Abstract
Exposure to bisphenolic chemicals during pregnancy occurs in >90% of pregnancies. Bisphenolic compounds can cross the placental barrier reaching fetal circulation. However, the effects of emerging bisphenolic compounds, such as bisphenol S (BPS), on placental function remain untested. The aim was to determine if bisphenol A (BPA) or BPS, at an environmentally relevant dose, impairs placental function. Pregnant sheep were randomly distributed into three treatment groups (n=7–8 / group): control, BPA, and BPS. All animals received daily injections of corn oil (control), BPA, or BPS (0.5 mg/kg; s.c.; internal fetal dose were ~2.6 ng/mL unconjugated BPA and ~7.7 ng/mL of BPS) from gestational day (GD) 30 to 100. After a 20 day wash-out period, placentas were weighed and placentomes collected. Placental endocrine function was assessed on biweekly maternal blood samples. Gestational exposure to BPS, but not BPA, reduced maternal circulating pregnancy-associated glycoproteins without change in placental weight or placental stereology. BPS-exposed placentas had 50% lower e-cadherin protein expression, ~20% fewer binucleate cells, and ~3-fold higher glial cell missing-1 (GCM1) protein expression. BPA placentas were not affected highlighting the intrinsic differences among bisphenolic chemicals. This is the first study to demonstrate that gestational BPS can result in placental endocrine dysfunction and points to a dysregulation in the fusogenic trophoblast signaling pathway.
Keywords: gestational exposure, placenta, trophoblast, sheep, endocrine disruptor, bisphenol S
Introduction
Worldwide production of plastics has reached 300 million tons annually, many of which are considered to contain endocrine disrupting chemicals (EDCs). The burden of EDCs exposures has risen in the past decades (Skakkebaek et al. 2011), and so has the concern that these chemicals may pose a risk to human health (Heindel et al. 2015). Bisphenols are among the most prevalent chemicals worldwide (Vandenberg et al. 2010) used in the production of plastics and manufacturing of consumer products. Regulations on use of bisphenol A (BPA) in certain consumer products has increased the demand for other substitutes, such as bis (4-hydroxyphenyl) sulfone, commonly known as bisphenol S (BPS) (Chen et al. 2016). BPS can be found in personal care products, food, and paper products (Liao and Kannan 2013; Liao and Kannan 2014; Liao et al. 2012a). Notably, BPS is detected in >80% of human urine samples (Asimakopoulos et al. 2016; Liao et al. 2012b; Ye et al. 2015) and fetal cord blood (Liu et al. 2017), and the exposure range is lower, but in the same order of magnitude to that reported for BPA (Liao et al. 2012a; Ye et al. 2015). At the current rate, BPS may become as widespread as BPA (Liao et al. 2012a). In fact, it has already surpassed BPA in certain geographic areas (Asimakopoulos et al. 2016; Liao et al. 2012a).
Exposure to EDCs is of concern especially during vulnerable periods of development, such as fetal life (Schneider et al. 2014; Stel and Legler 2015). During pregnancy, the placenta partly serves as the lungs, kidneys, liver, endocrine, and immune systems to the developing fetus and plays a critical role in ensuring normal fetal growth and development by acting as the immediate interface between maternal and fetal blood circulation (Benirschke et al. 2012). Many factors can impair this feto-placental-maternal balance, including maternal diet, stress, lifestyle modifications and/or exposure to EDCs (Fowden et al. 2015; Reynolds et al. 2013; Tait et al. 2015; Vaughan et al. 2011). Because most steroid receptors are expressed in the placenta (Fowden et al. 2015), it is an especially vulnerable target to xenosteroids.
As it relates to bisphenolic compounds, placental studies thus far have been restricted to BPA. To date, one in vivo study reported that BPA (50 mg/kg) reduces the placental layer size (Tait et al. 2015), while in vitro studies showed effects on placental invasion (Lan et al. 2017; Spagnoletti et al. 2015), hydroxysteroid dehydrogenase (HSD) activity (Rajakumar et al. 2015), expression of transporters (Sieppi et al. 2016), and miRNA expression (Avissar-Whiting et al. 2010). Receptor affinity differences among bisphenols (Rosenmai et al. 2014) and the emerging use of other bisphenols (BPB, BPE, BPF, and BPS), warrant their study as it relates to their impact on placental function. Among all BPA analogues, BPS is the most distinct compared to BPA with the weakest estrogenic and anti-androgenic activity, but the highest progestogenic activity (Grignard et al. 2012; Molina-Molina et al. 2013; Rosenmai et al. 2014). These marked functional differences call for toxicological risk assessment of BPA analogues. Thus far, toxicological studies of BPS during prenatal life have been restricted to water flea (Chen et al. 2002), zebrafish (Ji et al. 2013; Kinch et al. 2015; Naderi et al. 2014), rats (Castro et al. 2015) and mice (Catanese and Vandenberg 2017; LaPlante et al. 2017), but there is virtually no information on the effects of BPS on placental function.
The unique structure of the placenta allows for the transfer of gases, water, inorganic and organic molecules, including hormones. The placenta’s endocrine capacity is responsible for the majority of maternal and fetal circulating progesterone, which holds a key role in pregnancy maintenance for most mammalian species (Wooding et al. 2008a). In humans, progesterone is synthesized by the placental syncytiotrophoblast, which forms the outer most layer of the chorionic villi where feto-maternal exchange occurs. The syncytiotrophoblast forms by fusion of villous cytotrophoblasts, which are located basal to the syncytiotrophoblast layer. E-cadherin expressing cytotrophoblasts in contact with neighboring cytotrophoblasts can form adherens junctions. These junctions allow for cell-to-cell transfer of pro-fusogenic transcription factors, such as glial cell missing factor 1 (GCM1); which in turn regulate the activation of the protein syncytin-1, encoded by endogenous retrovirus envelope genes (ERVW) (Black et al. 2010; Huang et al. 2014). Syncytin-1, then, promotes cytotrophoblast homokaryonic fusion events, forming the multinucleated syncytiotrophoblast layer.
Because of the complexity of the placenta, EDC studies on placental function to date are limited, and often rely on placental cell studies that utilize either human primary cultures or cell lines (Gorrochategui et al. 2014; Huuskonen et al. 2015; Meruvu et al. 2016; Vitku et al. 2016; Zhang et al. 2015; Zhao et al. 2014). Although in vitro approaches inform about specific pathways involved, animal in vivo studies provide a more holistic understanding of exposure effects during pregnancy. In this study, we have used a comparative in vivo approach using sheep as an animal model to understand the effects of BPA and BPS on placental function. Sheep, like humans, can be monovulatory, which reduces potential confounding factors seen in litter-bearing species, such as the intrauterine fetal position phenomenon (vom Saal et al. 1999). Despite inherent differences in placentation between primates and ruminants (Fowden et al. 2015; Wooding et al. 2008a), sheep are also considered excellent models to study placental function (Fowden et al. 2015; Mourier et al. 2017), allow for temporal monitoring of placental endocrine changes (Roberts et al. 2017), and offer advantages critical for research that focus on the feto-maternal transfer of EDCs (Corbel et al. 2015). Similar to the human syncytiotrophoblast placental layer, the sheep placenta also has multinucleate cells (referred to as binucleate cells) in the placental trophoblast layer (Igwebuike 2006; Wooding et al. 2008b). The formation of these multinucleated cells has been proposed to occur through a similar fusogenic process to that of humans involving enJSRV, another endogenous retrovirus envelope gene (Black et al. 2010; Huang et al. 2014; Koshi et al. 2012). Additionally, these binucleate cells are responsible for the endocrine capacity of the sheep placenta; producing progesterone and pregnancy-specific glycoproteins, both of which are involved in pregnancy maintenance.
The objectives of this study were to assess if gestational exposure the bisphenolic compounds, particularly, BPA and BPS, would result in placental disruption. Specifically, we investigated their effect on placental endocrine changes, including progesterone and pregnancy-associated glycoproteins (PAGs). In addition, we have investigated their effect on placental morphology, binucleate cell number, and expression of proteins and genes involved in trophoblast cell fusion.
Methods
Animals
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Michigan State University (MSU), are consistent with the National Research Council’s Guide for the Care and Use of Laboratory Animals, and meet the ARRIVE guidelines for reporting animal research (Kilkenny et al. 2010). The study was conducted at the MSU Sheep Research Facility (East Lansing, MI; 42.7° N, 84.4° W) using an in-house flock of a cross between Polypay x Dorset breeds. Female sheep (n = 23) at first parity were bred using a time mated pregnancy strategy using three vasectomized rams as previously described (Pu et al. 2017). After estrus detection by vasectomized rams, females were moved with a fertile ram; only one fertile purebred Polypay ram was used in this experiment to reduce paternal variability. Fertility of the ram had been previously tested using a breeding soundness exam. Once mated, the females were randomly assigned to the different treatment groups, blocking for body condition and weight.
Starting 3 weeks before and continuing until 30 days after breeding, breeder ewes were fed a total mixed ration to allow unlimited consumption of a diet providing an energy concentration of 2.9 Mcal/kg of digestible energy and crude protein concentration of 12%. This diet was designed to provide a level of energy and protein intake that would optimize conditions for embryo survival. From days 30 to 100 of gestation, energy concentration was reduced to 2.6 Mcal/kg of digestible energy. Beginning 7 weeks before lambing (100 days of gestation), ewes were fed a total mixed ration designed to maximize dry matter intake (40% and 70% neutral detergent fiber digestibility). This diet was enriched in energy (3.0 Mcal/kg digestible energy) and crude protein (14%) to exceed the requirements of fetal growth and minimize loss of maternal energy and protein reserves as previously described (Pu et al. 2017).
Gestational treatments
Gestational BPA and BPS treatment consisted of daily subcutaneous injections of BPA (0.5 mg/kg/day; purity ≥99%, Cat# 239658; Lot# MKBQ5209V; Aldrich Chemical Co., Milwaukee, WI, USA) or BPS (4,4’-sulfonyl diphenol, Cat# 146915000, Lot# A0337011, Acros Organics, Geel, Belgium) in corn oil from days 30 through 100 of gestation (term: ~ 147 days). Control (C) mothers received corn oil vehicle. The use of a large animal model limited the study to a single dose per chemical. The internal dose of BPA achieved in umbilical arterial samples using the 0.5 mg/kg/day dose has already been published (Veiga-Lopez et al. 2013) and is targeted to produce maternal blood levels of BPA to that of the median level of BPA measured in maternal circulation of US women (Veiga-Lopez et al. 2015). The same dose of BPS was used to match the BPA exposure dose used in the study. Although main exposure route or bisphenol exposures is oral (Liao and Kannan 2013), the use of sheep as a model, a ruminant species, requires the use of an alternative exposure route to avoid confounding factors from ruminal metabolism. The choice of the s.c. route relates to previous work that has demonstrated that both oral and s.c. administration of BPA exhibit nearly identical phenotypes in a neonatal rat model for prostate health (Prins et al. 2011). The window of exposure starting at GD30 was aimed to avoid confounding effects during early embryonic development and implantation. In addition, the discontinuation of exposure at GD100 allowed for a washout period to avoid confounding effects from the continuous exposure until collection of placental tissue. Number of breeders used were 8, 8, and 7, in C, BPA, and BPS groups, respectively. Number of singleton:twin pregnancies was 6:2, 5:3, 6:1, in C, BPA, and BPS groups, respectively. Number of female:male fetuses were 6:4, 6:5, 4:4, in C, BPA, and BPS groups, respectively. The mother was considered the experimental unit.
Sample collection and placental characterization
Maternal serum samples were collected in all pregnant females (n = 23) every 2 weeks from gestational day (GD) 30 to 120 for hormonal, protein, and biochemical analyses, and frozen until assayed. To assess placental endocrine function, pregnancy associated glycoprotein 1 (PAG1) and pregnancy specific protein B (PSPB) were measured in all maternal samples. In addition, progesterone was assayed at GD45, GD75, and GD105 of pregnancy. To determine effects of bisphenols on maternal physiological status, a serum biochemistry panel was carried out in GD75 samples. Details of these assays are described below.
Placental samples were collected on gestational day 120 ± 1 (term: ~147 days). All mothers were euthanized with a barbiturate overdose (sodium pentobarbital, i.v.; Fatal Plus; Henry Schein, Melville, NY, USA). A midline incision was performed, the uterus exposed, and the placenta harvested until further processing. All placentas were weighed, number of placentomes counted and classified as types A, B, C, or D, as previously described (Beckett et al. 2014). A type A placentome next to the umbilicus was collected and the maternal and fetal portions of the placentome separated and frozen immediately. The placenta was then perfused with Carnoy’s fixative. A placentome near the umbilicus was placed in Carnoy’s fixative for 24 hours and processed for paraffin embedding. All placentomes (one per pregnancy) were sectioned (5 µm) with a microtome and center cross-sections of the placentome used for histological (placental stereology and binucleate cell counts) and immunohistochemistry (e-cadherin and GCM1) studies. Frozen placentomes were used to assess gene expression.
Hormonal, biochemical, and protein circulating concentrations
Two commercially available assays were used to measure PAGs in this study; the enzyme-linked immunosorbent assay (ELISA) BioPRYN assay (BioTracking Inc., Moscow, ID, USA) and the Bovine Pregnancy Test Kit (IDEXX Laboratories, Westbrook, ME, USA). Ruminant species have over 20 variants of PAGs and each of these ELISA kits detects a different subset of PAG variants.
The presence of pregnancy-specific protein B (PSPB) in serum was determined using the commercially available quantitative ELISA BioPRYN assay as previously described (Roberts et al. 2017). In brief, serum samples (150 µL) were incubated in a 96-well plate coated with PSPB antibodies overnight. After washing and dry blotting, each well was incubated with the detector solution (secondary labeled antibody) for 1 hour, washed and incubated with the enhancer solution (anti-IgG horseradish peroxidase conjugate) for 1 hour. After washing, each well was incubated with 3,3,5,5’-tetramethylbenzidine for 15 min and the reaction stopped with 1N HCl. Within 30 min, the plate was read at 630 nm (SpectraMax 384 PLUS, Molecular Devices, Sunnyvale, CA, USA). All procedures were done at room temperature. Standard line was accepted with a fit R2 > 0.989. Mean intra-assay coefficient of variation (CV) based on two quality control pools were < 1 and 2%. The inter-assay CV for the same quality control were 4.7 and 14.2%, respectively.
The Bovine Pregnancy Test Kit (IDEXX Laboratories) was used to semi-quantitatively determine PAG1 and has been validated for ovine species as previously described (Roberts et al. 2017). This kit detects the variants PAG-4, PAG-6, PAG-9, PAG-16, and PAG-19. Here, we use the term PAG1 to refer to the group of modern PAGs detected by this assay and secreted by the binucleate cells of the placenta (Sousa et al. 2006). In brief, maternal serum (100 µL) and assay controls (positive and negative) were pipetted into 96-well, anti-PAG antibody coated plates along with sample diluent, sealed, and incubated for 60 min at 37 ºC in a forced air incubator (Mini-Hybridization Oven, Hybaid, Franklin, MA, USA). Between assay steps, plates were washed 4 times with wash solution (405 LS Washer, BioTek, Winooski, VT, USA). The detector solution (anti-PAG antibody) was added to each well, covered, and incubated for 30 min at room temperature. The plates were incubated for 30 min at room temperature with conjugate solution (anti-IgG-horseradish peroxidase) followed by an incubation with substrate solution (tetramethylbenzidine) for 15 minutes at room temperature. Finally, stop solution was added and absorbance (450 nm) determined on a microtiter plate spectrophotometer (Elx808, BioTek, Winooski, VT, USA). Sample values were reported as serum sample minus negative controls after subtracting the mean absorbance value of the negative controls from the absorbance of each sample value. The intra-plate and inter-plate CVs for the positive controls were 2.7 and 6.3%, respectively.
Progesterone concentrations in serum were determined using a commercially available direct, competitive ELISA assay (Ridgeway Science, Gloucestershire, United Kingdom) following manufacturer instructions. Briefly, standards (0.6 to 15.0 ng/mL), serum samples and a plate control (10 ng/mL) were diluted 1:20 with progesterone-enzyme conjugate and 200 µL pipetted into anti-progesterone-coated 96-well plates in duplicate. Plates were sealed with adhesive plate seals and incubated at 37 °C for 2 hours on a rotating shaker at 250 rpm/min. After incubation, plates were washed five times, allowing buffer to sit in plates two minutes between each wash. After washing, 200 µL of the substrate was added and plates incubated for 30 min at room temperature in the dark. Absorbance (550 nm) was read using a microtiter plate spectrophotometer (Elx808, BioTek, Winooski, VT, USA). Progesterone concentrations for each sample and plate control were calculated from the standard curve generated with a 4-parameter logistic regression model (Gen5 2.06.10, BioTek, Winooski, VT, USA). Intra- and inter-plate CV for the plate control were 3.6 and 8.1%, respectively.
Full biochemistry analyses (total protein, albumin, globulin, serum ions (Na+, K+, Cl-, P, Mg2+, Ca2+, and I-), total bilirubin, anion gap, osmolarity, cholesterol, creatinine, urea nitrogen, alkaline phosphatase, aspartate aminotransferase, creatine kinase, and γ-glutamyltransferase) were performed at the American Association of Veterinary Laboratory Diagnosticians (AAVLD) accredited Diagnostic Center for Population and Animal Health (DCPAH) at Michigan State University.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from placental tissue was extracted from the maternal portion of a type A placentome (most common type in all pregnancies) using a RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The fetal portion of the placentome is highly vascularized and was not used for mRNA extraction. A Nanodrop (Thermo Fisher Science, Wilmington, NC, USA) was used to assess RNA quality and concentration. RNA (1 µg; A260/A280: 2.0 ± 0.05; RNA concentration: 300 ± 50 ng/μL) was reverse transcribed into cDNA using a High Capacity cDNA Reverse Transcription Kit (Promega, Madison, WI, USA). Quantitative real time PCR (ABI-Quant Studio 7 Flex Real-Time PCR System, ThermoFisher, Carlsbad, CA, USA) was performed to examine the mRNA expression of the genes related to trophoblast fusion: envelop Jaagsiekte sheep retrovirus (enJSRV) and hyaluronoglucosaminidase 2 (HYAL2). Primer sequences are shown in Supplemental Table 1 and designed using the Ovis aries genome. Fast start SYBR green master mix (Invitrogen, Warrington, UK) was used for qPCR. The amplification reaction included template denaturation and polymerase activation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. Melt curve analyses were performed for all genes, and the specificity, as well as the integrity of the PCR products was confirmed by the presence of a single peak. For all genes, an agarose gel was run to assess single product amplification. The levels of mRNA encoding the indicated genes were normalized against housekeeping genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and ribosomal protein L27 (RPL27) to calculate relative fold change to that of the control using the 2-ΔΔCT method (Schmittgen and Livak 2008). Results using both housekeeping genes provided with the same results, but only GAPDH data are shown.
Histology and immunohistochemistry
Staining was performed on 5 µm serial cross-sections of paraffin embedded placentomes. One section from each placentome was stained with hematoxylin and eosin for histological examination. In brief, sections were deparaffinized in xylene, rehydrated, incubated in Mayer’s hematoxylin for 3 min and eosin for 30 sec, dehydrated in ethanol and mounted with acrytol mounting medium. For immunohistochemistry, sections were deparaffinized in xylene, rehydrated through an ethanol series, treated with 3% hydrogen peroxide, and antigen retrieved using an EMS 2100 Retriever (Hatfield, PA, USA) with sodium citrate buffer. Sections were then subjected to a streptavidin and biotin block (SP-2002, Vector Laboratories, Burlingame, CA, USA), followed by 4% normal goat serum in phosphate buffered saline (PBS) block. Details of antibody information are listed in Supplemental Table 2. Mouse anti-e-cadherin and mouse anti-GCM1 primary antibodies were incubated at 4 °C overnight, and biotinylated Wisteria floribunda lectin was incubated at room temperature for 30 min. Biotinylated goat anti-mouse IgG secondary antibody was applied to e-cadherin and GCM1 sections. All sections were subjected to Vectastain ABC peroxidase kit (#PK-6100, Vector Laboratories, Burlingame, CA, USA) and visualized with a 2% 3,3’-diaminobenzidine (DAB) substrate solution. DAB development time was equal across all treatment groups. All sections for the same antibody were incubated at the same time so that staining intensity could be compared among samples of different treatment groups. Slides were then rehydrated through ethanol and xylene washes and mounted with acrytol mounting medium (#13518, EMS, Hatfield, PA, USA).
Image analysis
All images were captured using an Olympus BX41 microscope with an Olympus DP71 camera or a Leica DMLB microscope with a Leica DFC480 camera on a 20X or 40X objective lens. All imaging and analyses were conducted blind to treatment group. Unless otherwise noted, all images were taken within the feto-maternal interface of the placentome. The selection of the interface for image acquisition was done to avoid skewed contributions from the maternal or fetal compartments. Systematic random images (10 per placentome section/animal) of hematoxylin and eosin stained placentome sections were obtained across the placentome for stereological examination. Using a 256-square grid overlay with a tissue area of 140.6 mm2 the contribution of intervillous space area and area of maternal and fetal compartments was assessed. At each grid intersection, the tissue was classified as fetal, maternal, or intervillous space. Results for all 256 intersections were summed for each individual, averaged to treatment group, and presented as a percent relative to the total area at the feto-maternal interface. Systematic random images (10 per placentome section/animal) were captured for e-cadherin, GCM1, and lectin (stain for binucleate cells). Image analyses for e-cadherin and GCM1 were performed by selection and identification of DAB-positive stain area using the Fiji software immunohistochemistry analysis plug-in “IHC toolbox” (Schindelin et al. 2012). Binucleate cells were manually identified and counted as such when lectin stain was present in uni- or binucleate cells or absent in binucleate cells.
Maternal-to-fetal transfer of BPS
A pilot study to assess maternal-to-fetal placental transfer of BPS was conducted. At gestational day 117–118, two pregnant sheep (both carrying a singleton male fetus) underwent surgery to place catheters in the descending aorta and inferior vena cava as previously described (Ehrhardt et al. 2002). In brief, fasted animals were sedated using xylazine (0.2 mg/kg) and anesthetically induced with ketamine (8 mg/kg). Anesthesia was maintained using inhaled isoflurane (2% vaporized). After sterile preparation of surgical site, a midline laparotomy was performed for uterine access. Through a small hysterotomy in the uterine horn, the fetal hind limb was isolated by external uterine palpation, and exposed past the tibio-tarsal joint. The cranial tibial vein was isolated with fine forceps, and catheterized with a polyvinyl catheter (Saint-Gobain Performance Plastics, Beaverton, MI, USA; inner diameter: 0.86 mm, outer diameter: 1.37 mm). The catheter was threaded through the cranial tibial vein into the inferior vena cava (20 cm) and secured laterally to the fetal hind limb using a non-absorbable suture (silk, USP 3–0). The uterine incision was closed with an absorbable monofilament suture (polyglycolic acid, USP 0) using an inverted pattern finished with a single Cushing suture to avoid peri-uterine adhesion. Catheters were exteriorized through a small incision (1 cm) in the flank of the animal nearest to the hysterotomy. Muscle and peritoneum were closed using absorbable suture material (polyglycolic, USP 1) in an interrupted pattern. Midline skin incision was closed using non-absorbable suture (silk, USP 0) in an interrupted mattress pattern. Catheter patency was maintained through daily flushing with sterile saline, followed by heparinized saline. Fetuses all received injections (i.m.) and amniotic infusions of penicillin G (300,000 U) at the time of surgery, and every 2 days after surgery. Additionally, all ewes received penicillin G (6,000 U/kg BW) and gentamycin sulfate (2 mg/kg BW) 24 hours before surgery, at the time of the surgery, and every 12 hours for 3 days after surgery. One week after surgery, a single injection of BPS (0.5 mg/kg BW, s.c.) was administered to the pregnant female and fetal arterial blood samples were collected 1 hour after the BPS administration. Fetal arterial blood samples collected prior to maternal BPS administration were used as their baseline control. In addition, blank samples (double distilled water) were collected to assess potential BPS contamination from sampling collection materials. All samples were processed in glass vials with glass Pasteur pipets, stored in glass vials, and kept frozen until assayed.
Bisphenol S analysis
BPS (analytical standard) and the internal standard 13C12-BPS were purchased from Sigma Aldrich (St. Louis, MO, USA) and Cambridge Isotope Laboratories (Andover, MA, USA), respectively. An API 4500™ electrospray QTRAP mass spectrometer (ESI-MS/MS; Applied Biosystems, AB Sciex, Framingham, MA, USA) interfaced with an Agilent 1260 HPLC (Agilent Technologies Inc., Santa Clara, CA) was used for the analysis. A Zorbax SB-Aq (150 mm x 2.1 mm, 3.5 µm; Agilent Technologies Inc., Santa Clara, CA) column serially connected to a Javelin guard column (Betasil® C18, 20 × 2.1 mm, 5 µm) was used for the chromatographic separation of BPS using methanol and water as mobile phases. An isotopic dilution method was used for the quantification of BPS. BPS was extracted from sheep serum and other fluids by a liquid-liquid extraction method (Liao et al. 2012a) with slight modifications. Briefly, 250 µL of serum was transferred into a 15-mL polypropylene tube and spiked with 20 ng of the internal standard (IS). Five hundred microliters of 1.0 M ammonium acetate buffer (pH = 5.5) that contained 22 units of β-glucuronidase was added and digested at 37° C overnight (~15 h) in an incubator shaker. The samples were extracted twice with 10 mL (2 × 5 mL) of ethyl acetate by shaking in a mechanical shaker for 2 h. The extracts were combined and washed with 1 mL of ultra-pure water and centrifuged at 4500 x g for 10 min. The ethyl acetate layer was transferred into another 15-mL tube and concentrated to near-dryness under a gentle nitrogen stream. Then, 250 μL of methanol was added, vortex mixed, and transferred into a vial for HPLC–MS/MS analysis. For every 15 samples, a procedural blank, a matrix blank (human sera, Sigma Aldrich, St. Louis, MO, USA) and a matrix spike experiment were performed. The matrix spike recoveries ranged between 85.0 and 98.9% and the mean absolute recovery for the assay of IS was 90%. The instrumental LOD and LOQ were 0.02 ng/mL and 0.07 ng/mL, respectively. Trace level (0.005 ng/mL) of BPS was found in procedural blanks (n = 4) and that value was subtracted from all samples.
Statistical analysis
All data are presented as mean ± SEM. Appropriate transformations were applied, as needed, to account for normality of data. Comparisons among the three treatment groups were analyzed by a mixed model or ANOVA with Tukey posthoc tests with number of fetuses as a covariate using PASW Statistics for Windows release 18.0.1. Differences were considered significant at P<0.05.
Results
Gestational BPS disrupts placental endocrine function
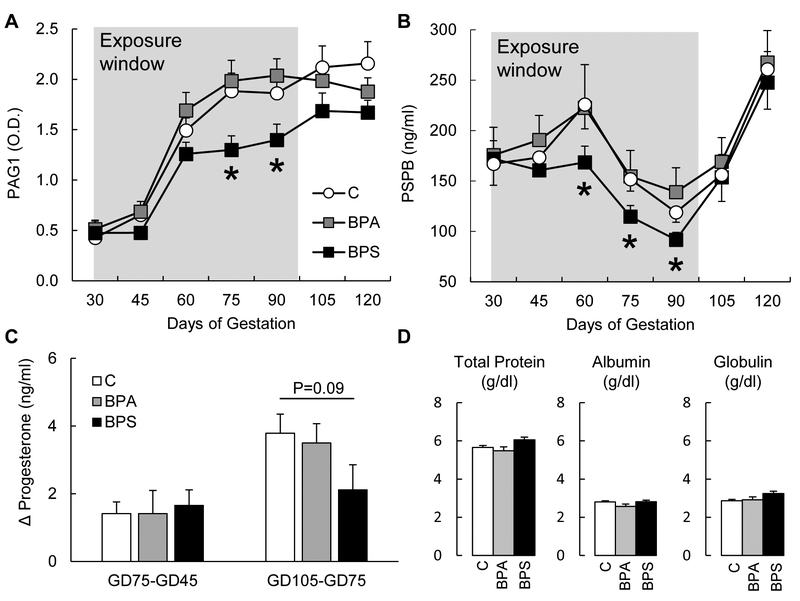
Pregnant sheep exposed to BPS daily from GD30 to GD100 had reduced maternal serum concentrations of trophoblastic proteins PAG1 and PSPB (Fig. 1) reflective of impaired placental endocrine function. This effect was significant from GD75 to GD90 for PAG1 (Fig. 1A; P<0.05) and from GD60 to GD90 for PSPB (Fig. 1B; P<0.05). Gestational BPA exposure did not have an effect on maternal PAG or PSPB plasma concentrations. Discontinuation of the BPS exposure at GD100 resulted in a partial recovery of both PAG1 and PSBP maternal serum concentrations. During late gestation, serum progesterone concentrations increase when progesterone secretion shifts from corpus luteum to placental origin. This normal physiological increase in serum progesterone levels tended to be lower in the BPS group in comparison to that of the control group (Fig. 1C). Progesterone concentrations were unaffected by BPA exposure. Serum biochemistry analyses at GD75 did not find any significant differences in total protein, albumin, and globulin (Fig. 1D). BPA- and BPS-exposed females had lower phosphorous compared to that of the control and creatinine was higher in BPS-exposed females compared to control and BPA-exposed females, although both phosphorous and creatinine were within normal reference values (Supplemental Table 3). All other biochemistry parameters studied were not significantly different among groups.
Figure 1.
Maternal serum (mean ± SE) pregnancy associated glycoprotein 1 (PAG1; (A)) and pregnancy specific protein B (PSPB (B)) in control (open circles), BPA- (gray squares) and BPS- (closed squares) exposed females. Change in maternal serum progesterone between GD45 and GD75 and between GD75 and GD105 (C) in control (open bars), BPA- (gray bars) and BPS- (closed bars) exposed females. Maternal serum (mean ± SE) total protein, albumin, and globulin at GD75 (D) in control (open bars), BPA- (gray bars) and BPS- (closed bars) exposed females. N=6–7/group. Asterisks denote statistical differences between control and BPS-exposed group at P<0.05.
Maternal-to-fetal transfer of BPS
One hour after a single injection of vehicle (corn oil) or BPS (0.5 mg/kg BW, s.c.) to the pregnant animal, BPS concentrations in fetal arterial blood samples were 0.02 ng/mL (LOD) in both control fetuses and 4.9 and 10.6 ng/mL in the two BPS fetuses. Mean BPS concentration in blank samples that had not been in contact with collection materials was 0.020 ± 0 ng/mL. Mean BPS concentration in blank samples that had been in contact with collection materials (fetal catheter and collection syringe) was 0.038 ± 0.017 ng/mL.
Gestational BPS does not affect placental gross morphology and histology
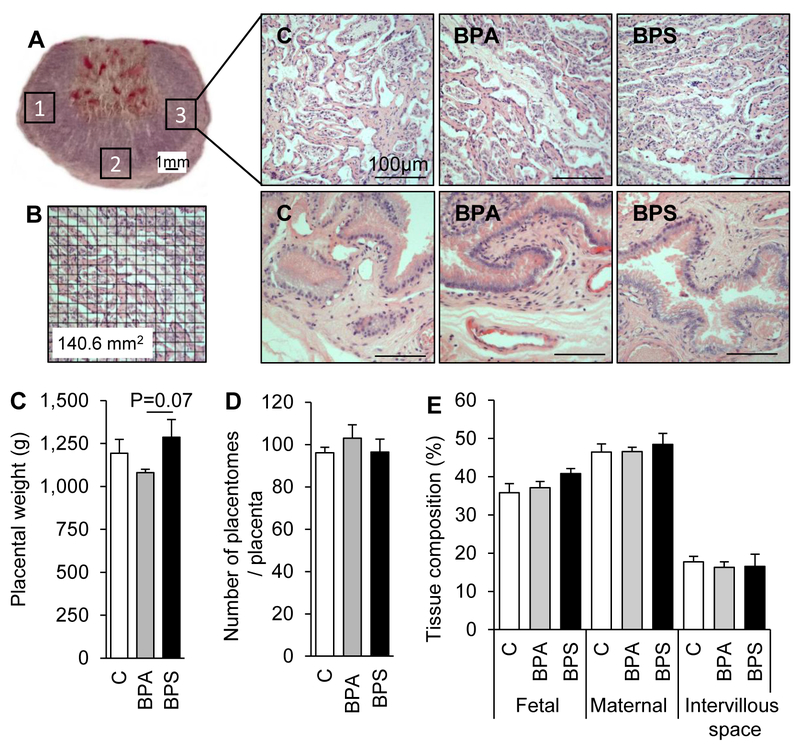
Histology of placentomes and placental membranes did not reveal pathological signs of fibrosis, picnosis, necrosis, or active inflammation (Fig. 2; top right). Gestational exposure to BPA or BPS did not alter placental weight when compared to that of the control group, but tended to be higher in BPS vs. BPA group (P=0.07; Fig. 2C). Placentome count assessed at the time of collection showed no significant difference in the number of placentomes among treatment groups (Fig. 2D). All placentomes were of types A for all placentas with the exception of one BPS-exposed placenta that had ~20% type C or D placentomes (data not shown). There was no difference in fetal or maternal tissue contribution or intervillous space at the feto-maternal interface among treatment groups (Fig. 2E). We have previously reported that gestational BPS exposure resulted in lower biparietal diameter in male fetuses, without fetal body weight differences (Pu et al. 2017).
Figure 2.
(A) Placentome cross-section stained with hematoxylin and eosin. Numbered squares (1, 2, and 3) denote approximate areas where images were taken for analysis of placental stereology and immunohistochemistry of e-cadherin, GCM1, and lectin (Figures 3, 4, and 5). Representative images of placentome cross-sections (top) and intercotyledonary membranes (bottom) in control (left), BPA- (middle) and BPS- (right) exposed females at gestational day (GD) 120 (20 days after discontinuation of exposure). (B) Example of placentome image with grid overlay used for placental stereology (Figure 2E). (C) Mean (± SE) placental weight, in control (open bars), BPA- (gray bars) and BPS- (closed bars) exposed females. (D) Number of placentomes per placenta. All placentome types were included (see text for details). (E) Placental tissue distribution, depicting percent intervillous space and fetal and maternal tissue contributions in control (open bars), BPA (gray bars), and BPS (black bars) exposed placentas. N=6–7/group. Four to five images studied per placentome.
Gestational BPS impairs the syncytialization signaling pathway
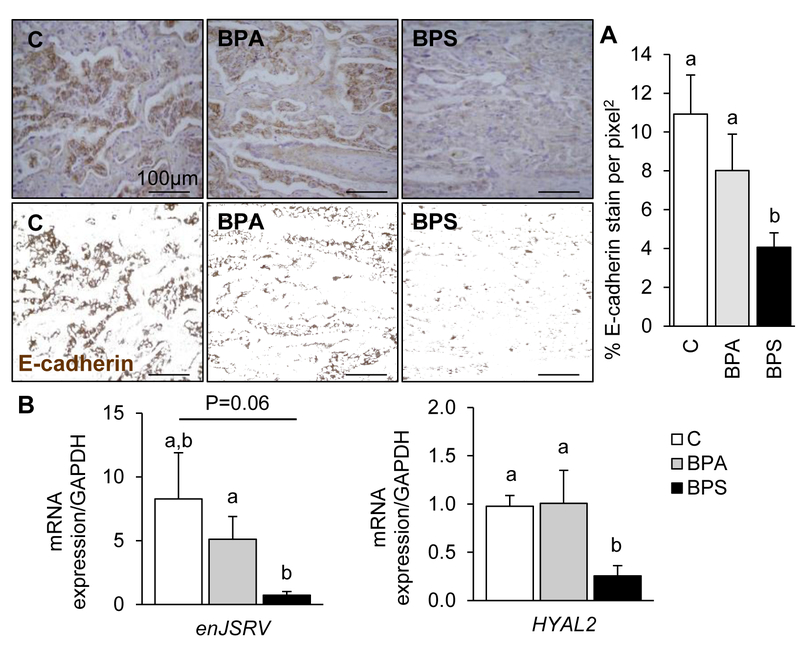
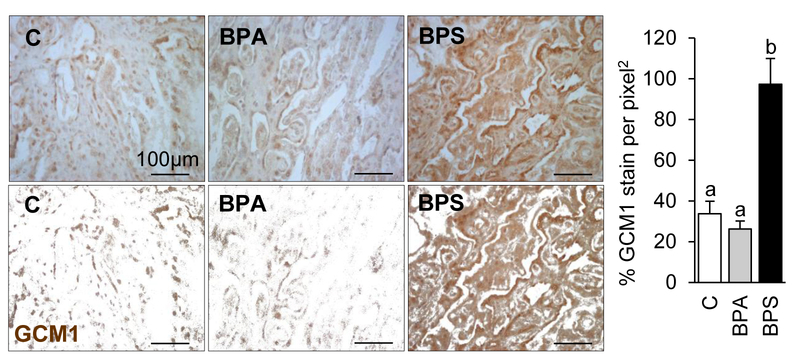
To examine if the reduction in maternal placental function reflected a reduced trophoblastic function in vivo, the syncytialization signaling pathway was explored in GD120 placentomes (20 days after chemical exposure had ended). Primarily localized in the fetal trophoblastic region of the placentome, e-cadherin immunostain was significantly reduced in the BPS group (Fig. 3A) compared to the control and BPA-exposed groups. mRNA expression of genes involved in trophoblast cell fusion (enJSRV (P=0.06) and HYAL2 (P<0.05)) were also downregulated in BPS-exposed placentas compared to that of the control group (Fig. 3B). GCM1 immunostain was significantly increased in prenatal BPS exposure when compared to the control and BPA-exposed groups (Fig. 4).
Figure 3.
Representative images of placentome cross-sections immunostained against e-cadherin (top) and respective processed images for imaging analyses (bottom) in control (left), BPA- (middle) and BPS- (right) exposed females at gestational day (GD) 120 (20 days after discontinuation of exposure). (A) E-cadherin protein (mean ± SE) quantification in control (open bars), BPA- (gray bars) and BPS- (closed bars) exposed females by immunohistochemistry (histogram). N=6–7/group. Ten images studied per placentome. Different letters denote statistical differences among treatment groups at P<0.05. (B) mRNA expression (mean ± SE) of envelop Jaagsiekte sheep retrovirus (enJSRV) and hyaluronoglucosaminidase 2 (HYAL2) in control (open bars), BPA- (gray bars), and BPS- (closed bars) exposed females. N=6–7/group. Different letters denote statistical differences among treatment groups at P<0.05.
Figure 4.
Representative images of placentome cross-sections immunostained against GCM1 (top) and respective processed images for imaging analyses (bottom) in control (left), BPA- (middle) and BPS- (right) exposed females at gestational day (GD) 120 (20 days after discontinuation of exposure). GCM1 protein (mean ± SE) quantification in control (open bars), BPA- (gray bars) and BPS- (closed bars) exposed females by immunohistochemistry. N=6–7/group. Ten images studied per placentome. Different letters denote statistical differences among treatment groups at P<0.05.
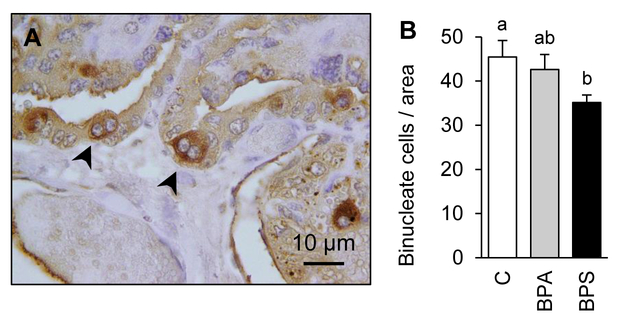
Gestational BPS reduces binucleate cell population
To assess the role that changes observed in cell adhesion and fusogenic proteins expression had on steroidogenic placental cell populations, binucleate cells were identified with lectin stain (Fig. 5A) and quantified in GD120 placentomes. A significant reduction (22.8%; P<0.02) in trophoblast-derived binucleate cell number was observed in BPS-exposed placentas when compared to the control group (Fig. 5B). No significant change was observed in the BPA-exposed group.
Figure 5.
(A) Lectin immunostained binucleate cell (arrows denote binucleate cells). (B) Binucleate cell number (mean ± SE) quantification in control (open bars), BPA- (gray bars) and BPS- (closed bars) exposed females. N=6–7/group. Binucleate cell number count ranged from 25–73 cells per image. Ten images studied per placentome. Different letters denote statistical differences among treatment groups at P<0.05.
Discussion
The effects of endocrine disrupting chemicals on placental function remain poorly understood. In this study, we evaluated placental endocrine and trophoblast fusogenic function in sheep exposed to BPA or BPS during pregnancy. Our comparative experimental design demonstrates for the first time the deleterious effects that BPS can exert on placental function. We have demonstrated that mid-gestation exposure to BPS results in impaired endocrine placental function, reduction of binucleate cells, downregulation of the cell to cell adhesion protein e-cadherin, genes involved in trophoblast fusion (enJSRV and HYAL2) and compensatory upregulation of the fusogenic transcription factor GCM1 (Fig. 6). Importantly, at the same exposure dose, gestational exposure to BPA did not result in an abnormal placental phenotype. We also demonstrated that BPS can cross the placental barrier and reach the developing fetus. Overall, this study proves that the placenta is susceptible to BPS, highlights the intrinsic differences among bisphenolic chemicals, and the need to further investigate the safety of BPA analogue exposures on placental function in humans.
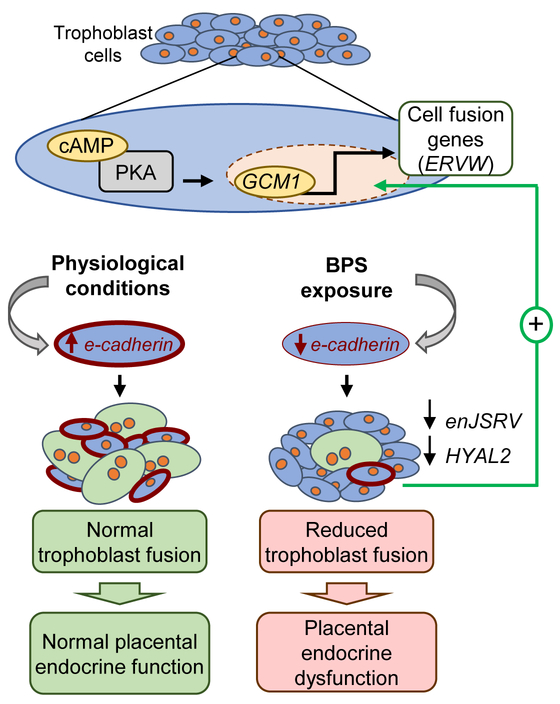
Figure 6.
BPS-induced placental dysfunction working model. Trophoblast cell fusion is a cAMP-activated of the protein kinase A (PKA) pathway which triggers the expression of the transcription factor glial cell missing factor 1 (GCM1). GCM1 in turn stimulates transcription activity of fusogenic genes, such as endogenous retrovirus envelope genes (ERVW). ERVW genes, such as enJSRV in sheep, can promote cell fusion, resulting in binucleate cell formation. However, before cell fusion can occur, cell to cell communication between two adjacent cells has to take place. E-cadherin, a transmembrane protein, facilitates cell to cell communication by the formation of adherens junctions in trophoblast cells (red cellular membranes). Under physiological conditions (left side), e-cadherin expression facilitates cell-to-cell communication enabling trophoblast fusion to occur and binucleate cells to form, resulting in normal placental endocrine function. Our findings demonstrate that gestational BPS exposure (right side) results in lower placental e-cadherin expression, preventing the formation of adherens junctions required for cell fusion and thus reducing the number of binucleate cells and expression of fusogenic genes (enJSRV and HYAL2). Altogether, this results in placental endocrine dysfunction. We hypothesize that the upregulation of GCM1 expression is a compensatory mechanism to overcome reduced trophoblast fusion rate.
Gestational BPS impairs placental endocrine function
Gestational exposure to BPS reduced maternal circulating levels of pregnancy associated glycoproteins (PAG1 and PSPB). PAGs are proteins from the aspartic proteinase family that are secreted by the trophoblast layer of the ruminant placenta (Sousa et al. 2006). Although PAGs biological significance remains elusive, these proteins are required for pregnancy maintenance in ruminants (Wallace et al. 2015). Because factors such as heat stress can reduce circulating PAGs (Thompson et al. 2013) and reduction in circulating PAGs have been associated with early and late pregnancy loss in cattle (Pohler et al. 2016), PAGs have been proposed as biomarkers for pregnancy health in ruminant species (Wallace et al. 2015). Our results further support this premise given that mothers were otherwise healthy (no alterations in the biochemical profile). The recent discovery of the presence of an aspartic proteinase in the human placenta, homologous to PAGs in other domestic species (Majewska et al. 2017), opens the opportunity to use PAGs as biomarkers in the context of human placental pathology.
The effect of BPS on circulating progesterone further supports a compromise in placental endocrine function. However, this effect was time-dependent. BPS did not affect plasma progesterone concentration during early mid-gestation, but rather during late mid-gestation. The lack of change in the early mid-gestation phase (GD45-GD75) is likely due to a relatively stable secretion of progesterone until GD75 in sheep (Bedford et al. 1972) of mainly ovarian origin (Harrison and Heap 1978). Under physiological conditions, progesterone continues to increase through the late mid-gestation phase reflecting a physiological shift from ovarian to placental production of progesterone at mid-gestation (Harrison and Heap 1978) and continues to increase until birth (Bedford et al. 1972). BPS exposure halted progesterone’s physiological increase through the late mid-gestation phase (GD75 to GD105) supportive of an impaired placental endocrine function. Such an effect in progesterone concentrations during pregnancy has only been reported in a few EDCs in vivo. For instance, triclosan reduces progesterone leading to abortion, but only at high exposure doses (600mg/kg/day) (Feng et al. 2016). In humans and sheep, low maternal serum progesterone is linked to pregnancy complications of unknown etiologies, such as preterm birth and spontaneous abortions (Arck et al. 2007; Fylling et al. 1973; Schindler 2005; Van Calster et al. 2016). Additional studies are necessary to elicit the involvement of EDCs in the development of these pregnancy complications. This study could not address if uninterrupted gestational exposure to BPS through pregnancy would have resulted in preterm delivery. To note is that progesterone supplementation is used in humans for preterm birth prevention in at-risk pregnancies (Newnham et al. 2014; Rundell and Panchal 2017), which highlights the significance of placental progesterone homeostasis on pregnancy maintenance.
The longitudinal experimental design allowed us to track progression of the placental defect. Although the reduction in PAGs was not significantly different until GD60 (30 days after the start of the exposure), PAG1 concentrations began to be numerically lower two weeks (GD45) after the exposure begun. This indicates that a minimum exposure of two weeks for BPS to elicit a detectable change in placental endocrine function. Importantly, the discontinuation of the exposure (GD100) before tissue harvest (GD120), resulted in partial and full recovery in PAG1 and PSPB circulating concentrations, respectively. This is supportive of the reversibility of the observed effect. Since the number of binucleate cells were lower in BPS-exposed placentas at GD120, we hypothesize that the placenta has the ability to buffer a significant loss (∼20%) of endocrine cells while continuing to partially maintain endocrine function and pregnancy. This is similar to other organs such as the liver and kidney that can continue to compensate homeostatic balance despite significant cell loss (Barai et al. 2010; Shoup et al. 2003). More information is required to understand the limitations of this buffering ability in the placenta and the threshold to successfully overcome a significant loss of placental endocrine cells.
We have demonstrated that BPS can cross the ovine placental barrier and reach fetal circulation. In this study, BPS fetal concentrations reached ~7 ng/mL (one hour after maternal administration), which is 100-fold higher compared to human fetal circulation (0.03–0.12 ng/mL) (Liu et al. 2017). These findings support human transplacental transfer of BPS reaching fetal circulation (Liu et al. 2017) and are similar to those observed in BPA-exposed fetuses (Veiga-Lopez et al. 2013). We have previously reported that fetal biparietal diameter tended to be lower in BPS-exposed males, but not females (Pu et al. 2017) despite a discontinuation of the exposure for 20 days prior to pregnancy termination. This suggests that fetal development may be directly affected by BPS (BPS can cross the placental barrier) or indirectly via placental endocrine dysfunction. Additional work is required to determine if BPS-induced placental defects result in long-term consequences for the progeny. Interestingly, we noticed that BPS had a stronger effect in placentas from male fetuses for many of the outcomes studied (binucleate cell number, GCM1, e-cadherin) than in placentae from female fetuses. However, due to the number of animals per group (n=7–8) results have been presented for all pregnancies combined.
Gestational BPS disrupts trophoblast fusogenic function
In sheep, placental endocrine function at the cellular level is dependent on trophectoderm derived uni- and bi-nucleate trophoblast cells. During placentation, ovine trophoblast cells undergo homokaryonic fusion to form binucleate trophoblast cells through a similar event to that of human syncytiotrophoblast formation (Huang et al. 2014; Koshi et al. 2012). One of the first events in cell fusion is the formation of adherens junctions between adjacent cells. This process is facilitated by the transmembrane protein e-cadherin, whose expression is reduced upon gestational exposure to BPS. We have not investigated the mechanism by which BPS results in e-cadherin downregulation. To date, underlying mechanisms by which bisphenols can modulate e-cadherin expression in the placenta remain unclear (Borman et al. 2017; Borman et al. 2015; Wang et al. 2015), although inhibition experiments have pointed out to the estrogen receptor 1 (ESR1) as the mechanism by which BPA modulates e-cadherin in hemangioma cells (Zhai et al. 2016).
Loss of e-cadherin naturally occurs in human pregnancies between the first and second trimester (Zhou et al. 1997). Lower than normal expression has also been reported in placenta accreta (Duzyj et al. 2015), placenta percreta (Incebiyik et al. 2016) and placentas of somatic cell nuclear transfer derived embryos (Kohan-Ghadr et al. 2011). Additionally, a down regulation of genes encoding cadherin-associated protein (CTNNAL) have also been reported in preeclamptic placentas complicated with hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome (Buimer et al. 2008). The loss of e-cadherin hampers the fusogenic ability of the placenta resulting in aberrant cell adhesion, initiation of an invasive phenotype, and ultimately may result in trophoblast cells undergoing epithelial to mesenchymal transition (Kokkinos et al. 2010). This is a common phenomenon observed in carcinoma invasiveness (Peinado et al. 2007; Schmalhofer et al. 2009). In this work, we have not further explored the tumorigenic nature of the exposure to BPS. However, other EDCs such as phthalates and BPA have been reported to trigger epithelial to mesenchymal transition in colorectal cancer cells (Chen et al. 2015) and ovarian cells (Kim et al. 2015). Although e-cadherin expression is required prior to cell fusion, its expression is reduced during cell fusion (Coutifaris et al. 1991). Given the reduced number of binucleate cells observed upon gestational BPS exposure, the lower e-cadherin expression is unlikely to be reflective of higher cell fusion events.
The observed reduction in e-cadherin protein and mRNA expression was accompanied by an upregulation of the transcription factor GCM1 in BPS-exposed placentas. Trophoblast cell fusion is controlled by a cAMP-mediated process which activates the transcription factor GCM1. GCM1, in turn, stimulates transcription activity of fusogenic genes, such as syncytin-1, encoded by endogenous retrovirus envelope genes (ERVW). The ovine homolog for syncytin-1 is enJSRV (Black et al. 2010), which tended to be downregulated by BPS. HYAL2, a cellular receptor for enJSRV (Rai et al. 2001) and exclusively expressed in binucleate cells (Dunlap et al. 2005) was also downregulated. The cell-to-cell fusion cascade is triggered by ERVW genes in both humans and ruminants (Black et al. 2010; Huang et al. 2014). Blocking the expression of ERVW in sheep results in a slower trophectoderm growth and inhibition of binucleate cell differentiation during the preimplantation period (Dunlap et al. 2006). GCM1 is a critical factor for normal placentation across mammalian species, as homozygous GCM1 knockout mice are embryonic lethal, present with an absent or amorphous placental labyrinth, and do not form a syncytiotrophoblast layer (Anson-Cartwright et al. 2000). Additionally, expression of GCM1 is repressed during the trophoblast fusion process (syncytialization) in humans (Kashif et al. 2011), which is supportive of our findings and proposed compensatory mechanism (lower binucleate cell number and higher GCM1 expression; Fig. 6). Effects of GCM1 overexpression vary depending on the placental cell types (Hughes et al. 2004); it arrests trophoblast stem cells proliferation, blocks differentiation of trophoblast giant cells, but is not sufficient to induce formation of the syncytiotrophoblast (Hughes et al. 2004). Additional research is required to predict the phenotype that BPS-induced GCM1 overexpression may have on the human placenta.
Because binucleate trophoblast cells are responsible for the production of hormones such as progesterone, and pregnancy associated glycoproteins in ruminant species (Wallace et al. 2015), BPS-induced low binucleate cell number is likely the direct cause of the observed reduction in PAG1, PSPB, and progesterone. The direct effect of BPS on placental endocrine function is further supported by the recovery observed in circulating PAG1 and PSPB after the discontinuation of the BPS treatment. Importantly, this cellular loss was not associated with placental weight reduction, changes in placental gross morphology or histopathology; reflective of a specific endocrine disrupting effect. Loss of syncytiotrophoblasts, the human homolog for the ovine binucleate cells, occurs in reactive oxygen species states and has been observed in preeclampsia and placentas of IUGR pregnancies (Wu et al. 2015). Additionally, apoptosis of syncytiotrophoblasts can be induced in hypoxic or reperfusion states (Wu et al. 2015). Macroscopic and histologic evaluation of BPS-exposed placentas and placentomes, did not reveal any evidence of hypoxia or necrosis. Further studies are required to evaluate the direct cause that lead to the loss of binucleate cells observed upon BPS exposure.
Gestational BPA does not affect placental endocrine function
In this study, BPA did not affect any of the endocrine or placental aspects investigated. The comparative experimental design and similar dose exposure levels used for both bisphenols help support previously reported differences in the mechanism of action and receptor affinities of both of these bisphenolic compounds (Grignard et al. 2012; Molina-Molina et al. 2013; Rosenmai et al. 2014). However, this needs to be further investigated in the context of placental cells. The lack of effects on the placenta differs from previous work where mice exposed to BPA (50 mg/kg/day) during early pregnancy resulted in reduction of the spongiotrophoblast layer of the placenta (Tait et al. 2015). The difference between studies is likely due to the 100-fold difference in dose exposure, but could also relate to the species or route of exposure. Other aspects of placental function reported to be affected upon BPA exposure in vitro (Avissar-Whiting et al. 2010; Lan et al. 2017; Rajakumar et al. 2015; Spagnoletti et al. 2015) need further validation in an in vivo model.
Conclusion
This study demonstrates, for the first time, detrimental effects of gestational BPS, but not BPA, exposure on placental development and endocrine function. This work adds to prior work demonstrating that BPS can affect endocrine organs (LaPlante et al. 2017) and highlights intrinsic differences between the two bisphenolic compounds, BPA and BPS. Further research is required to evaluate the safety of BPA analogues.
Supplementary Material
Acknowledgments
Acknowledgments: We thank Michigan State University (MSU) Sheep Teaching and Research Farm for help with animal husbandry, Dr. Ramona Ehrhardt for help with fetal catheterization, the MSU Diagnostic Center for Population and Animal Health for help with biochemistry analyses, Ms. Lindsay Hannah and Ms. Gabriela Saldana for their help during animal experimentation, and Ms. Madilyn Johnson for help with stereological analyses of the placenta.
Funding source: Research reported in this publication was supported the National Institute of Environmental Health Sciences of the National Institute of Health (1K22ES026208 to A.V-L.), Michigan State University (MSU), MSU AgBioResearch, and the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (Hatch MICL02383). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclosure statement: Authors have nothing to disclose
References
- Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC (2000) The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet 25(3):311–4 doi: 10.1038/77076 [DOI] [PubMed] [Google Scholar]
- Arck P, Hansen PJ, Mulac Jericevic B, Piccinni MP, Szekeres-Bartho J (2007) Progesterone during pregnancy: endocrine-immune cross talk in mammalian species and the role of stress. Am J Reprod Immunol 58(3):268–79 doi: 10.1111/j.1600-0897.2007.00512.x [DOI] [PubMed] [Google Scholar]
- Asimakopoulos AG, Xue J, De Carvalho BP, et al. (2016) Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environmental research 150:573–81 doi: 10.1016/j.envres.2015.11.029 [DOI] [PubMed] [Google Scholar]
- Avissar-Whiting M, Veiga KR, Uhl KM, et al. (2010) Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reproductive toxicology 29(4):401–6 doi: 10.1016/j.reprotox.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barai S, Gambhir S, Prasad N, Sharma RK, Ora M (2010) Functional renal reserve capacity in different stages of chronic kidney disease. Nephrology (Carlton) 15(3):350–3 doi: 10.1111/j.1440-1797.2010.01291.x [DOI] [PubMed] [Google Scholar]
- Beckett EM, Astapova O, Steckler TL, Veiga-Lopez A, Padmanabhan V (2014) Developmental programing: impact of testosterone on placental differentiation. Reproduction 148(2):199–209 doi: 10.1530/REP-14-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford CA, Harrison FA, Heap RB (1972) The metabolic clearance rate and production rate of progesterone and the conversion of progesterone to 20 -hydroxypregn-4-en-3-one in the sheep. The Journal of endocrinology 55(1):105–18 [DOI] [PubMed] [Google Scholar]
- Benirschke K, Burton G, Baergen RN (2012) Pathology of the human placenta, 6th edn Springer, Berlin ; London [Google Scholar]
- Black SG, Arnaud F, Palmarini M, Spencer TE (2010) Endogenous retroviruses in trophoblast differentiation and placental development. Am J Reprod Immunol 64(4):255–64 doi: 10.1111/j.1600-0897.2010.00860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman ED, Foster WG, deCatanzaro D (2017) Concurrent administration of diethylhexyl phthalate reduces the threshold dose at which bisphenol A disrupts blastocyst implantation and cadherins in mice. Environ Toxicol Phar 49:105–111 doi: 10.1016/j.etap.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Borman ED, Foster WG, Greenacre MKE, Muir CC, deCatanzaro D (2015) Stress lowers the threshold dose at which bisphenol A disrupts blastocyst implantation, in conjunction with decreased uterine closure and e-cadherin. Chem-Biol Interact 237:87–95 doi: 10.1016/j.cbi.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Buimer M, Keijser R, Jebbink JM, et al. (2008) Seven placental transcripts characterize HELLP-syndrome. Placenta 29(5):444–53 doi: 10.1016/j.placenta.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Castro B, Sanchez P, Torres JM, Ortega E (2015) Bisphenol A, bisphenol F and bisphenol S affect differently 5alpha-reductase expression and dopamine-serotonin systems in the prefrontal cortex of juvenile female rats. Environmental research 142:281–7 doi: 10.1016/j.envres.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Catanese MC, Vandenberg LN (2017) Bisphenol S (BPS) Alters Maternal Behavior and Brain in Mice Exposed During Pregnancy/Lactation and Their Daughters. Endocrinology 158(3):516–530 doi: 10.1210/en.2016-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Kannan K, Tan HL, et al. (2016) Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environmental science & technology 50(11):5438–5453 doi: 10.1021/acs.est.5b05387 [DOI] [PubMed] [Google Scholar]
- Chen MY, Ike M, Fujita M (2002) Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environmental toxicology 17(1):80–6 [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Yang XL, Liu H, et al. (2015) Bisphenol A modulates colorectal cancer protein profile and promotes the metastasis via induction of epithelial to mesenchymal transitions. Arch Toxicol 89(8):1371–81 doi: 10.1007/s00204-014-1301-z [DOI] [PubMed] [Google Scholar]
- Corbel T, Perdu E, Gayrard V, et al. (2015) Conjugation and deconjugation reactions within the fetoplacental compartment in a sheep model: a key factor determining bisphenol A fetal exposure. Drug metabolism and disposition: the biological fate of chemicals 43(4):467–76 doi: 10.1124/dmd.114.061291 [DOI] [PubMed] [Google Scholar]
- Coutifaris C, Kao LC, Sehdev HM, et al. (1991) E-cadherin expression during the differentiation of human trophoblasts. Development 113(3):767–77 [DOI] [PubMed] [Google Scholar]
- Dunlap KA, Palmarini M, Adelson DL, Spencer TE (2005) Sheep endogenous betaretroviruses (enJSRVs) and the hyaluronidase 2 (HYAL2) receptor in the ovine uterus and conceptus. Biol Reprod 73(2):271–9 doi: 10.1095/biolreprod.105.039776 [DOI] [PubMed] [Google Scholar]
- Dunlap KA, Palmarini M, Varela M, et al. (2006) Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc Natl Acad Sci U S A 103(39):14390–5 doi: 10.1073/pnas.0603836103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzyj CM, Buhimschi IA, Motawea H, et al. (2015) The invasive phenotype of placenta accreta extravillous trophoblasts associates with loss of E-cadherin. Placenta 36(6):645–51 doi: 10.1016/j.placenta.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Ehrhardt RA, Bell AW, Boisclair YR (2002) Spatial and developmental regulation of leptin in fetal sheep. Am J Physiol Regul Integr Comp Physiol 282(6):R1628–35 doi: 10.1152/ajpregu.00750.2001 [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhang P, Zhang Z, Shi J, Jiao Z, Shao B (2016) Endocrine Disrupting Effects of Triclosan on the Placenta in Pregnant Rats. PLoS One 11(5):e0154758 doi: 10.1371/journal.pone.0154758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ, Sferruzzi-Perri AN, Burton GJ, Vaughan OR (2015) Review: Endocrine regulation of placental phenotype. Placenta 36 Suppl 1:S50–9 doi: 10.1016/j.placenta.2014.11.018 [DOI] [PubMed] [Google Scholar]
- Fylling P, Sjaastad OV, Velle W (1973) Mid-Term Abortion Induced in Sheep by Synthetic Corticoids. Journal of Reproduction and Fertility 32(2):305–306 [DOI] [PubMed] [Google Scholar]
- Gorrochategui E, Perez-Albaladejo E, Casas J, Lacorte S, Porte C (2014) Perfluorinated chemicals: differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol Appl Pharmacol 277(2):124–30 doi: 10.1016/j.taap.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Grignard E, Lapenna S, Bremer S (2012) Weak estrogenic transcriptional activities of Bisphenol A and Bisphenol S. Toxicology in vitro : an international journal published in association with BIBRA 26(5):727–31 doi: 10.1016/j.tiv.2012.03.013 [DOI] [PubMed] [Google Scholar]
- Harrison FA, Heap RB (1978) Progesterone secretion during pregnancy in sheep with an autotransplanted adrenal and an autotransplanted ovary. J Reprod Fertil 54(1):153–7 [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Balbus J, Birnbaum L, et al. (2015) Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology 156(10):3416–21 doi: 10.1210/EN.2015-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Chen H, Li J, et al. (2014) Epigenetic and non-epigenetic regulation of syncytin-1 expression in human placenta and cancer tissues. Cell Signal 26(3):648–56 doi: 10.1016/j.cellsig.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Hughes M, Dobric N, Scott IC, et al. (2004) The Hand1, Stra13 and Gcm1 transcription factors override FGF signaling to promote terminal differentiation of trophoblast stem cells. Dev Biol 271(1):26–37 doi: 10.1016/j.ydbio.2004.03.029 [DOI] [PubMed] [Google Scholar]
- Huuskonen P, Auriola S, Pasanen M (2015) Zearalenone metabolism in human placental subcellular organelles, JEG-3 cells, and recombinant CYP19A1. Placenta 36(9):1052–5 doi: 10.1016/j.placenta.2015.06.014 [DOI] [PubMed] [Google Scholar]
- Igwebuike UM (2006) Trophoblast cells of ruminant placentas--A minireview. Anim Reprod Sci 93(3–4):185–98 doi: 10.1016/j.anireprosci.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Incebiyik A, Kocarslan S, Camuzcuoglu A, Hilali NG, Incebiyik H, Camuzcuoglu H (2016) Trophoblastic E-cadherin and TGF-beta expression in placenta percreta and normal pregnancies. J Matern Fetal Neonatal Med 29(1):126–9 doi: 10.3109/14767058.2014.989203 [DOI] [PubMed] [Google Scholar]
- Ji K, Hong S, Kho Y, Choi K (2013) Effects of bisphenol s exposure on endocrine functions and reproduction of zebrafish. Environmental science & technology 47(15):8793–800 doi: 10.1021/es400329t [DOI] [PubMed] [Google Scholar]
- Kashif M, Hellwig A, Kolleker A, et al. (2011) p45NF-E2 represses Gcm1 in trophoblast cells to regulate syncytium formation, placental vascularization and embryonic growth. Development 138(11):2235–47 doi: 10.1242/dev.059105 [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS biology 8(6):e1000412 doi: 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Hwang KA, Hyun SH, Nam KH, Lee CK, Choi KC (2015) Bisphenol A and nonylphenol have the potential to stimulate the migration of ovarian cancer cells by inducing epithelial-mesenchymal transition via an estrogen receptor dependent pathway. Chem Res Toxicol 28(4):662–71 doi: 10.1021/tx500443p [DOI] [PubMed] [Google Scholar]
- Kinch CD, Ibhazehiebo K, Jeong JH, Habibi HR, Kurrasch DM (2015) Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A 112(5):1475–80 doi: 10.1073/pnas.1417731112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan-Ghadr HR, Fecteau G, Smith LC, Murphy BD, Lefebvre RC (2011) Endocrine profiles of somatic nuclear transfer-derived pregnancies in dairy cattle. Theriogenology 76(5):911–20 doi: 10.1016/j.theriogenology.2011.04.022 [DOI] [PubMed] [Google Scholar]
- Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF (2010) Cadherins in the human placenta--epithelial-mesenchymal transition (EMT) and placental development. Placenta 31(9):747–55 doi: 10.1016/j.placenta.2010.06.017 [DOI] [PubMed] [Google Scholar]
- Koshi K, Suzuki Y, Nakaya Y, et al. (2012) Bovine trophoblastic cell differentiation and binucleation involves enhanced endogenous retrovirus element expression. Reprod Biol Endocrinol 10:41 doi: 10.1186/1477-7827-10-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Fu LJ, Zhang J, et al. (2017) Bisphenol A exposure promotes HTR-8/SVneo cell migration and impairs mouse placentation involving upregulation of integrin-beta1 and MMP-9 and stimulation of MAPK and PI3K signaling pathways. Oncotarget 8(31):51507–51521 doi: 10.18632/oncotarget.17882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlante CD, Catanese MC, Ruby Bansal R, Vandenberg LN (2017) Bisphenol S alters the lactating mammary gland and nursing behaviors in mice exposed during pregnancy and lactation Endocrinology en.2017–00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Kannan K (2013) Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. Journal of agricultural and food chemistry 61(19):4655–62 doi: 10.1021/jf400445n [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K (2014) A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food additives & contaminants Part A, Chemistry, analysis, control, exposure & risk assessment 31(2):319–29 doi: 10.1080/19440049.2013.868611 [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Alomirah H, et al. (2012a) Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environmental science & technology 46(12):6860–6 doi: 10.1021/es301334j [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Guo Y, et al. (2012b) Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environmental science & technology 46(16):9138–45 doi: 10.1021/es302004w [DOI] [PubMed] [Google Scholar]
- Liu JY, Li JG, Wu YN, et al. (2017) Bisphenol A Metabolites and Bisphenol S in Paired Maternal and Cord Serum. Environmental science & technology 51(4):2456–2463 doi: 10.1021/acs.est.6b05718 [DOI] [PubMed] [Google Scholar]
- Majewska M, Lipka A, Panasiewicz G, et al. (2017) Identification of Novel Placentally Expressed Aspartic Proteinase in Humans. Int J Mol Sci 18(6) doi: 10.3390/ijms18061227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruvu S, Zhang J, Bedi YS, Choudhury M (2016) Mono-(2-ethylhexyl) phthalate induces apoptosis through miR-16 in human first trimester placental cell line HTR-8/SVneo. Toxicology in vitro : an international journal published in association with BIBRA 31:35–42 doi: 10.1016/j.tiv.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Molina-Molina JM, Amaya E, Grimaldi M, et al. (2013) In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol 272(1):127–36 doi: 10.1016/j.taap.2013.05.015 [DOI] [PubMed] [Google Scholar]
- Mourier E, Tarrade A, Duan J, et al. (2017) Non-invasive evaluation of placental blood flow: lessons from animal models. Reproduction 153(3):R85–R96 doi: 10.1530/Rep-16-0428 [DOI] [PubMed] [Google Scholar]
- Naderi M, Wong MY, Gholami F (2014) Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquatic toxicology 148:195–203 doi: 10.1016/j.aquatox.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Newnham JP, Dickinson JE, Hart RJ, Pennell CE, Arrese CA, Keelan JA (2014) Strategies to prevent preterm birth. Front Immunol 5:584 doi: 10.3389/fimmu.2014.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7(6):415–28 doi: 10.1038/nrc2131 [DOI] [PubMed] [Google Scholar]
- Pohler KG, Pereira MH, Lopes FR, et al. (2016) Circulating concentrations of bovine pregnancy-associated glycoproteins and late embryonic mortality in lactating dairy herds. J Dairy Sci 99(2):1584–94 doi: 10.3168/jds.2015-10192 [DOI] [PubMed] [Google Scholar]
- Prins GS, Ye SH, Birch L, Ho SM, Kannan K (2011) Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reproductive toxicology 31(1):1–9 doi: 10.1016/j.reprotox.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Gingrich J, Steibel JP, Veiga-Lopez A (2017) Sex-specific modulation of fetal adipogenesis by gestational bisphenol A and bisphenol S exposure. Endocrinology doi: 10.1210/en.2017-00615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai SK, Duh FM, Vigdorovich V, Danilkovitch-Miagkova A, Lerman MI, Miller AD (2001) Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci U S A 98(8):4443–8 doi: 10.1073/pnas.071572898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumar C, Guan H, Langlois D, Cernea M, Yang K (2015) Bisphenol A disrupts gene expression in human placental trophoblast cells. Reproductive toxicology 53:39–44 doi: 10.1016/j.reprotox.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Vonnahme KA, Lemley CO, et al. (2013) Maternal stress and placental vascular function and remodeling. Curr Vasc Pharmacol 11(5):564–93 [DOI] [PubMed] [Google Scholar]
- Roberts JN, May KJ, Veiga-Lopez A (2017) Time-dependent changes in pregnancy-associated glycoproteins and progesterone in commercial crossbred sheep. Theriogenology 89:271–279 doi: 10.1016/j.theriogenology.2016.10.029 [DOI] [PubMed] [Google Scholar]
- Rosenmai AK, Dybdahl M, Pedersen M, et al. (2014) Are structural analogues to bisphenol a safe alternatives? Toxicol Sci 139(1):35–47 doi: 10.1093/toxsci/kfu030 [DOI] [PubMed] [Google Scholar]
- Rundell K, Panchal B (2017) Preterm Labor: Prevention and Management. Am Fam Physician 95(6):366–372 [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–82 doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AE (2005) Endocrinology of pregnancy: consequences for the diagnosis and treatment of pregnancy disorders. J Steroid Biochem Mol Biol 97(5):386–8 doi: 10.1016/j.jsbmb.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Schmalhofer O, Brabletz S, Brabletz T (2009) E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev 28(1–2):151–66 doi: 10.1007/s10555-008-9179-y [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C-T method. Nature Protocols 3(6):1101–1108 doi: 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Schneider JE, Brozek JM, Keen-Rhinehart E (2014) Our stolen figures: the interface of sexual differentiation, endocrine disruptors, maternal programming, and energy balance. Horm Behav 66(1):104–19 doi: 10.1016/j.yhbeh.2014.03.011 [DOI] [PubMed] [Google Scholar]
- Shoup M, Gonen M, D’Angelica M, et al. (2003) Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 7(3):325–30 [DOI] [PubMed] [Google Scholar]
- Sieppi E, Vahakangas K, Rautio A, Ietta F, Paulesu L, Myllynen P (2016) The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures. Mol Cell Endocrinol 429:41–9 doi: 10.1016/j.mce.2016.03.034 [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Toppari J, Soder O, Gordon CM, Divall S, Draznin M (2011) The exposure of fetuses and children to endocrine disrupting chemicals: a European Society for Paediatric Endocrinology (ESPE) and Pediatric Endocrine Society (PES) call to action statement. The Journal of clinical endocrinology and metabolism 96(10):3056–8 doi: 10.1210/jc.2011-1269 [DOI] [PubMed] [Google Scholar]
- Sousa NM, Ayad A, Beckers JF, Gajewski Z (2006) Pregnancy-associated glycoproteins (PAG) as pregnancy markers in the ruminants. J Physiol Pharmacol 57 Suppl 8:153–71 [PubMed] [Google Scholar]
- Spagnoletti A, Paulesu L, Mannelli C, et al. (2015) Low concentrations of Bisphenol A and para-Nonylphenol affect extravillous pathway of human trophoblast cells. Mol Cell Endocrinol 412:56–64 doi: 10.1016/j.mce.2015.05.023 [DOI] [PubMed] [Google Scholar]
- Stel J, Legler J (2015) The Role of Epigenetics in the Latent Effects of Early Life Exposure to Obesogenic Endocrine Disrupting Chemicals. Endocrinology 156(10):3466–72 doi: 10.1210/en.2015-1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S, Tassinari R, Maranghi F, Mantovani A (2015) Bisphenol A affects placental layers morphology and angiogenesis during early pregnancy phase in mice. J Appl Toxicol 35(11):1278–91 doi: 10.1002/jat.3176 [DOI] [PubMed] [Google Scholar]
- Thompson IM, Tao S, Branen J, Ealy AD, Dahl GE (2013) Environmental regulation of pregnancy-specific protein B concentrations during late pregnancy in dairy cattle. J Anim Sci 91(1):168–73 doi: 10.2527/jas.2012-5730 [DOI] [PubMed] [Google Scholar]
- Van Calster B, Bobdiwala S, Guha S, et al. (2016) Managing pregnancy of unknown location based on initial serum progesterone and serial serum hCG levels: development and validation of a two-step triage protocol. Ultrasound Obstet Gynecol 48(5):642–649 doi: 10.1002/uog.15864 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G (2010) Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118(8):1055–70 doi: 10.1289/ehp.0901716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan OR, Sferruzzi-Perri AN, Coan PM, Fowden AL (2011) Environmental regulation of placental phenotype: implications for fetal growth. Reprod Fertil Dev 24(1):80–96 doi: 10.1071/RD11909 [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V (2015) Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. The Journal of clinical endocrinology and metabolism 100(11):E1394–403 doi: 10.1210/jc.2015-1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V (2013) Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 154(5):1873–84 doi: 10.1210/en.2012-2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitku J, Starka L, Bicikova M, et al. (2016) Endocrine disruptors and other inhibitors of 11beta-hydroxysteroid dehydrogenase 1 and 2: Tissue-specific consequences of enzyme inhibition. J Steroid Biochem Mol Biol 155(Pt B):207–16 doi: 10.1016/j.jsbmb.2014.07.007 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Clark MM, Galef BG Jr, Drickamer LC, Vandenbergh JG (1999) The intrauterine position (IUP) phenomenon In: Knobil E, Neill J (eds) Encyclopedia of reproduction. vol 2 Academic Press, New York, p 893–900 [Google Scholar]
- Wallace RM, Pohler KG, Smith MF, Green JA (2015) Placental PAGs: gene origins, expression patterns, and use as markers of pregnancy. Reproduction 149(3):R115–26 doi: 10.1530/REP-14-0485 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Lu J, Zhang YZ, Zhang M, Liu T, Qu XL (2015) Effect of Bisphenol A on invasion ability of human trophoblastic cell line BeWo. Int J Clin Exp Patho 8(11):14355–14364 [PMC free article] [PubMed] [Google Scholar]
- Wooding P, Burton G, SpringerLink (Online service) (2008a) Comparative Placentation : Structures, Functions and Evolution. doi: 10.1007/978-3-540-78797-6 [DOI] [Google Scholar]
- Wooding P, Burton G, SpringerLink (Online service) (2008b) Synepitheliochorial Placentation: Ruminants (Ewe and Cow) Comparative Placentation : Structures, Functions and Evolution Springer Berlin Heidelberg, Berlin, Heidelberg, p 133–167 [Google Scholar]
- Wu F, Tian FJ, Lin Y (2015) Oxidative Stress in Placenta: Health and Diseases. Biomed Res Int 2015:293271 doi: 10.1155/2015/293271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM (2015) Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environmental science & technology 49(19):11834–9 doi: 10.1021/acs.est.5b02135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai D, He J, Li X, Gong L, Ouyang Y (2016) Bisphenol A regulates Snail-mediated epithelial-mesenchymal transition in hemangioma cells. Cell Biochem Funct 34(6):441–8 doi: 10.1002/cbf.3206 [DOI] [PubMed] [Google Scholar]
- Zhang N, Wang W, Li W, et al. (2015) Inhibition of 11beta-HSD2 expression by triclosan via induction of apoptosis in human placental syncytiotrophoblasts. The Journal of clinical endocrinology and metabolism 100(4):E542–9 doi: 10.1210/jc.2014-4376 [DOI] [PubMed] [Google Scholar]
- Zhao M, Zhang Y, Zhuang S, Zhang Q, Lu C, Liu W (2014) Disruption of the hormonal network and the enantioselectivity of bifenthrin in trophoblast: maternal-fetal health risk of chiral pesticides. Environmental science & technology 48(14):8109–16 doi: 10.1021/es501903b [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, et al. (1997) Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? The Journal of clinical investigation 99(9):2139–51 doi: 10.1172/JCI119387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.