Abstract
Background
Preterm premature rupture of membranes (PPROM) is a leading contributor to maternal and neonatal morbidity and mortality. Epidemiologic and experimental studies have demonstrated that thrombin causes fetal membrane weakening and subsequently PPROM. Although blood is suspected as the likely source of thrombin in fetal membranes and amniotic fluid of patients with PPROM, this has not been proven. Ureaplasma Parvum (U. parvum) is emerging as a pathogen involved in prematurity, including PPROM, but until now, prothrombin production directly induced by bacteria in fetal membranes has not been described.
Objectives
This study was designed to investigate whether U. parvum exposure can induce prothrombin production in fetal membranes cells.
Study Design
Primary fetal membrane cells (amnion epithelial, chorion trophoblast, and decidua stromal) or full-thickness fetal membrane tissue explants from elective, term, uncomplicated cesarean deliveries were harvested. Cells or tissue explants were infected with live U. parvum (1 × 105, 1 × 106, or 1 × 107 colony forming units (cfu)/ml) or lipopolysaccharide (Escherichia coli J5, L-5014, Sigma, 100 ng/ml or 1000 ng/ml) for 24 hours. Tissue explants were fixed for immunohistochemistry staining of thrombin/prothrombin. Fetal membrane cells were fixed for confocal immunofluorescent staining of the biomarkers of fetal membrane cell types and thrombin/prothrombin. Protein and mRNA were harvested from the cells and tissue explants for Western blot or qRT-PCR to quantify thrombin/prothrombin protein or mRNA production, respectively. Data are presented as mean values ± standard errors of mean. Data were analyzed using one-way ANOVA with post hoc Dunnett’s test.
Results
Prothrombin production and localization was confirmed by Western blot and immunostainings in all primary fetal membrane cells and tissue explants. Immunofluorescence observations revealed a perinuclear localization of prothrombin in amnion epithelial cells. Localization of prothrombin in chorion and decidua cells was perinuclear and cytoplasmic. Prothrombin mRNA and protein expression in fetal membranes was significantly increased by U. parvum, but not lipopolysaccharide, treatments in a dose-dependent manner. Specifically, U. parvum at a dose of 1×107 cfu/ml significantly increased both prothrombin mRNA (fold changes in amnion: 4.1±1.9; chorion: 5.7±4.2; decidua: 10.0±5.4; FM: 9.2±3.0) and protein expression (fold changes in amnion: 138.0±44.0; chorion: 139.6±15.1; decidua: 56.9±29.1; fetal membrane: 133.1±40.0) compared to untreated controls. U. parvum at a dose of 1×106 cfu/ml significantly upregulated prothrombin protein expression in chorion cells (fold change: 54.9±5.3) and prothrombin mRNA expression in decidua cells (fold change: 4.4±1.9).
Conclusions
Our results demonstrate that prothrombin can be directly produced by fetal membrane amnion, chorion, and decidua cells. Further, prothrombin production can be stimulated by U. parvum exposure in fetal membranes. These findings represent a potential novel underlying mechanism of U. parvum-induced rupture of fetal membranes.
Keywords: preterm birth, amnion, chorion, decidua, endotoxin, lipopolysaccharide, Ureaplasma parvum, prothrombin
Introduction
Preterm birth (PTB) remains a major public health problem and complicates approximately 10% of deliveries in the US1. Preterm premature rupture of membranes (PPROM) accounts for approximately one third of all PTB2. Prevention of pregnancy complications through enhanced understanding and appropriate intervention is of critical importance to improve both maternal and neonatal outcomes. The causes of PPROM are multifactorial and include: pathologic anatomical remodeling3, 4, altered membrane morphology5–10, complications from invasive procedures11, inflammation12–14, and genetic factors15–17. Although the mechanisms of PPROM are not well-understood, infection in the fetal membranes has been implicated as an early event in the pathogenesis of PPROM and/or preterm labor18–25. Histological chorioamnionitis complicates almost half of all PPROM cases26. The propagation of bacteria is an important contributing factor not only in PPROM, but also in adverse neonatal and maternal complications following PPROM.
Ureaplasma spp. are among the organisms frequently implicated in prematurity-linked conditions and chorioamnionitis27–34. The two species of Ureaplasma known to colonize humans are U. urealyticum and U. parvum. Of these, U. parvum is the most common species isolated from the genital tract of men and women29, 35, 36. Increasing evidence suggests that U. parvum is an significant pathogen in pregnancy and is associated with PPROM, PTB, and chorioamnionitis37. Specifically, U. parvum is the most frequently isolated pathogen in the amniotic fluid of women who deliver preterm22, 31, 32, 35 and its colonization in neonates is inversely related to gestational age at delivery28, 38. A recent study of the human microbiota during pregnancy indicated that elevated vaginal Ureaplasma, when combined with poor vaginal colony state, was associated with PTB39. The presence of U. parvum, but not U. urealyticum, in vaginal fluid during pregnancy was significantly associated with higher risk of spontaneous PTB in an Australian cohort of pregnant women40. Another study demonstrated that human placenta infection with Ureaplasma is associated with histological chorioamnionitis and adverse outcomes in moderate- and late-preterm infants41. The strongest evidence linking U. parvum to preterm labor is from experiments in animal models. Intra-amniotic inoculation of U. parvum resulted in chorioamnionitis in Rhesus macaques42, 43 and sheep40 and also promoted preterm delivery in the former42. However, to date, the pathogenicity of U. parvum in preterm birth is poorly understood44. Using our in vitro fetal membrane infection model, we have demonstrated that U. parvum adheres to fetal membrane cells and induces inflammation45, 46.
Using the same model, we harvested the condition media to identify the proteins stimulated by U. parvum infection using a proteomics-based approach. Prothrombin was detected in U. parvum-treated condition media but not control condition media. This preliminary finding indicated that U. parvum has the potential to induce prothrombin production in fetal membrane cells. We postulate that infection-induced prothrombin production (and subsequent conversion to thrombin) might be a novel mechanism of U. parvum pathogenicity in preterm birth47–54. Thrombin generation has been associated with fetal membrane weakening and PPROM50, 54, and treatment of amnion explants with thrombin results in increased levels of matrix metallopeptidase 9 (MMP9) and mechanical weakening55. However, thrombin generation in fetal membranes and the source of thrombin in amniotic fluid is not well described47, 56. Decidual hemorrhage associated with either intrauterine infection57 or bleeding in pregnancy58 is thought to be a primary source of thrombin. The ability of fetal membrane cells (amnion, chorion and decidua) to produce prothrombin and respond to infection through regulating prothrombin production is not established.
In this study, we hypothesize that U. parvum exposure stimulates prothrombin production in fetal membrane amnion, chorion, and decidua cells. Our objectives were two-fold: 1) Confirm the prothrombin localization in fetal membrane cells with immunostaining in ex vivo fetal membrane tissues and primary cultured fetal membrane cells and 2) Quantify prothrombin expression with Western blot analysis and PCR in fetal membrane tissues and cells with/without U. parvum exposure. In addition, each pathogen may elicit a unique response through specific signaling mechanisms. In this study, Gram-negative bacterial lipopolysaccharide (LPS) was used as a comparison. Gram-negative bacteria are also frequently associated with intra-amniotic infections, and LPS is often used to induce preterm birth in animal models.
Materials and Methods
This study used human fetal membrane tissues collected following planned, uncomplicated cesarean delivery at term without rupture of membranes or labor and a genital isolate U. parvum strain as described previously45.
Fetal membrane tissue explant and primary cell cultures
For tissue explant culture, fetal membranes were collected following planned, uncomplicated cesarean delivery at term (39 weeks of gestation) without rupture of membranes or labor. All subjects had no previous preterm birth and were not on progesterone therapy. All subjects had no pregnancy or medical complications. Membranes were washed in warm media, and blood clots were removed. Full-thickness fetal membrane tissue was cut into 1 × 1 cm pieces and cultured in DMEM/F12 media supplemented with 10% fetal bovine serum (FBS) in 6-well plates. The fetal membrane fragments were collected away from the area near the disk or the rupture site.
For primary cell culture, fetal membranes were cut into 2 × 2 inch pieces. The reflected amnion layer was removed manually. Amnion epithelial cells were harvested using a modification of a technique which was previously described45, 59. Briefly, the amnion tissue was minced and digested in DMEM/F12 containing 0.2% trypsin (Sigma) at 37°C for 30 min with periodic agitation. The mixture was then filtered using a tissue strainer to separate the dispersed amnion epithelial cells from the tissue fragments. This process was repeated three times, and the dispersed epithelial cells were combined and counted. The yield was 8–12 million amnion epithelial cells/g of amnion tissue; viability was 90% (assessed by trypan blue dye exclusion).
Separation of the decidua and chorion involved blunt dissection with forceps and scalpel. Separated chorion and decidua layers were minced and digested in DMEM/F-12 containing 0.125% trypsin and 0.2% collagenase (Sigma) at 37°C for 30–90 min with periodic agitation. Cells were filtered through 70 μm nylon cell filters (Falcon). A cell-separation gradient was prepared to purify the cells as described previously 60. Cells were plated in the same culture conditions as amnion cells for 48 h. Purity of primary cells was confirmed using immunofluorescence staining for cytokeratin and vimentin to distinguish amnion/chorion from decidua cells.
All cell types or tissue explants were confirmed to be free of Mycoplasma and Ureaplasma contamination using a chemiluminescent-labelled single-stranded DNA probe hybridization method (MTC-NI kit, Millipore).
Tissue explants (0.5 cm × 0.5 cm) and primary cells (2×106 cells/well in 6-well plates) were treated with and without live U. parvum (1×105, 1×106, or 1×107 cfu/ml) or lipopolysaccharide (LPS, 100ng/ml or 1000ng/ml, Escherichia coli J5, L-5014, Cat# L5014, Sigma). After 24h, culture media was collected for ELISA and cell or tissue lysates were harvested for Western blot. Total RNA was extracted from the cells or tissue explants using the RNeasy Mini Kit (Qiagen) for PCR.
Immunohistochemistry (IHC) staining
To determine basal prothrombin protein localization in fetal membranes, freshly harvested, full-thickness fetal membranes without U. parvum infection were fixed in 10% formalin for IHC. Paraffin sections were de-waxed and rehydrated. After heat-induced antigen retrieval, the sections were stained using the anti-mouse HRP-DAB Cell & Tissue Staining Kit following manufacturer’s instruction (R&D Systems). Mouse anti-human thrombin antibody (Abcam) was used at a 1:500 dilution in PBS with 1% BSA and 5% goat serum. Placenta tissue was used as positive control. For each batch of staining, one slide was incubated with normal mouse IgG (Abcam) instead of the primary antibody as a negative control. Images were taken using a Zeiss Axio Observer (20X). Three rounds of IHC staining were performed using fetal membrane tissues collected from three subjects.
Immunofluorescence staining
To determine the localization of thrombin/prothrombin protein in fetal membrane cells, primary cell cultures without U. parvum infection were fixed with cold methanol at −20°C for 10 min. The cells were permeabilized and blocked with 1% BSA, 5% normal goat serum, and 0.1% Tween-20 in PBS for 1h. After blocking, the slides were incubated with primary antibodies overnight at 4°C. Primary mouse anti-cytokeratin and anti-vimentin monoclonal antibodies (Dako) were used at 1:200 and rabbit anti-thrombin polyclonal antibody (Abcam) was used at 1:100. Anti-mouse and rabbit IgG antibodies were used as negative controls (R&D Systems). Goat anti-mouse secondary antibody Alexa Fluor 488 conjugate and goat anti-rabbit secondary antibody Alexa Fluor 594 (Life Technologies) were used at 1:500. Slides were mounted using mounting medium for fluorescence with DAPI (Vector Laboratories). Three rounds of immunofluorescence staining were performed using fetal membrane tissues collected from three subjects.
Real-time quantitative reverse transcription q(RT)-PCR
Total RNA (1 μg) was reverse-transcribed into cDNA using SuperScript III and Oligo dT (Life Technologies). Pre-validated thrombin Taqman® gene expression probes (Life Technologies, assay ID: Hs01011988_m1 F2) were used. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was measured by the SYBR® Green detection method (Bio-Rad). IQ supermix and IQ SYBR® Green supermix cocktail (Bio-Rad) were used for these assays, respectively. Primers used for GAPDH were forward (CATGAGAAGTATGACAACAGCCT) and reverse (AGTCCTTCCACGATACCAAAGT). Samples were run in duplicate and fold changes were calculated using ΔΔCt method after normalization. Six replicates were performed using fetal membrane tissues or primary fetal membrane cells collected from six subjects.
Western blot
Cell lysates were obtained using radioimmunoprecipitation assay (RIPA) buffer (Sigma) with the complete mini-protease inhibitor cocktail (Roche). For frozen tissue explants and placenta (positive control for Western blots), tissues (~500 mg) were pulverized then homogenized in 1.0 ml of RIPA buffer containing protease inhibitor. Purified plasma prothrombin (Enzyme Research Laboratories) was used as the positive control. Western blot analysis was performed using a standard procedure. Rabbit anti-human GAPDH antibody (1:20,000, Cell Signaling Technology), mouse anti-human thrombin antibody (1:2000, Abcam), and the secondary antibodies (1:2500, Cell Signaling Technology) were used. The primary human thrombin antibody detects both prothrombin and thrombin. Film development was optimized to maintain bands within the linear range. Band intensity was measured using ImageJ analysis software (National Institute of Health [NIH], Bethesda, MD). The densitometry of the protein bands were normalized to GAPDH and then compared and presented as ratios. Five replicates were performed using fetal membrane tissues or primary fetal membrane cells collected from five subjects.
Enzyme-Linked Immunosorbent Assay (ELISA)
The conditioned media were analyzed for prothrombin/thrombin protein concentrations by Human Thrombin ELISA Kit (Factor II;61 Abcam). Samples without dilution were run in duplicate with serial dilutions of recombinant human thrombin as standards. The sensitivity of this Kit is approximately 0.3 ng/ml. The intra- and inter-assay coefficients of variation are 4.7% and 7.2%, respectively. The absorbance was measured at an optical density (OD) of 450 nm with correction at an OD of 540 nm. Eight replicates were performed using fetal membrane tissues or primary cells collected from eight subjects.
Data analysis
Experiments performed using tissue explant or primary cell culture from one subject was counted as one repeat. All experiments were repeated more than five times (N > 5). Data are presented as means ± SEMs. The one-way ANOVA with the post-hoc Dunnett’s test was used for multiple comparisons. P < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 6.0 (La Jolla, CA).
Results
Prothrombin/thrombin protein detected in fetal membrane cells
To demonstrate the localization of thrombin/prothrombin in the fetal membrane cells, we performed IHC in full-thickness fetal membrane tissues and immunofluorescence staining in primary cultured fetal membrane cells. Positive prothrombin/thrombin staining was consistently observed in the amnion and chorion layers of fetal membranes (Figure 1). Prothrombin/thrombin staining in the decidua layer of fetal membranes was inconsistent, with negative staining observed in the fetal membranes of some subjects and positive staining observed in others. Even within the same sample, there were inconsistencies among stained cells in the decidual layer. Additionally, using the same staining conditions (same batch), the intensity of prothrombin/thrombin staining in all three layers of fetal membranes varied between subjects (Figure 1A, weak staining in Subject 1 vs strong staining in Subject 2). Intracellular staining indicated the presence of prothrombin, but extracellular prothrombin/thrombin staining was also observed.
Figure 1. Prothrombin/thrombin staining in fetal membranes.
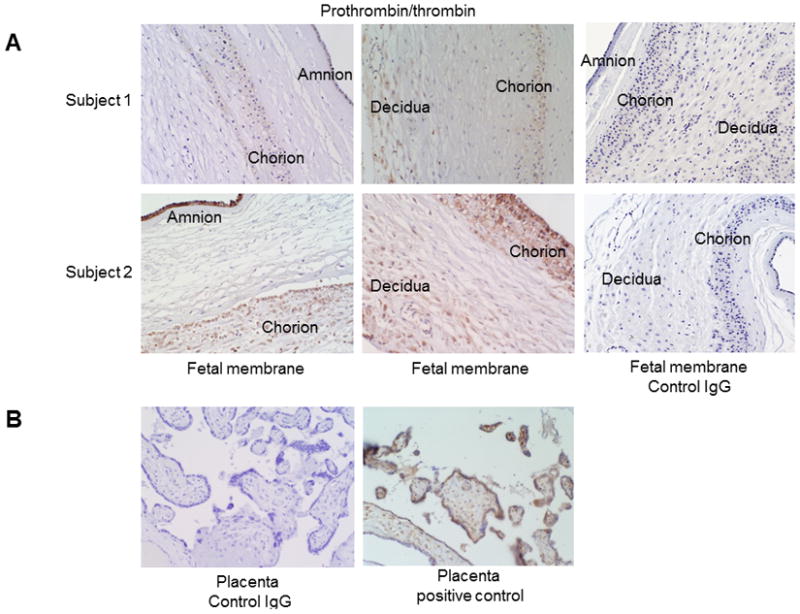
(A) Prothrombin/thrombin protein was present in the amnion, chorion, and decidua layers of the fetal membranes. Prothrombin/thrombin immunostaining intensity in Subject 2 was much higher than in Subject 1, which represented the variable expression of this protein among term fetal membranes. Negative control fetal membranes were stained negative. (B) Negative control and positive control of prothrombin/thrombin immunostaining in placenta tissues. Thrombin/prothrombin staining in placentas was in syncytiotrophoblasts and fetal vessels.
Using immunofluorescence, we further verified prothrombin/thrombin localization in primary cultured fetal membrane cells (Figure 2). In amnion cells, prothrombin/thrombin staining was predominately perinuclear whereas in chorion and decidua cells, the staining was diffuse in the cytoplasm (Figure 2).
Figure 2. Prothrombin/thrombin expression in primarily cultured fetal membrane cells.
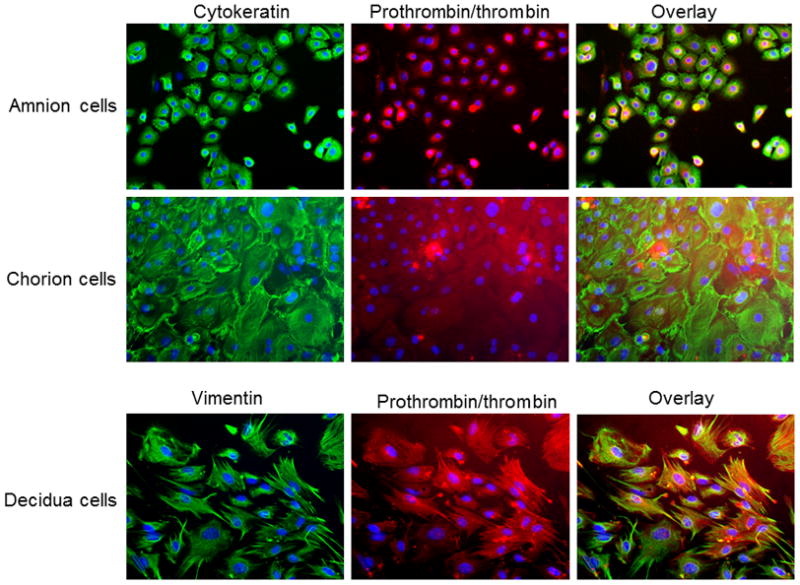
Cytokeratin and vimentin stained in green, prothrombin/thrombin stained in red, and DAPI stained in blue.
Prothrombin mRNA and protein expression was upregulated following U. parvum exposure in fetal membrane explants and cells
While not statistically significant at all doses, both prothrombin transcripts and protein expression were upregulated following U. parvum exposure in a dose-dependent manner. The lack of statistical significance for some of the lower doses may result from individual subject variability and the low virulence of U. parvum at low dose in fetal membranes.
U. parvum at the highest dose [1×107 cfu/ml] consistently and significantly increased prothrombin/thrombin mRNA expression in tissue explants (P < 0.01) and primary fetal membrane cells (P < 0.05, Figure 3, N = 6). Treatments with LPS (100ng/ml or 1000ng/ml) and lower doses (1×105 or 1×106 cfu/ml) of U. parvum did not significantly change the prothrombin/thrombin mRNA expression in tissue explants and primary fetal membrane cells, with the exception of a dose of U. parvum 1×106 cfu/ml in decidua cells (P < 0.05). The results are summarized in Table 1.
Figure 3. Prothrombin/thrombin transcriptional levels in fetal membrane cells and tissue explants (qRT-PCR analysis).
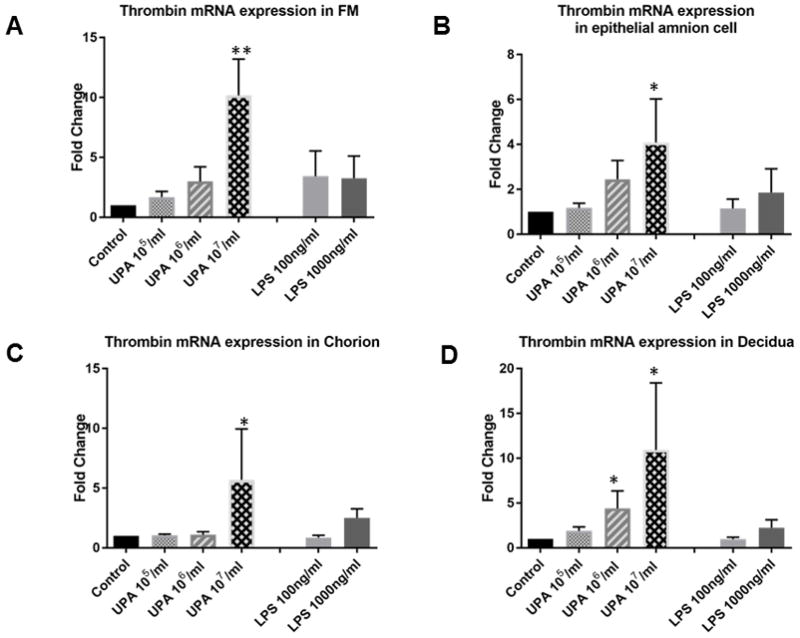
Control vs. U. parvum at 107 cfu/ml, P <0.05 in amnion, chorion, decidua cells, and fetal membrane explants. Other comparisons were not statistically significant (N = 6). N represents the number of replicates or subjects. A. FM; B. Amnion; C. Chorion; D. Decidua.
Table 1.
Prothrombin mRNA and protein expression in fetal membrane cells.
| Amnion (fold change) | ||||||
|---|---|---|---|---|---|---|
| Prothrombin | Control | UPA (105cfu/ml) | UPA (106cfu/ml) | UPA (107cfu/ml) | LPS (100ng/ml) | LPS (1000ng/ml) |
| mRNA | 1 | 1.2±0.2 | 2.4±0.8 | 4.1±1.9* | 1.2±0.4 | 1.9±1.1 |
| Protein | 1 | 33.5±16.4 | 83.4±39.3 | 138.0±44.0* | 1.0±0.1 | 1.4±0.3 |
| Chorion (fold change) | ||||||
| mRNA | 1 | 1.1±0.1 | 1.1±0.3 | 5.7±4.2* | 0.9±0.2 | 2.5±0.7 |
| Protein | 1 | 18.1±8.2 | 54.9±5.3*** | 139.6±15.1*** | 0.9±0.2 | 1.1±0.4 |
| Decidua (fold change) | ||||||
| mRNA | 1 | 1.9±0.4 | 4.4±1.9* | 10.0±5.4* | 1.0±0.2 | 2.3±0.9 |
| Protein | 1 | 1.9±1.6 | 4.0±1.9 | 56.9±29.1* | 0.7±0.3 | 0.5±0.3 |
| FM (fold change) | ||||||
| mRNA | 1 | 1.7±0.5 | 3.0±1.2 | 9.2±3.0** | 3.4±2.1 | 3.3±1.8 |
| Protein | 1 | 7.1±4.6 | 67.4±23.1 | 133.1±40.0* | 1.1±0.2 | 1.1±0.1 |
P<0.05;
P < 0.01;
P < 0.001
A band at approximately 75 kDa was detected in Western blots for all fetal membrane samples. A single band at approximately 72 kDa was identified for purified plasma prothrombin (Figure 4), which further demonstrated the specificity of the antibody. The slight molecular weight difference might be due to the modifications in prothrombin prior to secretion, such as signal-peptide cleavage62, complex glycosylation63, and disulphide-bridge formation64.
Figure 4. Prothrombin protein levels in fetal membrane cells and tissue explants (Western blotting analysis).
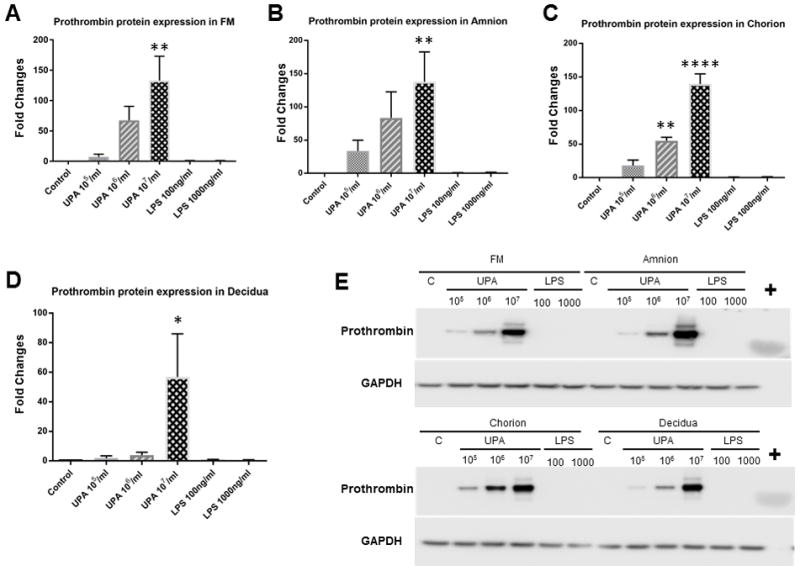
Control vs. U. parvum at 107 cfu/ml, P < 0.01 in amnion cells and fetal membrane tissue explants, P < 0.001 in chorion cells, P < 0.05 in decidua cells. Control vs. U. parvum at 106 cfu/ml, P < 0.01 in chorion cells. Other comparisons were not statistically significant (N = 5). A. FM bar graph; B. Amnion bar graph; C. Chorion bar graph; D. Decidua bar graph; E. Representative Western blot images. + = purified plasma prothrombin; C = control; N represents the number of replicates or subjects.
The densitometry analysis of Western blots (N = 5) is consistent with the qRT-PCR findings which demonstrated that U. parvum upregulated prothrombin protein expression in a dose-dependent manner. Chorion cells were more susceptible than amnion or decidua cells to U. parvum exposure-induced prothrombin protein expression, as demonstrated by significant induction at both high (1×107 cfu/ml, P < 0.001) and medium (1×106 cfu/ml, P < 0.001) U. parvum doses. In amnion and decidua cells, as well as tissue explants, prothrombin protein expression was significantly induced by U. parvum at 1×107 cfu/ml (P < 0.05), but not at lower doses.
The prothrombin/thrombin protein in conditioning media was not significantly induced by U. parvum exposure
Prothrombin/thrombin was detectable in culture supernatants collected from fetal membrane cells and tissue explant cultures at approximately 100 and 2000 pg/ml, respectively (Figure 5). In amnion and chorion cell culture supernatants, U. parvum exposure at 1×107 cfu/ml resulted in increased prothrombin/thrombin concentration, but results were not statistically significant (N = 8).
Figure 5. Prothrombin/thrombin protein in conditioned culture media (ELISA analysis).
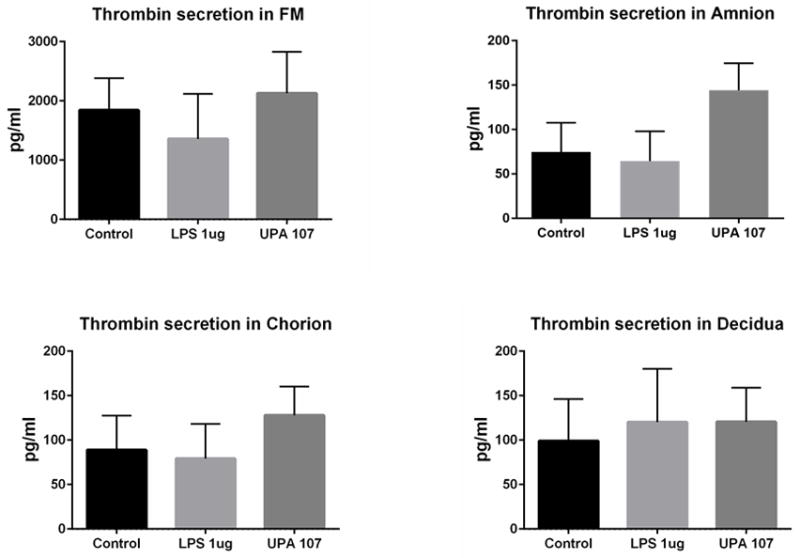
In fetally derived amnion and chorion cell culture supernatants, U. parvum exposure at 107 cfu/ml resulted in increased concentrations of prothrombin/thrombin. However, no comparisons were statistically significant (N=8). N represents the number of replicates or subjects.
Comment
Principal Findings of the study
In this study, we characterized prothrombin expression in cells of the fetal membranes and demonstrated that U. parvum exposure induced prothrombin production by fetal membrane cells. Interestingly, we were not able to demonstrate that LPS-induced prothrombin production either in fetal membranes or in cells derived from fetal membranes.
Clinical and Research Implications
These findings suggest: 1) A connection between microorganisms and thrombin production in cells of the fetal membranes and 2) A possible pathogenicity of Ureaplasma which is not completely understood. This novel discovery significantly contributes to our understanding of the pathogenicity of U. parvum and U. parvum infection-induced PTB and PPROM. Although numerous clinical observational studies regarding Ureaplasma spp. infection during pregnancy have been conducted in the past three decades, its clinical significance is still debated65, 66. It is a commonly held belief that Ureaplasma spp. are not pathogenic because they are common commensal organisms in the female genital tract and often detected in gestational tissues. Ureaplasma spp. are considered to be low-virulence bacteria. In fact, studies evaluating the impact on pregnancy of treatment of Ureaplasma and Mycoplasma spps have had mixed results67, 68. Our findings support a pathogenic mechanism for Ureaplasma by which it stimulates prothrombin production in gestational tissues. The previously reported Ureaplasma virulence factors include adhesion molecules69, IgA protease70, urease71, phospholipases A and C72, and production of hydrogen peroxide73. However, examination of the genome of multiple serovars of Ureaplasma failed to reveal genes encoding these enzymes74. The multiple-banded antigen (MBA) of Ureaplasma is the predominant antigen recognized during the infection process and may be involved in the stimulation of host inflammatory response75. Our findings indicate that there are potential undiscovered Ureaplasma virulence factors that regulate host coagulation and hemostasis. Coagulation modifications induced by Ureaplasma in fetal membranes indicate that Ureaplasma has the potential to act as an opportunistic pathogen during pregnancy.
Our discovery sheds new light on the long-established yet poorly understood link between infection/inflammation and PTB: U. parvum-induced thrombin production. Thrombin acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin and also catalyzes many other coagulation-related reactions. Beyond its key role in the dynamic process of thrombus formation, thrombin has pronounced pro-inflammatory properties acting via its specific cell membrane protease-activated receptors (PARs)76–81. PARs, which control important physiologic and disease-relevant tissue-specific processes, are an emerging therapeutic target for major diseases such as burns and gastric ulcers82. The mechanisms of actions of PARs are poorly understood in gestational tissues. Fetal membrane cells express PAR-183, 84 and thrombin induces inflammation and weakens fetal membranes50, 54, 55, 83, 84, and alternative mechanisms of thrombin action have also been demonstrated. For example, one study reported that thrombin weakens the amnion extracellular matrix (ECM) directly rather than through PARs84. Protein- and/or peptide-based therapies targeted to limit thrombin enzyme activities and ultimately reduce the risk of PPROM should be explored.
Following bacterial infection, the resultant inflammatory reaction activates a coagulation cascade resulting in the formation of fibrin, which effectively walls off the infection85, 86. Mackman et al. postulate that coagulation is an important component of the inflammatory response and necessary to eliminate the infection87. The prevailing view is that coagulation factor production is restricted to three compartments: the liver, where most coagulation factor production takes place; the endothelium, which is characterized, for example, by high levels of von Willebrand Factor (vWF); and the platelets, which, despite being devoid of nuclei, are capable of substantial coagulation factor production88. However, there is evidence to refute this simplistic model of coagulation factor synthesis. For instance, in the lung, alveolar macrophages express FVIIa89, epithelial cells express tissue factor (TF)90, factor VIIa (FVIIa)91, and factor Xa (FXa)92, and fibroblasts express tissue factor pathway inhibitor (TFPI)93. Our current study also challenges the standard paradigm of coagulation factor production by confirming prothrombin biosynthesis in human fetal membrane cells including amnion epithelial cells, chorion trophoblasts, and decidua stroma cells. Among these cells, there are differences in the distribution of prothrombin and the susceptibility to prothrombin stimulation following U. parvum exposure. Our findings suggest a different functional relevance of prothrombin in these cell types. Based on the size of the single band detected by Western blot, the stimulated prothrombin was not activated within the intracellular and pericellular spaces of these cells. Although prothrombin mRNA and protein were consistently upregulated following U. parvum exposure, the prothrombin levels in culture supernatants were extremely variable, ranging from below detection to a 5-fold increase in U. parvum-treated groups. The individual patient variability is not easily explained, but this may be a result of the complexity of prothrombin secretion and the instability of prothrombin in media. Additionally, as it is impossible to avoid blood contamination in the culture media of full-thickness fetal membranes, this could explain in part the variable results in tissue explants. Our objective in this current study was to demonstrate the ability of gestational tissues to synthesize prothrombin and respond to U. parvum exposure. Future studies to understand the individual patient variability and downstream effects of fetal membrane prothrombin biosynthesis and secretion induced by U. parvum are warranted.
Strengths and Limitations
A standard bacterial inoculum is critically important for in vivo and in vitro studies. We have previously demonstrated that the quantity of bacteria present in the fetal membranes is correlated with chorion thinning, suggesting that bacterial presence may incrementally evoke a host response that leads to chorion cell death and tissue degradation94. There is now evidence that the dose and variation of the MBA of U. parvum might affect the severity of chorioamnionitis in pregnant sheep95. Therefore, we optimized the doses of U. parvum and treatment duration to avoid cell death as observed in our previous studies45, 96. The optimized dose range was chosen because: 1) the consistent and effective induction of interleukin 8 (IL-8), cyclooxygenase 2 (COX2), prostaglandin E2 (PGE2) and MMP9 in these cultures was observed; 2) cell death was not observed for the treatment duration; 3) the multiplicity of infection (MOI) of 5 (for example, 107 cfu U. parvum to 2 × 106 fetal membrane cells) was within the range of positive adherence to fetal membrane cells; and 4) the MOI of U. parvum in this study is consistent with other studies in human amniotic epithelial cells97. In this study, 108 cfu U. parvum infected 107 amniotic epithelial cells with a MOI of 1097. Based on methods previously published, we have established standardized experimental procedures to ensure that the experiments can be directly compared45. Overgrowth of U. parvum in cell culture is not a concern because of their unique growth conditions: high concentrations of urea, a variety of amino acid supplementation, and acidic conditions of the culture media.
Live U. parvum was used in this study for several reasons. The ability of U. parvum to have pathogenicity is dependent on its adherence to human cells. The proposed major virulence factor of U. parvum, MBA, is a surface protein which is easily altered by heat unlike endotoxin, the major virulence factor of Escherichia coli (E. coli). Heat inactivation can affect the surface of U. parvum and therefore attenuate adherence and damage the protein structure of MBA, both of which will result in variations in host-microbe interactions. As expected, our preliminary data suggests that heat inactivation attenuated the ability of U. parvum ability to adhere to human cells. A previous study using heat-inactivated Ureaplasma demonstrated a mild inflammatory response in fetal membranes98.
Specific limitations of our study include the use of LPS instead of live E. coli to compare to U. Parvum-induced thrombin generation. LPS was selected due to the rapid overgrowth of live E. coli in in vitro culture models which results in significant cell death and limits the interpretation of findings. LPS, also known as lipoglycans and endotoxins, are large molecules consisting of a lipid and a polysaccharide composed of O-antigen, outer core, and inner core joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria and elicit strong immune responses and coagulopathy including thrombin generation in animals99–103 and humans100, 101. Although we cannot conclude that E. coli does not stimulate prothrombin production in fetal membranes, we extrapolated this based on our finding that LPS treatment failed to induce prothrombin production. LPS has, however, been shown to stimulate the production of cytokines (such as IL-1 and TNF) which may be associated with coagulation. The exact relationship between cytokines and coagulation in fetal membranes is poorly understood.
The other limitation of this study is that we did not demonstrate a direct link between prothrombin production and fetal membrane rupture. Due to the complexity of the activation of prothrombin, it is difficult to replicate prothrombin activation in vitro. Many coagulation factors necessary for the activation of prothrombin are not present in the cell culture system. Future in vivo studies are warranted.
Conclusions
Our findings demonstrate a potential pathway to PPROM and PTB: U. parvum exposure-induced prothrombin production in fetal membrane cells. Ureaplasma spp. is understudied yet recognized as an important pathogen during pregnancy and in the preterm neonate. Our findings contribute to the field of microbiology and maternal fetal medicine by identifying a novel molecular event resulting from the interactions of U. parvum and cells of the fetal membranes. Most importantly, the understanding of coagulation regulation as one of the U. parvum virulence factors in fetal membranes will likely yield novel therapeutic interventions to limit onset and progression of PTB and PPROM.
Implications and Contributions.
Epidemiologic and experimental studies have demonstrated that thrombin causes fetal membrane weakening and PPROM. However, the sources of thrombin in the fetal membranes and amniotic fluid of PPROM patients were previously unknown. This study aimed to investigate whether thrombin production by fetal membrane cells can be induced by U. parvum exposure.
The key findings are two-fold: 1) Prothrombin can be directly produced by fetal membrane amnion, chorion, and decidua cells and 2) Prothrombin production can be stimulated by U. parvum exposure in fetal membranes.
We discovered a potential novel mechanism by which U. parvum infection induce alteration of fetal membranes.
Acknowledgments
Sources of Funding: This study was supported by the Charles Hammond Fund of the Department of Obstetrics and Gynecology, Duke University (PI: Liping Feng) and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001115 (Terrence K. Allen). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Dr. Andra James for her helpful discussions.
Footnotes
Disclosure: The authors report no conflict of interests.
Previous Presentation: This research was presented in part at the 63rd Annual Meeting of the Society for Reproductive Investigation, 16–19 March 2016, Montreal, Canada.
Author Contributions
Liping Feng designed the study, performed experiments, analyzed and interpreted the data, and prepared the manuscript. William Marinello performed experiments. Terrence Allen provided the primary amnion cells and contributed to the manuscript preparation. Amy Murtha contributed to study design, discussion, and manuscript preparation.
References
- 1.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Mathews TJ. Births: final data for 2011. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;62:1–69. 72. [PubMed] [Google Scholar]
- 2.Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstet Gynecol Clin North Am. 2005;32:411–28. doi: 10.1016/j.ogc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Quintero RA, Morales WJ, Kalter CS, et al. Transabdominal intra-amniotic endoscopic assessment of previable premature rupture of membranes. American journal of obstetrics and gynecology. 1998;179:71–6. doi: 10.1016/s0002-9378(98)70252-2. [DOI] [PubMed] [Google Scholar]
- 4.Makieva S, Dubicke A, Rinaldi SF, Fransson E, Ekman-Ordeberg G, Norman JE. The preterm cervix reveals a transcriptomic signature in the presence of premature prelabor rupture of membranes. American journal of obstetrics and gynecology. 2017;216:602.e1–02e21. doi: 10.1016/j.ajog.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Maymon E, Romero R, Pacora P, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. American journal of obstetrics and gynecology. 2000;183:914–20. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 6.Athayde N, Edwin SS, Romero R, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. American journal of obstetrics and gynecology. 1998;179:1248–53. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 7.Maymon E, Romero R, Pacora P, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. American journal of obstetrics and gynecology. 2000;183:887–94. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Chaiworapongsa T, Espinoza J, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. American journal of obstetrics and gynecology. 2002;187:1125–30. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. American journal of obstetrics and gynecology. 1989;161:336–41. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Maymon E, Pacora P, et al. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. American journal of obstetrics and gynecology. 2000;183:1070–7. doi: 10.1067/mob.2000.108885. [DOI] [PubMed] [Google Scholar]
- 11.Gratacos E, Sanin-Blair J, Lewi L, et al. A histological study of fetoscopic membrane defects to document membrane healing. Placenta. 2006;27:452–6. doi: 10.1016/j.placenta.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PloS one. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. Journal of perinatal medicine. 2010;38:261–8. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28:1394–409. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, Friel LA, Velez Edwards DR, et al. A genetic association study of maternal and fetal candidate genes that predispose to preterm prelabor rupture of membranes (PROM) American journal of obstetrics and gynecology. 2010;203:361.e1–61e30. doi: 10.1016/j.ajog.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tchirikov M, Schlabritz-Loutsevitch N, Maher J, et al. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. Journal of perinatal medicine. 2017 doi: 10.1515/jpm-2017-0027. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Sammel MD, Tromp G, et al. A 12-bp deletion in the 5′-flanking region of the SERPINH1 gene affects promoter activity and protects against preterm premature rupture of membranes in African Americans. Hum Mutat. 2008;29:332. doi: 10.1002/humu.9522. [DOI] [PubMed] [Google Scholar]
- 18.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125.e1–25e15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 20.Murtha AP, Nieves A, Hauser ER, et al. Association of maternal IL-1 receptor antagonist intron 2 gene polymorphism and preterm birth. American journal of obstetrics and gynecology. 2006;195:1249–53. doi: 10.1016/j.ajog.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Seminars in reproductive medicine. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. American Journal of Obstetrics and Gynecology. 1992;166:1576–87. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. American Journal of Obstetrics and Gynecology. 1988;159:661–66. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 24.Musilova I, Bestvina T, Hudeckova M, et al. Vaginal fluid interleukin-6 concentrations as a point-of-care test is of value in women with preterm prelabor rupture of membranes. American journal of obstetrics and gynecology. 2016;215:619.e1–19e12. doi: 10.1016/j.ajog.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Prince AL, Ma J, Kannan PS, et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. American journal of obstetrics and gynecology. 2016;214:627.e1–27e16. doi: 10.1016/j.ajog.2016.01.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. American journal of reproductive immunology. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breugelmans M, Vancutsem E, Naessens A, Laubach M, Foulon W. Association of abnormal vaginal flora and Ureaplasma species as risk factors for preterm birth: a cohort study. Acta obstetricia et gynecologica Scandinavica. 2010;89:256–60. doi: 10.3109/00016340903418769. [DOI] [PubMed] [Google Scholar]
- 28.Cassell GH, Davis RO, Waites KB, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10:294–302. [PubMed] [Google Scholar]
- 29.Cassell GH, Waites KB, Watson HL, Crouse DT, Harasawa R. Ureaplasma urealyticum intrauterine infection: role in prematurity and disease in newborns. Clin Microbiol Rev. 1993;6:69–87. doi: 10.1128/cmr.6.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cultrera R, Seraceni S, Germani R, Contini C. Molecular evidence of Ureaplasma urealyticum and Ureaplasma parvum colonization in preterm infants during respiratory distress syndrome. BMC infectious diseases. 2006;6:166. doi: 10.1186/1471-2334-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. The Journal of infectious diseases. 2003;187:518–21. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg RL, Andrews WW, Goepfert AR, et al. The Alabama Preterm Birth Study: Umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. American Journal of Obstetrics and Gynecology. 2008;198:43.e1–43.e5. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namba F, Hasegawa T, Nakayama M, et al. Placental features of chorioamnionitis colonized with Ureaplasma species in preterm delivery. Pediatric research. 2010;67:166–72. doi: 10.1203/PDR.0b013e3181c6e58e. [DOI] [PubMed] [Google Scholar]
- 34.Viscardi RM. Ureaplasma species: role in diseases of prematurity. Clin Perinatol. 2010;37:393–409. doi: 10.1016/j.clp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knox CL, Timms P. Comparison of PCR, nested PCR, and random amplified polymorphic DNA PCR for detection and typing of Ureaplasma urealyticum in specimens from pregnant women. J Clin Microbiol. 1998;36:3032–9. doi: 10.1128/jcm.36.10.3032-3039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlicht MJ, Lovrich SD, Sartin JS, Karpinsky P, Callister SM, Agger WA. High Prevalence of Genital Mycoplasmas among Sexually Active Young Adults with Urethritis or Cervicitis Symptoms in La Crosse, Wisconsin. Journal of Clinical Microbiology. 2004;42:4636–40. doi: 10.1128/JCM.42.10.4636-4640.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. American Journal of Obstetrics and Gynecology. 1998;179:1254–60. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 38.Cassell GH, Crouse DT, Waites KB, Rudd PT, Davis JK. Does Ureaplasma-Urealyticum Cause Respiratory-Disease in Newborns. Pediatric Infectious Disease Journal. 1988;7:535–41. [PubMed] [Google Scholar]
- 39.DiGiulio DB, Callahan BJ, McMurdie PJ, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A. 2015;112:11060–5. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne MS, Ireland DJ, Watts R, et al. Ureaplasma parvum genotype, combined vaginal colonisation with Candida albicans, and spontaneous preterm birth in an Australian cohort of pregnant women. BMC pregnancy and childbirth. 2016;16:312. doi: 10.1186/s12884-016-1110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweeney EL, Kallapur SG, Gisslen T, et al. Placental Infection With Ureaplasma species Is Associated With Histologic Chorioamnionitis and Adverse Outcomes in Moderately Preterm and Late-Preterm Infants. J Infect Dis. 2016;213:1340–7. doi: 10.1093/infdis/jiv587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as Sole Pathogens Cause Chorioamnionitis, Preterm Delivery, and Fetal Pneumonia in Rhesus Macaques. Reproductive Sciences. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 43.Senthamaraikannan P, Presicce P, Rueda CM, et al. Intra-amniotic Ureaplasma parvum-Induced Maternal and Fetal Inflammation and Immune Responses in Rhesus Macaques. The Journal of infectious diseases. 2016;214:1597–604. doi: 10.1093/infdis/jiw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiGiulio DB. Diversity of microbes in amniotic fluid. Seminars in fetal & neonatal medicine. 2012;17:2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Feng L, Ransom CE, Nazzal MK, et al. The Role of Progesterone and a Novel Progesterone Receptor, Progesterone Receptor Membrane Component 1, in the Inflammatory Response of Fetal Membranes to Ureaplasma parvum Infection. PLoS One. 2016;11:e0168102. doi: 10.1371/journal.pone.0168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potts LC, Feng L, Seed PC, et al. Inflammatory Response of Human Gestational Membranes to Ureaplasma parvum Using a Novel Dual-Chamber Tissue Explant System. Biology of reproduction. 2016;94:119. doi: 10.1095/biolreprod.115.137596. [DOI] [PubMed] [Google Scholar]
- 47.Chaiworapongsa T, Espinoza J, Yoshimatsu J, et al. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:368–73. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- 48.Funderburk SJ, Guthrie D, Meldrum D. Outcome of pregnancies complicated by early vaginal bleeding. British journal of obstetrics and gynaecology. 1980;87:100–5. doi: 10.1111/j.1471-0528.1980.tb04500.x. [DOI] [PubMed] [Google Scholar]
- 49.Nagy S, Bush M, Stone J, Lapinski RH, Gardo S. Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstetrics and gynecology. 2003;102:94–100. doi: 10.1016/s0029-7844(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 50.Rosen T, Kuczynski E, O’Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. The Journal of maternal-fetal medicine. 2001;10:297–300. doi: 10.1080/714904361. [DOI] [PubMed] [Google Scholar]
- 51.Signore CC, Sood AK, Richards DS. Second-trimester vaginal bleeding: correlation of ultrasonographic findings with perinatal outcome. American journal of obstetrics and gynecology. 1998;178:336–40. doi: 10.1016/s0002-9378(98)80022-7. [DOI] [PubMed] [Google Scholar]
- 52.Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. American journal of obstetrics and gynecology. 2001;185:1059–63. doi: 10.1067/mob.2001.117638. [DOI] [PubMed] [Google Scholar]
- 53.Vidaeff AC, Monga M, Saade G, Bishop K, Ramin SM. Prospective investigation of second-trimester thrombin activation and preterm birth. American journal of obstetrics and gynecology. 2012;206:333.e1–6. doi: 10.1016/j.ajog.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Mackenzie AP, Schatz F, Krikun G, Funai EF, Kadner S, Lockwood CJ. Mechanisms of abruption-induced premature rupture of the fetal membranes: Thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol. 2004;191:1996–2001. doi: 10.1016/j.ajog.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Kumar D, Schatz F, Moore RM, et al. The effects of thrombin and cytokines upon the biomechanics and remodeling of isolated amnion membrane, in vitro. Placenta. 2011;32:206–13. doi: 10.1016/j.placenta.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erez O, Romer R, Vaisbuch E, et al. Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J Matern Fetal Neonatal Med. 2009;22:971–82. doi: 10.3109/14767050902994762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez R, Romero R, Nien JK, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2005;18:31–7. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 58.Lockwood CJ, Toti P, Arcuri F, et al. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin-enhanced interleukin-8 expression in term decidua. The American journal of pathology. 2005;167:1443–9. doi: 10.1016/S0002-9440(10)61230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casey ML, MacDonald PC. Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biology of reproduction. 1996;55:1253–60. doi: 10.1095/biolreprod55.6.1253. [DOI] [PubMed] [Google Scholar]
- 60.Mills AA, Yonish B, Feng L, Schomberg DW, Heine RP, Murtha AP. Characterization of progesterone receptor isoform expression in fetal membranes. American journal of obstetrics and gynecology. 2006;195:998–1003. doi: 10.1016/j.ajog.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 61.Liu JF, Hou SM, Tsai CH, Huang CY, Yang WH, Tang CH. Thrombin induces heme oxygenase-1 expression in human synovial fibroblasts through protease-activated receptor signaling pathways. Arthritis research & therapy. 2012;14:R91. doi: 10.1186/ar3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Degen SJ, MacGillivray RT, Davie EW. Characterization of the complementary deoxyribonucleic acid and gene coding for human prothrombin. Biochemistry. 1983;22:2087–97. doi: 10.1021/bi00278a008. [DOI] [PubMed] [Google Scholar]
- 63.Mizuochi T, Yamashita K, Fujikawa K, Kisiel W, Kobata A. The carbohydrate of bovine prothrombin. Occurrence of Gal beta 1 leads to 3GlcNAc grouping in asparagine-linked sugar chains The Journal of biological chemistry. 1979;254:6419–25. [PubMed] [Google Scholar]
- 64.Magnusson S, Sottrup-Jensen L, Claeys H, Zajdel M, Petersen TE. Proceedings: Complete primary structure of prothrombin. Partial primary structures of plasminogen and hirudin Thromb Diath Haemorrh. 1975;34:562–3. [PubMed] [Google Scholar]
- 65.Sung TJ. Ureaplasma infections in pre-term infants: Recent information regarding the role of Ureaplasma species as neonatal pathogens. Korean journal of pediatrics. 2010;53:989–93. doi: 10.3345/kjp.2010.53.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Seminars in fetal & neonatal medicine. 2009;14:190–9. doi: 10.1016/j.siny.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Eschenbach DA, Nugent RP, Rao AV, et al. A randomized placebo-controlled trial of erythromycin for the treatment of Ureaplasma urealyticum to prevent premature delivery. The Vaginal Infections and Prematurity Study Group Am J Obstet Gynecol. 1991;164:734–42. doi: 10.1016/0002-9378(91)90506-m. [DOI] [PubMed] [Google Scholar]
- 68.Vouga M, Greub G, Prod’hom G, et al. Treatment of genital mycoplasma in colonized pregnant women in late pregnancy is associated with a lower rate of premature labour and neonatal complications. Clin Microbiol Infect. 2014;20:1074–9. doi: 10.1111/1469-0691.12686. [DOI] [PubMed] [Google Scholar]
- 69.Saada AB, Terespolski Y, Adoni A, Kahane I. Adherence of Ureaplasma urealyticum to human erythrocytes. Infect Immun. 1991;59:467–9. doi: 10.1128/iai.59.1.467-469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kilian M, Brown MB, Brown TA, Freundt EA, Cassell GH. Immunoglobulin A1 protease activity in strains of Ureaplasma urealyticum. Acta pathologica, microbiologica, et immunologica Scandinavica Section B, Microbiology. 1984;92:61–4. doi: 10.1111/j.1699-0463.1984.tb02794.x. [DOI] [PubMed] [Google Scholar]
- 71.Ligon JV, Kenny GE. Virulence of ureaplasmal urease for mice. Infection and immunity. 1991;59:1170–1. doi: 10.1128/iai.59.3.1170-1171.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng XT, Teng LJ, Watson HL, Glass JI, Blanchard A, Cassell GH. Small Repeating Units within the Ureaplasma-Urealyticum Mb Antigen Gene Encode Serovar Specificity and Are Associated with Antigen Size Variation. Infection and Immunity. 1995;63:891–98. doi: 10.1128/iai.63.3.891-898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Somerson NL, Walls BE, Chanock RM. Hemolysin of Mycoplasma pneumoniae: tentative identification as a peroxide. Science. 1965;150:226–8. doi: 10.1126/science.150.3693.226. [DOI] [PubMed] [Google Scholar]
- 74.Glass JI, Lefkowitz EJ, Glass JS, Heiner CR, Chen EY, Cassell GH. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature. 2000;407:757–62. doi: 10.1038/35037619. [DOI] [PubMed] [Google Scholar]
- 75.Dando SJ, Nitsos I, Kallapur SG, et al. The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy? PLoS One. 2012;7:e29856. doi: 10.1371/journal.pone.0029856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen D, Dorling A. Critical roles for thrombin in acute and chronic inflammation. Journal of thrombosis and haemostasis: JTH. 2009;7(Suppl 1):122–6. doi: 10.1111/j.1538-7836.2009.03413.x. [DOI] [PubMed] [Google Scholar]
- 77.O’Sullivan CJ, Allen NM, O’Loughlin AJ, Friel AM, Morrison JJ. Thrombin and PAR1-activating peptide: effects on human uterine contractility in vitro. American journal of obstetrics and gynecology. 2004;190:1098–105. doi: 10.1016/j.ajog.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 78.Maner WL, Garfield RE, Maul H, Olson G, Saade G. Predicting term and preterm delivery with transabdominal uterine electromyography. Obstetrics and gynecology. 2003;101:1254–60. doi: 10.1016/s0029-7844(03)00341-7. [DOI] [PubMed] [Google Scholar]
- 79.Erez O, Kim SS, Kim JS, et al. Over-expression of the thrombin receptor in the placenta of patients with preeclampsia: The intersection between coagulation and inflammation. American journal of obstetrics and gynecology. 2006;195:S136–S36. [Google Scholar]
- 80.O’Sullivan CJ, Allen NM, O’Loughlin AJ, Friel AM, Morrison JJ. Thrombin and PAR1-activating peptide: Effects on human uterine contractility in vitro. American journal of obstetrics and gynecology. 2004;190:1098–105. doi: 10.1016/j.ajog.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 81.Allen NM, O’Brien M, Friel AM, Smith TJ, Morrison JJ. Expression and function of protease-activated receptor 4 in human myometrium. American journal of obstetrics and gynecology. 2007;196:169.e1–6. doi: 10.1016/j.ajog.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 82.Lundblad RL, Bradshaw RA, Gabriel D, Ortel TL, Lawson J, Mann KG. A review of the therapeutic uses of thrombin. Thrombosis and haemostasis. 2004;91:851–60. doi: 10.1160/TH03-12-0792. [DOI] [PubMed] [Google Scholar]
- 83.Mogami H, Keller PW, Shi H, Word RA. Effect of thrombin on human amnion mesenchymal cells, mouse fetal membranes, and preterm birth. The Journal of biological chemistry. 2014;289:13295–307. doi: 10.1074/jbc.M114.550541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puthiyachirakkal M, Lemerand K, Kumar D, et al. Thrombin weakens the amnion extracellular matrix (ECM) directly rather than through protease activated receptors. Placenta. 2013;34:924–31. doi: 10.1016/j.placenta.2013.07.064. [DOI] [PubMed] [Google Scholar]
- 85.Bouchard BA, Tracy PB. Platelets, leukocytes, and coagulation. Current opinion in hematology. 2001;8:263–9. doi: 10.1097/00062752-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Nicolaes GA, Dahlback B. Factor V and thrombotic disease: description of a janus-faced protein. Arteriosclerosis, thrombosis, and vascular biology. 2002;22:530–8. doi: 10.1161/01.atv.0000012665.51263.b7. [DOI] [PubMed] [Google Scholar]
- 87.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesthesia and analgesia. 2009;108:1447–52. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barile CJ, Herrmann PC, Tyvoll DA, Collman JP, Decreau RA, Bull BS. Inhibiting platelet-stimulated blood coagulation by inhibition of mitochondrial respiration. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2539–43. doi: 10.1073/pnas.1120645109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilcox JN, Noguchi S, Casanova J. Extrahepatic synthesis of factor VII in human atherosclerotic vessels. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:136–41. doi: 10.1161/01.atv.0000043418.84185.3c. [DOI] [PubMed] [Google Scholar]
- 90.Shetty S, Bhandary YP, Shetty SK, et al. Induction of tissue factor by urokinase in lung epithelial cells and in the lungs. American journal of respiratory and critical care medicine. 2010;181:1355–66. doi: 10.1164/rccm.200901-0015OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shinagawa K, Ploplis VA, Castellino FJ. A severe deficiency of coagulation factor VIIa results in attenuation of the asthmatic response in mice. American journal of physiology Lung cellular and molecular physiology. 2009;296:L763–70. doi: 10.1152/ajplung.90638.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scotton CJ, Krupiczojc MA, Konigshoff M, et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. The Journal of clinical investigation. 2009;119:2550–63. doi: 10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bajaj MS, Steer S, Kuppuswamy MN, Kisiel W, Bajaj SP. Synthesis and expression of tissue factor pathway inhibitor by serum-stimulated fibroblasts, vascular smooth muscle cells and cardiac myocytes. Thrombosis and haemostasis. 1999;82:1663–72. [PubMed] [Google Scholar]
- 94.Fortner KB, Grotegut CA, Ransom CE, et al. Bacteria localization and chorion thinning among preterm premature rupture of membranes. PloS one. 2014;9:e83338. doi: 10.1371/journal.pone.0083338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knox CL, Dando SJ, Nitsos I, et al. The severity of chorioamnionitis in pregnant sheep is associated with in vivo variation of the surface-exposed multiple-banded antigen/gene of Ureaplasma parvum. Biology of reproduction. 2010;83:415–26. doi: 10.1095/biolreprod.109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Potts LC, Feng L, Seed PC, et al. Inflammatory Response of Human Gestational Membranes to Ureaplasma parvum Using a Novel Dual-Chamber Tissue Explant System. Biology of reproduction. 2016 doi: 10.1095/biolreprod.115.137596. [DOI] [PubMed] [Google Scholar]
- 97.Triantafilou M, De Glanville B, Aboklaish AF, Spiller OB, Kotecha S, Triantafilou K. Synergic activation of toll-like receptor (TLR) 2/6 and 9 in response to Ureaplasma parvum & urealyticum in human amniotic epithelial cells. PloS one. 2013;8:e61199. doi: 10.1371/journal.pone.0061199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Menon R, Peltier MR, Eckardt J, Fortunato SJ. Diversity in cytokine response to bacteria associated with preterm birth by fetal membranes. American journal of obstetrics and gynecology. 2009;201:306.e1–6. doi: 10.1016/j.ajog.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 99.Giebelen IA, Leendertse M, Dessing MC, et al. Endogenous beta-adrenergic receptors inhibit lipopolysaccharide-induced pulmonary cytokine release and coagulation. American journal of respiratory cell and molecular biology. 2008;39:373–9. doi: 10.1165/rcmb.2007-0439OC. [DOI] [PubMed] [Google Scholar]
- 100.Hellum M, Ovstebo R, Brusletto BS, Berg JP, Brandtzaeg P, Henriksson CE. Microparticle-associated tissue factor activity correlates with plasma levels of bacterial lipopolysaccharides in meningococcal septic shock. Thrombosis research. 2014;133:507–14. doi: 10.1016/j.thromres.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 101.Khakpour S, Wilhelmsen K, Hellman J. Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate immunity. 2015;21:827–46. doi: 10.1177/1753425915606525. [DOI] [PubMed] [Google Scholar]
- 102.Wang B, Wu SM, Wang T, et al. Pre-treatment with bone marrow-derived mesenchymal stem cells inhibits systemic intravascular coagulation and attenuates organ dysfunction in lipopolysaccharide-induced disseminated intravascular coagulation rat model. Chinese medical journal. 2012;125:1753–9. [PubMed] [Google Scholar]
- 103.Wang Y, Hwaiz R, Luo L, Braun OO, Norstrom E, Thorlacius H. Rac1 regulates bacterial toxin-induced thrombin generation. Inflammation research: official journal of the European Histamine Research Society [et al] 2016;65:405–13. doi: 10.1007/s00011-016-0924-3. [DOI] [PubMed] [Google Scholar]


