Abstract
To study the vestibular system or the vestibular compensation process, a number of methods have been developed to cause vestibular damage, including surgical or chemical labyrinthectomy and vestibular neurectomy. Surgical labyrinthectomy is a relatively simple, reliable, and rapid method. Here, we describe the surgical technique for rat labyrinthectomy. A postauricular incision is made under general anesthesia to expose the external auditory canal and the tympanic membrane, after which the tympanic membrane and the ossicles are removed without the stapes. The stapes artery, which is located between the stapes and the oval window, is a vulnerable structure and must be preserved to obtain a clear surgical field. A hole to fenestrate the vestibule is made with a 2.1-mm drill bur superior to the stapes. Then, 100% ethanol is injected through this hole and aspirated several times. Meticulous dissection under a microscope and careful bleeding control are essential to obtain reliable results. Symptoms of vestibular loss, such as nystagmus, head tilting, and a rolling motion, are seen immediately after surgery. The rotarod or rotation chair test can be used to objectively and quantitatively evaluate the vestibular function.
Keywords: Medicine, Issue 135, Compensation, dizziness, inner ear, labyrinthectomy, rat, vertigo, vestibule
Introduction
The vestibular organ is essential for balance and eye control. A normal vestibular function depends upon symmetrical afferent signals from the vestibular organs in the two inner ears. Vestibular hypofunction or loss induces dizziness, nystagmus, and postural imbalance. After acute damage, the vestibular function recovers spontaneously within several days, a process known as vestibular compensation1,2. The vestibular compensation of static deficits is a process of recovery related to the imbalance of spontaneous resting activity between the ipsilateral and contralateral vestibular nuclei. The vestibular compensation of dynamic deficits is achieved principally via sensory and behavioral substitutions (using visual or somatosensory inputs)3.These processes are attractive for neuronal plasticity studies4,5.
A number of methods have been developed to study the vestibular system and the mechanisms underpinning neuronal plasticity during vestibular compensation, such as surgical and chemical labyrinthectomy and vestibular neurectomy5,6,7,8. Vestibular neurectomy is a certain way to induce complete vestibular loss, but it is a more difficult and invasive procedure and may induce brain damage8,9. This method requires greater surgical skill and takes more time than labyrinthectomy. Chemical labyrinthectomy including gentamycin, arsanilate, and tetracaine, is easier and can yield reliable results10,11,12. However, the cochlea may also be damaged and vestibular loss may develop over time11. Additionally, the effects of the chemicals on the brain, which should be preserved for accurate evaluation, are unclear. Surgical labyrinthectomy was first introduced in animal studies in 184215 and was first reported in the rat in 193616. This technique has since been used in many animal studies5,17,18,19. Surgical labyrinthectomy is a specific, reliable, and relatively simple method.13,14 Moreover, the symptoms of vestibular damage are seen immediately after surgery. Here, we describe our surgical technique for rat labyrinthectomy.
Protocol
This study was performed in accordance with the Institutional Animal Care and Use Committee of Seoul National University Hospital (14-0148-C1A1), which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
NOTE: The experiments were performed on Sprague-Dawley (SD) male rats of 7 - 8 weeks old (200/250 g). Each animal was acclimatized to the laboratory conditions for 1 week prior to the start of the experiment. The animals were housed in a temperature- and humidity-controlled room with a constant 12-h:12-h light:dark cycle with free access to food and water.
1. Labyrinthectomy
Cover the autoclaved surgical instruments with a sterile pad. Disinfect the operating area with 70% ethanol. Use a sterile drape and maintain sterile conditions during the surgery.
Wear a gown, mask (to cover nose and mouth), cap (to cover head), and a pair of sterile gloves. The surgical instruments must only come in contact with sterile surfaces.
Inject tiletamine-zolazepam anesthetic (40 mg/kg body weight) intramuscularly into the medial thigh or intraperitoneally, and xylazine (10 mg/kg body weight) intramuscularly or intraperitoneally. NOTE: Other modes of general anesthesia, including isoflurane inhalation, can also be used.
Place the rat on a warming pad (initially set at 42 °C). Apply lubricant eye ointment to both eyes of the rat to prevent eye dryness while under anesthesia.
Place the rat on its right side. Shave the fur in the surgical area with hair clippers. Disinfect the site with 70% ethanol.
Inject 1% lidocaine hydrochloride subcutaneously into the left retroauricular area.
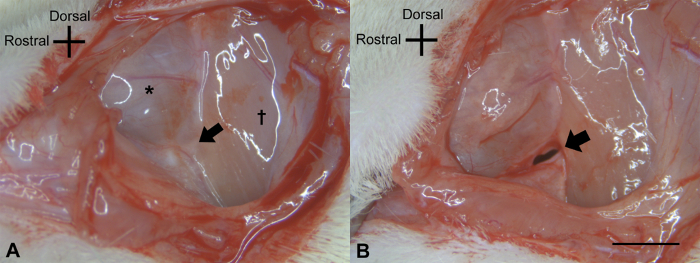
Using a scalpel blade #15, make a ~5.0 cm retroauricular incision. Separate the muscle and fascia to expose the external auditory canal with Iris scissors (Figure 1A). Slightly open the external auditory canal using the blade #15 or the Iris scissors (Figure 1B). Widely expose the tympanic membrane (Figure 2A).
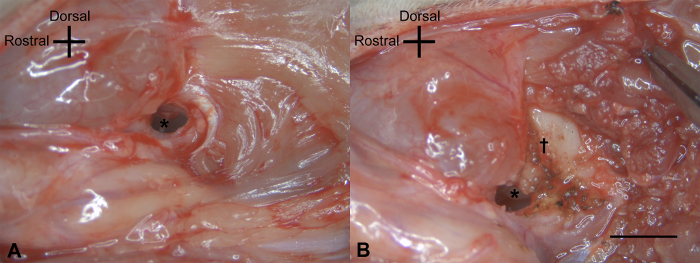
Under a microscope (7.5X or 12.5X), remove the tympanic membrane and the ossicles with the exception of the stapes, using the forceps. Detach the muscles on the lambdoidal ridge with the blade #15 or the Iris scissors (Figure 2B).
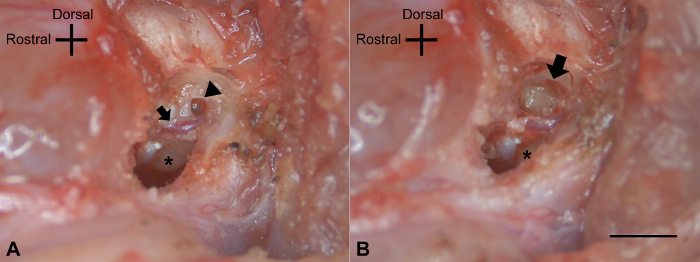
Using a drill at low speed (<3000 rpm) with a diamond bur (2.1 or 1.4 mm), drill the tympanic bulla bone around the point of exit of the facial nerve (Figure 3A). Identify the stapedial artery. Take care not to damage the stapedial artery during the drilling process.
Continue drilling the vestibule superior to the stapedial artery and expose the round window and the bony lateral semicircular canal. Open the lateral semicircular canal near its ampulla. Continue drilling in the plane of the lateral semicircular canal and drill the ampulla of the superior semicircular canal.
Aspirate the contents of the vestibule using a 18-G or 22-G needle. Inject and aspirate 1 cm3 of 100% ethanol 3x.
Close the muscles and the skin in two layers with simple interrupted sutures.
Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. Keep the rat warm until it wakens using a heating pad or a heat lamp. After awakening, move the rat to an individual cage in a room featuring the usual environmental conditions.
Administer antibiotics (e.g., Cefazoline 20 mg/kg, trimethoprim-sulfonamide 100 µL subcutaneously, or penicillin 300,000 IU) as needed on day 1; an analgesic (not an opioid; e.g., Metacam 2 mg/kg, Carprofen 5 mg/kg subcutaneously) can also be given.
2. Sham Surgery
For the sham surgery as a control, perform the same surgical and post-surgical procedures (as per section 1), including the exposure of the external auditory canal and the tympanic membrane (step 1.7), the removal of the tympanic membrane and the ossicles, and the detachment of the muscles on the lambdoidal ridge (step 1.8), but do not open the semicircular canal or inject ethanol.
3. Check the Loss of Vestibular Function
NOTE: The loss of vestibular function can be evaluated using either behavioral or vestibular function tests.13,17,18 Behavioral tests include the evaluation of postural asymmetry and nystagmus.
Identify postural asymmetry which includes spontaneous or evoked barrel-rolling, falling to the left side, or moving around while leaning toward the lesioned side18. See Video 1.
Identify nystagmus by a visual inspection of voluntary eye movement. When spontaneous nystagmus is absent in the resting state, puff air gently over the head of the animal18. See Video 2.
Representative Results
The success of the surgery was validated by behavioral tests. All animals exhibited the typical behavior of a unilateral loss of vestibular function. Spontaneous barrel-rolling was evident immediately after surgery, being evoked by an air puff over the head or a light touch to the body in the early recovery phase (Video 1). 3 d after surgery, the animals moved around leaning toward the lesioned side with occasional falls to the left side. Spontaneous nystagmus was observed within 2 d (Video 2). Evoked nystagmus was observed within 3 d.
The surgery usually required <30 min. Over the course of 50 surgeries, 2 rats died during the operation and 3 within 1 d of the surgery. Overall, the loss rate was 10% (5 deaths during/after 50 surgeries). All deaths were caused by an injury to the stapedial artery. We noted no delayed death and no infection within 1 week. For several days after the surgery, the rats can exhibit a poor oral intake and weight loss.
Figure 1: Post-auricular incision of the left ear. (A) A postauricular incision was made, and the facial nerve (↑) was identified between the temporalis muscle (*) and the sternomastoid muscle (†). (B) Then, the external auditory canal (↑) was opened slightly and the cartilage of the outer ear was separated from the pyramidal bone. The scale bar of 2 mm applies to all panels. Please click here to view a larger version of this figure.
Figure 2: Exposure of the bony wall of the vestibule. (A) After opening the external auditory canal wide, the malleus (*) was seen beneath the tympanic membrane. (B) After the removal of the tympanic membrane and the ossicles (with the exception of the stapes), the cochlear promontory (*) was identified and the muscles on the lambdoidal ridge elevated (†). The scale bar of 2 mm applies to all panels. Please click here to view a larger version of this figure.
Figure 3: Vestibular fenestration. (A) After the removal of the posterior lateral wall of the tympanic cavity, the bone around the point of exit of the facial nerve was drilled. The stapedial artery (↑) and the round window (Δ) above the cochlear promontory (*) were exposed. (B) After drilling out the lateral vestibule, the ampulla of the semicircular canals was apparent in the vestibular remnant (↑), as was the inferior location of the cochlear promontory (*). The scale bar of 2 mm applies to all panels. Please click here to view a larger version of this figure.
 Video 1. Spontaneous barrel rolling. The SD rat that underwent labyrinthectomy 2 d previously showed spontaneous barrel rolling. Please click here to view this video. (Right-click to download.)
Video 1. Spontaneous barrel rolling. The SD rat that underwent labyrinthectomy 2 d previously showed spontaneous barrel rolling. Please click here to view this video. (Right-click to download.)
 Video 2. Spontaneous nystagmus. The SD rat that underwent labyrinthectomy 2 d previously showed spontaneous nystagmus. Please click here to view this video. (Right-click to download.)
Video 2. Spontaneous nystagmus. The SD rat that underwent labyrinthectomy 2 d previously showed spontaneous nystagmus. Please click here to view this video. (Right-click to download.)
Discussion
This technique is a useful method for creating sudden, permanent, and complete vestibular function loss. This could be used to study vestibular pathologies, such as vestibular neuritis, an acoustic tumor, and Meniere's disease. Many studies have used this technique to study the neuronal plasticity of vestibular nuclei or the related central process5,17,18,19.
The most critical steps for successful surgery are 1) the preservation of the stapedial artery, 2) a careful drilling at the precise position, and 3) meticulous bleeding control. A large postauricular incision can cause a large external wound, which disturbs the leg movement on the treated side. However, a small incision limits the surgical field and causes the operation to take longer. A proper retraction is essential to maintain a sufficient surgical view13. After the postauricular incision, the facial nerve can be identified above the bulla and between the digastric muscle and the sternocleidomastoid muscle, running anterior-inferiorly to the face. The retroauricular artery and the greater auricular nerve run in the opposite direction. Any damage to the facial nerve causes facial palsy after the procedure. To achieve a wider surgical view and avoid a stapes artery injury, we prefer to remove the superior external auditory canal wall and then approach superior to the stapes artery17,19. This approach is associated with a greater risk of infection compared to the ventrolateral approach, which does not feature the opening of the external auditory canal wall or the removal of the tympanic membrane (which exposes the middle ear to the external environment). In our experience, the long-term sequelae (the general animal performance, its feeding and drinking habits, the resumption of weight gain, and the infection status) do not differ notably between animals subjected to the transtympanic approach described here and the ventrolateral approach17,20. Opening the bulla can damage the attached muscle and sometimes result in some bleeding. In the rat, the stapes artery crosses close to the site of vestibular fenestration. In humans, however, the stapes artery disappears during embryogenesis. When the surgical field is narrow, it is not easy to control the bleeding, and the field can rapidly fill with blood. To avoid this, some authors coagulate the stapes artery before making the hole. Although its role is not clear, some authors prefer not to coagulate the stapes artery14 because it may supply the inner ear, central nerve system (the geniculate ganglion, medial lemniscus, and trapezoid body), and facial nerve in the rat14,21. If bleeding occurs, we compress the artery with a small cotton swab and then use electrical coagulation with sufficient suction.
A small-sized burr is useful for making a hole in the vestibule. A small hook or needle could be used instead of a burr. A mechanical drilling system results in less fenestration trauma and helps to obtain consistent results. Without ethanol irrigation, we observed variable behavioral responses after the labyrinthectomy13,17. Ethanol irrigation and suction ensure that the labyrinth is terminally damaged.13 Blunt electrical coagulation of the vestibular nerve can be performed via this hole22. Proper magnification is essential, and a microscope is mandatory in most cases.
The middle and inner ear are also damaged during the labyrinthectomy, resulting in deafness. There is no way to preserve the inner ear safely. Chemical labyrinthectomy can preserve the animal's hearing but yields variable results8. Vestibular neurectomy can also cause damage to the cochlear nerve8. To perform the sham operation in the control group, we removed the tympanic membrane and ossicles without the stapes, which resulted in mild conductive hearing without any vestibular damage13.
Several behavioral and vestibular function tests can be used to confirm that the labyrinthectomy has been performed successfully13,14. Immediately after the procedure, a skew deviation of the eye can be observed. After the recovery from the anesthesia, spontaneous nystagmus, a rolling motion, and head tilting can be seen. Rotation by tail hanging is the most reliable test17,20. These deficits recover slowly within several days17,20. Spontaneous nystagmus with rapid movement to the contralateral side will disappear within 3 - 4 d after the procedure. The head tilting will remain for several months. Vestibular function tests, such as a rotation chair test or a rotarod test, can provide more objective and quantitative data23,24.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C2651).
References
- Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. Journal of Vestibular Research. 1995;5(2):67–107. [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Research. Brain Research Reviews. 1989;14(2):155–180. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Lacour M, Helmchen C, Vidal PP. Vestibular compensation: the neuro-otologist's best friend. Journal of Neurology. 2016;263(Suppl 1):S54–S64. doi: 10.1007/s00415-015-7903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington CL, Smith PF. Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Progress in Neurobiology. 2000;62(3):313–325. doi: 10.1016/s0301-0082(00)00002-2. [DOI] [PubMed] [Google Scholar]
- Shinder ME, Ramanathan M, Kaufman GD. Asymmetric gene expression in the brain during acute compensation to unilateral vestibular labyrinthectomy in the Mongolian gerbil. Journal of Vestibular Research. 2006;16(4-5):147–169. [PubMed] [Google Scholar]
- Dutheil S, Brezun JM, Leonard J, Lacour M, Tighilet B. Neurogenesis and astrogenesis contribution to recovery of vestibular functions in the adult cat following unilateral vestibular neurectomy: cellular and behavioral evidence. Neuroscience. 2009;164(4):1444–1456. doi: 10.1016/j.neuroscience.2009.09.048. [DOI] [PubMed] [Google Scholar]
- Gunther L, et al. N-acetyl-L-leucine accelerates vestibular compensation after unilateral labyrinthectomy by action in the cerebellum and thalamus. PLoS One. 2015;10(3):e0122015. doi: 10.1371/journal.pone.0120891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericat D, Farina A, Agavnian-Couquiaud E, Chabbert C, Tighilet B. Complete and irreversible unilateral vestibular loss: a novel rat model of vestibular pathology. Journal of Neuroscience Methods. 2017;283:83–91. doi: 10.1016/j.jneumeth.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Cass SP, Goshgarian HG. Vestibular compensation after labyrinthectomy and vestibular neurectomy in cats. Otolaryngology-Head and Neck Surgery. 1991;104(1):14–19. doi: 10.1177/019459989110400104. [DOI] [PubMed] [Google Scholar]
- Vignaux G, et al. Evaluation of the chemical model of vestibular lesions induced by arsanilate in rats. Toxicology and Applied Pharmacology. 2012;258(1):61–71. doi: 10.1016/j.taap.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Berryhill WE, Graham MD. Chemical and physical labyrinthectomy for Meniere's disease. Otolaryngologic Clinics of North America. 2002;35(3):675–682. doi: 10.1016/s0030-6665(02)00025-7. [DOI] [PubMed] [Google Scholar]
- Morgenstern C, Mori N, Arnold W. Experimental studies on the effect of labyrinth anesthesia. Archives of Oto-Rhino-Laryngology. 1983;237(3):255–261. doi: 10.1007/BF00453730. [DOI] [PubMed] [Google Scholar]
- Nadasy GL, Raffai G, Feher E, Schaming G, Monos E. A simple standard technique for labyrinthectomy in the rat: a methodical communication with a detailed description of the surgical process. Physiology International. 2016;103(3):354–360. doi: 10.1556/2060.103.2016.3.8. [DOI] [PubMed] [Google Scholar]
- Hitier M, Besnard S, Vignaux G, Denise P, Moreau S. The ventrolateral surgical approach to labyrinthectomy in rats: anatomical description and clinical consequences. Surgical and Radiologic Anatomy. 2010;32(9):835–842. doi: 10.1007/s00276-010-0690-9. [DOI] [PubMed] [Google Scholar]
- Flourens MJ. Recherches experimentales sur les propriétés et les fonctions du système nerveux dans les animaux vertébrés. Paris: Crevot; 1824. [Google Scholar]
- T'Ang Y, Wu CF. The effects of unilateral labyrinthectomy in the albino rat. Chinese Journal of Physiology. 1936;10:571–598. [Google Scholar]
- Chang MY, et al. MicroRNAs 218a-5p, 219a-5p, and 221-3p regulate vestibular compensation. Scientific Reports. 2017;7(1):8701. doi: 10.1038/s41598-017-09422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist F, Ludwig M, Dutia MB. Role of the commissural inhibitory system in vestibular compensation in the rat. The Journal of Physiology. 2008;586(18):4441–4452. doi: 10.1113/jphysiol.2008.155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron SA, Dutia MB. Cellular basis of vestibular compensation: changes in intrinsic excitability of MVN neurones. NeuroReport. 1997;8(11):2595–2599. doi: 10.1097/00001756-199707280-00035. [DOI] [PubMed] [Google Scholar]
- Park MK, Lee BD, Lee JD, Jung HH, Chae SW. Gene profiles during vestibular compensation in rats after unilateral labyrinthectomy. Annals of Otology, Rhinology & Laryngology. 2012;121(11):761–769. doi: 10.1177/000348941212101110. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Tominaga M, Sone M, Nakashima T. Contribution of stapedial artery to blood flow in the cochlea and its surrounding bone. Hearing Research. 2003;186(1-2):69–74. doi: 10.1016/s0378-5955(03)00310-1. [DOI] [PubMed] [Google Scholar]
- Potegal M, Abraham L, Gilman S, Copack P. Technique for vestibular neurotomy in the rat. Physiology & Behavior. 1975;14(2):217–221. doi: 10.1016/0031-9384(75)90169-9. [DOI] [PubMed] [Google Scholar]
- Tung VW, Burton TJ, Dababneh E, Quail SL, Camp AJ. Behavioral assessment of the aging mouse vestibular system. Journal of Visualized Experiments. 2014. p. e51605. [DOI] [PMC free article] [PubMed]
- de Jeu M, De Zeeuw CI. Video-oculography in mice. Journal of Visualized Experiments. 2012;65(65):e3971. doi: 10.3791/3971. [DOI] [PMC free article] [PubMed] [Google Scholar]


