Abstract
Fear is considered an integral part of the brain’s defensive mechanism that evolved to protect animals and humans from predation and other ecological threats. Hence, it is logical to study fear from the perspective of antipredator-survival behaviors and circuits by sampling a range of threatening situations that organisms are likely to encounter in the wild. In the past several decades, however, mainstream fear research has focused on the importance of associative learning; that is, how animals become frightened of innocuous cues as consequences of their contingent pairing with aversive events. While significant discoveries have been made, contemporary fear models derived from learning studies are likely to provide only a partial picture of the brain’s fear system because they cannot simulate the dynamic range of risky situations in nature that require various adaptive actions and decisions. This review considers two different approaches to study fear, grounded on behaviorism and ethology and examines their contributions in revealing the naturalistic workings of fear in guiding and shaping behavior as animals make real-world choices.
Keywords: Fear conditioning, Ethology, Amygdala, Predators, Decision-making
“Every man gets a narrower and narrower field of knowledge in which he must be an expert in order to compete with other people. The specialist knows more and more about less and less and finally knows everything about nothing.” Konrad Lorenz [1]
Introduction
Inside the crypt of Christ Church Cathedral (c. 1030) in Dublin, Ireland, there is a display featuring a mummified cat and rat, which were discovered during a renovation (Fig. 1). As the story goes, sometime in the 1850s, a rat (presumably foraging for food) encountered a cat, became frightened and fled into an organ pipe. The cat pursued the rat into the organ pipe where it got stuck, thereby blocking the only passage for the rat. In this impasse, both rat and the cat were eternally trapped and became naturally preserved. Two traditional schools of thought—behaviorism and ethology—offer alternate hypotheses as to why the rat took flight from the cat. A behavioristic view emphasizes the survival value of fear learning (i.e., Pavlovian or classical conditioning) [2,3], and would conjecture that the rat must have previously encountered a predator, and consequently some elements of the predator (conditioned stimulus; CS) became “associated” with a predatory attack (unconditioned stimulus; US) that ultimately evoked a reflexive fear/pain response (unconditioned response; UR) [4]. Surviving this life-or-death experience, upon subsequent encounter with the cat, the predatory CS (e.g., visual/auditory/olfactory cues) evoked the memory of the prior perilous interaction with the predatory (attack) US, which then triggered learned or conditioned fear responses (CR) in the rat. In contrast, ethology emphasizes the competitive advantages of animals’ genetically pre-programmed (innate) fear responses, hardwired to respond to simple yet evolutionarily-reliable indicators (sign stimulus or releaser) of threat. Such innate fear would prevent physical contact with predators in the first place [5–7], and the rat would instinctively flee from the cat—independent of prior predatory experience. This view contends that if associative trial-and-error learning—which can be time-consuming and hazardous—were the primary fear (defensive) mechanism, most animals would be killed before they learned which predators and risky situations are to be avoided [8]. In the real world, it is likely that both innate and learned fear mechanisms play critical roles in guiding and shaping behaviors that help organisms adapt to various ecological challenges [6,9].
Figure 1. The Cat and the Rat.
The cat – presumably in chase – and the rat – in flight – were trapped in an organ pipe in the 1850s and became mummified. The photograph courtesy of Christ Church Cathedral, Dublin, Ireland.
At the outset, we acknowledge that the word ‘fear’ is routinely used as a scientific term that applies to both animals and humans. This conventional view has been questioned recently on the ground that the anthropomorphic sense of fear requires higher cognitive capacities, such as consciousness, and thus evolved relatively recently among the phylogeny of defensive-survival mechanisms [10,11]. Hence, the editors of this Special Issue logically question whether ‘fear’ in animals is isomorphic to fear in humans and suggest the use of ‘survival behaviors and circuits’ as a scientific framework in animal research. However, there should also be caution in ascribing preeminent importance of ‘consciousness’ in fear when consciousness itself is ill-defined and poorly understood scientifically. For example, some have argued that there is “suggestive evidence” of, at least, simple consciousness in animals, including rodents [12]. With this caveat in mind, this review nonetheless uses the term ‘fear’ in the manner that ‘decision-making,’ ‘empathy,’ ‘wanting vs. liking,’ ‘episodic memory,’ etc. are used as lingua franca in other animal models of human behaviors. It should also be noted that Darwin described ‘fear’ behavior of nestling birds and chickens [13] and that Tinbergen used ‘fear’ in the context of territorial behavior in animals to potentially understand war and peace in man [14].
Pavlovian fear conditioning
For the past several decades, the majority of basic fear research has employed fear conditioning paradigms in rodents to understand the behavioral principles and biological mechanisms underlying conditioned fear memories, with the goal of translating this knowledge into effective treatments for anxiety, panic, phobia and posttraumatic stress disorders [15–21]. This model systems approach—where the CS and US are well-defined and can be precisely controlled—has been successful in delineating the fear conditioning circuit, with the amygdala functioning as the core (Fig. 2) that integrates acquisition, expression, generalization, extinction, and return of a specific fear CR at behavioral, systems, circuit and molecular-genetics levels. However, the tradeoff is that fear conditioning studies cannot address the fact that animals rely on a multitude of actions and decisions to survive a breadth of risky situations in nature [6,15,22]. Accordingly, even with the use of ultramodern molecular, genetic, imaging, and recording techniques, the in-depth knowledge gained from researching fear conditioning circuits and molecular mechanisms will provide an incomplete picture of the brain’s fear system and limit insights to the etiology and treatment of fear disorders. Indeed, the meta-analysis evidence supporting abnormal fear conditioning processes in patients with anxiety disorders varies [23]. Hence, we cannot safely reach firm conclusions on the brain’s fear system and its normal/abnormal functioning simply based on behavioral and biological manipulations of CR expression in restrictive, unnatural contexts. Clearly, there is a need to complement fear conditioning studies with ecologically-relevant fear research that, by better approximating real-world threat situations, can provide different perspectives on the complexity of human fear disorders, which encompass various symptoms of cognition, mood, avoidance, arousal, and reactivity [24].
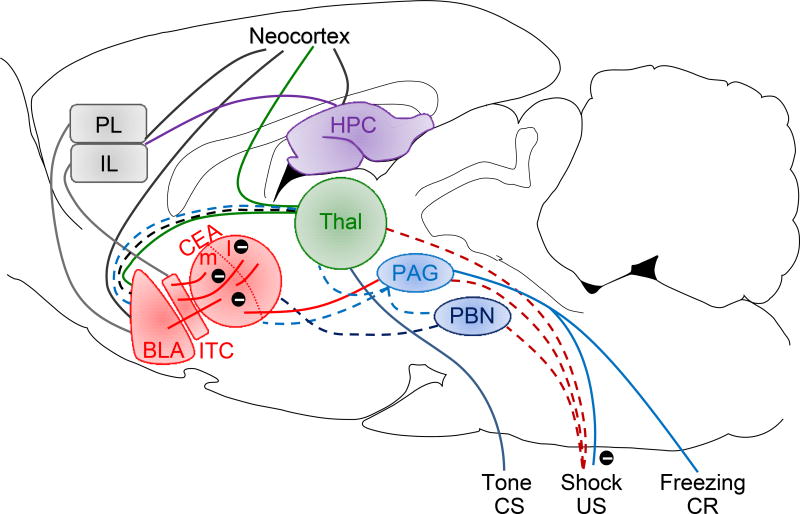
Figure 2. A contemporary model of fear conditioning.
The CS and US information are relayed through their respective sensory pathways and converge in the amygdala where the crucial CS-US association formation occurs. It is further hypothesized that different CSs (e.g., tones, lights, contexts) develop association with the US (typically footshock) in distinct subnuclei of the amygdala. The long-term storage of conditioned fear memories seems to reside in different areas of the neocortex but still require the amygdala for the manifestation of fear responses. The PL and IL are thought to be involved in the expression and extinction of CRs. Abbreviations: BLA, basolateral complex of the amygdala; CEA, central nucleus of the amygdala; ITC, intercalated cells of the amygdala; PL and IL, prelimbic and infralimbic regions of the medial prefrontal cortex; HPC, hippocampus; Thal, thalamus; PAG, periaqueductal gray; PBN, parabrachial nucleus; CS, conditioned stimulus; US, unconditioned stimulus; CR, conditioned response. Encircled minus symbols represent inhibitory pathways. Reprinted from Publication “What can ethobehavioral studies tell us about the brain’s fear system” (2016) Trends Neurosci 39, 420–431, with permission from Elsevier.
Ethological fear paradigms
In nature, animals constantly face the risk of predation as they navigate their environment in search of primary resources, such as food, water, and conspecifics for mating [7,25,26]. To survive, animals must immediately react to present threats via genetically determined, species-specific defense reactions [8], as well as to future dangers by altering their exploration and exploitation strategies to avoid areas of high predation likelihood (Fig. 3) [7,27]. Hence, there is a need to scrutinize the utility of fear conditioning circuits and mechanisms beyond that of simple CRs. For example, recent efforts to integrate various ecological risk factors, such as pain, predators, and aggressive conspecifics, provide the richness of learned and innate fear behaviors and circuits [22]. Here, we consider several ethologically-inspired behavioral paradigms that employ odor-, sound- and visual-based threat sign stimuli/releasers to simulate a range of risky situations that animals are likely to encounter in the wild. Unlike real predators, these putative predatory threat sign stimuli are completely controllable (in duration, frequency and intensity, which allow precise time-stamped behavioral and neurophysiological analyses), consistent between subjects and across trials, and pose no physical harm or pain to the subjects. Furthermore, they can be tested in combinations to better approximate natural threats.
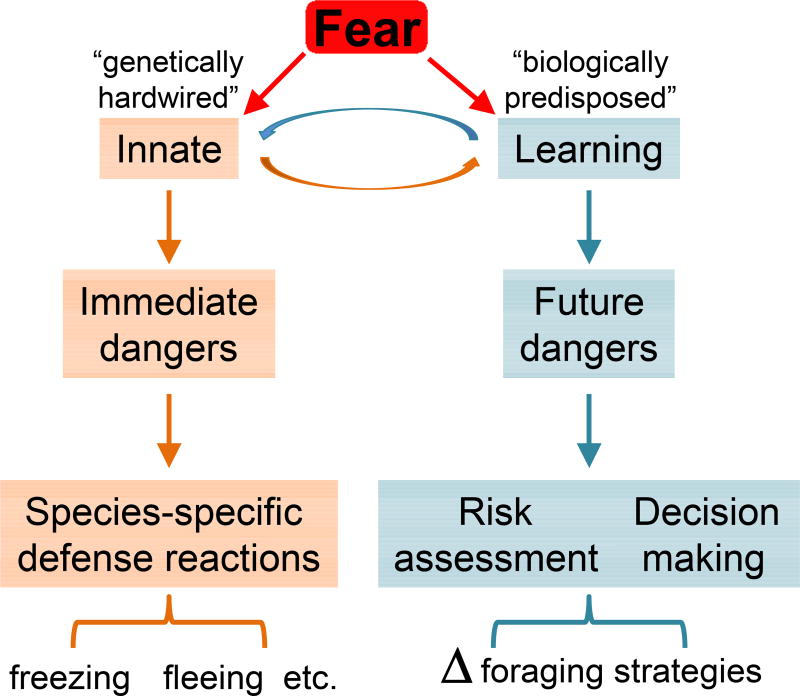
Figure 3. A simple diagram of the natural structure of fear.
The fear system is functionally divisible to genetically hardwired innate fear and biologically predisposed learned fear. Innate responses, such as freezing and escaping, counter immediate threats, while learned responses, such as alterations in foraging area and time, lessen/prepare encountering future threats. Naturally, the two fear systems interact to maximize survivability.
Olfactory threat signals
Olfaction plays a crucial function in navigational (and other) behaviors in rodents [28]. Both field and laboratory studies have found that chemical signals associated with natural predators, such as cat fur, saliva, bobcat, weasel, ferret and fox urine/feces [29,30], can produce unconditioned freezing and suppress foraging, feeding and grooming in mice and rats. The inborn ability to recognize biologically significant predator odorants presents valuable “nonlethal” information about places (where) and times (when) to avoid predators. Since the 1980s, the synthetic compound 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) has received considerable interest as the active fear-producing analog of predator odors [31–33]. A recent optogenetic study [34] reported that the hypothalamus-lateral habenula-dorsal tegmentum circuit regulates innate fear responses to TMT in mice. Contrary to odorants directly emitted from predators, however, the evidence supporting TMT as the olfactory sign stimulus of predatory threat is debated [35] and may depend on the specific experimental setting in which it is tested. Furthermore, the report of TMT serving as the aversive US to support fear conditioning seems to be “the exception rather than the rule” [36]. There are other predator kairomones that have been reported to cause aversion in mice, via activating distinct subpopulations of olfactory sensory neurons [37].
Auditory threat signals
Laboratory rodents emit a range of ultrasonic vocalizations (USV) [38] that are believed to convey the animal’s affective state to conspecifics [39]. In rats, the 22-kHz USV (discrete 100–1500-msec calls), which has been associated with distress state, has been hypothesized to convey social signaling of imminent danger (an auditory Schreckstoff) that benefits group survival [40]. Consistent with this view, a replay of 22-kHz USV has been shown to increase neuronal activities in the amygdala and other brain structures implicated in fear/defensive behavior [41,42]. Behaviorally, when a small group of rats in a semi-natural visible burrow system were exposed to a cat, they fled from the open space to burrows and emitted 22-kHz USV that continued for several minutes after the removal of the cat [43]. Interestingly, when rats individually confronted the cat, they failed to emit 22-kHz USV, suggesting that USV occurs only under social situations. While amygdalar stimulation [44] and lesion/inactivation can produce (in the absence of aversive experience) and block (in response to aversive experience) 22-kHz USV, respectively [45], it’s putative function as a social alarm signal remains unclear because naïve rats exposed to conspecific’s 22-kHz USV distress calls do not reliably exhibit fear behavior.
Visual threat signals
Many animals, including humans, show defensive behaviors instinctively to visual ‘looming’ stimuli [46]. For example, in mice freely moving about in a chamber, an overhead visual display (~30 cm above the floor) of a rapidly expanding dark disc (2 degrees increasing to 20 degrees of visual angle in 250 msec.) that approximates a shadow cast by an imminent aerial predator (e.g., owls) [47] triggers either flight (if animals are close to the nest) or freezing (if animals are distant from the nest) responses [48]. The trajectory and velocity of the looming stimulus (a constant small black disk, 2.5 cm, 5 degrees) also influences whether mice will exhibit escape (in response to a fast-moving disk, 84 degrees/sec) or freezing (to a slower moving disk, <42 degrees/sec) behavior [49]. Presumably, such freezing and escape behaviors have been evolutionarily successful in countering aerial predator’s reconnaissance (simulated by a slow-moving disk) and pursue/attack behavior (simulated by a fast-moving disk), respectively. These findings are consistent with Fanselow and Lester’s hypothesis [50] that predatory imminence determines the “topography of defensive behavior”; freezing is an adaptive strategy for distant predators because it is harder to detect a motionless (rather than moving) animal, whereas escape is adaptive for fast approaching predators that can easily detect a freezing animal. A subset of “OFF” retinal ganglion cells have been found to respond selectively to stimuli approaching or increasing in size, compared with laterally moving or shrinking stimuli, allowing for rapid detection of imminent threats without the need for cortical processing [51]. The circuit involving these motion-sensitive retinal ganglion cells is comprised of connections between the superior colliculus, lateral posterior thalamus, and basolateral nucleus of the amygdala, and appears to mediate the defensive responses to looming stimuli [52,53].
Rodents also face terrestrial predators, such as wild canines, felines and snakes [54]. One study simulated terrestrial threats using a robotic “predator” in a naturalistic “approach food-avoid predator” situation and found that rats foraging for food in an open area reacted with a coordinated set of defensive behaviors [55]. In response to the surging robot, rats reflexively fled and froze in the safe nest (fear responses) area, followed by a stretched posture anchored inside the nest opening to scan and monitor the foraging area (risk-assessment). Subsequently, rats cautiously ventured out, pausing and moving toward the food (decision-making) until the robot’s surge retriggered the rat’s fear responses. Animals were successful in obtaining food when it was placed near the nest but not near the surging robot. To note, hippocampal place cells might be involved in delineating the boundaries of danger and safety because their place fields were stable in/near the safe nest but unstable in around the threat area [49]. Both behavioral and neural correlates of a distance gradient of fear were abolished by amygdalar lesions and inactivation. Conversely, amygdalar stimulation was sufficient to cause foraging rats to flee to the nest in the absence of external threat [56]. The same amygdalar stimulation, however, elicited freezing and 22-kHz USV when rats were in a typical conditioning chamber. An important implication of these findings is that recently identified, distinct populations of neurons and circuits in the amygdala derived from fear conditioning studies may function much differently in naturalistic settings.
Genes do not supply the brain with detailed information about diverse potential predators. Most likely, genes encode for neural networks that detect looming stimuli, which are simple, evolutionarily-reliable signals of danger; all predators must approach their prey for consumption. Interestingly, terrestrial and aerial threat information may not be processed by a common innate fear circuit, at least at the sensory processing level, because overhead visual stimuli that effectively produce fear responses in rats are no longer effective when presented from the side [57].
Conclusion
Much of our knowledge on fear circuits and mechanisms are based on fear conditioning-centric studies assessing specific behaviors (e.g., freezing) in a small chamber that restricts the animal’s repertoire of defensive behavior. Accordingly, the continued reliance on applying the latest (e.g., optogenetics, optical imaging) and future techniques to fear conditioning will eventually lead to a scientific cul-de-sac [1]. This limitation also applies to modern brain imaging techniques used in human studies where the emphasis seems to be on the technology to advance the spatial-temporal resolution of neuronal activation while continuously employing variations of fear conditioning paradigms. Parallel progress in the use of ecologically valid behavioral paradigms, which closely simulate real-world threat situations, will lead to a better understanding of the natural structure of the brain’s fear system [5]. Ecological fear studies may also illuminate whether human fear and animal ‘fear’ are qualitatively different or not on the continuum of defensive-survival mechanisms, and in doing so may provide a deeper insight into human fear disorders that are abnormal amalgamations of innate/learned fear, risk-assessment, and decision-making processes.
Highlights.
Fear elicits coordinated defensive behaviors to counter immediate threats and alter foraging (purposive) behaviors to evade future threats.
Behaviorism and Ethology offer opposing viewpoints on how fear operates in nature.
Fear disorders are complex products of innate/learned fear, risk-assessment, and decision-making processes.
Acknowledgments
This manuscript preparation was supported by the the National Institutes of Health grant MH099073 and the Korean Federation of Science and Technology, Brain Pool Program (J.J.K.). We thank Bryan Schuessler for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
● of special interest
●● of outstanding interest
- 1.Casadevall A, Fang FC. Specialized Science. Infection and Immunity. 2014;82:1355–1360. doi: 10.1128/IAI.01530-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson JB, Rayner R. Conditioned emotional reactions. J Exp Psychol. 1920;3:1–14. doi: 10.1037//0003-066x.55.3.313. [DOI] [PubMed] [Google Scholar]
- 3.Fanselow MS. What Is Conditioned Fear. Trends in Neurosciences. 1984;7:460–462. [Google Scholar]
- 4.Hull CL. A functional interpretation of the conditioned reflex. Psychol Rev. 1929;36:498–511. [Google Scholar]
- 5.Mobbs D, Kim JJ. Neuroethological studies of fear, anxiety, and risky decision-making in rodents and humans. Current Opinion in Behavioral Sciences. 2015;5:8–15. doi: 10.1016/j.cobeha.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellman BA, Kim JJ. What can ethobehavioral studies tell us about the brain's fear system? Trends in neurosciences. 2016;39:420–431. doi: 10.1016/j.tins.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7●●.Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology. 1990;68:619–640. A milestone paper presenting a connectionist model of various prey-predator interaction scenarios and strategies. [Google Scholar]
- 8.Bolles RC. Species-Specific Defense Reactions and Avoidance Learning. Psychological Review. 1970;77:32–48. [Google Scholar]
- 9●.Mobbs D, Hagan CC, Dalgleish T, Silston B, Prévost C. The ecology of human fear: survival optimization and the nervous system. Frontiers in Neuroscience. 2015;9:55. doi: 10.3389/fnins.2015.00055. This paper presents a novel conceptual model, Survival Optimization System, that bridges ecological threat scenarios and human affective science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10●.LeDoux JE. Coming to terms with fear. Proceedings of the National Academy of Sciences. 2014;111:2871–2878. doi: 10.1073/pnas.1400335111. This paper provides a succinct history of Pavlovian fear conditioning research and critically contrasts fear in humans versus ‘fear’ in animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeDoux JE, Brown R. A higher-order theory of emotional consciousness. Proceedings of the National Academy of Sciences. 2017 doi: 10.1073/pnas.1619316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin DR, Speck GB. New evidence of animal consciousness. Animal Cognition. 2004;7:5–18. doi: 10.1007/s10071-003-0203-x. [DOI] [PubMed] [Google Scholar]
- 13.Darwin C, Bynum WF. The origin of species by means of natural selection: or, the preservation of favored races in the struggle for life. 2009 [Google Scholar]
- 14.Tinbergen N. On War and Peace in Animals and Man. Science. 1968;160:1411–1418. doi: 10.1126/science.160.3835.1411. [DOI] [PubMed] [Google Scholar]
- 15.Beckers T, Krypotos A-M, Boddez Y, Effting M, Kindt M. What's wrong with fear conditioning? Biological Psychology. 2013;92:90–96. doi: 10.1016/j.biopsycho.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience. 2013;14:417. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh L-F, Watanabe M, Sulkes-Cuevas J, Johansen JP. Dysregulation of aversive signaling pathways: a novel circuit endophenotype for pain and anxiety disorders. Current Opinion in Neurobiology. 2018;48:37–44. doi: 10.1016/j.conb.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends in neurosciences. 2014;37:455–464. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 21.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience and Biobehavioral Reviews. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross CT, Canteras NS. The many paths to fear. Nature Reviews Neuroscience. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- 23.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Lader M. Generalized Anxiety Disorder. In: Stolerman IP, Price LH, editors. Encyclopedia of Psychopharmacology. Springer; Berlin Heidelberg: 2015. pp. 699–702. [Google Scholar]
- 25.Clinchy M, Sheriff MJ, Zanette LY. Predator-induced stress and the ecology of fear. Functional Ecology. 2013;27:56–65. [Google Scholar]
- 26.Bednekoff PA. Foraging: behaviour and ecology. University of Chicago Press; Chicago: 2007. Foraging in the face of danger; pp. 305–329. [Google Scholar]
- 27.Bach DR, Dayan P. Opinion: Algorithms for survival: a comparative perspective on emotions. Nat Rev Neurosci. 2017 doi: 10.1038/nrn.2017.35. advance online publication. [DOI] [PubMed] [Google Scholar]
- 28.Slotnick B. Animal cognition and the rat olfactory system. Trends in Cognitive Sciences. 2001;5:216–222. doi: 10.1016/s1364-6613(00)01625-9. [DOI] [PubMed] [Google Scholar]
- 29.Papes F, Logan DW, Stowers L. The Vomeronasal Organ Mediates Interspecies Defensive Behaviors through Detection of Protein Pheromone Homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrero DM, Lemon JK, Fluegge D, Pashkovski SL, Korzan WJ, Datta SR, Spehr M, Fendt M, Liberles SD. Detection and avoidance of a carnivore odor by prey. Proceedings of the National Academy of Sciences. 2011;108:11235–11240. doi: 10.1073/pnas.1103317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vernet-Maury E, H P, Polak E, Demael A. Stracture-activity relationship of stress-inducing odorants in the rat. 1984;10 doi: 10.1007/BF00987509. [DOI] [PubMed] [Google Scholar]
- 32.Wallace KJ, Rosen JB. Predator odor as an unconditioned fear stimulus in rats: Elicitation of freezing by trimethylthiazoline, a component of fox feces. Behavioral Neuroscience. 2000;114:912–922. doi: 10.1037//0735-7044.114.5.912. [DOI] [PubMed] [Google Scholar]
- 33.Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neuroscience & Biobehavioral Reviews. 2005;29:1145–1156. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Yang J, Xi W, Hao S, Luo B, He X, Zhu L, Lou H, Yu Y-q, Xu F, et al. Laterodorsal tegmentum interneuron subtypes oppositely regulate olfactory cue-induced innate fear. Nature Neuroscience. 2016;19:283. doi: 10.1038/nn.4208. [DOI] [PubMed] [Google Scholar]
- 35.McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behavioural Brain Research. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- 36.Fendt M, Endres T. 2,3,5-Trimethyl-3-thiazoline (TMT), a component of fox odor – Just repugnant or really fear-inducing? Neuroscience & Biobehavioral Reviews. 2008;32:1259–1266. doi: 10.1016/j.neubiorev.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Gómez A, Bleymehl K, Stein B, Pyrski M, Birnbaumer L, Munger Steven D, Leinders-Zufall T, Zufall F, Chamero P. Innate Predator Odor Aversion Driven by Parallel Olfactory Subsystems that Converge in the Ventromedial Hypothalamus. Current Biology. 2015;25:1340–1346. doi: 10.1016/j.cub.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson JW. The Production of Ultrasonic Sounds by Laboratory Rats and Other Mammals. Science. 1954;119:808–809. doi: 10.1126/science.119.3101.808. [DOI] [PubMed] [Google Scholar]
- 39.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychological Bulletin. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 40.Litvin Y, Blanchard DC, Blanchard RJ. Rat 22kHz ultrasonic vocalizations as alarm cries. Behavioural Brain Research. 2007;182:166–172. doi: 10.1016/j.bbr.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 41.Beckett SRG, Duxon MS, Aspley S, Marsden CA. Central C-Fos Expression Following 20kHz/Ultrasound Induced Defence Behaviour in the Rat. Brain Research Bulletin. 1997;42:421–426. doi: 10.1016/s0361-9230(96)00332-2. [DOI] [PubMed] [Google Scholar]
- 42.Furtak SC, Allen TA, Brown TH. Single-Unit Firing in Rat Perirhinal Cortex Caused by Fear Conditioning to Arbitrary and Ecological Stimuli. The Journal of Neuroscience. 2007;27:12277–12291. doi: 10.1523/JNEUROSCI.1653-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiology & Behavior. 1991;50:967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- 44.Kim EJ, Horovitz O, Pellman BA, Tan LM, Li QL, Richter-Levin G, Kim JJ. Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14795–14800. doi: 10.1073/pnas.1310845110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45●.Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5:e15077. doi: 10.1371/journal.pone.0015077. This study presents a novel ‘auto-conditioning’ model of how 22-kHz USV develops into social signaling of fear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bronson GW. The fear of novelty. Psychological Bulletin. 1968;69:350–358. doi: 10.1037/h0025706. [DOI] [PubMed] [Google Scholar]
- 47.Brown JS, Kotler BP, Smith RJ, Wirtz WO. The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia. 1988;76:408–415. doi: 10.1007/BF00377036. [DOI] [PubMed] [Google Scholar]
- 48●●.Yilmaz M, Meister M. Rapid innate defensive responses of mice to looming visual stimuli. Current Biology. 2013;23:2011–2015. doi: 10.1016/j.cub.2013.08.015. This study elegantly demonstrated that an overhead display of rapidly expanding dark disk produces escape or freezing in mice depending on the proximity to a nest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Franceschi G, Vivattanasarn T, Saleem Aman B, Solomon Samuel G. Vision Guides Selection of Freeze or Flight Defense Strategies in Mice. Current Biology. 2016;26:2150–2154. doi: 10.1016/j.cub.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. 1988 [Google Scholar]
- 51.Münch TA, Da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nature neuroscience. 2009;12:1308–1316. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- 52.Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53●.Wei P, Liu N, Zhang Z, Liu X, Tang Y, He X, Wu B, Zhou Z, Liu Y, Li J. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nature communications. 2015;6:6756. doi: 10.1038/ncomms7756. This study revealed a novel circuit that mediates innate defensive behavior to visual looming stimuli in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andersson M, Erlinge S. Influence of Predation on Rodent Populations. Oikos. 1977;29:591–597. [Google Scholar]
- 55.Choi JS, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc Natl Acad Sci U S A. 2010;107:21773–21777. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim EJ, Park M, Kong M-S, Park SG, Cho J, Kim JJ. Alterations of Hippocampal Place Cells in Foraging Rats Facing a “Predatory” Threat. Current Biology. 2015;25:1362–1367. doi: 10.1016/j.cub.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JN. Rats maintain an overhead binocular field at the expense of constant fusion. Nature. 2013 doi: 10.1038/nature12153. [DOI] [PubMed] [Google Scholar]