S A. 2000;97:4023–4028. doi: 10.1073/pnas.97.8.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kefas BA, Cai Y, Ling Z, Heimberg H, Hue L, Pipeleers D, Van de Casteele M. AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol. 2003;30:151–161. doi: 10.1677/jme.0.0300151. [DOI] [PubMed] [Google Scholar]
- 17.Kefas BA, Heimberg H, Vaulont S, Meisse D, Hue L, Pipeleers D, Van de Casteele M. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia. 2003;46:250–254. doi: 10.1007/s00125-002-1030-3. [DOI] [PubMed] [Google Scholar]
- 18.Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, Foufelle F, Hue L. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett. 2002;526:38–42. doi: 10.1016/s0014-5793(02)03110-1. [DOI] [PubMed] [Google Scholar]
- 19.Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol. 2004;67:2005–2011. doi: 10.1016/j.bcp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Gil M, Pesi R, Perna S, Allegrini S, Giannecchini M, Camici M, Tozzi MG. 5'-aminoimidazole-4-carboxamide riboside induces apoptosis in human neuroblastoma cells. Neuroscience. 2003;117:811–820. doi: 10.1016/s0306-4522(02)00836-9. [DOI] [PubMed] [Google Scholar]
- 21.Su RY, Chao Y, Chen TY, Huang DY, Lin WW. 5-Aminoimidazole-4-carboxamide riboside sensitizes TRAIL- and TNF{alpha}-induced cytotoxicity in colon cancer cells through AMP-activated protein kinase signaling. Mol Cancer Ther. 2007;6:1562–1571. doi: 10.1158/1535-7163.MCT-06-0800. [DOI] [PubMed] [Google Scholar]
- 22.Campas C, Lopez JM, Santidrian AF, Barragan M, Bellosillo B, Colomer D, Gil J. Acadesine activates AMPK and induces apoptosis in B-cell chronic lymphocytic leukemia cells but not in T lymphocytes. Blood. 2003;101:3674–3680. doi: 10.1182/blood-2002-07-2339. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 24.Richards SK, Parton LE, Leclerc I, Rutter GA, Smith RM. Over-expression of AMP-activated protein kinase impairs pancreatic {beta}-cell function in vivo. J Endocrinol. 2005;187:225–235. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- 25.Aoki CA, Borchers AT, Ridgway WM, Keen CL, Ansari AA, Gershwin ME. NOD mice and autoimmunity. Autoimmun Rev. 2005;4:373–379. doi: 10.1016/j.autrev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Sibley RK, Sutherland DE, Goetz F, Michael AF. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985;53:132–144. [PubMed] [Google Scholar]
- 27.Itoh N, Hanafusa T, Miyazaki A, Miyagawa J, Yamagata K, Yamamoto K, Waguri M, Imagawa A, Tamura S, Inada M. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest. 1993;92:2313–2322. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallone R, Martinuzzi E, Blancou P, Novelli G, Afonso G, Dolz M, Bruno G, Chaillous L, Chatenoud L, Bach JM, van Endert P. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613–621. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 29.Savinov AY, Tcherepanov A, Green EA, Flavell RA, Chervonsky AV. Contribution of Fas to diabetes development. Proc Natl Acad Sci U S A. 2003;100:628–632. doi: 10.1073/pnas.0237359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. S97-107. [DOI] [PubMed] [Google Scholar]
- 31.Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA., Jr CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J Exp Med. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, Shastri N, Pamer EG, Janeway CA., Jr Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 33.Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345(Pt 3):437–443. [PMC free article] [PubMed] [Google Scholar]
- 34.Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim WH, Lee JW, Gao B, Jung MH. Synergistic activation of JNK/SAPK induced by TNF-alpha and IFN-gamma: apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell Signal. 2005;17:1516–1532. doi: 10.1016/j.cellsig.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Mellado-Gil JM, Aguilar-Diosdado M. High glucose potentiates cytokine- and streptozotocin-induced apoptosis of rat islet cells: effect on apoptosis-related genes. J Endocrinol. 2004;183:155–162. doi: 10.1677/joe.1.05542. [DOI] [PubMed] [Google Scholar]
- 38.Nikulina MA, Sandhu N, Shamim Z, Andersen NA, Oberson A, Dupraz P, Thorens B, Karlsen AE, Bonny C, Mandrup-Poulsen T. The JNK binding domain of islet-brain 1 inhibits IL-1 induced JNK activity and apoptosis but not the transcription of key proapoptotic or protective genes in insulin-secreting cell lines. Cytokine. 2003;24:13–24. doi: 10.1016/s1043-4666(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 39.Van de Casteele M, Kefas BA, Cai Y, Heimberg H, Scott DK, Henquin JC, Pipeleers D, Jonas JC. Prolonged culture in low glucose induces apoptosis of rat pancreatic beta-cells through induction of c-myc. Biochem Biophys Res Commun. 2003;312:937–944. doi: 10.1016/j.bbrc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Kefas BA, Cai Y, Kerckhofs K, Ling Z, Martens G, Heimberg H, Pipeleers D, Van de Casteele M. Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochem Pharmacol. 2004;68:409–416. doi: 10.1016/j.bcp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 41.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong FS, Moustakas AK, Wen L, Papadopoulos GK, Janeway CA., Jr Analysis of structure and function relationships of an autoantigenic peptide of insulin bound to H-2K(d) that stimulates CD8 T cells in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 2002;99:5551–5556. doi: 10.1073/pnas.072037299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu D, Pavlovic D, Chen MC, Flodstrom M, Sandler S, Eizirik DL. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS-/-) Diabetes. 2000;49:1116–1122. doi: 10.2337/diabetes.49.7.1116. [DOI] [PubMed] [Google Scholar]
- 44.Cattan P, Berney T, Schena S, Molano RD, Pileggi A, Vizzardelli C, Ricordi C, Inverardi L. Early assessment of apoptosis in isolated islets of Langerhans. Transplantation. 2001;71:857–862. doi: 10.1097/00007890-200104150-00006. [DOI] [PubMed] [Google Scholar]
- 45.Chang I, Cho N, Kim S, Kim JY, Kim E, Woo JE, Nam JH, Kim SJ, Lee MS. Role of calcium in pancreatic islet cell death by IFN-gamma/TNF-alpha. J Immunol. 2004;172:7008–7014. doi: 10.4049/jimmunol.172.11.7008. [DOI] [PubMed] [Google Scholar]
- 46.Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, et al. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 47.Forcet C, Etienne-Manneville S, Gaude H, Fournier L, Debilly S, Salmi M, Baas A, Olschwang S, Clevers H, Billaud M. Functional analysis of Peutz-Jeghers mutations reveals that the LKB1 C-terminal region exerts a crucial role in regulating both the AMPK pathway and the cell polarity. Hum Mol Genet. 2005;14:1283–1292. doi: 10.1093/hmg/ddi139. [DOI] [PubMed] [Google Scholar]
- 48.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 49.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 50.Jambal P, Masterson S, Nesterova A, Bouchard R, Bergman B, Hutton JC, Boxer LM, Reusch JE, Pugazhenthi S. Cytokine-mediated down-regulation of the transcription factor cAMP-response element-binding protein in pancreatic beta-cells. J Biol Chem. 2003;278:23055–23065. doi: 10.1074/jbc.M212450200. [DOI] [PubMed] [Google Scholar]
- 51.Cai Y, Martens GA, Hinke SA, Heimberg H, Pipeleers D, Van de Casteele M. Increased oxygen radical formation and mitochondrial dysfunction mediate beta cell apoptosis under conditions of AMP-activated protein kinase stimulation. Free Radic Biol Med. 2007;42:64–78. doi: 10.1016/j.freeradbiomed.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 52.Kim WH, Lee JW, Suh YH, Lee HJ, Lee SH, Oh YK, Gao B, Jung MH. AICAR potentiates ROS production induced by chronic high glucose: roles of AMPK in pancreatic beta-cell apoptosis. Cell Signal. 2007;19:791–805. doi: 10.1016/j.cellsig.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Dudek NL, Thomas HE, Mariana L, Sutherland RM, Allison J, Estella E, Angstetra E, Trapani JA, Santamaria P, Lew AM, Kay TW. Cytotoxic T-cells from T-cell receptor transgenic NOD8.3 mice destroy beta-cells via the perforin and Fas pathways. Diabetes. 2006;55:2412–2418. doi: 10.2337/db06-0109. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki A, Kusakai G, Kishimoto A, Shimojo Y, Miyamoto S, Ogura T, Ochiai A, Esumi H. Regulation of caspase-6 and FLIP by the AMPK family member ARK5. Oncogene. 2004;23:7067–7075. doi: 10.1038/sj.onc.1207963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.