Abstract
Previous studies have reported both positive and negative effects of culture of islets at high glucose concentrations on regulated insulin secretion. Here, we have reexamined this question in mouse islets and determined the role of changes in lipid synthesis in the effects of glucose. Glucose-stimulated insulin secretion (GSIS) and gene expression were examined in islets from C57BL/6 mice or littermates deleted for sterol regulatory element binding protein-1 (SREBP1) following four days culture at elevated glucose concentrations. Culture of control islets at 30 vs. 8 mmol/l glucose led to enhanced secretion at both basal (3 mmol/l) and stimulatory (17 mmol/l) glucose concentrations, and to enhanced triacylglycerol (TG) accumulation. These changes were associated with increases in the expression of genes involved in glucose sensing (Slc2a2, Gck, Abcc8, Kcnj11), differentiation (Pdx1), and lipogenesis (Srebp1, Fas, Acc1, Scd1). When cultured at either 8 or 30 mmol/l glucose, SREBP1-/- islets displayed reduced GSIS and TG content compared to normal islets. Correspondingly, glucose induction of the above genes in control islets was no longer observed in SREBP1-/- mouse islets. We conclude that enhanced lipid synthesis mediated by SREBP1c-dependent genes is required for the adaptive changes in islet gene expression and insulin secretion at elevated glucose concentrations.
Supplementary key words: islets, SREBP1c, insulin secretion, Pdx1, triacsin C
Introduction
The effects of chronic hyperglycemia on the function of wild type β-cells have been investigated in several earlier studies (1; 2). Chronically-elevated glucose concentrations have been proposed to cause a progressive inhibition of GSIS in vivo, and in in vitro studies on islets from human (3; 4) and rat (5), as well as in insulinoma cells (6). By contrast, other studies have reported that chronic culture at high glucose concentrations can lead to a left shift in the response to glucose of mouse islets (7–9).
Elevated glucose concentrations stimulate the expression of several genes likely to impact on the differentiated function of β-cells. These include genes involved in regulating glycolytic flux (Slc2a2 coding for glucose transporter 2, (10); glucokinase, Gck (11)), lipogenesis (fatty acid synthase, Fas (12); acetyl-CoA carboxylase 1, Acc1 (13); stearoyl-CoA desaturase, Scd1 (14); carbohydrate-responsive element-binding protein, Chrebp (15; 16)) and electrical activity (Abcc8 and Kcnj11 coding for the ATP-sensitive potassium channel subunits and the sulfonylurea receptor1, (14)). Underlying these changes, high glucose concentrations increase the levels (17) and nuclear accumulation (18) of pancreatic duodenal homeobox 1 (PDX1). Furthermore, in rat islets (19) and clonal β-cell lines (20; 21) glucose increases the expression of the lipogenic transcription factor sterol regulatory element binding protein 1c (SREBP1c).
SREBP1c belongs to a family of sterol-regulated factors also including SREBP1a and SREBP-2 (22). Whereas SREBP-2 is involved in the regulation of genes implicated in the sterol synthesis (23), SREBP1c controls the expression of genes involved in triglyceride synthesis (24). SREBPs are helix-loop-helix leucine zipper (bHLH-Zip) factors, and are synthesised as a precursor protein bound to the endoplasmic reticulum (ER) and nuclear membranes. When required, a SREBP cleavage-activating protein (25) escorts SREBPs from the ER to the Golgi, where SREBPs are sequentially cleaved by Site-1 and 2 proteases. The processed, mature SREBPs then enter the nucleus to activate the promoters of specific genes.
Several in vitro studies have shown that over-expression of SREBP1c in β-cells induces the lipogenic genes Fas and Acc1, leading to an accumulation of triglycerides and an inhibition of glucose-stimulated insulin secretion (GSIS) (20; 26). Recent studies in a model cellular system implicated SREBP1 in β-cell glucolipotoxicity (21), and microarray gene expression profiles of rat islets over expressing SREBP1 using adenoviruses showed changes in the expression of a number of pro- but also anti-apoptotic genes (27).
The above observations have suggested that up-regulation of SREBP1 in hyperglycaemic states is likely principally to exert a deleterious effect on β-cell function. However, we have recently found that SREBP1c inactivation in Zucker diabetic fatty rat islets failed to normalise GSIS in this model of lipotoxicity β-cell dysfunction (28) implying that small increases in SREBP1 level and triglycerides content are not the principal cause of defective secretion.
Culture of mouse islets at high glucose concentrations has previously been shown to cause hypersecretion of insulin (29; 30), though the mechanisms involved are unclear. Here, we assessed whether SREBP1 induction in response to high glucose concentrations may be important for the enhanced expression of genes which then mediate the adaptive response to hyperglycaemia of mouse islets. We have also explored the impact of SREBP1 deletion on the ability of islets from this species to respond on an extended period at high glucose concentrations with enhanced insulin secretion. Specifically, we have used islets from wild type C57BL/6J mice or littermates deleted for SREBP1 by homologous recombination (31).
We show that culture for 96 h at 8 or 30 mmol/l glucose markedly (>50%) impairs GSIS, and triglyceride content, in islets from SREBP1-/- versus wild type mice. This difference is associated with the loss, in islets lacking SREBP1, of the induction by high glucose not only of lipogenic genes (Fas, Acc1, Scd1) but, unexpectedly, of genes involved in β-cell differentiation, glucose sensing and electrical activity. Triacsin C, which prevents the synthesis and oxidation of fatty acyl-CoA (32), also blocked the induction of several of the above genes and decreased the triglyceride (TG) content and glucose responsiveness of cultured islets. We propose that adequate lipid synthesis is a requirement for the adaptive changes of mouse islets to high glucose concentrations.
Materials and Methods
Materials
Collagenase was obtained from Serva (Heidelberg, Germany). Culture medium (Dulbecco’s modified Eagles’ medium; DMEM), FCS (foetal calf serum) and glutamine were obtained from Gibco BRL (Paisley, Renfrewshire, Scotland, U.K.). Antibiotics were from Sigma (Poole, Dorset, U.K.).
Animals and genotyping
SREBP1-/-, SREBP1+/-, and SREBP1+/+ mice were generated as described and bred in the animal facility of the University of Bristol. The mice were fed a normal rodent diet and were housed in colony cages, maintained on a 12-h light/12-h dark cycle. Mice were genotyped by PCR on tail genomic DNA with specific primers. For the triacsin C experiments (Figure 3 and 5), islets were isolated from 3-4 month old C57BL/6J mice from a separate colony (Harlan, Bicester, U.K.). All animal procedures were carried out in accordance with U.K. Home Office welfare guidelines and project license restrictions.
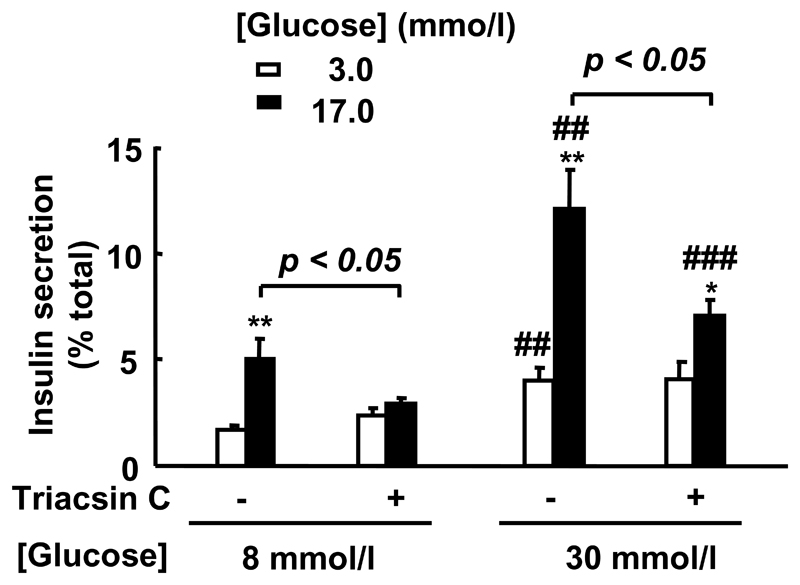
Figure 3. Effect of triacsin C on glucose-induced insulin secretion in islets exposed to long-term culture at high [glucose].
After isolation, islets from wild type mice (n=3/genotype) were cultured for 96 h at 8 or 30 mmol/l glucose in the presence or absence of triacsin C (10 μmol/l) before measuring GSIS (5 determinations/experiment). *p<0.05, **p<0.01 for effect of 17 mmol/l glucose effect. ## p<0.01; ### p<0.001 for the chronically elevated glucose effect.
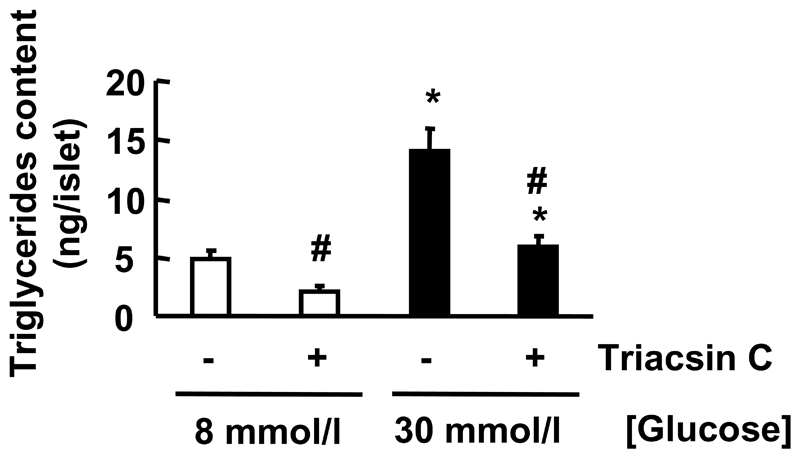
Figure 5. Effect of Triacsin C on TG content in islets exposed to long-term culture at high [glucose].
After isolation, islets from wild type mice (n=3/genotype) were cultured for 96h at 8 or 30 mmol/l glucose in the presence or absence of Triacsin C (10 μmol/l). TG content was measured as described in Material and Methods. p<0.05 for the effect of chronically elevated [glucose]. # p<0.05 for the Triacsin C effect.
Blood glucose and plasma insulin measurements
Tail blood was assayed for glucose concentration using a Glucometer Accu-chek™ (Roche). Plasma insulin was measured using a rat insulin kit (Chrystal Chem Inc., Downers Grove, IL, U.S.A.).
Isolation and culture of pancreatic islets
Mice (3-4 month) were killed by cervical dislocation and islets isolated as previously described (33). Briefly, pancreata were digested with collagenase and hand-picked. The medium used for islet isolation was a bicarbonate-buffered solution (120 mmol/l NaCl, 4.8 mmol/l KCl, 2.5 mmol/l CaCl2, 1.2 mmol/l MgCl2, 24 mmol/l NaHCO3, 10 mmol/l glucose and 1 mg/ml bovine serum albumin). It was gassed with O2/CO2 (95/5%) and equilibrated at pH 7.4. For culture in chronically elevated glucose concentrations, islets were incubated for 16 h in DMEM containing 10% (v/v) FCS, 11 mmol/l glucose, 2 mmol/l glutamine, 100 units/ml penicillin and 100 mg/ml streptomycin, incubated at 37°C with 95% air and 5% CO2. Then islets were cultured for 96 h at 8 or 30 mmol/l glucose in presence or absence of triacsin C (10 μmol/l, Biomol, Exeter, UK) before use. Furthemore, previous studies have shown that such conditions of chronic high glucose culture had no effect on C57BL/6 mouse islets viability (30) or DNA content (34).
Insulin secretion by static incubation
Cultured islets were incubated for 60 min. in a shaking water bath at 37°C in 1 ml of Krebs Ringer bicarbonate buffer (KRBH; 130 mmol/l NaCl, 3.6 mmol/l KCl, 1.5 mmol/l CaCl2, 0.5 mmol/l MgSO4, 0.5 mmol/l KH2PO4, 2.0 mmol/l NaHCO3 and 10 mmol/l Hepes) supplemented with 11 mmol/l glucose/ 0.1% (w/v) BSA. KRBH was equilibrated with O2/CO2 (95/5%), pH 7.4. Batches of three islets were handpicked and incubated for 30 min. in 0.5 ml of KRBH as above, containing either 3 or 17 mmol/l glucose. Medium was collected for insulin secretion measurement and islets were harvested with acidified ethanol to determine cellular insulin content. Insulin was measured by radio-immunoassay (Linco Research, St. Charles, MO, U.S.A.).
RNA extraction and TaqMan® real-time PCR assay
Total RNA was isolated by cell lysis in TRIzol (Gibco) according to the manufacturer’s instructions. RNA samples were treated with DNA-free™ (Ambion, Austin, TX, U.S.A.) to remove any genomic DNA contamination, and quantified by RiboGreen assay (Molecular Probes). cDNA (100 μl) was synthesized from 1 μg of total RNA, using random hexamer primers and Moloney-murine-leukaemia virus reverse transcriptase (Applied Biosystems, Warrington, U.K.). Quantitative real-time PCR (Taqman®) was performed using 25 ng of reverse-transcribed total RNA with 300 nmol/l of sense and antisense primers, 100 nmol/l of probe (Table 1), 12.5 μl of Master Mix (Qiagen, Crawley, U.K.) in a total volume of 25 μl in an ABI PRISM 7700 Sequence Detection System Instrument. Probes were labelled with 6-carboxyfluororescein (FAM) and 6-carboxy-N, N, N’, N”-tetramethylrhodamine (TAMRA). Standard curves were constructed by amplifying serial dilutions of untreated mice islet cDNA (50 ng-0.64 pg) and plotting cycle threshold (Ct) values as a function of starting reverse-transcribed RNA, the slope of which was used to calculate relative expression of the target gene.
Table 1.
Primer and probe sequences used in quantitative real time RT-PCR analysis (TaqMan®).
Probes were labelled with 6-carboxyfluororescein (FAM) and 6-carboxy-N, N, N’, N” Tetramethyl-rhodamine (TAMRA).
| Genes | Accession number | Forward Primer (5’ - 3’) | Reverse Primer (5′ - 3′) | Probe (5′ - 3′) |
|---|---|---|---|---|
| Srebp1c | NM_011480 | CCACTAGAGGTCGGCATGGT | TCCCTTGAGGACCTTTGTCATT | TGCTTGTCAGGCTCACCCTCTGGAA |
| Cyclophilin | NM_017101 | TATCTGCACTGCCAAGACTGA | CCACAATGCTCATGCCTTCTTTCA | CCAAAGACCACATGCTTGCCATCCA |
| Gapdh | M32599 | GTCGTGGATCTGACGTGCC | GATGCCTGCTTCACCACCTT | CCTGGAGAAACCTGCCAAGTATGATGACAT |
| Fas | NM_007988 | CCCTTGATGAAGAGGGATCA | ACTCCACAGGTGGGAACAAG | TCTTTCTCACCAACCTTGGCAAGGT |
| Acc1 | AY451393 | TTCTGAATGTGGCTATCAAGACTG | TGCTGGGTGAACTCTCTGAACA | CGATATTGAGGATGACAGGCTTGCAGCT |
| Chrebp | NM_021455 | CAACTCAGCACTTCCACAAG | TGGAAACTTTCACCAGGATT | CTGACTGACCCCAGCCTTGT |
| Scd1 | NM_009127 | CCTCCGGAAATGAACGAGAG | CAGGACGGATGTCTTCTTCCA | AGG TGA AGA CGG TGC CCC TCC AC |
| Scl2a2 | NM_031197 | CCCTGGGTACTCTTCACCAA | GCCAAGTAGGATGTGCCAAT | TGGCCCTTGTCACAGGCATTCT |
| Gck | L38990 | TCCCTGTAAGGCACGAAGACAT | ATTGCCACCACATCCATCTCA | CTCTTGATAGCATCTCGGAGAAGTCCCA |
| Pdx1 | NM_008814 | GAAGAGCCCAACCGCGT | TTGTTTTCCTCGGGTTCCG | CTCCTGCCCACTGGCCTTTCCA |
| Abcc8 | L40624 | CCCTCTACCAGCACACCAAT | CAGTCAGCATGAGGCAGGTA | CTTTCTCGGCTCTGGATGTCCATCT |
| Kcnj11 | D50581 | TACCACGTCATCGACTCCAA | GTTTCTACCACGCCTTCCAA | ACCACCAGGACCTGGAGATCATTGT |
| Ngn3 | NM_009719 | TGCAGCCACATCAAACTCTC | GGTCACCCTGGAAAAAGTGA | TGAGTCTGCCCTCATTCAAATCTGC |
| NKx 6.1 | NM_144955 | TTCGGAGAATGAGGAGGATGA | ACCGCTCGATTTGTGCTTTT | ACAAACCTCTGGACCCGAACTCTGACG |
Triglyceride measurements
Total lipids were extracted from 50 islets using chloroform/methanol (2:1, v/v) (35). Extracted lipids were air-dried and 10 μl of a detergent (Thesit; Fluka, Gillingham, Dorset, U.K.) was added to the dry pellet. Samples were air-dried again and resuspended in 30 μl of water (36). TG was measured using a commercial kit (Infinity™ Triglyceride Reagent, Sigma) and a standard curve of triolein (Sigma) treated in parallel with the samples.
Total islet protein assay
Total protein (10 islets) was extracted using radio-immunoprecipitation assay buffer, comprising PBS supplemented with 1.0% (v/v) Nonidet P40, 0.5% (w/v) sodium deoxycholate and 0.1% (w/v) SDS. Protein concentration was determined using BCA kit (Pierce, Rockford, IL, U.S.A.).
Statistics
Data are quoted as mean ± S.E.M and statistical analysis performed by one-way ANOVA followed by Newman-Keuls.
Results
Metabolic parameters
We observed no significant differences in body weight, blood glucose or insulin concentrations between SREBP1-/-, SREBP1+/- and wild type mice at 3-4 months of age in either the fasting or the fed state (Table 2). Other parameters are discussed below and in the Supplementary section.
Table 2.
Serum insulin and glucose levels in SREBP1-/-, SREBP1+/- and wild type mice at 18h fasted and fed state
| Mice genotype | n | Weight (g) |
Insulin (ng/ml) |
Glucose (mmol/l) |
|
|---|---|---|---|---|---|
| Fed state | |||||
| SREBP1 +/+ | 9 | 25.3 ± 0.55 | 1.27 ± 0.42 | 8.94 ± 0.46 | |
| SREBP1 +/- | 12 | 24.5 ± 0.54 | 0.82 ± 0.17 | 7.86 ± 0.56 | |
| SREBP1 -/- | 8 | 24.7 ± 0.54 | 1.09 ± 0.42 | 9.27 ± 0.68 | |
| Fasted state | |||||
| SREBP1 +/+ | 10 | 24.4 ± 1.1 | 0.40 ± 0.06 | 4.73 ± 0.28 | |
| SREBP1 +/- | 13 | 22.7 ± 0.8 | 0.34 ± 0.03 | 5.46 ± 0.30 | |
| SREBP1 -/- | 8 | 23.2 ± 1.0 | 0.53 ± 0.09 | 5.20 ± 0.27 |
Effects of culture of wild type or SREBP1 deficient islets at elevated glucose concentrations on glucose-stimulated insulin secretion, and TG content
Islets were cultured at glucose concentrations representing either severe hyperglycemia (30 mmol/l) or at level in the physiological range for fed mice (8 mmol/l) (see also Table 2); the effects of lower glucose concentrations (eg 5.5 mmol/l), which correspond to the starved state (Table 2), were not examined here, since our own (F. Diraison and G.A. Rutter, unpublished) and others’ (37; 38) observations indicate that these are associated with increased apoptosis during extended islet culture. Culture of wild type islets at 30 versus 8 mmol/l glucose concentrations increased basal (3 mmol/l) and high (17 mmol/l) glucose-stimulated insulin secretion (Figure 1). In contrast to freshly isolated islets, where a small increase in the extend of glucose-stimulated (17 versus 3 mmol/l) insulin secretion was apparent (supplementary data 1), SREBP1-/- islets displayed a significantly lower fold change in the acute stimulation of insulin secretion by glucose after culture at either 8 or 30 mmol/l glucose (Figure 1A, 1B). A smaller and non-significant tendency towards impaired GSIS was also seen in SREBP1-/+ islets (Figure 1A, 1B).
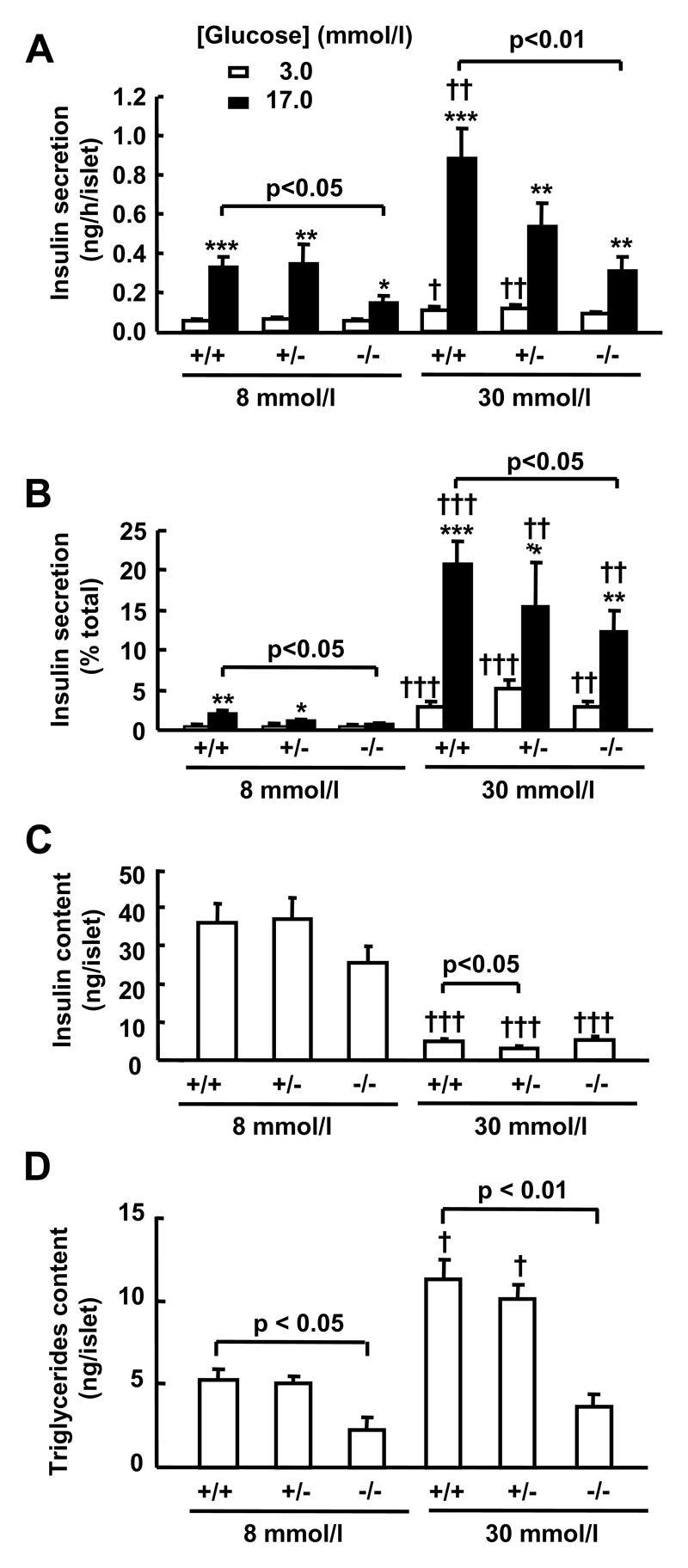
Figure 1. Effects of glucose and SREBP1 deletion on glucose-stimulated insulin secretion and triglyceride content after chronic exposure of mouse islets to high [glucose].
After isolation, islets (n=3/genotype) were cultured for 96 h at 8 or 30 mmol/l glucose before measuring GSIS (5 determinations/experiment) and TG content, as described in Material and Methods. A, B: Insulin release. C: Insulin content. D: TG content. *p<0.05; ** p<0.01; *** p<0.001 for the 17 mmol/l glucose effect. † p <0.05; †† p <0.01; ††† p <0.001 for the effect of chronically elevated [glucose].
However, if we compared GSIS between genotypes, we observed that there were no significant differences between the fold-stimulation of insulin secretion acutely by 17 mmol/l versus 3 mmol/l glucose for islets of the same genotype cultured at either 8 or 30 mmol/l (Figure 1A, 1B).
Culture for four days at 30 mmol/l compared to 8 mmol/l glucose also decreased total insulin content by > 85 % in each genotype (Figure 1C), presumably reflecting the sustained stimulation of insulin release under these conditions.
TG accumulation was significantly enhanced by culture of wild type or SREBP1+/- islets at 30 vs. 8 mmol/l glucose (Figure 1D). Furthermore, with respect to islets from wild type or SREBP+/- mice, SREBP1-/- mouse islets displayed a substantially (60%) decreased TG content after culture at 8 mmol/l glucose, and no further TG increase was seen in these islets after culture at 30 mmol/l glucose (Figure 1D).
Effect of chronic exposure to elevated glucose concentrations on gene expression in wild type or SREBP1 deficient islets
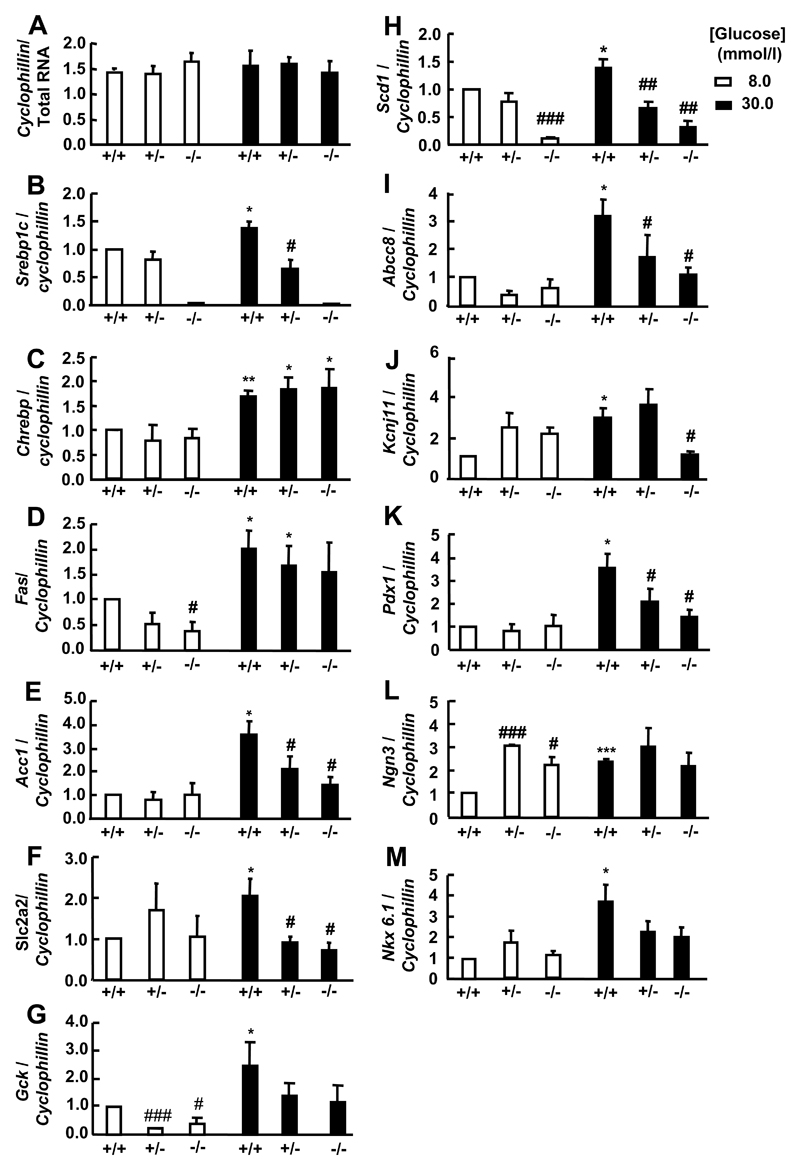
To analyse in more detail the mechanisms that may be responsible for the decreases in glucose-stimulated insulin secretion in SREBP1-/- vs. wild-type islets, we measured the expression of candidate genes using quantitative real time PCR (Taqman®) (Figure 2). Deletion of SREBP1 had complex effects on the changes in the lipogenic and other gene expression observed during culture at elevated glucose concentrations. Thus, Fas and Gck gene expression was decreased in SREBP1-deleted compare to wild type islets after four days culture at 8 but not 30 mmol/l glucose (Figure 2D, 2G). By contrast, Acc1, Slc2a2, Abcc8, Kcnj11 and Pdx1 mRNA levels were decreased in SREBP1 knockout versus wild type islets at 30 mmol/l glucose but not at 8 mmol/l (Figure 2E, 2F, 2I, 2J, 2K). Scd1 mRNA levels were decreased at 30 and 8 mmol/l (Figure 2H) whereas SREBP 1 deletion had no effect on Chrebp or NKx6.1 gene expression (Figure 2C, 2M). In most cases, the levels of gene expression in heterozygote mice were intermediate between those in wild type and SREBP1-/- islets at each glucose concentration.
Figure 2. Gene expression in islets cultured for 96 h at 8 or 30 mmol/l glucose.
After isolation, islets (n=3/genotype) were cultured for 96 h at 8 or 30 mmol/l glucose before RNA extraction. #p<0.05; ### p<0.001 for the genotype effect. *p<0.05; ***p<0.001 for the effect of chronically elevated [glucose].
When cultured at 8 mmol/l glucose, Ngn3 gene expression was significantly increased in SREBP1+/- and SREBP1-/- mouse islets (Figure 2L). We observed no changes in the expression of cyclophillin D (Figure 2A) to which other genes were normalised, or another ”housekeeping” gene, Gapdh (data not shown) excluding the effects of glucose as being non-specific.
Effect of triacsin C on glucose-stimulated insulin secretion, TG content and gene expression in islets cultured in chronic high glucose concentrations
To examine the hypothesis that there may be a potential role of acyl-CoA or TG synthesis in the long term regulation of gene expression and insulin secretion by glucose we used Triacsin C. This pharmacological agent is an inhibitor of long-chain acyl-CoA synthetase and thus of de novo triglyceride synthesis and acyl-CoA oxidation (32).
We first determined whether triacsin C may mimic the effects of SREBP1 deletion on glucose-stimulated insulin secretion observed in islets cultured in the same conditions (Figure 3). Islets from wild type mice were cultured for 96 h at 8 mmol/l or 30 mmol/l glucose in the presence or absence of 10 μmol/l triacsin C, before measuring GSIS. Addition of triacsin C decreased GSIS by 42.3% and 41.5% when islets were cultured for 96 h at 8 mmol/l or 30 mmol/l glucose respectively, but had no effect on basal insulin secretion (Figure 3).
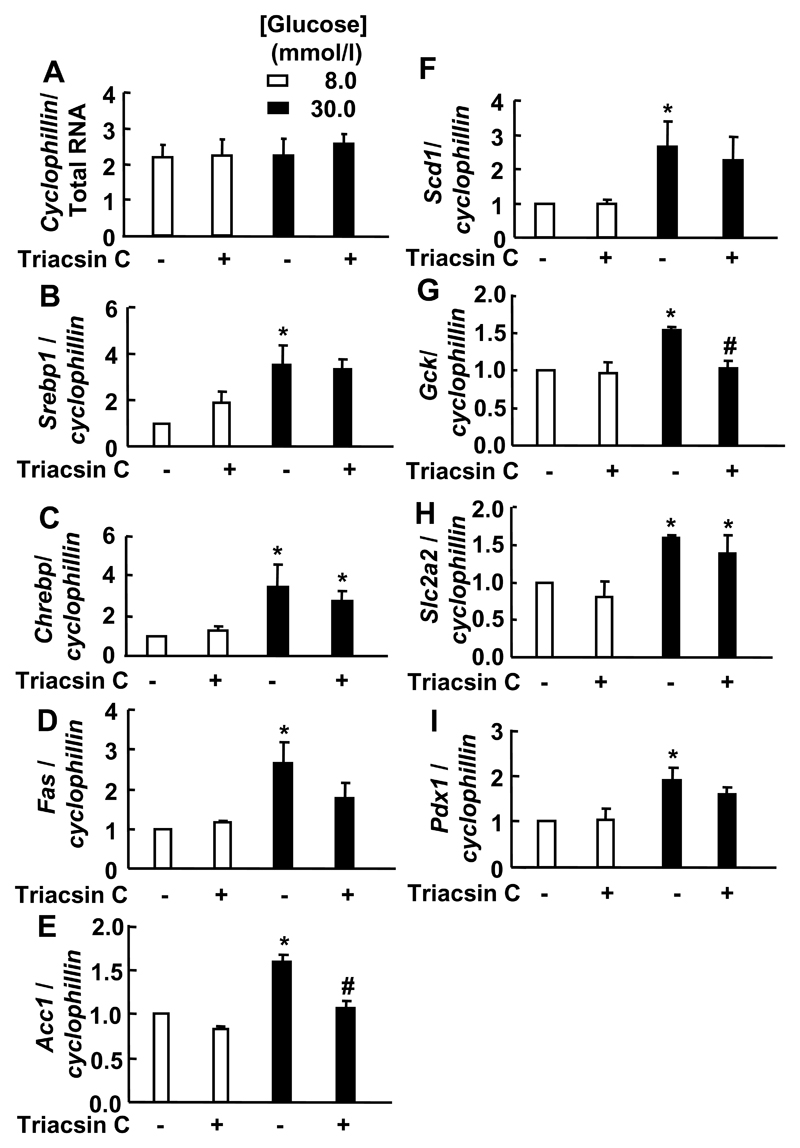
Under the same conditions, triacsin C had no effect on Srebp1, Chrebp, Fas, Acc1, Scd1, Pdx1, Gck or Slc2a2 mRNA levels in islets cultured at 8 mmol/l glucose. However, with the exception of Slc2a2 and Chrebp mRNA, which was still induced by 30 mmol/l glucose, up-regulation of Acc1 (p<0.05), Gck (p<0.05), Srebp1, Fas, Scd1 and Pdx1 gene expression was decreased in the presence of triacsin C (Figure 4). Again, we observed no changes in the expression of Cyclophillin D (Figure 4) or Gapdh (data not shown).
Figure 4. Effect of triacsin C on gene expression in islets exposed to long-term culture at high [glucose].
(A-F): After isolation, islets from wild type mice (n=3/genotype) were cultured for 96 h at 8 or 30 mmol/l glucose in the presence or absence of triacsin C (10 μmol/l) before RNA extraction. *p<0.05 for the chronically elevated glucose effect. # p<0.05 for the triacsin C effect.
We also measured the effect of this drug on TG content in islets cultured under the same conditions. Triacsin C decreased TG content when islets (versus non-treated islets) were cultured for 96h at 8 mmol/l or 30 mmol/l glucose respectively (Figure 5).
Discussion
The principal aims of this study were (a) to re-examine the effects of extended culture at elevated glucose concentrations on basal and glucose-stimulated insulin secretion from mouse islets and (b) to determine whether the induction by glucose of SREBP1 (20), and enhanced fatty acid and/or triglyceride synthesis, might contribute to any effects observed.
In line with findings from Khaldi et al (7) of a “left shift” in the dose response to glucose of mouse islets incubated at high glucose concentrations, we show firstly that both basal and glucose-stimulated insulin secretion are enhanced by culture of C57BL/6 mouse islets at 30 versus 8 mmol/l glucose (Figure 1). Culture at 8 mmol/l glucose essentially preserved the secretory responses observed in freshly isolated islets (compare Figure 1A and supplementary data 1A), when rates of release were compared at 3 or 17 mmol/l glucose in each case. Whilst glucose has been shown to stimulate growth and proliferation of β-cells, especially in foetal islets (39), it would seem unlikely that the enhanced secretion of insulin observed in the present study simply reflects an increased in β-cell mass per islet after culture at 30 versus 8 mmol/l.
The present data thus confirm that, in the mouse, culture at mildly or strongly elevated glucose concentrations fails to elicit evident “glucotoxic” effects but rather elevates basal insulin secretion whilst preserving glucose-stimulated secretion. In this respect, the response to long term high glucose treatment of C57BL/6 mouse islets appears to be quite distinct to that of isolated rat islet β-cells, where even relatively short term (24 h) exposure to 30 mmol/l glucose caused marked decreases in glucose-stimulated insulin secretion (40; 7).
Although SREBP1-/- mice displayed a slight increase in glycaemia following an intraperitoneal glucose tolerance test (supplementary data 3), it seems possible that this may reflect, at least in part, altered insulin sensitivity rather than defective insulin secretion. Indeed, GSIS was slightly enhanced in freshly isolated islets from mice deleted for both Srebp1 alleles versus wild type mice (supplementary data 1), consistent with earlier results (26), this difference was reversed upon culture at either 8 or 30 mmol/l glucose. Thus, a clear impairment of GSIS was apparent in SREBP1-/- (but not SREBP1+/-) islets after culture at either 8 or 30 mmol/l glucose (Figure 1) and this change was associated with decreased islet TG content in SREBP1-/- islets (Figure 1D). These results also show that even if the fold-stimulation of insulin secretion for islets of the same genotype were similar when cultured at 8 or 30 mmol/l glucose (Figure 1A, 1B), SREBP1-/- islets tolerated less well than wild type islets prolonged culture at either glucose concentration.
The above results thus challenge the physiological relevance of previous findings from ourselves (19) and others (21; 26) which showed that forced over-expression of SREBP1 in β-cells or islets increased lipogenic gene expression, leading to an accumulation of triglycerides and an inhibition of GSIS, as well as an ER stress response and enhanced cell death (41). Nevertheless, it might be argued that more substantial increases in SREBP1 expression, for example in the combined presence of elevated glucose and free fatty acid levels (ie “glucolipotoxic” conditions), might contribute to β-cell dysfunction. Arguing against this view, expression in ZDF islets (42) of a dominant-negative form of SREBP1c (28) failed to significantly reverse defective glucose-stimulated insulin secretion, whilst substantially decreasing islet TG content and reversing the expression of lipogenic genes.
We also show here that SREBP1 is required for the induction by 30 versus 8 mmol/l glucose, as expected, of lipogenic (Fas, Acc1, Scd1) genes (Figure 2) given the previously described role of this factor in the control of lipogenic genes (20, 43) consequently for enhanced TG accumulation (Figure 1D). More surprising was the apparent action of SREBP1 knockout to block the induction by 30 versus 8 mmol/l on several other genes involved in glucose sensing (Slc2a2, Gck, Abcc8, Kcnj11) (Figure 2).
It was also observed here that Pdx1 gene expression was increased in normal mouse islets cultured at elevated glucose concentrations (Figure 2), and that this effect was inhibited in islets from mice deleted for SREBP1. PDX1 is a key transcription factor involved in pancreatic development (44) and in the maintenance of the β-cell phenotype (45), serving to control insulin (46), Slc2a2 (47) and Nkx6.1 (48) gene expression. LXR (Liver X receptors, α and β) is a member of a nuclear receptor superfamily of ligand-activated transcription factors. In a recent study (49), down-regulation of SREBP1 expression in INS-1 cells by RNA interference blocked Liver X Receptor (LXR)-induced expression of Pdx1, compatible with the view that SREBP1 is involved in the regulation of Pdx1. Decreases in Pdx1 levels in SREBP1-/- following culture at 30 mmol/l glucose islets may subsequently underlie the loss of glucose-stimulated expression of Slc2a2 and Nkx6.1. By contrast, Pdx1 gene expression was higher in freshly isolated islets from SREBP1-/- versus SREBP1+/+ mice (supplementary data 2) a finding consistent with a negative role for SREBP1c in the control of basal Pdx1 gene expression. Perhaps reconciling these observations, a recent study proposed that the effects of SREBP1 on β-cells function depend of the level and the duration of its activation (49).
Interestingly, SREBP1 deletion had no effect on Chrebp mRNA levels, confirming previous in vitro data in an insulinoma cell line (41) and showing that there were no compensatory increases in the expression of the latter transcription factor in SREBP1-/- islets.
In a complementary approach, we also used Triacsin C here to study the potential role of acyl-CoA or TG synthesis in the long term regulation of gene expression and insulin secretion by high glucose. Blockage of acyl-CoA synthesis using Triacsin C affects both fatty acid oxidation and the synthesis of triglycerides (32). Importantly, using the same culture conditions (four days at 8 or 30 mmol/l glucose) we could mimic the effects of SREBP1 deletion on both glucose-stimulated insulin secretion (Figure 3) and TG content (Figure 5). Interestingly, the resistance of Slc2a2 mRNA induction to the effects of triacsin C (Figure 4H), compared to the complete abolition of glucose-induced increases in this gene in SREBP1-/- islets (Figure 2F) may reflect a direct binding of SREBP1c to the Slc2a2 promoter, as reported in primary rat hepatocytes (50). Nevertheless, it seems reasonable to conclude that the augmentation of basal and glucose-stimulated insulin secretion by culture at elevated glucose concentrations (Figures 1, 3) may reflect enhanced fatty acyl-CoA synthesis resulting in the up-regulation of several genes involved in glucose metabolism or sensing.
In summary, the present results demonstrate a requirement for SREBP1 in the hypersecretion of insulin resulting from chronic exposure of mouse islets to high glucose concentrations in vitro. In addition to the requirement for SREBP1 in the induction of lipogenic genes, SREBP1 is also shown, unexpectedly, to be necessary for the up-regulation of genes directly involved in the expression of β-cell enriched genes (Pdx1) and in genes whose products are central to glucose sensing (Slc2a2, Gck, Kcnj11, Abcc81). Induction of SREBP1c and enhanced lipid synthesis may therefore play a key role in adaptive insulin hypersecretion observed in some models of hyperglycemia.
Supplementary Material
Acknowledgments
We thank the Medical Research Council (Research Grant G0401641), the Wellcome Trust (Programme Grants 081958/Z/07/Z and 067081/Z/02/Z), The Juvenile Diabetes Research Fund, EU FP6 Consortium “SaveBeta” and Diabetes U.K. for financial support. GAR was supported by a Wellcome Trust Research Leave Fellowship. We thank Dr Isabelle Leclerc for assistance in mouse genotyping.
Abbreviations used
- Acc1
acetyl-coenzyme A carboxylase 1
- bHLH-Zip
basic helix-loop-helix leucine zipper
- Chrebp
carbohydrate-responsive element-binding protein
- ER
endoplasmic reticulum
- Fas
fatty acid synthase
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- Gck
glucokinase
- Slc2a2
glucose transporter 2
- GSIS
glucose-stimulated insulin secretion
- IPGTT
intraperitoneal glucose tolerance test
- KATP
ATP sensitive K+ channel
- Kcnj11
inwardly rectifying K+ channel 6.2
- KRBH
Krebs-Ringer bicarbonate buffer
- LXR
liver X Receptors
- Ngn3
neurogenin 3
- Pdx1
pancreatic duodenal homeobox 1
- Scd1
stearoyl-CoA desaturase 1
- SRE
sterol response element
- SREBP
sterol regulatory element-binding protein
- TG
triglyceride
References
- 1.Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- 2.Topp BG, McArthur MD, Finegood DT. Metabolic adaptations to chronic glucose infusion in rats. Diabetologia. 2004;47:11602–1610. doi: 10.1007/s00125-004-1493-5. [DOI] [PubMed] [Google Scholar]
- 3.Marshak S, Leibowitz G, Bertuzzi F, Socci C, Kaiser N, Gross DJ, Cerasi E, Melloul D. Impaired beta-cell functions induced by chronic exposure of cultured human pancreatic islets to high glucose. Diabetes. 1999;48:1230–1236. doi: 10.2337/diabetes.48.6.1230. [DOI] [PubMed] [Google Scholar]
- 4.Ling Z, Pipeleers DG. Prolonged exposure of human beta cells to elevated glucose levels results in sustained cellular activation leading to a loss of glucose regulation. J Clin Invest. 1996;98:2805–2812. doi: 10.1172/JCI119108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling Z, Kiekens R, Mahler T, Schuit FC, Pipeleers-Marichal M, Sener A, Kloppel G, Malaisse WJ, Pipeleers DG. Effects of chronically elevated glucose levels on the functional properties of rat pancreatic beta-cells. Diabetes. 1996;45:1774–1782. doi: 10.2337/diab.45.12.1774. [DOI] [PubMed] [Google Scholar]
- 6.Roche E, Assimacopoulos-Jeannet F, Witters LA, Perruchoud B, Yaney G, Corkey B, Asfari M, Prentki M. Induction by glucose of genes coding for glycolytic enzymes in a pancreatic beta-cell line (INS-1) J Biol Chem. 1997;272:3091–3098. doi: 10.1074/jbc.272.5.3091. [DOI] [PubMed] [Google Scholar]
- 7.Khaldi MZ, Guiot Y, Gilon P, Henquin JC, Jonas JC. Increased glucose sensitivity of both triggering and amplifying pathways of insulin secretion in rat islets cultured for 1 wk in high glucose. Am J Physiol Endocrinol Metab. 2004;287:E207–E217. doi: 10.1152/ajpendo.00426.2003. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Najafi H, Smith RM, Zimmerman EC, Magnuson MA, Tal M, Matschinsky FM. Concordant glucose induction of glucokinase, glucose usage, and glucose-stimulated insulin release in pancreatic islets maintained in organ culture. Diabetes. 1992;41:792–806. doi: 10.2337/diab.41.7.792. [DOI] [PubMed] [Google Scholar]
- 8.Liu YQ, Moibi JA, Leahy JL. Chronic high glucose lowers pyruvate dehydrogenase activity in islets through enhanced production of long chain acyl-CoA: prevention of impaired glucose oxidation by enhanced pyruvate recycling through the malate-pyruvate shuttle. J Biol Chem. 2004;279:7470–7475. doi: 10.1074/jbc.M307921200. [DOI] [PubMed] [Google Scholar]
- 10.Schuit F, Flamez D, De Vos A, Pipeleers D. Glucose-regulated gene expression maintaining the glucose-responsive state of beta-cells. Diabetes. 2002;51(Suppl 3):S326–S332. doi: 10.2337/diabetes.51.2007.s326. [DOI] [PubMed] [Google Scholar]
- 11.Noma Y, Bonner-Weir S, Latimer JB, Davalli AM, Weir GC. Translocation of glucokinase in pancreatic ß-cells during acute and chronic hyperglycemia. Endocrinology. 1996;137:1485–1491. doi: 10.1210/endo.137.4.8625927. [DOI] [PubMed] [Google Scholar]
- 12.Roche E, Farfari S, Witters LA, Assimacopoulos-Jeannet F, Thumelin S, Brun T, Corkey BE, Saha AK, Prentki M. Long-term exposure of beta-INS cells to high glucose concentrations increases anaplerosis, lipogenesis, and lipogenic gene expression. Diabetes. 1998;47:1086–1094. doi: 10.2337/diabetes.47.7.1086. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Shaoying, Kim Ki-Han. Essential Role of Acetyl-CoA Carboxylase in the Glucose-Induced Insulin Secretion in a Pancreatic ß-Cell Line. Cell Signal. 1998;10:35–42. doi: 10.1016/s0898-6568(97)00070-3. [DOI] [PubMed] [Google Scholar]
- 14.Flamez D, Berger V, Kruhoffer M, Orntoft T, Pipeleers D, Schuit FC. Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes. 2002;51:2018–2024. doi: 10.2337/diabetes.51.7.2018. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Wollheim CB. ChREBP rather than USF2 regulates glucose stimulation of endogenous L-pyruvate kinase expression in insulin-secreting cells. J Biol Chem. 2002;277:32746–32752. doi: 10.1074/jbc.M201635200. [DOI] [PubMed] [Google Scholar]
- 16.da Silva Xavier G, Rutter GA, Diraison F, Andreolas C, Leclerc I. ChREBP binding to fatty acid synthase and L-type pyruvate kinase genes is stimulated by glucose in pancreatic beta-cells. J Lipid Res. 2006;47:2482–2491. doi: 10.1194/jlr.M600289-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.da Silva Xavier G, Rutter J, Rutter GA. Involvement of Per-Arnt-Sim (PAS) kinase in the stimulation of preproinsulin and pancreatic duodenum homeobox 1 gene expression by glucose. Proc Natl Acad Sci U S A. 2004;101:8319–8324. doi: 10.1073/pnas.0307737101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafiq I, Kennedy HJ, Rutter GA. Glucose-dependent translocation of insulin promoter factor-1 (IPF-1) between the nuclear periphery and the nucleoplasm of single MIN6 beta-cells. J Biol Chem. 1998;273:23241–23247. doi: 10.1074/jbc.273.36.23241. [DOI] [PubMed] [Google Scholar]
- 19.Diraison F, Parton L, Ferre P, Foufelle F, Briscoe CP, Leclerc I, Rutter GA. Over-expression of sterol-regulatory-element-binding protein-1c (SREBP1c) in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release: modulation by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) Biochem J. 2004;378(Pt 3):769–778. doi: 10.1042/BJ20031277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreolas C, da Silva Xavier G, Diraison F, Zhao C, Varadi A, Lopez-Casillas F, Ferre P, Foufelle F, Rutter GA. Stimulation of acetyl-CoA carboxylase gene expression by glucose requires insulin release and sterol regulatory element binding protein 1c in pancreatic MIN6 beta-cells. Diabetes. 2002;51:536–545. doi: 10.2337/diabetes.51.8.2536. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Maechler P, Antinozzi PA, Herrero L, Hagenfeldt-Johansson KA, Bjorklund KA, Wollheim CB. The transcription factor SREBP-1c is instrumental in the development of beta-cell dysfunction. J Biol Chem. 2003;278:16622–16629. doi: 10.1074/jbc.M212488200. [DOI] [PubMed] [Google Scholar]
- 22.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99:838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 24.Shimano H. Sterol regulatory element-binding protein-1 as a dominant transcription factor for gene regulation of lipogenic enzymes in the liver. Trends Cardiovasc Med. 2000;10:275–278. doi: 10.1016/s1050-1738(00)00079-7. [DOI] [PubMed] [Google Scholar]
- 25.Nohturfft A, DeBose-Boyd RA, Scheek S, Goldstein JL, Brown MS. Sterols regulate cycling of SREBP cleavage-activating protein (SCAP) between endoplasmic reticulum and Golgi. Proc Natl Acad Sci U S A. 1999;96:11235–11240. doi: 10.1073/pnas.96.20.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi A, Motomura K, Kato T, Yoshikawa T, Nakagawa Y, Yahagi N, Sone H, Suzuki H, Toyoshima H, Yamada N, Shimano H. Transgenic mice overexpressing nuclear SREBP-1c in pancreatic beta-cells. Diabetes. 2005;54:492–499. doi: 10.2337/diabetes.54.2.492. [DOI] [PubMed] [Google Scholar]
- 27.Diraison F, Motakis E, Parton LE, Nason GP, Leclerc I, Rutter GA. Impact of adenoviral transduction with SREBP1c or AMPK on pancreatic islet gene expression profile: analysis with oligonucleotide microarrays. Diabetes. 2004;53(Suppl 3):S84–S91. doi: 10.2337/diabetes.53.suppl_3.s84. [DOI] [PubMed] [Google Scholar]
- 28.Parton LE, McMillen PJ, Shen Y, Docherty E, Sharpe E, Diraison F, Briscoe CP, Rutter GA. Limited role for SREBP-1c in defective glucose-induced insulin secretion from Zucker diabetic fatty rat islets: a functional and gene profiling analysis. Am J Physiol Endocrinol Metab. 2006;291:E982–E994. doi: 10.1152/ajpendo.00067.2006. [DOI] [PubMed] [Google Scholar]
- 29.Svensson C, Sandler S, Hellerstrom C. Lack of long-term beta-cell glucotoxicity in vitro in pancreatic islets isolated from two mouse strains (C57BL/6J; C57BL/KsJ) with different sensitivities of the beta-cells to hyperglycaemia in vivo. J Endocrinol. 1993;136:289–296. doi: 10.1677/joe.0.1360289. [DOI] [PubMed] [Google Scholar]
- 30.Kooptiwut S, Kebede M, Zraika S, Visinoni S, Aston-Mourney K, Favaloro J, Tikellis C, Thomas MC, Forbes JM, Cooper ME, Dunlop M, et al. High glucose-induced impairment in insulin secretion is associated with reduction in islet glucokinase in a mouse model of susceptibility to islet dysfunction. J Mol Endocrinol. 2005;35:39–48. doi: 10.1677/jme.1.01720. [DOI] [PubMed] [Google Scholar]
- 31.Shimano H, Shimomura I, Hammer RE, Herz J, Goldstein JL, Brown MS, Horton JD. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J Clin Invest. 1997;100:2115–2124. doi: 10.1172/JCI119746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB. Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J Biol Chem. 1998;273:16146–16154. doi: 10.1074/jbc.273.26.16146. [DOI] [PubMed] [Google Scholar]
- 33.Ravier MA, Henquin JC. Time and amplitude regulation of pulsatile insulin secretion by triggering and amplifying pathways in mouse islets. FEBS Lett. 2002;530:215–219. doi: 10.1016/s0014-5793(02)03491-9. [DOI] [PubMed] [Google Scholar]
- 34.Svensson C, Hellerstrom C. Long-term effects of a high glucose concentration in vitro on the oxidative metabolism and insulin production of isolated rat pancreatic islets. Metabolism. 1991;40:513–518. doi: 10.1016/0026-0495(91)90233-m. [DOI] [PubMed] [Google Scholar]
- 35.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 36.Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 2001;50:315–321. doi: 10.2337/diabetes.50.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van de Casteele M, Kefas BA, Cai Y, Heimberg H, Scott DK, Henquin JC, Pipeleers D, Jonas JC. Prolonged culture in low glucose induces apoptosis of rat pancreatic beta-cells through induction of c-myc. Biochem Biophys Res Commun. 2003;312:937–944. doi: 10.1016/j.bbrc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, Berggren PO. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- 39.Swenne I. Glucose-stimulated DNA replication of the pancreatic islets during the development of the rat fetus. Effects of nutrients, growth hormone, and triiodothyronine. Diabetes. 1985;34:803–807. doi: 10.2337/diab.34.8.803. [DOI] [PubMed] [Google Scholar]
- 40.Tsuboi T, Ravier MA, Parton LE, Rutter GA. Sustained exposure to high glucose concentrations modifies glucose signaling and the mechanics of secretory vesicle fusion in primary rat pancreatic beta-cells. Diabetes. 2006;55:1057–1065. doi: 10.2337/diabetes.55.04.06.db05-1577. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci. 2005;118(Pt 17):3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- 42.Shimabukuro M, Zhou YT, Lee Y, Unger RH. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J Biol Chem. 1998;273:3547–3550. doi: 10.1074/jbc.273.6.3547. [DOI] [PubMed] [Google Scholar]
- 43.Tabor DE, Kim JB, Spiegelman BM, Edwards PA. Identification of conserved cis-elements and transcription factors required for sterol-regulated transcription of stearoyl-CoA desaturase 1 and 2. J Biol Chem. 1999;274:20603–20610. doi: 10.1074/jbc.274.29.20603. [DOI] [PubMed] [Google Scholar]
- 44.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 45.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peers B, Leonard J, Sharma S, Teitelman G, Montminy MR. Insulin expression in pancreatic islet cells relies on cooperative interactions between the helix loop helix factor E47 and the homeobox factor STF-1. Mol Endocrinol. 1994;8:1798–1806. doi: 10.1210/mend.8.12.7708065. [DOI] [PubMed] [Google Scholar]
- 47.Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol. 1996;10:1327–1334. doi: 10.1210/mend.10.11.8923459. [DOI] [PubMed] [Google Scholar]
- 48.Watada H, Mirmira RG, Leung J, German MS. Transcriptional and translational regulation of beta-cell differentiation factor Nkx6.1. J Biol Chem. 2000;275:34224–34230. doi: 10.1074/jbc.M004981200. [DOI] [PubMed] [Google Scholar]
- 49.Zitzer H, Wente W, Brenner MB, Sewing S, Buschard K, Gromada J, Efanov AM. Sterol regulatory element-binding protein 1 mediates liver X receptor-beta-induced increases in insulin secretion and insulin messenger ribonucleic acid levels. Endocrinology. 2006;147:3898–3905. doi: 10.1210/en.2005-1483. [DOI] [PubMed] [Google Scholar]
- 50.Im SS, Kang SY, Kim SY, Kim HI, Kim JW, Kim KS, Ahn YH. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes. 2005;54:1684–1691. doi: 10.2337/diabetes.54.6.1684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.