Abstract
Background
Higher grip strength (GS) is associated with lower mortality risk. However, whether this association is independent of adiposity is uncertain.
Objective
The purpose of this study was to examine the associations between GS, adiposity and mortality.
Design
The UK Biobank study is an ongoing prospective cohort of >0.5 million UK adults aged 40-69 years. Baseline data collection (2006-2010) included measurements of GS and adiposity indicators including body mass index (BMI). Age- and gender-specific GS quintiles were used. BMI was classified according to clinical cut-points.
Results
Data from 403,199 participants were included in analyses. Over a median 7.0-year follow-up, 8,287 all-cause deaths occurred. The highest GS quintile had 32% (95% confidence interval [CI]: 26%, 38%) and 25% (95% CI: 16%, 33%) lower all-cause mortality risks in men and women, respectively, compared with the lowest GS quintile, after adjustment for confounders and BMI. Obesity class II (BMI≥35) was associated with greater all-cause mortality risks. Compared with the highest GS and normal weight category, the highest GS and Obesity class II category showed relatively higher all-cause mortality hazards (not statistically significant in men); however, the increased risk was relatively lower than the risk for the lowest GS and Obesity class II category. All-cause mortality risks were generally lower for obese but stronger individuals than for non-obese but weaker individuals. Similar patterns of associations were observed for cardiovascular mortality.
Conclusions
Lower grip strength and excess adiposity are both independent predictors of higher mortality risk. The higher mortality risk associated with excess adiposity is attenuated, although not completely attenuated, by greater GS. Interventions/polices should focus on improving muscular strength of the population regardless of their adiposity levels.
Keywords: grip strength, adiposity, muscle strength, obesity, mortality, UK Biobank
Introduction
Obesity is a global public health concern (1). Excess adiposity is known to be associated with greater risk of mortality as well as cardiovascular disease (CVD) such as heart failure, hypertension, and coronary heart disease (2). However, substantial evidence (3) suggests greater aerobic fitness can lower the risk of death and CVD associated with greater fatness.
Muscular fitness, a complementary aspect of fitness, has also been found to be a strong predictor of mortality (4). As such, grip strength (GS), as a simple inexpensive measure of overall muscular strength (5–7), has been recognized as a useful prognostic indicator of mortality (8, 9) as well as adverse health outcomes, such as sarcopenia and frailty (10). A few studies (11–14) have attempted to further explore the “fit-fat” paradigm in relation to mortality and muscle strength, suggesting that mortality risk may be reduced in individuals with higher muscle strength irrespective of weight status. However, the evidence on the associations of muscle strength and fatness with mortality has been predicated primarily upon data with a relatively small sample size (<8000) of men (11, 12) or older adults (13). So, findings from these studies provide limited evidence on the relative risk of mortality for the combination of muscle strength and fatness for general adult populations. Furthermore, the majority of the studies have used body mass index (BMI) as a sole crude adiposity indicator (12–14). Abdominal adiposity defined by waist circumference (WC) predicts mortality independently of general adiposity (i.e. BMI, percent body fat [%BF]) (15). Hence, it is critical to discern the interactions of different adiposity indicators and muscle strength with mortality in general populations of men and women. Therefore, the purpose of the present analysis is to examine the relative risk of all-cause and CVD mortality for GS, various clinical adiposity measures (BMI, WC, %BF) and their interactions in middle-aged and older men and women.
Subjects and Methods
Study design and participants
UK Biobank is an ongoing UK national cohort of over half a million adults aged 40-69 years at recruitment. Individuals were contacted who were registered with the National Health Service and living <25 miles away from one of 22 assessment centers across the UK. Of those, >500 000 individuals performed baseline data collection (2006-2010) that included a wide variety of physical measurements and biological samples, as well as questionnaires on socio-demographic factors, family history/early-life exposures, general health/disabilities, environmental/lifestyle factors, and psychological/cognitive state. The UK Biobank methodology is described in detail elsewhere (16). All participants signed informed written consent prior to participation, and the protocol of the UK Biobank project was approved by the North West Multi-Centre Research Ethics Committee.
Exposures
Grip strength (GS)
GS was assessed once in each hand using a Jamar J00105 hydraulic hand dynamometer, which can measure isometric grip force up to 90 kilograms (calibrated by staff at the start of each measurement day) showing good reliability and reproducibility (17). The handle of the device was adjustable to five grip positions between 1-3/8 and 3-3/8 inches. Participants were allowed to choose a grip position that they felt most comfortable with. Each participant was asked to grasp the handle of the device in their right hand while sitting upright on a chair with their forearm on the armrest. They were required to maintain a 90° angle of their elbow adjacent to their side so that their thumb would face upwards while squeezing the handle as strongly as possible for about 3 seconds. The same protocol was undertaken with the left hand. For the current analysis, values from the two hands were averaged if available; otherwise, the value from a single hand was used in a small subsample (n=1,177).
Adiposity measures
BMI was calculated as measured weight (kg) divided by measured height (m) squared. WC was measured using a tape measure at the level of the umbilicus. Fat-free mass was assessed with the Tanita BC-418MA bio-impedance analyzer, from which %BF was calculated as 1 minus fat-free mass divided by body weight. BMI was categorized into normal weight (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2), obesity class I (30.0-34.9 kg/m2) and obesity class II (≥35.0 kg/m2). The following sex-specific clinical cut-offs were applied to create three groups of WC and %BF: WC<94cm, 94-102cm or ≥102cm for men; WC<80cm, 80-88cm, or ≥88cm for women (1); %BF≤20%, 20-25% or >25% for men; and %BF≤30%, 30-33% or >33% for women (18).
Outcomes
Participants were followed up for mortality until February 15th 2016 through linkage with death records from the National Health Service Information Centre and the Scottish Morbidity Record. CVD mortality was defined as the International Classification of Diseases-10 codes F01 and I00-I99. The median follow-up period was 7 years (interquartile range: 6.3 and 7.6 years).
Covariates
The following variables that could confound GS-mortality associations were included as covariates in the analyses: ethnicity (White, mixed, Asian/Asian British, Black/Black British, others), smoking status (never, previous, current), employment (unemployed, employed), Townsend Deprivation Index (a composite score of employment, car ownership, home ownership and household overcrowding, with higher values indicating a given area’s higher degree of deprivation), statin use (yes/no), hormone replacement therapy (yes/no; women only), alcohol consumption (never, previous, currently <3times/week, currently ≥3times/week), processed/red meat consumption (days/week), resting pulse rate (beats/min), and moderate-to-vigorous physical activity (MVPA) (minutes/day). MVPA time was estimated based on self-reported walking, transportation activities, occupational activities/walking, strenuous/other exercise, and do-it-yourself activities by calibrating them to heart rate and accelerometry data (19) from 12 435 UK adults participating in the Fenland project (20).
Statistical analyses
Cox regression models (with age as the underlying time scale) were used to estimate associations of GS and adiposity with all-cause and CVD mortality. First, models were fit to estimate associations between GS and mortality, with adjustment for potential confounders (Model 1). Further adjustments for each of the three adiposity indicators (BMI, WC or %BF) were made in three separate models (Models 2a, 2b and 2c). In parallel with the models using GS as an exposure variable, models using each adiposity measure as an exposure variable were also fitted with adjustment for the same covariates (Model 1) and additional adjustments for GS (Model 2). Models using per-5kg increment in GS as an exposure were fitted by personal/lifestyle risk factor and disease status. GS-mortality associations were stratified by each adiposity variable. Gender- and age-specific quintiles of GS (Q1-Q5) and different adiposity categories were combined to examine joint associations with mortality. All analyses were performed for men and women separately. Subgroup analyses and tests of interaction between GS and age, weight status, waist circumference, %BF, MVPA, TV viewing, smoking, alcohol consumption, hypertension and diabetes were performed. Log-log plots provided support for the proportional hazards assumptions for all covariates. Sensitivity analyses were performed 1) using the maximum GS from either hand, 2) GS normalized for body weight or fat-free mass to account for variation by body size, 3) excluding the first 2-year mortality follow-up, and 4) excluding individuals who had chronic obstructive pulmonary disease or were ‘current’/’previous’ smokers at baseline when examining adiposity as exposure (the latter two to minimize the risk of reverse causality). All analyses were performed in STATA/SE Version 14 (StataCorp LP, College Station, TX).
Results
Of an initial sample of 502,639 participants who undertook baseline data collection, individuals were excluded if they had a history of heart attack, stroke or cancer at baseline (n=55,401) to minimize the risk for reverse causality (8, 21), their censoring date was before the date of baseline data collection (n=3) or they had missing values on any of the variables (n=44,036), leaving 403,199 participants in the final analytic sample (Supplemental Figure 1).
Table 1 shows participants’ characteristics across quintiles of GS. The specific cut-points to create the gender- and age-specific quintiles of GS are shown in Supplemental Table 1. A total of 8,081 all-cause deaths occurred during 1,268,314 person-years of follow-up for men and 1,533,538 person-years for women. Differences in BMI, WC, and %BF across quintiles of GS and the correlations between these variables (Supplemental Table 2) were minimal.
Table 1. Participants’ characteristics.
| Variables | Men (n=183,006) | Women (n=220,193) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Quintiles of grip strength | All | Quintiles of grip strength | |||||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Grip strength, kg | 39.7 (8.8) | 27.7 (4.9) | 35.3 (26) | 39.3 (2.7) | 43.6 (2.9) | 51.2 (4.9) | 23.5 (6.2) | 14.7 (3.6) | 20.2 (2.1) | 22.9 (2.1) | 26.2 (2.1) | 31.2 (3.5) |
| Age, years | 56.2 (8.2) | 56.4 (8.4) | 55.8 (8.3) | 56.5 (8.1) | 56.5 (8.2) | 55.6 (8.1) | 56.0 (8.0) | 56.7 (8.0) | 55.8 (8.2) | 56.5 (8.0) | 55.7 (7.7) | 55.4 (8.1) |
| Ethnicity, % | ||||||||||||
| White | 94.4% | 89.6% | 93.7% | 95.5% | 96.3% | 96.6% | 94.4% | 90.9% | 94.0% | 95.1% | 95.9% | 95.6% |
| Mixed | 0.5% | 0.5% | 0.6% | 0.5% | 0.5% | 0.5% | 0.7% | 0.7% | 0.7% | 0.6% | 0.7% | 0.8% |
| Asian/Asian British | 2.6% | 6.3% | 3.2% | 2.0% | 1.2% | 0.7% | 2.1% | 5.0% | 2.5% | 1.7% | 1.2% | 0.6% |
| Black/Black British | 1.6% | 2.0% | 1.5% | 1.4% | 1.4% | 1.7% | 1.8% | 1.8% | 1.6% | 1.7% | 1.5% | 2.4% |
| Others | 0.9% | 1.6% | 1.0% | 0.7% | 0.7% | 0.5% | 1.0% | 1.6% | 1.1% | 0.9% | 0.8% | 0.6% |
| Smoking status, % | ||||||||||||
| Never | 50.4% | 51.4% | 50.9% | 50.7% | 49.6% | 49.7% | 60.3% | 61.6% | 61.4% | 60.5% | 59.6% | 58.8% |
| Previous | 37.3% | 35.0% | 36.5% | 37.1% | 38.7% | 38.9% | 31.0% | 29.2% | 29.9% | 31.3% | 31.7% | 32.5% |
| Current | 12.3% | 13.6% | 12.6% | 12.1% | 11.7% | 11.4% | 8.7% | 9.2% | 8.7% | 8.2% | 8.7% | 8.7% |
| Employment, % | ||||||||||||
| Unemployed | 35.9% | 42.2% | 35.3% | 36.0% | 35.6% | 30.9% | 43.0% | 50.2% | 42.4% | 44.4% | 39.3% | 39.9% |
| Townsend deprivation index | -1.33 (3.1) | -0.58 (3.4) | -1.16 (3.1) | -1.44 (3.0) | -1.62 (2.9) | -1.79 (2.8) | -1.39 (3.0) | -0.95 (3.2) | -1.28 (3.0) | -1.47 (3.0) | -1.57 (2.9) | -1.59 (2.9) |
| Statin use, % | 19.7% | 23.6% | 19.7% | 19.8% | 18.9% | 16.8% | 11.7% | 15.4% | 12.1% | 11.5% | 10.3% | 10.0% |
| Hormone replacement therapy (W), % | (N/A) | (N/A) | (N/A) | (N/A) | (N/A) | (N/A) | 7.5% | 7.4% | 7.2% | 7.2% | 7.7% | 7.9% |
| Alcohol Consumption, % | ||||||||||||
| Never | 2.7% | 4.7% | 2.8% | 2.4% | 1.9% | 1.6% | 5.6% | 9.1% | 6.1% | 5.1% | 4.4% | 4.1% |
| Previous | 3.3% | 5.0% | 3.4% | 2.9% | 2.7% | 2.3% | 3.4% | 4.8% | 3.7% | 3.2% | 2.9% | 2.7% |
| Current (<3times/week) | 41.8% | 44.3% | 42.7% | 41.3% | 40.3% | 40.7% | 53.7% | 56.4% | 55.1% | 53.4% | 52.9% | 51.5% |
| Current (≥3times/week) | 52.3% | 45.9% | 51.0% | 53.5% | 55.1% | 55.3% | 37.2% | 29.8% | 35.2% | 38.2% | 39.7% | 41.7% |
| Processed/red meat consumption, days/week | 1.04 (0.60) | 1.05 (0.64) | 1.04 (0.61) | 1.04 (0.59) | 1.03 (0.58) | 1.05 (0.58) | 0.78 (0.50) | 0.79 (0.53) | 0.78 (0.50) | 0.78 (0.50) | 0.78 (0.49) | 0.78 (0.49) |
| Resting pulse rate, beats/min | 68.3 (11.7) | 69.5 (12.3) | 68.4 (11.8) | 68.0 (11.6) | 67.9 (11.6) | 67.9 (11.5) | 70.1 (10.5) | 70.8 (10.7) | 70.1 (10.5) | 69.9 (10.4) | 69.8 (10.4) | 69.9 (10.6) |
| Self-reported MVPA time, min/day | 82.3 (22.9) | 78.4 (20.7) | 81.8 (22.2) | 82.4 (24.0) | 83.1 (23.0) | 85.3 (23.6) | 51.6 (19.5) | 49.2 (16.1) | 51.0 (18.8) | 51.3 (18.8) | 52.5 (19.5) | 53.5 (22.9) |
| BMI, kg/m2 | 27.7 (4.2) | 27.7 (4.6) | 27.6 (4.3) | 27.6 (4.1) | 27.7 (3.9) | 28.2 (3.9) | 27.0 (5.1) | 27.6 (5.5) | 26.9 (5.1) | 26.8 (5.0) | 26.8 (4.9) | 27.0 (5.0) |
| Normal weight, % | 25.0% | 28.0% | 27.8% | 25.9% | 24.3% | 19.6% | 39.2% | 35.0% | 39.9% | 40.6% | 41.0% | 39.1% |
| Overweight, % | 50.1% | 46.2% | 48.6% | 50.6% | 51.7% | 52.9% | 37.3% | 37.0% | 37.0% | 37.3% | 37.5% | 37.7% |
| Obesity class I, % | 19.4% | 19.1% | 18.0% | 18.5% | 19.3% | 22.0% | 15.8% | 17.9% | 15.7% | 15.2% | 14.8% | 15.5% |
| Obesity class II, % | 5.5% | 6.7% | 5.6% | 5.0% | 4.7% | 5.5% | 7.7% | 10.1% | 7.4% | 6.9% | 6.7% | 7.6% |
| WC, cm | 96.6 (11.1) | 97.0 (12.0) | 96.2 (11.4) | 96.2 (11.0) | 96.4 (10.7) | 97.3 (10.5) | 84.5 (12.4) | 86.0 (13.1) | 84.2 (12.3) | 83.9 (12.1) | 83.8 (12.1) | 84.6 (12.2) |
| <94cm(M); <80cm(W) | 45.3% | 44.8% | 47.5% | 46.6% | 46.0% | 41.8% | 42.5% | 37.9% | 43.2% | 44.1% | 44.4% | 42.1% |
| 94-102cm(M); 80-88cm(W) | 25.4% | 23.7% | 24.5% | 25.4% | 25.7% | 27.2% | 21.9% | 21.3% | 21.8% | 21.9% | 22.1% | 22.3% |
| ≥102cm(M); ≥88cm(W) | 29.4% | 31.5% | 28.1% | 28.0% | 28.3% | 30.9% | 35.6% | 40.8% | 34.9% | 34.0% | 33.5% | 35.6% |
| %BF | 25.1 (5.8) | 25.8 (6.1) | 25.2 (5.9) | 25.0 (5.7) | 24.8 (5.6) | 24.7 (5.5) | 36.4 (6.9) | 37.5 (7.0) | 36.5 (6.9) | 36.4 (6.8) | 36.1 (6.8) | 36.0 (6.9) |
| ≤20%(M); ≤30%(W) | 18.3% | 16.5% | 18.2% | 18.8% | 19.1% | 18.9% | 17.6% | 14.4% | 17.2% | 17.5% | 18.7% | 19.5% |
| 20-25%(M); 30-33%(W) | 30.9% | 27.5% | 30.3% | 31.0% | 32.1% | 33.1% | 12.8% | 11.2% | 13.0% | 12.8% | 13.5% | 13.4% |
| >25%(M); >33%(W) | 50.8% | 56.0% | 51.5% | 50.2% | 48.8% | 47.9% | 69.6% | 74.4% | 69.8% | 69.7% | 67.8% | 67.1% |
| Fat free mass, kg | 63.8 (7.8) | 61.0 (8.0) | 62.3 (7.5) | 63.3 (7.3) | 64.5 (7.1) | 67.3 (7.4) | 44.5 (5.0) | 43.4 (5.2) | 43.7 (4.8) | 44.0 (4.7) | 44.8 (4.7) | 46.3 (4.9) |
| Systolic blood pressure, mm Hg | 140.9 (17.3) | 139.2 (17.7) | 139.8 (17.2) | 141.1 (17.4) | 142.0 (17.2) | 142.3 (16.9) | 135.0 (19.2) | 134.3 (19.2) | 133.8 (19.2) | 135.2 (19.3) | 135.1 (19.0) | 136.1(19.1) |
| Diastolic blood pressure, mm Hg | 84.3 (9.9) | 83.2 (10.1) | 83.8 (10.0) | 84.3 (9.9) | 84.8 (9.9) | 85.4 (9.8) | 80.7 (10.0) | 80.2 (10.0) | 80.1 (10.0) | 80.5 (9.9) | 80.8 (9.9) | 81.5 (9.9) |
| Hypertension, % | 61.1% | 60.3% | 58.7% | 60.9% | 62.4% | 62.8% | 47.7% | 49.3% | 46.0% | 47.9% | 46.8% | 48.6% |
| Diabetes, % | 6.1% | 9.7% | 6.5% | 5.8% | 4.9% | 4.1% | 3.4% | 5.4% | 3.6% | 3.1% | 2.8% | 2.6% |
Note: The quintiles of grip strength were gender- and age-specific. Body Mass Index (BMI) was used to categorize participants into normal weight (18.5kg/m2≤BMI<25kg/m2), overweight (25kg/m2≤BMI<30kg/m2), obesity class I (30kg/m2≤BMI<35kg/m2) and obesity class II (BMI≥35kg/m2). Hypertension was defined as systolic/diastolic blood pressure ≥140/90mm Hg, reported physician diagnosis of hypertension, or reported medication use to regulate blood pressure. Participants were considered to have diabetes if they reported a physician diagnosis of diabetes, or taking glucose-lowering treatment.
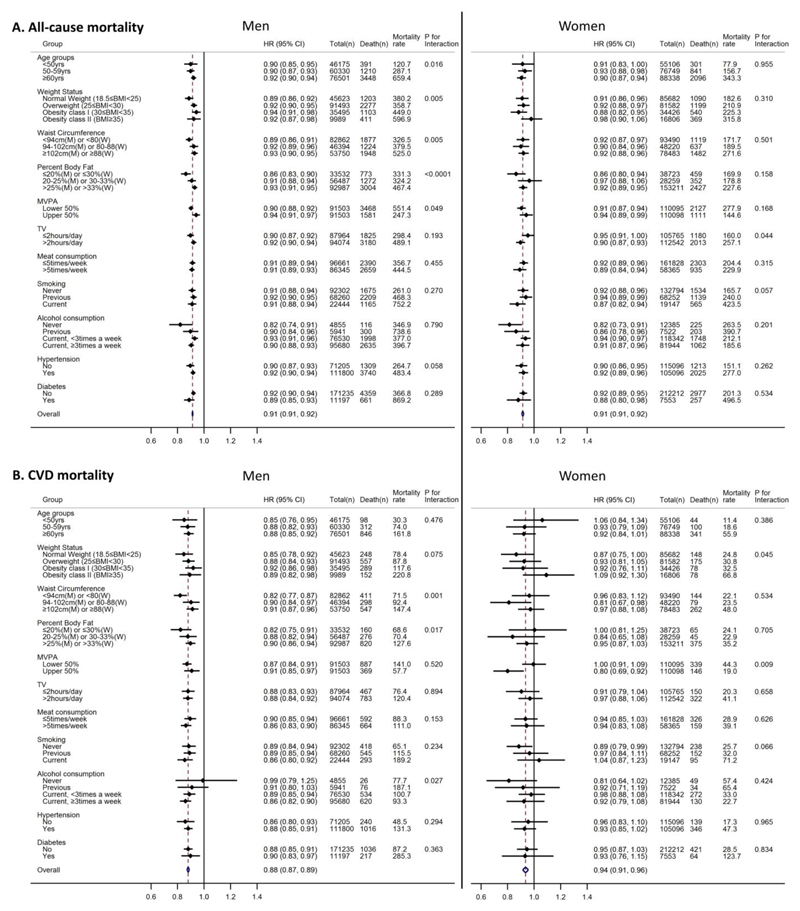
Table 2 summarizes associations between GS and all-cause mortality. Compared with the lowest quintile of GS, the highest quintile of GS had considerably lower risks of all-cause mortality in both men and women (except for Q2) after adjusting for confounders (Model 1) plus additional adjustments for each adiposity measure (Model 2): p-values for trends <0.0001. Specifically, hazards of all-cause mortality were approximately 32% (95% confidence interval [CI]: 26%, 38%) and 25% (95%CI: 16%, 33%) lower for men and women in Q5 of GS, respectively, compared with Q1 of GS after adjusting for confounders and BMI (Model 2a). The hazard ratios (HR) for per-5kg increase in GS was 0.92 for both men (95%CI: 0.90, 0.93) and women (95%CI: 0.89, 0.95) after adjusting for all confounders and BMI (Model 2a). Sensitivity analyses found similar associations with the maximal GS from either hand, and GS unnormalized or normalized for body weight or fat-free mass (Supplemental Figure 2). Another sensitivity analysis removing the first 2 years of follow-up yielded similar results (Supplemental Table 3). The pattern of associations of GS with CVD mortality was similar to the associations with all-cause mortality for men (Table 2). While the HRs were not statistically significant in women, p-values for linear trends were all less than 0.05. The associations of per-5kg increase in GS with all-cause and CVD mortality were significant (p-values<0.05) within almost all of the subgroups examined in both men and women (Figure 1), with some exceptions particularly for women.
Table 2. Independent associations of grip strength with all-cause and cardiovascular disease (CVD) mortality.
| Hazard ratios (95% confidence interval) for mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality outcome | Gender | Comparisons | Number of deaths | Person-years of follow-up | Mortality rate | Model 1 | Model 2a | Model 2b | Model 2c |
| All-cause | Men | 5,049 | 1,268,314 | 398.1 | |||||
| Quintiles of grip strength | |||||||||
| Q1 (Reference) | 1,389 | 241,358 | 575.5 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||
| Q2 | 933 | 232,139 | 401.9 | 0.80 (0.73, 0.87) | 0.81 (0.75, 0.88) | 0.80 (0.73, 0.87) | 0.80 (0.73, 0.87) | ||
| Q3 | 920 | 253,118 | 363.5 | 0.71 (0.65, 0.77) | 0.72 (0.66, 0.78) | 0.70 (0.65, 0.77) | 0.71 (0.65, 0.77) | ||
| Q4 | 972 | 268,240 | 362.4 | 0.72 (0.66, 0.78) | 0.73 (0.67, 0.79) | 0.72 (0.66, 0.78) | 0.72 (0.66, 0.78) | ||
| Q5 | 835 | 273,460 | 305.3 | 0.67 (0.62, 0.73) | 0.68 (0.62, 0.74) | 0.67 (0.61, 0.73) | 0.67 (0.62, 0.74) | ||
| P for linear trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| Per 5kg increment in grip strength | 0.91 (0.90, 0.93) | 0.92 (0.90, 0.93) | 0.91 (0.90, 0.93) | 0.91 (0.90, 0.93) | |||||
| Women | 3,238 | 1,533,538 | 211.1 | ||||||
| Quintiles of grip strength | |||||||||
| Q1 (Reference) | 746 | 270,638 | 275.6 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||
| Q2 | 652 | 274,981 | 237.1 | 0.96 (0.86, 1.06) | 0.97 (0.87, 1.08) | 0.97 (0.87, 1.07) | 0.96 (0.86, 1.06) | ||
| Q3 | 656 | 316,838 | 207.0 | 0.81 (0.73, 0.90) | 0.82 (0.74, 0.91) | 0.82 (0.74, 0.91) | 0.81 (0.73, 0.90) | ||
| Q4 | 592 | 323,506 | 182.0 | 0.79 (0.71, 0.88) | 0.80 (0.72, 0.89) | 0.80 (0.71, 0.89) | 0.79 (0.71, 0.88) | ||
| Q5 | 592 | 347,576 | 170.3 | 0.74 (0.67, 0.83) | 0.75 (0.67, 0.84) | 0.74 (0.67, 0.83) | 0.74 (0.67, 0.83) | ||
| P for linear trend | <0.0001 | <0.0001 | <0.0001 | <.0001 | |||||
| Per 5kg increment in grip strength | 0.91 (0.89, 0.94) | 0.92 (0.89, 0.95) | 0.91 (0.89, 0.94) | 0.91 (0.89, 0.94) | |||||
| CVD | Men | 1,256 | 1,268,314 | 99.0 | |||||
| Quintiles of grip strength | |||||||||
| Q1 (Reference) | 373 | 241,358 | 154.5 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||
| Q2 | 246 | 232,139 | 106.0 | 0.81 (0.69, 0.96) | 0.82 (0.70, 0.97) | 0.81 (0.69, 0.95) | 0.81 (0.69, 0.96) | ||
| Q3 | 222 | 253,118 | 87.7 | 0.66 (0.56, 0.78) | 0.67 (0.56, 0.79) | 0.67 (0.56, 0.79) | 0.67 (0.57, 0.79) | ||
| Q4 | 235 | 268,240 | 87.6 | 0.68 (0.58, 0.81) | 0.69 (0.58, 0.81) | 0.68 (0.58, 0.81) | 0.69 (0.58, 0.82) | ||
| Q5 | 180 | 273,460 | 65.8 | 0.58 (0.48, 0.69) | 0.57 (0.47, 0.68) | 0.57 (0.47, 0.68) | 0.58 (0.48, 0.70) | ||
| P for linear trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| Per 5kg increment in grip strength | 0.88 (0.84, 0.91) | 0.88 (0.85, 0.91) | 0.88 (0.85, 0.91) | 0.88 (0.86, 0.92) | |||||
| Women | 485 | 1,533,538 | 31.6 | ||||||
| Quintiles of grip strength | |||||||||
| Q1 (Reference) | 122 | 270,638 | 45.1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||
| Q2 | 98 | 274,981 | 35.6 | 0.93 (0.72, 1.22) | 0.93 (0.71, 1.21) | 0.95 (0.73, 1.24) | 0.94 (0.72, 1.23) | ||
| Q3 | 89 | 316,838 | 28.1 | 0.73 (0.56, 0.97) | 0.72 (0.55, 0.95) | 0.75 (0.57, 0.99) | 0.74 (0.56, 0.97) | ||
| Q4 | 92 | 323,506 | 28.4 | 0.85 (0.65, 1.12) | 0.83 (0.63, 1.10) | 0.86 (0.65, 1.13) | 0.85 (0.65, 1.12) | ||
| Q5 | 84 | 347,576 | 24.2 | 0.74 (0.56, 0.98) | 0.73 (0.55, 0.97) | 0.74 (0.56, 0.98) | 0.74 (0.56, 0.98) | ||
| P for linear trend | 0.028 | 0.021 | 0.021 | 0.027 | |||||
| Per 5kg increment in grip strength | 0.93 (0.87, 0.99) | 0.93 (0.86, 1.01) | 0.94 (0.87, 1.01) | 0.94 (0.87, 1.01) | |||||
Note: All Cox regression models used age as the underlying time variable. The quintiles of grip strength were gender- and age-specific. Mortality rate is crude mortality rate per 100,000-person years
Model 1: Adjusted for ethnicity (White, mixed, Asian/Asian British, Black/Black British, others), smoking status (never, previous, current), employment (unemployed, employed), Townsend Deprivation Index, statin use (yes/no), hormone replacement therapy (yes/no; women only), alcohol consumption (never, previous, currently <3times/week, currently ≥3times/week), processed/red meat consumption (days/week), resting pulse rate (beats/min), and moderate-to-vigorous physical activity time (minutes/day).
Model 2a: Adjusted for all confounders included in Model 1 plus body mass index. Cases with BMI<18.5 (n=369 for men; n=1,525 for women) were excluded.
Model 2b: Adjusted for all confounders included in Model 1 plus waist circumference.
Model 2c: Adjusted for all confounders included in Model 1 plus percent body fat.
Figure 1.
Associations of per-5kg increment of grip strength with all-cause and cardiovascular disease (CVD) mortality for men and women. Models (using age as the underlying time variable) were adjusted for ethnicity (White, mixed, Asian/Asian British, Black/Black British, others), smoking status (never, previous, current; except for models stratified by smoking status), employment (unemployed, employed), Townsend Deprivation Index, statin use (yes/no), hormone replacement therapy (yes/no; women only), alcohol consumption (never, previous, currently <3times/week, currently ≥3times/week; except for models stratified by alcohol consumption), processed/red meat consumption (days/week; except for models stratified by processed/red meat consumption), resting pulse rate (beats/min), moderate-to-vigorous physical activity (MVPA) time (minutes/day; except for models stratified by MVPA), and body mass index (BMI) (except for models stratified by BMI, waist circumference and percent body fat). Hypertension was defined as systolic/diastolic blood pressure ≥140/90mm Hg, reported physician diagnosis of hypertension, or reported medication use to regulate blood pressure. Participants were considered to have diabetes if they reported a physician diagnosis of diabetes, or taking glucose-lowering treatment. Mortality rate is crude mortality rate per 100,000-person years. Cases with BMI<18.5 (n=369 for men; n=1,525 for women) were excluded in the BMI-stratified models. Abbreviations: HR – hazard ratio; CI – confidence interval; M – men; W– Women.
Associations of adiposity measures with all-cause and CVD mortality after adjusting for confounders (Model 1) and GS (Model 2) are shown in Supplemental Table 4. There were ‘J-shaped’ associations between BMI and mortality risk (i.e. substantially lower all-cause mortality only in overweight men compared with normal weight men), which persisted even after excluding individuals who had chronic obstructive pulmonary disease and/or were ‘current’/’previous’ smokers at baseline (Supplemental Table 5). The highest categories of BMI (i.e. obesity class II) and WC (i.e. abdominal obesity in men) were associated with increased hazards of all-cause and CVD mortality.
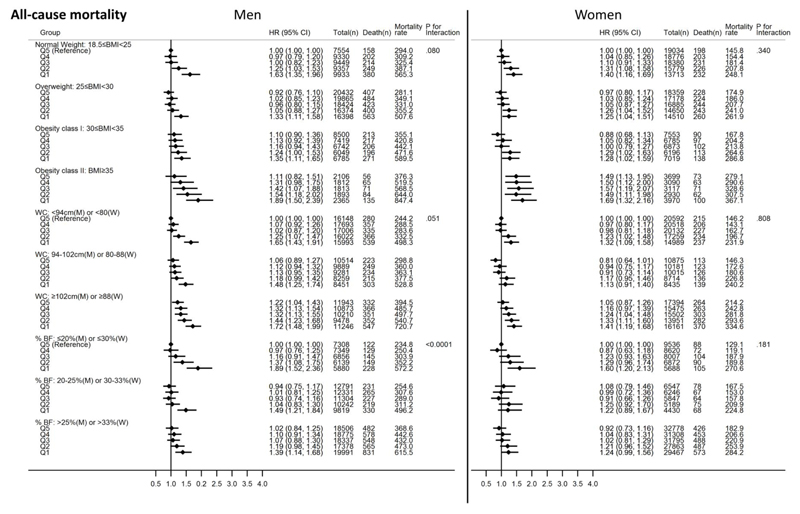
Figure 2 shows joint associations of GS quintiles and adiposity categories with all-cause mortality. Compared with normal weight men with the highest level of GS, more obese men with lower GS had higher risks of all-cause mortality. For example, men with the highest level of BMI (i.e. Obesity class II) and lowest level of GS had a 89% higher risk of all-cause mortality (HR: 1.89; 95%CI: 1.50, 2.39) compared with the normal weight men with the highest GS. A notable observation was the relatively higher mortality risks for normal weight men with lower GS in comparison with more obese men with higher GS. Similar trends of findings were observed with WC and %BF as adiposity indicators.
Figure 2.
Joint associations of grip strength and body mass index, waist circumference or percent body fat with all-cause mortality for men and women. All Cox regression models (using age as the underlying time variable) were adjusted for ethnicity (White, mixed, Asian/Asian British, Black/Black British, others), smoking status (never, previous, current), employment (unemployed, employed), Townsend Deprivation Index, statin use (yes/no), hormone replacement therapy (yes/no; women only), alcohol consumption (never, previous, currently <3times/week, currently ≥3times/week), processed/red meat consumption (days/week), resting pulse rate (beats/min), and moderate-to-vigorous physical activity time (minutes/day). The quintiles of grip strength were gender- and age-specific. Mortality rate is crude mortality rate per 100,000-person years. Cases with BMI<18.5 (n=369 for men; n=1,525 for women) were excluded in the models with BMI. Abbreviations: HR – hazard ratio; CI – confidence interval; M– men; W– Women.
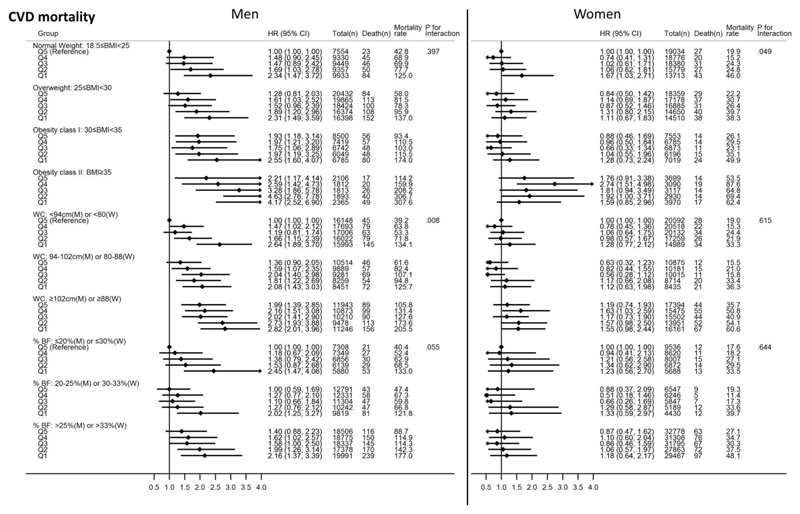
Similarly, more obese women with lower GS had generally higher all-cause mortality risks compared with normal weight women with higher GS. The HR for women with the highest BMI level (i.e. Obesity class II) and lowest GS was 1.69 (95%CI: 1.32, 2.16) compared with normal weight women with the highest GS. The higher GS quintiles in the Obesity class II category were associated with significantly higher risks of all-cause mortality compared with the reference group. Joint analyses with WC and %BF as adiposity indicators found more obese women with higher GS to have lower all-cause mortality risks compared with non-obese women with lower GS. This pattern of associations was, in general, similar to the associations observed for CVD mortality (Figure 3).
Figure 3.
Joint associations of grip strength and body mass index, waist circumference or percent body fat with cardiovascular disease (CVD) mortality for men and women. All Cox regression models (using age as the underlying time variable) were adjusted for ethnicity (White, mixed, Asian/Asian British, Black/Black British, others), smoking status (never, previous, current), employment (unemployed, employed), Townsend Deprivation Index, statin use (yes/no), hormone replacement therapy (yes/no; women only), alcohol consumption (never, previous, currently <3times/week, currently ≥3times/week), processed/red meat consumption (days/week), resting pulse rate (beats/min), and moderate-to-vigorous physical activity time (minutes/day). The quintiles of grip strength were gender- and age-specific. Mortality rate is crude mortality rate per 100,000-person years. Cases with BMI<18.5 (n=369 for men; n=1,525 for women) were excluded in the models with BMI. Abbreviations: HR – hazard ratio; CI – confidence interval; M– men; W– Women.
The lower GS quintiles had relatively higher all-cause (Supplemental Figure 3) and CVD mortality (Supplemental Figure 4) risks compared with the highest GS quintile within each adiposity stratum in both men and women.
Discussion
This study investigated the complex interplay of GS and various clinical adiposity measures with mortality from all causes and CVD in middle-aged and older men and women. Overall, greater GS was strongly associated with lower all-cause mortality risks, independently of adiposity measures. Moreover, every 5kg increment in GS was associated with about 8% lower hazard of mortality across nearly all subgroups defined by demographic and lifestyle risk factors or disease status. In contrast, adiposity measures had non-significant and/or inconsistent associations with mortality, although obesity class II and abdominal obesity were strong predictors of mortality, independent of GS. The mortality risk was highest for men and women with the lowest level of GS and the highest level of adiposity in the combined analyses. More importantly, obese individuals with greater GS had lower or similar mortality risks compared with non-obese individuals with lower GS. The pattern of associations between GS and CVD mortality was comparable to the findings for all-cause mortality. Overall, our findings provide compelling rationales for developing interventions and policies to improve muscular strength and reduce excess adiposity to minimize mortality risk.
The findings of this study are consistent with previous research by Leong at al. (9), which also demonstrated the high prognostic value of GS for various mortality and adverse health outcomes in 139,691 adults from 17 countries of different economic status. The HR of all-cause mortality for every 5kg reduction was 1.16 in Leong et al. study (9) but 1.08 (i.e. 1/0.92) in the present study. Some potential reasons for the difference are the use of gender- and age-specific quintiles of GS to take into account the inherent variation of GS by gender and age since GS is higher in men and younger individuals, and the exclusion of baseline medical conditions to minimize potential bias due to underlying subclinical conditions on GS and mortality in the present study. Furthermore, the use of a substantially larger sample allowed for comprehensive subgroup analyses by a number of lifestyle risk factors as well as disease status.
The present study is generally consistent with the previous studies (11–14) in terms of the independent and joint associations of GS and adiposity with mortality outcomes. For instance, greater muscle strength predicted mortality independent of adiposity (11–14). In addition, the highest mortality risk was observed in individuals with the lowest muscle strength level and the highest adiposity level, implying the interactive impacts of muscle strength and adiposity on mortality (11, 12, 14). However, a novel observation of the present study is that strong obese individuals had relatively lower mortality risks compared with weak non-obese individuals. This suggests that improving muscle strength may be a more important public health priority than reducing adiposity levels in decreasing mortality risks, although excessive adiposity itself is a strong risk factor for mortality (15). Another novel aspect of this study over the previous studies (11–14) is the use of a large cohort dataset, which enabled to create multiple sub-groups of GS and various clinical adiposity indicators in examining the joint associations with mortality in men and women separately.
The present study found that men had more consistent associations between GS and mortality (independent of adiposity) than women, which is in line with previous research (13). There is also evidence on the weaker associations of GS with all-cause mortality for women (22). In this regard, convincing evidence suggests an age-related decline in muscle strength in women (particularly after menopause) can be prevented through estrogen-hormone replacement therapy (23). However, none of the previous studies (13, 22) included estrogen-hormone replacement therapy as a potential confounder in the models for women whereas the present study did. Our study clearly demonstrated lower mortality rates for both men and women with greater GS. Moreover, given that current public health guidelines (24) recommend that both men and women do muscle-strengthening activities at least twice a week, interventions and policies should be designed and implemented in a way to encourage both genders to engage in regular muscle-strengthening activities, regardless of their adiposity levels.
Compelling evidence suggests that resistance exercise can result in improvements in muscle strength (including GS) and neuromotor functions in healthy and clinical adult populations (25). It appears that muscle strength gained through resistance exercise can diminish rapidly after the termination of training, but its effects on neuromotor functions can be sustained for a relatively long period of time even with a weekly session of moderate-to-vigorous intensity resistance exercise (25). We observed weak relationships between GS and adiposity measures, suggesting greater GS is determined based on better neuromotor functions rather than higher adiposity itself. Nonetheless, it is important to point out that the effects of resistance training are typically site-specific (26), so training solely GS may not necessarily yield favorable effects on other parts of the body. Thus, efforts should be placed on improving whole-body muscle strength as well as neuromuscular functions.
Effects of resistance training on reducing metabolic risk are also well-documented. Specifically, glucose metabolisms and insulin sensitivity can be enhanced in response to resistance exercise (27). In the present study, the prevalence of diabetes was lower in both men and women across incremental GS quintiles. It may be that participation in resistance training was higher in those with greater GS since people use their hands in most upper-body resistance training. This suggests individuals with greater muscle strength may sustain metabolically healthier lives.
Furthermore, a meta-analysis of randomized-controlled trials concluded that resistance training programs reduced levels of lipids and lipoproteins circulating in the blood stream (28). However, high-intensity resistance training may increase arterial stiffness (29), which may then increase the risk of mortality and CVD (30). More evidence is needed to determine the specific dose-response relationship between resistance training and health outcomes.
This study is not without limitations. First, the use of data from an observational prospective study cannot fully determine causal relationships between GS and mortality. However, we excluded individuals with critical medical conditions at baseline in the primary analysis, and further excluded individuals who died in the first 2 years of follow-up and individuals who had respiratory disease or were current or previous smokers at baseline in the sensitivity analysis, in order to minimize the risk for reverse causality. Second, due to the lack of sampling strategies for recruiting samples in UK Biobank, results of this study may only be generalizable to those of similar characteristics to the sample analyzed here. Another limitation is the measurement method for aerobic fitness, a strong mortality predictor (31). Ideally, this is measured as oxygen consumption during maximal exercise tests. We adjusted for resting pulse rate instead, which is strongly associated with maximal oxygen consumption (32). The relatively low number of death cases in the analysis for CVD mortality is another limitation. Finally, the use of self-reported data for some of the covariates may have increased the risk of residual confounding.
Conclusions
Men and women with greater GS had lower risks of all-cause and CVD mortality, independent of adiposity. While excess adiposity per se presents substantial risk of mortality, the risk associated with excess adiposity was reduced, although not completely eliminated, through greater GS. Public health efforts should aim to improve muscle strength of the population in all adiposity levels.
Supplementary Material
Acknowledgment
YK designed this study, performed statistical analysis, and drafted an initial version of the manuscript. KW, DCL, SJS, NW, and SB all contributed to conceptualizing the study idea and developing the analytical plans, and provided assistance with statistical analysis. All authors critically reviewed, approved of the final version of the manuscript, and agreed to be responsible for all facets of this work.
Sources of Support: This work was supported by the UK Medical Research Council [grant numbers MC_UU_12015/1 to NW and MC_UU_12015/3 to SB] and an Intermediate Basic Science Research Fellowship of the British Heart Foundation [grant number FS/12/58/29709 to KW]. This research has been conducted using the UK Biobank Resource under Application Number 408.
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- GS
grip strength
- HR
hazard ratio
- MVPA
moderate-to-vigorous physical activity
- %BF
percent body fat
- WC
waist circumference
Footnotes
The authors have no conflict of interest.
References
- 1.World Health Organization (WHO) Consultation. Obesity: Preventing and managing the global epidemic - Introduction. Who Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong YL, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss - An update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Barry VW, Baruth M, Beets MW, Durstine JL, Liu JH, Blair SN. Fitness vs. Fatness on All-Cause Mortality: A Meta-Analysis. Progress in cardiovascular diseases. 2014;56:382–390. doi: 10.1016/j.pcad.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz JR, Sui X, Lobelo F, Morrow JR, Jr, Jackson AW, Sjostrom M, Blair SN. Association between muscular strength and mortality in men: prospective cohort study. Bmj. 2008;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. Journal of applied physiology. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bohannon RW. Hand-grip dynamometry provides a valid indication of upper extremity strength impairment in home care patients. Journal of hand therapy : official journal of the American Society of Hand Therapists. 1998;11:258–60. doi: 10.1016/s0894-1130(98)80021-5. [DOI] [PubMed] [Google Scholar]
- 7.Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing. 1994;23:132–7. doi: 10.1093/ageing/23.2.132. [DOI] [PubMed] [Google Scholar]
- 8.Cooper R, Kuh D, Hardy R, Mortality Review G, Falcon, and Teams HAS Objectively measured physical capability levels and mortality: systematic review and meta-analysis. Bmj. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–73. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz JR, Sui X, Lobelo F, Lee DC, Morrow JR, Jr, Jackson AW, Hebert JR, Matthews CE, Sjostrom M, Blair SN. Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:1468–76. doi: 10.1158/1055-9965.EPI-08-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol a-Biol. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 13.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36:228–35. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 14.Stenholm S, Mehta NK, Elo IT, Heliovaara M, Koskinen S, Aromaa A. Obesity and muscle strength as long-term determinants of all-cause mortality-a 33-year follow-up of the Mini-Finland Health Examination Survey. Int J Obesity. 2014;38:1126–1132. doi: 10.1038/ijo.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction An Individual Participant Meta-Analysis of Prospective Observational Data From 17,635 Subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UK Biobank Coordinating Centre. UK Biobank: Protocol for a large-scale prospective epidemiological resource. Design. 2007;06:1–112. [Google Scholar]
- 17.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 18.Bray GA. Fat distribution and body weight. Obes Res. 1993;1:203–5. doi: 10.1002/j.1550-8528.1993.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 19.Brage S, Westgate K, Franks PW, Stegle O, Wright A, Ekelund U, Wareham NJ. Estimation of Free-Living Energy Expenditure by Heart Rate and Movement Sensing: A Doubly-Labelled Water Study. Plos One. 2015;10 doi: 10.1371/journal.pone.0137206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor L, Brage S, Griffin SJ, Wareham NJ, Forouhi NG. The cross-sectional association between snacking behaviour and measures of adiposity: the Fenland Study, UK. Brit J Nutr. 2015;114:1286–1293. doi: 10.1017/S000711451500269X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen K, Rasmussen F, Held C, Neovius M, Tynelius P, Sundstrom J. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1.1 million young Swedish men: cohort study. Bmj. 2015;351:h4543. doi: 10.1136/bmj.h4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katzmarzyk PT, Craig CL. Musculoskeletal fitness and risk of mortality. Med Sci Sports Exerc. 2002;34:740–4. doi: 10.1097/00005768-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle Weakness in Women Occurs at an Earlier Age Than in Men, but Strength Is Preserved by Hormone Replacement Therapy. Clin Sci. 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 24.Global Recommendations on Physical Activity for Health. Geneva: 2010. [PubMed] [Google Scholar]
- 25.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports M American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 26.Layne JE, Nelson ME. The effects of progressive resistance training on bone density: a review. Med Sci Sport Exer. 1999;31:25–30. doi: 10.1097/00005768-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Strasser B, Pesta D. Resistance Training for Diabetes Prevention and Therapy: Experimental Findings and Molecular Mechanisms. Biomed Res Int. 2013 doi: 10.1155/2013/805217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Preventive medicine. 2009;48:9–19. doi: 10.1016/j.ypmed.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. British journal of sports medicine. 2013;47:393–6. doi: 10.1136/bjsports-2012-090488. [DOI] [PubMed] [Google Scholar]
- 30.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness A Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 31.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women A Meta-analysis. Jama-J Am Med Assoc. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 32.Blair SN, Wei M, Lee CD. Cardiorespiratory fitness determined by exercise heart rate as a predictor of mortality in the Aerobics Center Longitudinal Study. J Sport Sci. 1998;16:S47–S55. doi: 10.1080/026404198366678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.