Abstract
Zinc (Zn2+) ions are increasingly recognised as playing an important role in cellular physiology. Whereas the free Zn2+ concentration in the cytosol has been established to be 0.1-1 nM, the free Zn2+ concentration in subcellular organelles is not well-established. Here, we extend the eCALWY family of genetically-encoded Förster Resonance Energy Transfer (FRET) Zn2+ probes to permit measurements in the endo(sarco)plasmic reticulum (ER) and mitochondrial matrix. Deployed in a range of mammalian cell types, these probes reveal resting mitochondrial free [Zn2+] values of ~ 300 pM, somewhat lower than in the cytosol but three orders of magnitude higher than recently reported using an alternative FRET-based sensor. By contrast, free ER [Zn2+] was found to be ≥5 nM, which is >5000-fold higher than recently reported, but consistent with the proposed role of the ER as a mobilisable Zn2+ store. Treatment of β-cells or cardiomyocytes with sarco(endo)plasmic reticulum Ca2+-ATPase inhibitors, mobilization of ER Ca2+ after purinergic stimulation with ATP, or manipulation of ER redox, exerted no detectable effects on [Zn2+]ER. These findings question the previously proposed role of Ca2+ in Zn2+ mobilization from the ER and suggest that high ER Zn2+ levels may be an important aspect of cellular homeostasis.
Zinc is an essential trace element in all living cells, binding to as many as 3,000 proteins (~10% of human proteome).1 In addition to its structural role in proteins, zinc is also an important cofactor in more than 300 metalloenzymes2 and redox sensors.3 Because of its role in fundamental cellular processes such as transcription, cell proliferation, apoptosis, and possibly signal transduction,2,4 precise regulation of the uptake, storage and availability of zinc ions is vital. To regulate intracellular free Zn2+ concentrations, an elaborate system exists in mammalian cells involving metal binding proteins such as metallothionein (MT),3,5 zinc transporters (ZnT, encoded by Slc30a1-10) and zinc importers (ZiP, Zrt- and Irt-like proteins encoded by Slc39a1-14).6 High Zn2+ concentrations (total and free) within the endoplasmic reticulum or other organelles may conceivably provide a mobilisable store (or buffer) for these ions which might be targeted by hormones or other stimuli to provoke changes in cytosolic free Zn2+ concentration.7–10 At present, however, evidence for such fluxes of zinc in individual cells is sparse and sometimes conflicting.
We have recently described a new generation of genetically-encoded Zn2+ probes termed “eCALWYs”.11 These Förster Resonance Energy Transfer (FRET)-based probes consist of two cysteine-containing metal binding domains (ATOX1 and WD4) connected by a long, flexible glycine-serine linker and flanked by GFP-derived cerulean and citrine fluorescent domains (Figure 1a). In the absence of zinc an intramolecular complex is formed between cerulean and citrine which results in high FRET. Conversely, when Zn2+ binds between ATOX and WD4, the interaction between cerulean and citrine is disrupted, lowering the citrine:cerulean fluorescence intensity ratio. Shortening of the linker length between the metal binding domains and/or mutation of one of the metal binding cysteines in the WD4 domain yielded a series of sensor variants (eCALWY1-6) with Zn2+ affinities ranging between 2 pM and 5 nM, while all displaying at least a two-fold change in emission ratio.11,12 Mid-range in this sensor toolbox, eCALWY-4 has a dissociation constant (Kd = 630 pM) that is appropriate for in cellulo measurements and has therefore been used successfully to determine cytosolic free zinc concentrations in HEK293, HeLa, primary and immortalised pancreatic β-cells.11–13
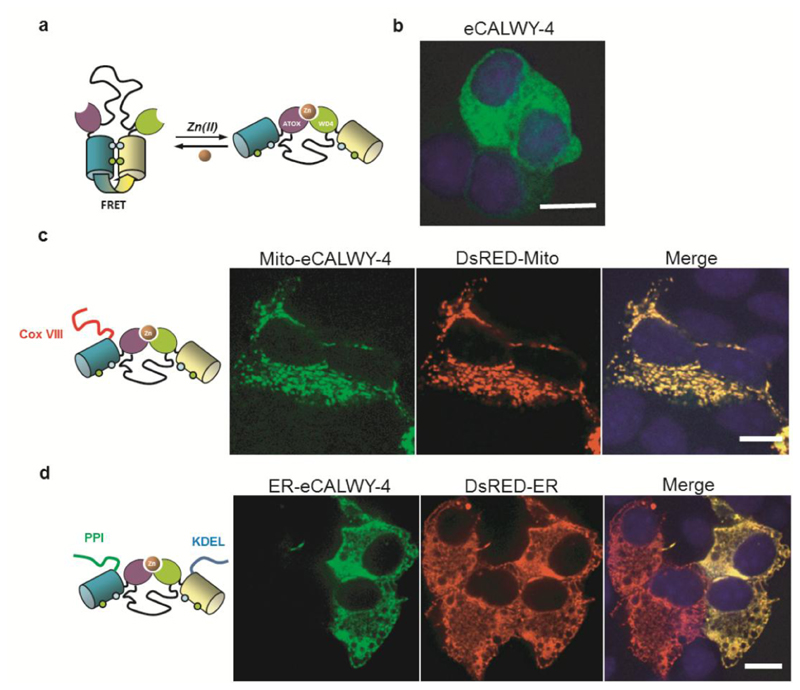
Figure 1. Design and subcellular localisation of mito-eCALWY-4 and ER-eCALWY-4 in MIN6 β-cells.
(a) eCALWY-4 sensor design11 and (b) localization in the cytosol in MIN6 cells. (c) MIN6 cells were co-transfected with an eCALWY-4 construct targeted to the mitochondria (mito-eCALWY-4) and with a mitochondrial marker (DsRED-Mito). (d) MIN6 cells were co-transfected with an eCALWY-4 construct targeted to the Endoplasmic Reticulum (ER-eCALWY-4) and with an ER marker (DsRED-ER). Images were acquired on a spinning-disk system with a 63x/NA1.4 objective. Merged images show a precise localisation in the targeted organelles. Scale bars represent 10 µm.
The high picomolar (400-1500 pM) values obtained for [Zn2+]cyt are close to those measured in other secretory and non-secretory cell types using synthetic, intracellularly-trappable fluorescent probes such as FluoZin-3,1,14 but considerably higher than measurements made using carbonic anhydrase-(CA) based sensors in PC12 cells.15,16 Subsequent studies17,18 using the ZapCY2 FRET-sensor protein reported values that, albeit somewhat lower, are in the same range as those determined using the eCALWY probes. These free Zn2+ levels are buffered by the cytosolic metallothionein system and are sufficient to supply Zn2+ to endogenous zinc binding proteins, yet below those levels that are known to inhibitory to several metabolic enzymes.1
Recent reports using the ZapCY1 FRET sensor17,19 have suggested that concentrations of free Zn2+ may be far lower in the ER, Golgi (~1 pM) and mitochondria (~0.1 pM) than those reported in the cytosol. A value of 0.2 pM has also been reported using a mitochondria-targeted CA-based sensor,16 while a substantially higher concentration of 72 pM was recently determined using a small molecule ratiometric fluorescent probe targeted to the mitochondria of NIH3T3 cells.20 The low pM values would appear to argue against a role for the latter pools in regulating cytosolic zinc levels, or providing a mobilisable source of these ions whose release could be triggered in response to extracellular stimuli or other environmental changes. Organellar Zn2+ measurements with the eCALWY probes, which provide a large range of Kd values and robust FRET responses, have so far only been reported for the secretory granule.11 Here, we report targeting of the eCALWYs to the endoplasmic reticulum or mitochondrial matrix in a variety of mammalian cell types including primary pancreatic β-cells and cardiomyocytes. Surprisingly, the eCALWY probes show free Zn2+ concentrations in the mitochondria (300 pM) and ER (>5 nM) that are different from the cytosol and three orders of magnitude higher than those reported with the ZapCY1 sensor. Importantly, these results suggest that ER/SR Zn2+ concentrations might be far higher and more resistant to the effects of cell stimulation and Ca2+ fluxes across the ER membrane, than proposed.17,19
Results and Discussion
Targeting eCALWY probes to the ER/SR and mitochondria
Examined in the murine pancreatic β-cells-derived line, MIN6, non-targeted eCALWY-4 displayed the expected cytosolic localisation (Figure 1b). We targeted the probe to the mitochondrial matrix by fusion with cytochrome c oxidase subunit VIII (Cox VIII) signal peptide,21 generating the new reporter mito-eCALWY-4. This sequence has previously been used to target other probes, including aequorin,21,22 to mitochondria and, after cleavage, is expected to lead to the liberation of the active soluble reporter in the matrix.
Co-expression of mito-eCALWY-4 with the genetically-encoded mitotracker DsRed-mito showed essentially complete overlap of fluorescence, confirming correct targeting of the zinc probe to this compartment (Figure 1c). Secondly, we targeted eCALWY-4 to the ER (ER-eCALWY-4) by the addition of an N-terminal preproinsulin (PPI) sequence as a signal peptide and a C-terminal Lys-Asp-Glu-Leu (KDEL) sequence, inducing probe retention within the ER lumen.23 Co-expression with a genetically-encoded ER-tracker (DsRed-ER) demonstrated precise localisation to the target compartment in MIN6 β-cells (Figure 1d). After packaging into adenoviral particles24, correct organellar localisation of the expressed targeted probes was also demonstrated in primary rat cardiomyocytes (Figure S1).
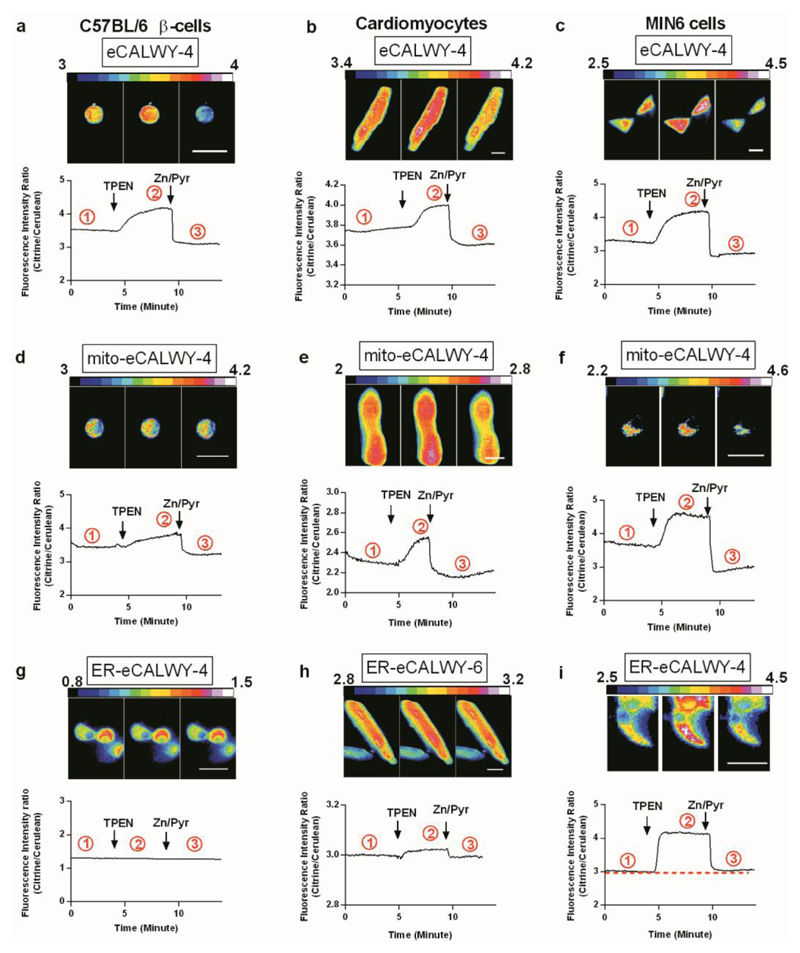
Figure 2 shows representative traces illustrating the behaviour of cytosolic eCALWY-4, mito-eCALWY-4 or ER-eCALWY-4/6 in primary murine β-cells (C57BL/6), primary rat cardiomyocytes and MIN6 cells. As discussed below, ER-eCALWY6 (Kd = 2.9 nM) was designed to extend the range of concentrations over which Zn2+ could be measured in this compartment. ER-eCALWY-6 probe localisation was identical to ER-eCALWY-4 localisation (not shown). Collected data from similar experiments in these and other cell types (Figure S2) are provided in Table 1.
Figure 2. Measurement of resting compartmentalised free Zn2+ concentrations in primary murine β-cells, primary rat cardiomyocytes and MIN6 cells.
Representative traces and ratiometric images are shown for primary murine β-cells (a,d,g), ventricular cardiomyocytes (b,e,h) and MIN6 cells (c,f,i) expressing cytosolic, mitochondrial or ER-targeted sensors. Murine β-cells and rat cardiomyocytes were transduced with adenoviral particles and MIN6 cells were transfected with plasmid constructs. Acquisitions were performed 16-24 h after transfection or infection, and during perifusion with KREBS-Hepes-Bicarbonate (KHB) buffer. Steady state fluorescence intensity ratio (Citrine/Cerulean) was first measured before the maximum ratio was obtained under perifusion with KHB buffer containing 50 µM TPEN (Zinc-free condition). Finally minimum ratio was obtained under perfusion with KHB buffer containing 5 µM pyrithione and 100 µM Zn2+ (Zinc-saturated condition). Ratio images were captured at the points (1,2,3) on the traces circled. Scale bars represent 20 µm.
Table 1. Cytosolic, mitochondrial and ER free Zn2+ concentrations measured in different cell types, using the indicated e-CALWY sensor.
N values indicate number of cells (non-saturated) used in quantitation of Zn2+ and total cells analysed (parentheses). Where 100 % of cells were saturated, values given are based on an estimate from the Kd of the probe used. Values are means (± S.E.M) - nd, not determined.
| Cell type | Cytosolic Concentration (nM) e-CALWY-4 | n value | Percentage of cells saturated | Mitochochondrial Concentration (nM) mito-eCALWY-4 | n value | Percentage of cells saturated | ER Concentration (nM) ER-eCALWY-4 |
n value | Percentage of cells saturated | ER Concentration (nM) ER-eCALWY-6 |
n value | Percentage of cells saturated |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HEK293 | 1.84 ± 0.18 | 37 (53) | 30% | 0.3 ± 0.04 | 5 (5) | 0% | 1.6 ± 0.26 | 7 (32) | 78% | 5.58 ± 0.7 | 6 (10) | 44% |
| HeLa | 1.18 ± 0.21 | 13 (20) | 35% | 0.23 ± 0.03 | 10 (12) | 16% | > 5 | 0 (9) | 100% | nd | ||
| MIN6 | 1.89 ± 0.28 | 22 (42) | 48% | 0.25 ± 0.07 | 10 (13) | 23% | > 5 | 0 (35) | 100% | 7.24 ± 1.0 | 2 (4) | 50% |
| Rat cardiomyocytes | 0.61 ± 0.07 | 42 (49) | 14% | 0.18 ± 0.04 | 18 (18) | 0% | 4.13 ± 1.75 | 3 (8) | 62% | 5.6 ± 1.1 | 6 (6) | 0% |
| C57BL/6 beta cells | 1.5 ± 0.3 | 13 (23) | 43% | 0.25 ± 0.03 | 10 (10) | 0% | N/A | N/A | ||||
| Human beta cells | 2.2 ± 0.6 | 18 (24) | 25% | nd | N/A | N/A | ||||||
Cells expressing eCALWY-4 and mito-eCALWY-4 displayed large changes in FRET ratio upon addition of the membrane permeable chelator TPEN and subsequent addition of excess ZnCl2 in the presence of the ionophore pyrithione (Figure 2a-f). This calibration allowed determination of [Zn2+]cyt for each individual cell, yielding values of 0.6-2.2 nM that are broadly consistent with previous reports with this probe in β-cell lines11 though slightly higher. A variable percentage of cells of each type analysed presented with a saturated response, meaning that in these cells [Zn2+]cyt was ≥5 nM (given a Kd value of the probe of 630 pM).11
The occupancy of the mito-eCALWY-4 probes was found to be ~80% in all cells examined. The Zn2+ affinity of the eCALWY probes is pH dependent11 and their use in different organelles requires that the Kd value is determined at the relevant pH. Since intramitochondrial pH values are typically ~7.825 we re-determined the Kd value of eCALWY-4 at this pH and obtained a value of 60 pM (Figure S3). Using this value, the mitochondrial zinc concentrations were determined to be 0.2-0.3 nM in each of the cell types interrogated (Table 1). While this concentration is somewhat lower than typically observed in the cytosol, these values are 3 orders of magnitude higher than recently reported using a mitochrondria-targeted ZapCY1 FRET sensor. In this case, the percentage of cells in which the Zn2+ probe was saturated was low, indicative of relatively low cell-cell variability (Table 1).
ER-eCALWY4 was found to be fully saturated at the start of the experiment in MIN6 (Figure 2i), HeLa and the great majority of HEK293 cells (80%) examined (Figure S2, Table 1), which means that [Zn2+]ER was ≥ 5 nM. Even in the small percentage of HEK293 cells and primary cardiomyocytes in which ER-eCALWY-4 appeared not completely saturated, the average sensor occupancy was still > 80%, corresponding to [Zn2+]ER = 2-4 nM. In each case, a population was also observed that did not respond to TPEN or Zn2+/pyrithione, possibly reflecting mis-folding of the probe in the ER lumen.
Unfortunately, adenovirus-mediated expression of ER-eCALWY-4 failed to lead to the production of a Zn2+-sensitive reporter in primary β-cells (61 cells examined from 5 mice; Figure 2g), and the adenovirally-delivered probe was similarly unresponsive in HEK293 and MIN6 cells. On the other hand expressed in primary cardiomyocytes, ER-eCALWY-4 responded to zinc changes in a substantial minority of cells examined (8/23 from 6 rats), whereas in the remaining cells the probe did not respond to TPEN or Zn2+/pyrithione (again, likely to reflect mis-folding of the probe in this compartment). The above value provides an estimate for free Zn2+ in the ER and/or SR of cardiomyocytes of ~4 nM or above (Table 1).
Given the observation that [Zn2+]ER values were close to, or above, levels where ER-eCALWY-4 was saturated in the majority of cell types examined, we turned next to the use of the less Zn2+-sensitive (Kd = 2.9 nM) probe, ER-eCALWY-6. In this case, the proportion of cells displaying probe saturation was substantially lower (44, 50 and 0% in HEK293, MIN6 and cardiomyocytes, respectively, Table 1) returning [Zn2+]ER values in the range 5-7 nM in each case. Of note, the change in emission ratio upon calibration was low with ER-eCALWY-6, limiting the precision of the measurements with this probe.
Free Zn2+ concentrations in the cytosol and ER remain stable in MIN6 cells, primary β-cells and cardiomyocytes during large excursions in ER calcium content and redox state
Using a zinc finger-based probe (ER-ZapCY1) Palmer and colleagues17 recently reported that [Zn2+]ER is lowered by mobilisation of Ca2+ from this store in HeLa cells. Similarly, others26 reported an action of Ca2+ store depletion to increase cytosolic Zn2+ in cortical neurones. We therefore used simultaneous imaging of Zn2+ alongside cytosolic Ca2+, monitored with Fura-2, to determine whether a similar phenomenon may be evident in excitable β-cells or heart cells.
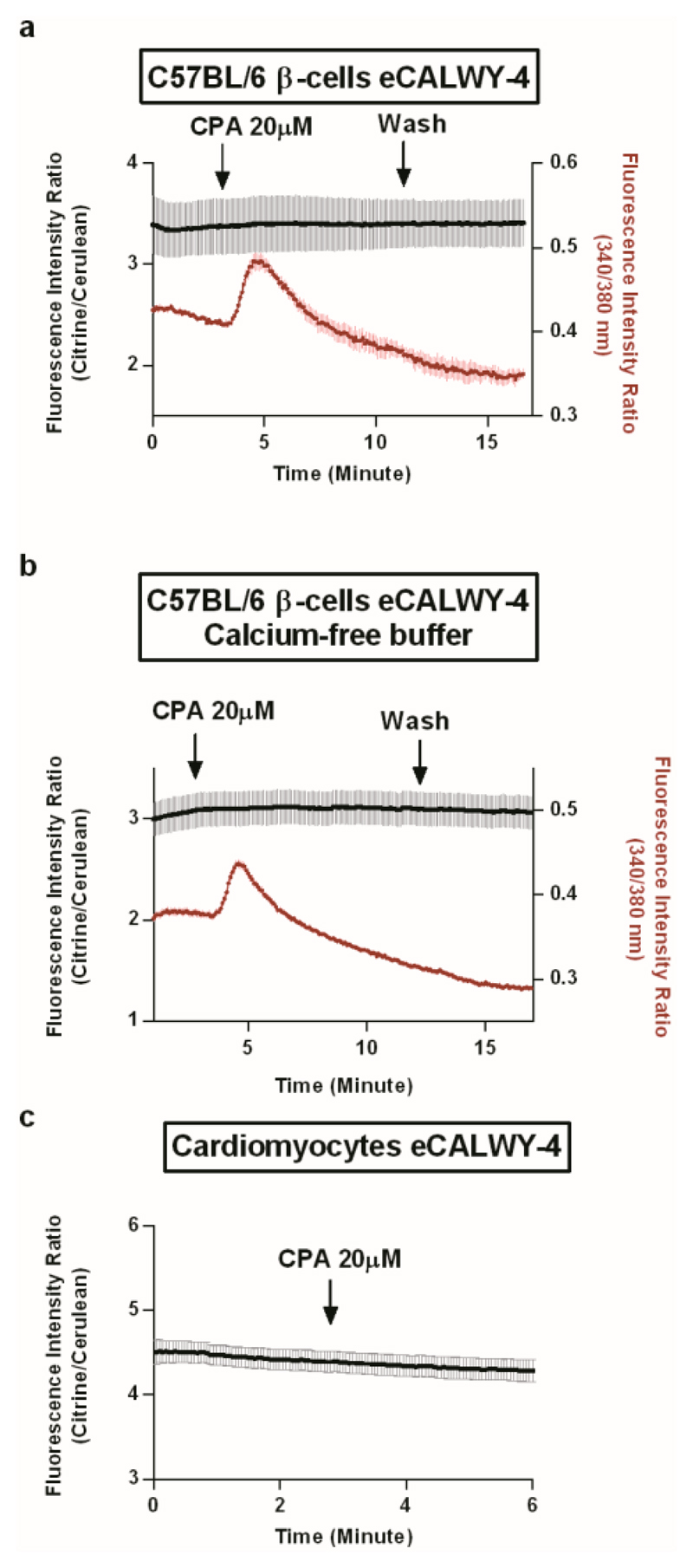
Contrary to this expectation, cyclopiazonic acid (CPA), a potent inhibitor of sarco(endo)plasmic reticulum Ca2+ATPase (SERCA) pumps, whose action leads to ER Ca2+ release via leak channels,27 exerted no significant effect on cytosolic Zn2+ in primary β-cells (Figure 3a,b) or myocytes (Figure 3c) whilst clearly mobilising ER Ca2+ (as measured indirectly via changes in cytosolic Ca2+ in β-cells [red traces] or via cell contraction in myocytes; not shown). Thus, the stability of the cytosolic Zn2+ concentration under these conditions strongly suggests the absence of zinc release from the ER.
Figure 3. SERCA inhibition with CPA and ER calcium depletion with has no effect on cytosolic free Zn2+ in primary murine β-cells and primary rat cardiomyocytes.
(a,b) Primary β-cells expressing eCALWY-4 were preincubated with Fura-2 (3µM) in KHB for 20 minutes before acquisition. Acquisitions rate was 12 images per minutes and fluorescence intensity ratios were determined sequentially for eCALWY-4 and Fura-2. Representative results are shown (a) Cells were perifused with KHB and after 3 minutes were treated with CPA 20 µM (n = 3 cells). (b) Cells were perifused with KHB containing 100 µM EGTA (Calcium-free buffer) and treated in the same conditions (n = 7 cells). (c) Cardiomyocytes expressing eCALWY-4 were perfused with KHB for 3 minutes then with KHB containing 20 µM CPA (n = 4 cells). Data are means ± S.E.M.
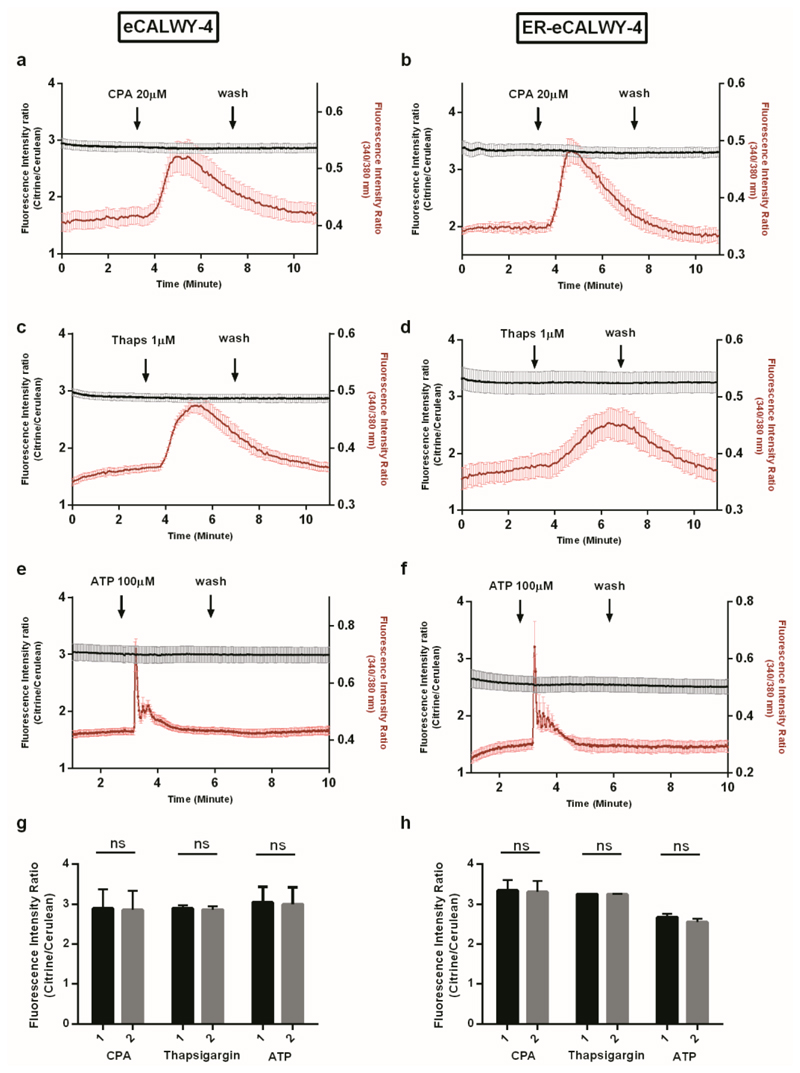
The latter result was unaffected by removal of calcium from the medium in primary β-cells (Figure 3b), a condition under which cytosolic calcium signals are not confounded by Ca2+ influx from the extracellular medium. Likewise, neither CPA nor thapsigargin, another SERCA pump inhibitor,27,28 substantially affected [Zn2+]cyt (Figure 4a,c,g) or caused [Zn2+]ER to fall below 5 nM (Figure 4b,d,h) in MIN6 β-cells. Again, similar observations were made in the absence of external calcium (Figure S4a,b). Furthermore, stimulation of MIN6 cells with the purinoreceptor agonist ATP,29 which prompts robust and rapid release of ER calcium through inositol 1,4,5 trisphosphate receptors, exerted no detectable effect on [Zn2+]cyt or in apparent [Zn2+]ER, either in the presence (Figure 4e-h) or in absence of external calcium (Figure S4c,d).
Figure 4. Simultaneous recording of free ER Zn2+ and cytosolic free Ca2+ in response to CPA, thapsigargin or ATP treatment of MIN6 cells.
MIN6 cells expressing eCALWY-4 (a,c,e) or ER-eCALWY-4 (b,d,f) were incubated with Fura-2 (3 µM) in KHB for 20 minutes before acquisition. Fluorescence intensity ratios were determined sequentially for eCALWY-4 sensors and Fura-2. After 3 minutes of perfusion with KHB, cells were treated with different drugs. Acquisition rates were 20 images per minutes for CPA and thapsigargin experiments, and 30 images per minute for ATP experiments. Results displayed are representatives of the responses obtained. (a) MIN6 cells expressing eCALWY-4 were treated with 20 µM CPA (n = 6 cells) (b) MIN6 cells expressing ER-eCALWY-4 were treated with 20 µM CPA (n = 6 cells) (c) MIN6 cells expressing eCALWY-4 were treated with 1 µM thapsigargin (n = 9 cells) (d) MIN6 cells expressing ER-eCALWY-4 were treated with 1 µM thapsigargin (n = 4 cells) (e) MIN6 cells expressing eCALWY-4 were treated with 100 µM ATP (n = 9 cells) (f) MIN6 cells expressing ER-eCALWY-4 were treated with 100 µM ATP (n = 3 cells). Data are means ± S.E.M.
(g,h) Mean ratios were calculated before (1) and during (2) calcium response for represented traces and for an acquisition time of 1 minute. (g) Mean ratios upon CPA, thapsigargin and ATP treatment for MIN6 cells expressing eCALWY-4. (h) Mean ratios upon CPA thapsigargin and ATP treatment for MIN6 cells expressing ER-eCALWY-4. Error bars represent S.D.
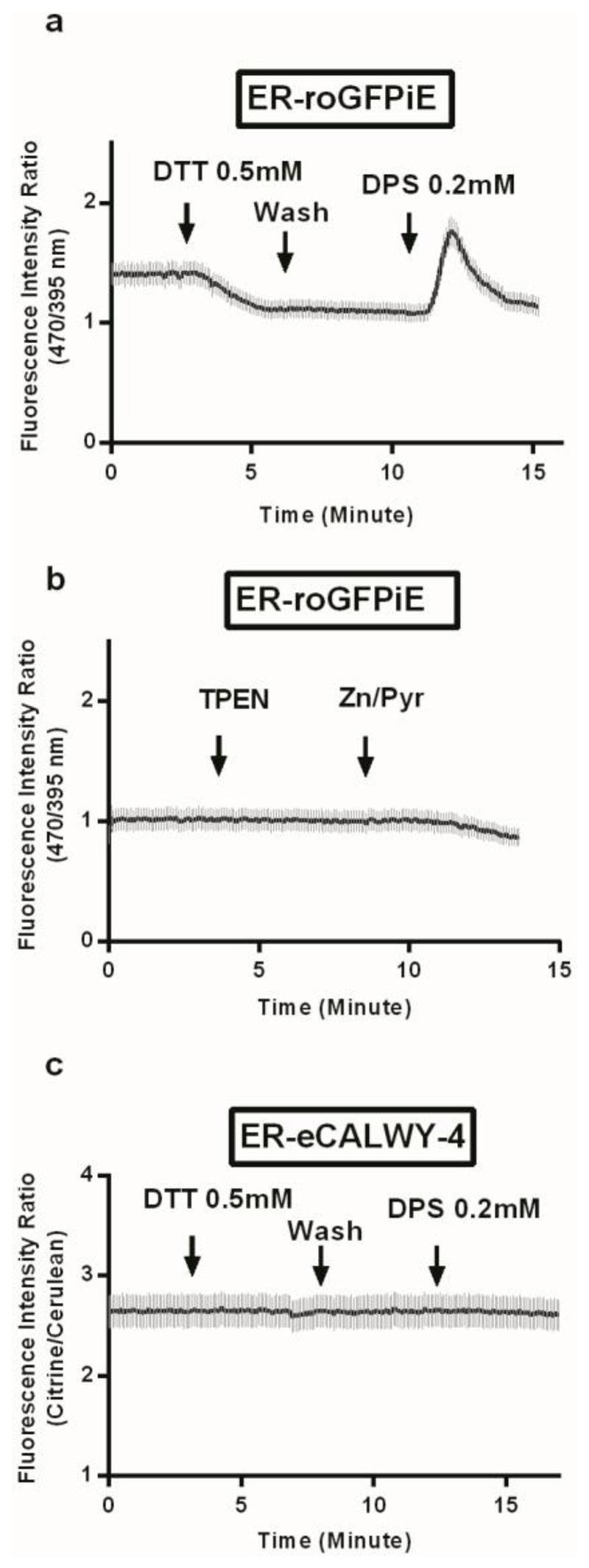
The ER redox state is known to be substantially more oxidising than the cytosol,30 providing a permissive environment for disulphide bond formation and protein folding. We therefore also determined whether fluctuations in ER redox, which may conceivably occur during the stimulation of pancreatic β-cells with fuels31 and might influence [Zn2+]ER. To test this possibility, cells were treated with the reducing agent dithiothreitol (DTT), or with the oxidising agent 2-2’-dithiodipyridine (DPS), manoeuvres which led to the expected changes in ER redox state, as monitored with the targeted redox probe ERroGFPiE32 (Figure 5a). By contrast, excursions in [Zn2+]ER elicited by the sequential addition of TPEN and Zn2+/pyrithione had no impact on ER redox state (Figure 5b). Likewise, forced changes in redox had no evident impact on ER-eCALWY-4 ratio (Figure 5c). Considering that the probe has been shown to be zinc-saturated under these conditions (Figure 2i) this indicates that [Zn2+]ER remains above 5 nM.
Figure 5. Forced changes in ER redox state do not impact ER free Zn2+ levels in HEK293 cells as reported with EReCALWY-4.
(a,b) HEK293 cells were transfected with a construct expressing the redox-sensitive GFP targeted to the ER, ERroGFPiE.32 (a) Redox status changes in the ER were monitored with ERroGFPiE: cells were perfused and treated as stated with reducing agent DTT and oxidising agent DPS (n = 8 cells). (b) TPEN and Zn2+/pyrithione treatment on ER-roGFPiE-expressing cells showed no impact on the ER redox state (n = 7 cells). (c) ER-eCALWY4 expressing HeK cells were perfused and treated by DTT and DPS, which had no effect on fluorescence intensity ratio (n = 8 cells). Data are means ± S.E.M.
Discussion
Our major aim in the present study was to compare the free concentration of Zn2+ ions in three subcellular locations: the cytosol, ER lumen and mitochondrial matrix, in a variety of mammalian cell types. To this end, we addressed the recently developed and robust eCALWY zinc sensors11 to these compartments.
The current experiments revealed, firstly, that cytosolic [Zn2+] values can vary between different cell types when assessed using an identical probe and the same imaging system. Thus, previous reports1 of variations reported in separate studies and using different probes may represent genuine differences between cell types.18 Moreover, we12 recently reported that long term (24 h) culture at elevated glucose concentrations increased free cytosolic Zn2+, as reported with eCALWY-4, showing that variations can exist in this parameter for a given cell type depending on culture conditions. We also noted during the course of the present studies that considerable cell-cell variation in cytosolic Zn2+ was apparent in cardiomyocytes, and was related to the apparent health of individual cells: those with abnormal (rounded) morphology tended to display higher [Zn2+]cyt values (Tuncay et al, in preparation).
We did, however, find a higher cytosolic zinc concentration in HEK293 cells than previously determined with the same probe (1.8 vs 0.4 nM).11 We suspect that this difference is due to several factors including: the use here of a different batch of HEK293 cells, improvements in the transfection, acquisition, background correction and analysis protocols, and correction for photobleaching.
Next, we demonstrated that the limiting membranes of both mitochondria and the ER sustain significant gradients of Zn2+ ions versus the cytosolic compartment, with lower and higher free zinc levels respectively pertaining in these organelles. Across all the cell types examined here the mitochondrial free [Zn2+] was ~2000-fold higher than the value of ~0.14 pM reported by Palmer and colleagues19 using the mito-ZapCY1 sensor in HeLa cells and the 0.2 pM obtained by McCranor et al using a CA-based sensor in PC12 cells.16 Our values are more in line with the 72 pM that was recently determined using a small-molecule ratiometric fluorescent probe targeted to the mitochondria of NIH3T3 cells.20 We based our calculation of mitochondrial free [Zn2+] on a mitochondrial pH value of 7.8. This pH may be less in pancreatic β-cells where lower intramitochondrial pH values have been reported (~7.3), at least in clonal insulinoma-derived INS1E cells at low glucose concentrations.33 Should the latter pH values pertain then the present data would translate into an even higher concentration of free Zn2+, suggesting that β-cells may be different from the other cell lines studies here in maintaining a smaller gradient (out vs in) for mitochondrial Zn2+. Elevated concentrations of Zn2+ such as those that occur following ischemia34 are known to inhibit mitochondrial energy metabolism, and several enzyme systems in respiratory metabolism have been reported to be inhibited by Zn2+, including the cytochrome bc1 complex and α-ketoglutarate dehydrogenase.35,36 Thus, the work of Brown and co-workers37 showed that the lipoamide dehydrogenase reaction catalysed by the latter enzyme is inhibited by Zn2+, whereas the oxidase reaction that results in the production of hydrogen peroxide is enhanced 5-fold. However, the apparent inhibition and activation constants for Zn2+ (0.15 µM and 0.09 µM, respectively) are still much higher than the 300 pM mitochondrial Zn2+ concentrations obtained in this work. To the best of our knowledge, there is no evidence of mitochondrial enzymes that are inhibited by sub-nanomolar Zn2+ concentrations.
Whilst we show that our new ER probes function well in cell lines after conventional transfection of plasmids, delivery of these probes using adenoviral vectors (necessary for efficient expression of the probe in primary cells) was more problematic. Although the reasons behind this are not obvious, we were able to exclude proteolysis by the absence of degraded products in Western analysis (not shown). Notably, infection was also problematic in MIN6 cells (data not shown), strongly indicating that stresses induced by infection are likely to be the cause of the unresponsiveness. Interestingly, probe localisation to the ER was unaffected while the steady-state emission ratio was unusually low (~1), suggesting mis-folding of the probe.
Successfully deployed in the clonal MIN6 cell line upon transfection, as well as two other transformed cell lines (HEK293, HeLa), the ER-eCALWY probes were saturated with Zn2+ in the majority of cells. These observations suggested that the concentration of Zn2+ ions in the ER was likely to be in excess of ~5 nM (i.e. some 8-10-fold above the Kd of the probe for Zn2+, ~600 pM). Measurements achieved with the less Zn2+-sensitive, but more weakly responding, ER-eCALWY-6, further indicated that ER/SR concentrations are likely to be around 5-8 nM in about 50 % of HEK293 and MIN6 cells, where the probe was not fully saturated and higher in the remaining cells. Likewise, in primary cardiomyocytes, [Zn2+]ER was ≥ 6 nM (Table 1). Taken together, across all the cell types tested here, we conclude that ER/SR free zinc concentrations are substantially higher than the cytosolic zinc values. The values reported here for the ER are more compatible with the known coordination chemistry of thiols which, through metallothionein in particular, create high affinity Zn2+ binding sites in the cytosol. In the oxidising environment of the ER (and Golgi), free thiols are not available to provide these high affinity Zn2+ binding sites, which means that one would have to invoke other mechanisms (e.g. vigorous pumping/exchange across the ER membrane) to maintain free Zn2+ concentrations of 1 pM or lower.
Importantly, the [Zn2+]ER values (5 nM or above) reported here seem more compatible with the view that the ER compartment may provide a sink or store for Zn2+ ions8 than might be surmised from measurements of <1 pM.17 Indeed, studies in the past few years have suggested that changes in cytosolic free Zn2+ may be an important means of transmitting signals generated at the plasma membrane across the cytoplasmic space, at least in immune cells.7–10 Similarly, an influx of Zn2+ across the plasma membrane has been reported in several studies,38 notably in response to depolarisation of excitable cells including primary β-cells39 (P.C., G.M., G.A.R., unpublished). However, more controversial has been the suggestion that the ER may serve as a mobilisable store of these ions in such cells. Indeed, the recent work by Qin et al17 using ER-ZapCY1 probe suggested that Zn2+ may decrease in the ER during Ca2+ mobilisation in HeLa cells (albeit an initial concentration lower than 1 pM). Moreover, a study26 reported an action of Ca2+ store depletion by thapsigargin to increase cytosolic Zn2+ in cortical neurones, and suggested a mobilisation of ER Zn2+. The present report failed to replicate the findings of the latter study in β-cells and cardiomyocytes.
In these primary cell types, changes in [Zn2+]ER were monitored indirectly by measuring [Zn2+]cyt using eCALWY-4 sensor. However, in MIN6 cells (as in other cell lines), ER-eCALWY-4 was deployable in order to follow a potential decrease in ER Zn2+.Thus, we found that a variety of manoeuvres causing drastic changes in cytosolic and/or ER calcium failed to decrease apparent [Zn2+]ER below 5 nM in MIN6 and primary β-cells as well as cardiomyocytes (Figure 4). Whilst we have no clear explanation for this difference between the present results and previous reports,17,19,26 we cannot exclude the possibility of differences in this respect between the relationship between ER Zn2+ and Ca2+ in HeLa17 or cortical neurones 26 and the cell types examined here. Furthermore, we note that an increase in ER Zn2+ would be difficult to detect with the ER probes used in this study, given their apparent near-saturation under resting conditions.
Both ER and mitochondrial zinc concentrations were drastically higher than recent estimates performed with the targeted ZapCY1 probes.17,19 This discrepancy cannot simply be explained by an error in determining the Zn2+ affinities of one of the sensors, since the eCALWY sensors and ZapCY1/CY2 sensors give much more consistent results when applied in the cytosol. To exclude the possibility that the high Ca2+ concentration in the ER (~ 200 µM40) affected the eCALWY-4 sensor, we performed a Ca2+ titration in vitro. However, no significant response was observed upon addition of up to 860 µM Ca2+ (Figure S5).
We presently do not have a convincing explanation as to why the eCALWY and ZapCY-based sensors behave so differently when targeted to the ER and mitochondria. We note, however, that in the case of either probe, the calculated values for free Zn2+ are dependent upon the Kd value in situ remaining identical to that measured in vitro. Whilst known variables such as pH can be accounted for, the binding of other species, or protein mis-folding, could affect these values. Unfortunately, precise measurements of in situ Kd for the organelle-targeted probes are very difficult to perform, as they would require equilibration of Zn2+ ions across two (or three in the case of mitochondria) separate membranes. Importantly, in the present study, we obtained no evidence that disulphide bridge formation in the oxidising environment of the ER may lead to a change in Zn2+ binding for ER-eCALWY4 (Figure 5 and PC, unpublished results). In addition, partial oxidation of the cysteines present in eCALWY4 would be expected to decrease its affinity for Zn2+, and would therefore not provide an explanation for the differences observed with the ZapCY1-probe. One way to resolve these discrepancies might be to develop and test FRET-sensors that use alternative binding mechanisms.
In summary, the present report revises upwards recent estimates17,19 of Zn2+ concentrations in the mitochondrial matrix and the ER lumen in a range of mammalian cell types and casts doubt over the suggestions that significant Zn2+ fluxes occur across the ER membrane in response to Ca2+ mobilization from the latter organelle. Release of Zn2+ mediated by other forms of stimulation8 remains conceivable, especially given higher free ER Zn2+ concentrations than have latterly been assumed.17
Methods
Supplemental methods are available in Supporting Information.
Generation of organelles-targeted eCALWY-4 and eCALWY-6
Cloning strategies to address eCALWY- probes to the mitochondrial matrix and ER is described in Supporting Information. Adenoviral vectors were generated using pAdeasy system as described earlier.41
Imaging of [Zn2+] by FRET measurement
Cells on coverslips were washed twice in Krebs-Hepes-bicarbonate (KHB) buffer (140 mM NaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.2 mM MgSO4, 1.5 mM CaCl2, 10 mM Hepes, 25 mM NaHCO3), which was warmed, bubbled with 95:5 O2:CO2, set to pH 7.4, and contained 3 mM glucose. Imaging of [Zn2+] using eCALWY sensors was carried out as optimized before.11,12 Briefly, cells were maintained at 37°C throughout with a heating stage (MC60, LINKAM, Scientific Instruments), and KHB was perifused (1.5 to 2 ml/minute) with additions as stated in the Figures. Images were captured at 433 nm monochromatic excitation wavelength (Polychrome IV, Till photonics) using an Olympus IX-70 wide-field microscope with a 40x/1.35NA oil immersion objective and a zyla sCMOS camera (Andor Technology) controlled by Micromanager software.42 Acquisition rate was 20 images/minute. Emitted light was splitted and filtered by a Dual-View beam splitter (Photometrics) equipped with a 505dcxn dichroic mirror and two emission filters (Chroma Technology - D470/24 for cerulean and D535/30 for citrine).
Image analysis was performed with ImageJ software43 using a home-made macro and the fluorescence emission ratios were derived after subtracting background. We observed that during acquisition, photobleaching gradually decreased the steady-state ratio with a linear kinetic (not shown). This drift was thus, when necessary, corrected in function of time with a constant factor. Steady-state fluorescence intensity ratio citrine/cerulean (R) was measured, then maximum and minimum ratios were determined to calculate free Zn2+ concentration using the following formula: [Zn2+] = Kd×(Rmax-R)/(R-Rmin). The maximum ratio (Rmax) was obtained upon intracellular zinc chelation with 50 µM TPEN and the minimum ratio (Rmin) was obtain upon zinc saturation with 100 μM ZnCl2 in the presence of the Zn2+ ionophore, pyrithione (5 µM).11
Simultaneous imaging of Zn2+ and Ca2+
eCALWY-expressing cells were preincubated with 3 µM Fura-2AM (Invitrogen) in KHB prior to imaging. eCALWYs signal was collected as described previously and Fura-2 signal was collected at 340 and 380 nm excitation wavelengths through the D535/30 emission filter. Fura-2 fluorescence emission ratio 340/380 was determined after subtracting background using a home-made macro. Acquisition rates are specified in the figure legends.
Statistics
Data are presented as mean ± S.E.M. unless otherwise stated. Differences were determined using Student’s t-test with Bonferroni correction for multiple comparisons as required using GraphPad Prism. P-values <0.05 were considered significant.
Supplementary Material
Additional Results and Methods. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgements
G.A.R. was supported by a Wellcome Trust Senior Investigator Award (WT098424AIA), an MRC (UK) Programme grant (MR/J0003042/1), a Royal Society Wolfson Research Merit Award, and project grants from the Biotechnology and Biological Sciences Research Council (BBSRC, UK, BB/J015873/1) and the European Association for the Study of Diabetes. The work leading to this publication has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n° 155005 (IMIDIA), resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution (G.A.R., P.M.). A.R.L. is supported by a British Heart Foundation Intermediate Research Fellowship (FS/11/67/28954) and the National Institute for Health Research-funded Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital. The work of A.M.H. and M.M. was supported by an ECHO grant from The Netherlands Organization of Scientific Research (700.59.013) and an ERC starting grant (ERC-2011-StG 280255). E. T. was supported by 2214-TUBITAK international doctoral research fellows programme. We thank Professor David Ron (University of Cambridge, U.K.) for the provision of plasmid encoding ERroGFPiE. We acknowledge Mr Andrew Cakebread, Franklin-Wilkins Building, King’s College London, for metal analysis by inductively coupled plasma mass spectrometry.
References
- 1.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Maret W. Redox biochemistry of mammalian metallothioneins. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. 2011;16:1079–1086. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 4.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. 2011;16:1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maret W, Krezel A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol Med. 2007;13:371–375. doi: 10.2119/2007-00036.Maret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, Hirano T. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor KM, Hiscox S, Nicholson RI, Hogstrand C, Kille P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci Signal. 2012;5:ra11. doi: 10.1126/scisignal.2002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaltenberg J, Plum LM, Ober-Blobaum JL, Honscheid A, Rink L, Haase H. Zinc signals promote IL-2-dependent proliferation of T cells. Eur J Immunol. 2010;40:1496–1503. doi: 10.1002/eji.200939574. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki S, Hasegawa A, Hojyo S, Ohashi W, Fukada T, Nishida K, Hirano T. A novel role of the L-type calcium channel alpha1D subunit as a gatekeeper for intracellular zinc signaling: zinc wave. PLoS One. 2012;7:e39654. doi: 10.1371/journal.pone.0039654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellomo EA, Meur G, Rutter GA. Glucose regulates free cytosolic Zn(2)(+) concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet beta-cells. The Journal of biological chemistry. 2011;286:25778–25789. doi: 10.1074/jbc.M111.246082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindenburg LH, Hessels AM, Ebberink EH, Arts R, Merkx M. Robust red FRET sensors using self-associating fluorescent domains. ACS Chem Biol. 2013;8:2133–2139. doi: 10.1021/cb400427b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Maret W. Transient fluctuations of intracellular zinc ions in cell proliferation. Exp Cell Res. 2009;315:2463–2470. doi: 10.1016/j.yexcr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Bozym RA, Thompson RB, Stoddard AK, Fierke CA. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS chemical biology. 2006;1:103–111. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- 16.McCranor BJ, Bozym RA, Vitolo MI, Fierke CA, Bambrick L, Polster BM, Fiskum G, Thompson RB. Quantitative imaging of mitochondrial and cytosolic free zinc levels in an in vitro model of ischemia/reperfusion. J Bioenerg Biomembr. 2012;44:253–263. doi: 10.1007/s10863-012-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci U S A. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin Y, Miranda JG, Stoddard CI, Dean KM, Galati DF, Palmer AE. Direct comparison of a genetically encoded sensor and small molecule indicator: implications for quantification of cytosolic Zn(2+) ACS Chem Biol. 2013;8:2366–2371. doi: 10.1021/cb4003859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JG, Qin Y, Galati DF, Palmer AE. New sensors for quantitative measurement of mitochondrial Zn(2+) ACS Chem Biol. 2012;7:1636–1640. doi: 10.1021/cb300171p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue L, Li G, Yu C, Jiang H. A ratiometric and targetable fluorescent sensor for quantification of mitochondrial zinc ions. Chemistry. 2012;18:1050–1054. doi: 10.1002/chem.201103007. [DOI] [PubMed] [Google Scholar]
- 21.Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 22.Rutter GA, Theler JM, Murgia M, Wollheim CB, Pozzan T, Rizzuto R. Stimulated Ca2+ influx raises mitochondrial free Ca2+ to supramicromolar levels in a pancreatic beta-cell line. Possible role in glucose and agonist-induced insulin secretion. The Journal of biological chemistry. 1993;268:22385–22390. [PubMed] [Google Scholar]
- 23.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 24.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholls D. Bioenergetics: an Introduction to the Chemiosmotic theory. 1982.
- 26.Stork CJ, Li YV. Zinc release from thapsigargin/IP3-sensitive stores in cultured cortical neurons. J Mol Signal. 2010;5:5. doi: 10.1186/1750-2187-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelangeli F, East JM. A diversity of SERCA Ca2+ pump inhibitors. Biochem Soc Trans. 2011;39:789–797. doi: 10.1042/BST0390789. [DOI] [PubMed] [Google Scholar]
- 28.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum PJ, Christensen SB, Hanley MR. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- 29.Hillaire-Buys D, Chapal J, Bertrand G, Petit P, Loubatieres-Mariani MM. Purinergic receptors on insulin-secreting cells. Fundam Clin Pharmacol. 1994;8:117–127. doi: 10.1111/j.1472-8206.1994.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 30.van Lith M, Tiwari S, Pediani J, Milligan G, Bulleid NJ. Real-time monitoring of redox changes in the mammalian endoplasmic reticulum. J Cell Sci. 2011;124:2349–2356. doi: 10.1242/jcs.085530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duprez J, Roma LP, Close AF, Jonas JC. Protective antioxidant and antiapoptotic effects of ZnCl2 in rat pancreatic islets cultured in low and high glucose concentrations. PLoS One. 2012;7:e46831. doi: 10.1371/journal.pone.0046831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avezov E, Cross BC, Kaminski Schierle GS, Winters M, Harding HP, Melo EP, Kaminski CF, Ron D. Lifetime imaging of a fluorescent protein sensor reveals surprising stability of ER thiol redox. J Cell Biol. 2013;201:337–349. doi: 10.1083/jcb.201211155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akhmedov D, Braun M, Mataki C, Park KS, Pozzan T, Schoonjans K, Rorsman P, Wollheim CB, Wiederkehr A. Mitochondrial matrix pH controls oxidative phosphorylation and metabolism-secretion coupling in INS-1E clonal beta cells. FASEB J. 2010;24:4613–4626. doi: 10.1096/fj.10-162222. [DOI] [PubMed] [Google Scholar]
- 34.Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 35.Link TA, von Jagow G. Zinc ions inhibit the QP center of bovine heart mitochondrial bc1 complex by blocking a protonatable group. The Journal of biological chemistry. 1995;270:25001–25006. doi: 10.1074/jbc.270.42.25001. [DOI] [PubMed] [Google Scholar]
- 36.Brown AM, Kristal BS, Effron MS, Shestopalov AI, Ullucci PA, Sheu KF, Blass JP, Cooper AJ. Zn2+ inhibits alpha-ketoglutarate-stimulated mitochondrial respiration and the isolated alpha-ketoglutarate dehydrogenase complex. The Journal of biological chemistry. 2000;275:13441–13447. doi: 10.1074/jbc.275.18.13441. [DOI] [PubMed] [Google Scholar]
- 37.Gazaryan IG, Krasinskaya IP, Kristal BS, Brown AM. Zinc irreversibly damages major enzymes of energy production and antioxidant defense prior to mitochondrial permeability transition. The Journal of biological chemistry. 2007;282:24373–24380. doi: 10.1074/jbc.M611376200. [DOI] [PubMed] [Google Scholar]
- 38.Kerchner GA, Canzoniero LM, Yu SP, Ling C, Choi DW. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J Physiol. 2000;528(Pt 1):39–52. doi: 10.1111/j.1469-7793.2000.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gyulkhandanyan AV, Lee SC, Bikopoulos G, Dai F, Wheeler MB. The Zn2+-transporting pathways in pancreatic beta-cells: a role for the L-type voltage-gated Ca2+ channel. The Journal of biological chemistry. 2006;281:9361–9372. doi: 10.1074/jbc.M508542200. [DOI] [PubMed] [Google Scholar]
- 40.Montero M, Brini M, Marsault R, Alvarez J, Sitia R, Pozzan T, Rizzuto R. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. The EMBO journal. 1995;14:5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ainscow EK, Zhao C, Rutter GA. Acute overexpression of lactate dehydrogenase-A perturbs beta-cell mitochondrial metabolism and insulin secretion. Diabetes. 2000;49:1149–1155. doi: 10.2337/diabetes.49.7.1149. [DOI] [PubMed] [Google Scholar]
- 42.Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. Computer control of microscopes using microManager. Current protocols in molecular biology / edited by Frederick M. Ausubel … [et al.] 2010;Chapter 14:Unit14 20. doi: 10.1002/0471142727.mb1420s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.