Abstract
With an estimated 6000 species worldwide, hoverflies are ecologically important as alternative pollinators to domesticated honeybees. However, they are also a useful scientific model to study motion vision and flight dynamics in a controlled laboratory setting. As the larvae develop in organically polluted water, they are useful models for investigating investment in microbial immunity. While large scale commercial breeding for agriculture already occurs, there are no standardized protocols for maintaining captive populations for scientific studies. This is important as commercial captive breeding programs focusing on mass output during peak pollination periods may fail to provide a population that is consistent, stable and robust throughout the year, as is often needed for other research purposes. Therefore, a method to establish, maintain and refresh a captive research population is required. Here, we describe the utilization of an artificial hibernation cycle, in addition to the nutritional and housing requirements, for long term maintenance of Eristalis tenax. Using these methods, we have significantly increased the health and longevity of captive populations of E. tenax compared to previous reports. We furthermore discuss small scale rearing methods and options for optimizing yields and manipulating population demographics.
Keywords: Environmental Sciences, Issue 135, Eristalis tenax, Insect rearing, Insect maintenance, Hoverflies, Insect physiology, Pollinators, Entomology
Introduction
Hoverflies are emerging as useful models for investigating a range of scientific questions, including flight behavior1, neural mechanisms underlying motion vision2, pollination efficiency3,4,5,6 and microbial immunity7. However, as opposed to some other dipteran models, such as Drosophila8, there are no standardized protocols for lab rearing of hoverflies for use in scientific research. Indeed, even if the current literature describes methods for breeding the hoverfly Eristalis tenax, many of these are developed for the mass cultivation of hoverflies for crop pollination, bio-degradation of organic waste, or anatomical studies9,10,11. Thus, they do not address the need for an easy protocol that provides a consistent supply of healthy robust hoverflies, whilst maintaining the genetic fitness of the population.
Following bees and bumblebees, hoverflies are one of the most important wild, generalist pollinator groups12,13. There are about 6000 hoverfly species worldwide14,15, with more than 300 species in 75 genera in Sweden16 and more than 300 species in 69 genera in India17,18,19. For example, the agriculturally important marmalade hoverfly Episyrphus balteatus and the drone fly, Eristalis tenax, which we focus on here, are found across Europe, America and Asia6,16,17,18,19,20,21,22,23,24,25. Hoverflies are not equally active throughout the year, nor throughout the day. Indeed, not only the season, and the time of day, but also fluctuations in light intensity, temperature, humidity and wind velocity, affect the activity patterns of hoverflies26,27. In the field, Eristalis can be found at any time of the year in Mediterranean climates11, but the numbers of active hoverflies are much lower in winter. Conversely in cold temperate climates, Eristalis hibernate over winter and are not found actively behaving in the field from around October through to March28.
Freely flying hoverflies can be collected by netting in the field. Indeed, in temperate climates they are found in the greatest abundance in the mid to late morning, on calm sunny days, at the end of summer and throughout autumn26,27. Alternatively, mature E. tenax larvae, second or third instar, can be identified and harvested from decaying organic matter, such as manure heaps or organically polluted streams10,11. Indeed, published techniques for lab rearing of E. tenax are all based on raising larvae in organically polluted water, either via some form of vegetative or fecal matter9,10,29,30,31,32,33. However, larvae collection is limited by season, and is only a viable collection tool from late spring to the start of autumn11. Furthermore, the abundance of larvae is affected by local weather patterns, as changes in ambient temperature can affect both the occurrence of oviposition and larval development rates9,28.
Therefore, strategies to maintain healthy stocks of hoverflies by rearing larvae and eggs within the laboratory are needed to ensure that experiments can be conducted year-round, irrespective of the season or local weather events. Importantly, the technique described here breeds the hoverflies from only wild-mated females.This is important as a study by Francuski, et al.10 found that the genetic diversity of a laboratory bred population of hoverflies, originally established from 120 mature larvae, was rapidly lost. They therefore suggested that to maintain genetic diversity in colonies to be used for commercial crop pollination purposes, these need to be replenished, or even completely re-established, with field collected individuals each spring10.
When working on vision, or other senses used in courtship and mating, we thus recommend maintaining genetic diversity, either by re-establishing the colony or by replenishing the colony with field collected individuals, regularly. This is important as sexual selection affects the genetic drift of the population. Indeed, in the wild, male hoverflies need to identify and intercept suitable mates, as well as compete with other males for mating rights by defending their territories34. This process ensures that males with the best vision and spatial attention are likely to be the most successful in mating, and hence these traits are passed on to the next generation. The resultant effect of these ongoing processes is, in part, demonstrated by the presence of sexual dimorphisms in the visual pathway of hoverflies35,36. In captivity males do not have the same obstacles to successful mating as in the field: firstly, females are readily available, and secondly the small, confined enclosure negates the effect of territorial behaviors, which act to deter the mating access of other competitive males. The experimental removal of sexual selection in Drosophila melanogaster, has been shown to have a significant effect on captive populations with a decrease in overall body size, testes size and sperm production37, and reduced rates of male courtship behaviors38. Thus, a captive breeding program, without any consideration of sexual selection, may have a profound effect on both the visual and behavioral studies subsequently conducted.
We here describe a simple and cost-effective solution that provides a consistent supply of healthy hoverflies. The protocol is flexible and easy to re-start and/or upscale, depending on the research demands.
Protocol
1. Establish Captive E. tenax Colony
Establish colony via the collection of mature larvae (Step 1.2) or alternatively via the collection of free-flying hoverflies (Step 1.3).
- Collection of Mature Larvae
- Collect second and third instar larvae from manure pits at cattle farms. NOTE: Mature larvae are easiest to find during the beginning of their migratory phase, as they are actively seeking a dark dry environment to pupate. This tends to be near the borders of the manure pits where damp manure is close to drier areas containing large amounts of straw. We collected under permission from a cattle farm near Uppsala, Sweden.
- Rear mature larvae in cow manure as outlined in Step 3.2.
- Collection of Wild Hoverflies
- Collect wild hoverflies by netting in the field, usually from botanic gardens and parks where there is an abundance of flowering plants. NOTE: We collected under permission from several locations including any of the three Adelaide Botanic Gardens, a dairy farm in Myponga, South Australia, and at various botanic gardens and parks throughout Uppsala, Sweden.
- House field collected hoverflies as outlined in Step 2.
2. Housing and Long-Term Maintenance of Hoverflies
House hoverflies in 30 cm x 45 cm plastic bags for groups of 20 or less, or in an insect rearing cage (25 cm x 25 cm x 25 cm) for larger groups.
- Provide food and water ad libitum, in the form of 10-20 grains of bee pollen and 2-3 mL honey placed on top of several moist cotton balls. NOTE: For housing in plastic bags, it is important that the cotton balls are moist but not overly saturated, as any accumulation of water inside the bag can be detrimental to survival rates. Conversely, for housing in insect rearing cages, the mesh sides allow for significant evaporation to occur. Cotton balls should therefore be placed in a shallow container and be fully saturated.
- Leave hoverflies to feed for 6 hours at room temperature.
- Place hoverflies with food and water in their housing, in the fridge at 8-10 °C and keep in complete darkness. NOTE: Storing hoverflies at 8-10 °C in darkness causes the hoverflies to enter a state of hibernation, with a reduction in activity and metabolic rate.
- Every 3-4 days remove hoverflies from the fridge, thus breaking the artificial hibernation and allowing both feeding and grooming to occur.
- Transfer hoverflies to a new plastic bag or clean insect cage with fresh food and water. This transfer can either be done manually, for small numbers of flies, or by utilizing phototaxis, for larger numbers.
- To utilize phototaxis, join a clean insect cage to the old one, ensure there is an opening for the hoverflies to move freely between the two without escaping. Cover the old insect cage with an opaque fabric. The hoverflies will move towards the light and into the clean insect cage.
Allow hoverflies to feed and groom at room temperature for 6 h.
Return hoverflies, with the food and water in their housing, into the fridge at 8-10 °C in complete darkness. This recommences the artificial hibernation of the hoverflies.
Continue the cyclical breaking of hibernation, every 3-4 days, to ensure the health and longevity of hoverflies for the duration of their captivity.
3. Laboratory Rearing of E. tenax
Rear mature larvae collected from cattle farms (Step 3.2) or alternatively rear eggs laid by gravid wild caught females (Step 3.3).
- Laboratory Rearing of Mature Larvae from Cattle Farms
- Place larvae collected in cow manure from cattle farms in 30 L buckets.
- Position the bucket, containing mature larvae, within a larger box or bag (minimum volume of 50-60 L) and place 20-30 L wood shavings up to the height of the rim of the bucket. NOTE: This allows 3rd instar larvae to crawl into the wood shavings and pupate.
- Hang a double layered mosquito net from the ceiling allowing it to drape over the boxes and/or bags, thus ensuring that neither larvae nor any emerging hoverflies can escape. NOTE: As a precaution, double-sided sticky tape can be used to surround the setup, as any escaping larvae will get stuck and pupate on this tape. If they do, remove the pupae before eclosion.
- House both larvae and pupae at room temperature (21.5 ± 2.5 °C), and expose to either indirect sunlight as well as room lights during office hours or keep at a light:dark cycle of 12 h light:12 h dark. NOTE: Exposure to 24 h light can be detrimental to survival rates. For larvae collected from cattle farms pupation time will vary from 1- 20 days after collection depending on their maturity at the time of collection.
- Provide food and water within the hanging mosquito net enclosure (as prepared in Step 2.2) prior to the expected eclosion date and replace every 2-3 days. Eclosion will occur 7-10 days after pupation.
- Allow emerging hoverflies to feed for 6 hours at room temperature before placing them in housing as outlined in Step 2.
- Laboratory Rearing of Eggs laid by Gravid Wild Caught Females
- Check hoverfly housing for eggs, both before changing housing (Step 2), and prior to returning them to the fridge. Oviposition by wild-caught gravid females occurs at both 8-10 °C and at room temperature.
- Place eggs in a 100 mm x 20 mm Petri dish containing 70 mL of tap water and keep at room temperature until hatching occurs, usually 2-3 days after oviposition.
- Place hatched larvae in a 2.3 L bucket containing 1 L of fresh rabbit feces and 1 L of tap water.
- Check slurry every 2-3 days and add additional tap water as required, to ensure that the slurry does not dry out before the 3rd instar larvae emerge.
- Position the bucket, containing the larvae in the rabbit feces slurry, within a larger box (minimum volume 30 L) containing 20 L of wood shavings. Ensure the wood shavings are up to the height of the rim of the bucket. NOTE: This allows 3rd instar larvae to crawl into the wood shavings and pupate.
- Place a double layered mosquito net over the box to ensure that neither larvae nor any emerging hoverflies can escape.
- House both larvae and pupae at room temperature (21.5 ± 2.5 °C), and expose to either indirect sunlight as well as room lights during office hours or keep at a light:dark cycle of 12 h light:12 h dark. NOTE: Exposure to 24 h light can be detrimental to survival rates.
- Expect pupation to occur after 15-20 days. Collect pupae and place in an insect cage, allowing hoverflies to eclose there.
- Provide food and water (as prepared in Step 2.2) prior to the expected eclosion date - eclosion will occur 6-10 days after pupation - and replace every 2-3 days.
- Allow emerging hoverflies to feed for 6 hours at room temperature before placing them in housing, as outlined in Step 2. NOTE: Both the pupation of larvae and the eclosion of pupae can be delayed by storage in darkness at 8-10 °C. For this purpose, store larvae in the rabbit feces slurry and pupae in wood shavings.
Representative Results
We have developed a three-way strategy that maintains a healthy population for both visual and behavioral studies (summarized in Figure 1). Our method starts with collection of hoverflies from the wild (Step 1, Figure 1). In our lab, hoverflies are housed in insect cages or plastic bags, under an artificial hibernation cycle (Step 2, Figure 1), substantially prolonging their life span. For increased numbers, offspring can be reared from wild-mated females (Step 3, Figure 1).
We have found that catching vast numbers of wild hoverflies is a time intensive endeavor, even when environmental conditions are favorable. In contrast, the successful rearing of mature larvae harvested from the manure pits of cattle farms is a far more efficient way to source large numbers of wild hoverflies (Step 1, Figure 1), with us collecting up to 700 larvae in 0.03 m3 of manure. Additionally, our techniques to rear eggs laid by captured gravid females have proved to be successful (Step 3, Figure 1). Females captured in a Mediterranean climate (Adelaide) during the autumn and winter months laid several batches of eggs, with 24 clusters observed from 19 females in a period of 20 weeks. Of these egg batches, 10 were placed into water, all of which were fertile and resulted in the hatching of larvae. 3 groups of larvae were then taken beyond this point, and placed in the rabbit feces slurry, resulting in 163 ± 34 (mean ± SD, N=3) emerged hoverflies, with no observed gender bias (Figure 2).
The health of these lab reared hoverflies was determined by a comparison of the weight and locomotor activity of female hoverflies compared to field captured individuals. General locomotor activity was assessed using a Locomotor Activity Monitoring system (LAMS), as described previously39. No significant differences in weight (Figure 3A) or activity (Figure 3B) were observed between lab reared and wild caught hoverflies after 4 months in captivity under our artificial hibernation cycle. When E. tenax were maintained in the laboratory without the use of an artificial hibernation cycle we saw a significant decrease in longevity, with a lifespan of 2.5 - 3 months (73 ± 7 days for 5 females and 79 ± 4 days for 11 males). When the hoverflies were kept in artificial hibernation they could live in excess of 12 months.
Additionally, the effect of long term maintenance, using our described methods, was further assessed by a comparison of weights over time for both sexes of lab reared hoverflies. We observed a significant increase in weight over a period of 4 months for both sexes, with females consistently weighing more than their male counterparts (p < 0.0001, two-way ANOVA, N=12, Figure 4).
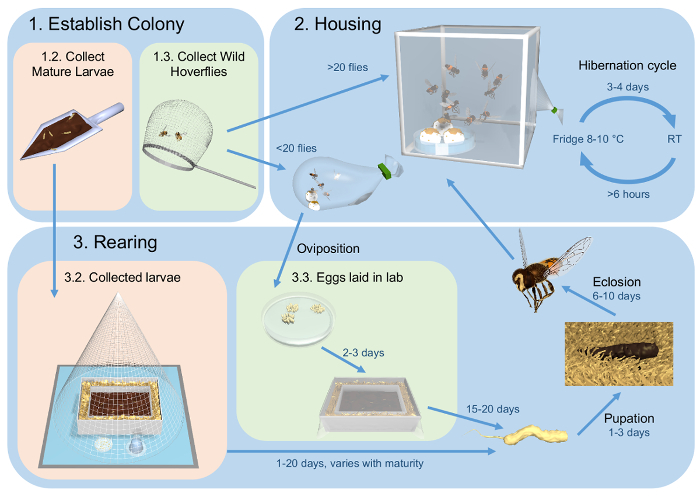
Figure 1: Flow diagram outlining the methods for maintenance of healthy captive population of E. tenax. (1) The procedure described here starts with collection of either mature larvae from cow dung (Step 1.2) or freely flying hoverflies (Step 1.3). (2) The hoverflies are housed in insect cages or plastic bags, dependent on numbers. They are kept in an artificial hibernation cycle at 8-10 °C, which is broken every 3-4 days. (3) Collected larvae are kept in their cow dung (Step 3.2). Eggs laid in the lab are placed in a rabbit feces slurry (Step 3.3). When reaching maturity, 3rd instar larvae crawl into the surrounding saw dust where they pupate. Eclosion occurs after 6-10 days, and the eclosed hoverflies are placed in the housing (Step 2). Please click here to download this file.
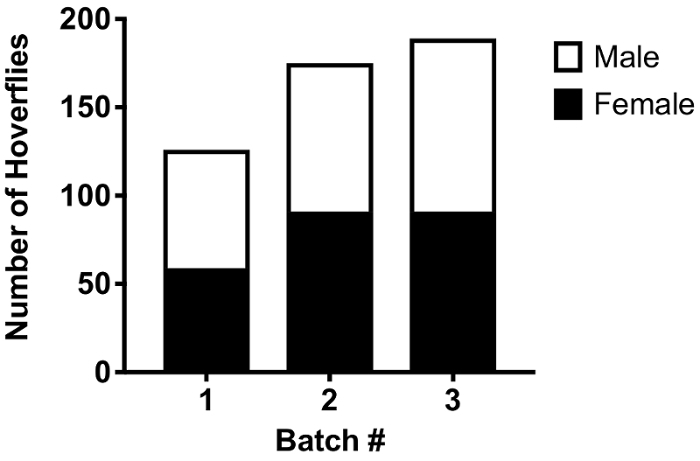
Figure 2: Number and sex ratio of E. tenax successfully reared from individual egg batches. The data show the numbers of E. tenax that eclosed from pupae developed from 3 batches of eggs laid in our lab. The eggs were laid by wild-caught females. The data are color coded for the sex of the flies. There is no significant difference in the ratio.
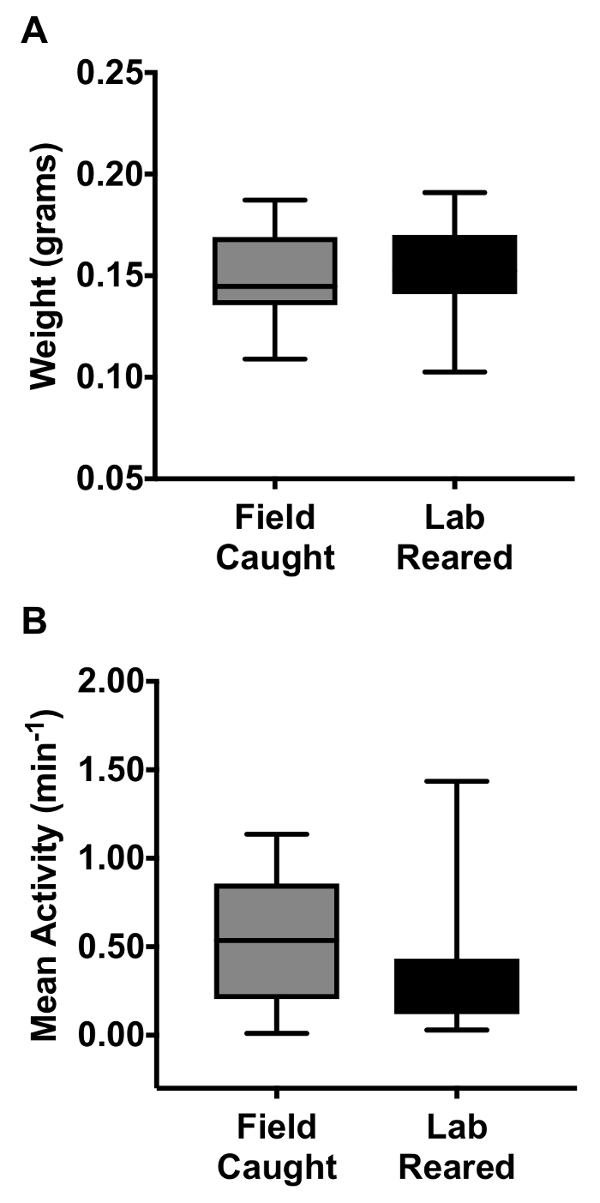
Figure 3: Assessment of population health in laboratory reared and field collected female hoverflies after long term captivity. (A) Weight comparison between lab reared and field captured female hoverflies after 4 months in captivity under artificial hibernation (N=12). (B) Activity levels of lab-reared and field captured female hoverflies after 4 months in captivity under artificial hibernation. The hoverflies' locomotor activity was measured in a Locomotor Activity System by them breaking an infrared beam during movement. As previously, we averaged the activity across 6.7 hours in the middle of the day, on the second full day in the Locomotor Activity Monitor System39 (Nfield caught=9, Nlab reared=12). The central mark of each boxplot shows the median, the edges of the box the 25th to 75th percentiles of the data, and the whiskers extend from the minimum to the maximum of the data.
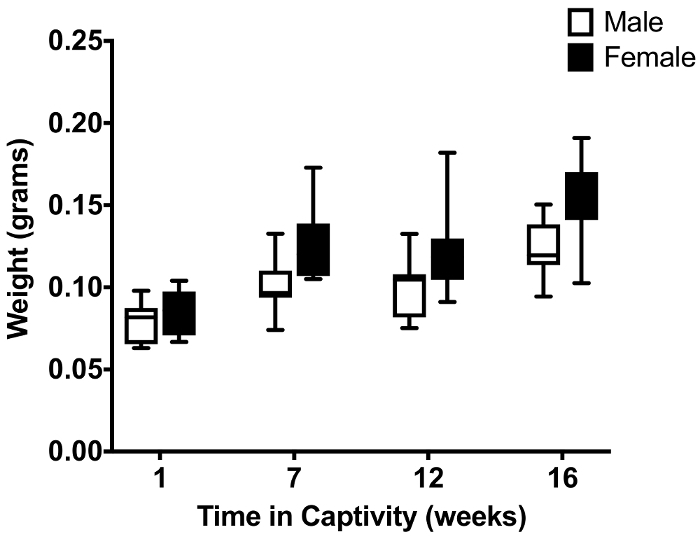
Figure 4: Comparison of the effect of long term maintenance on the weight of lab reared hoverflies of either sex. The data show the hoverfly weight as a function of time kept in captivity under artificial hibernation. As all hoverflies were reared from eggs laid in our lab (N=12 at each data point) and t=0 equals time of pupal hatching, the time in captivity is the same as the age of the animals. The central mark of each boxplot shows the median, the edges of the box the 25th to 75thpercentiles of the data, and the whiskers extend from the minimum to the maximum of the data.
Discussion
Using our techniques (Figure 1) hoverflies have been maintained in the laboratory for a period of over 1 year and successfully used in behavioral experiments after 7 months in captivity39. Indeed, even if it seems counterintuitive, keeping the hoverflies in a more natural environment, under 12 h light:12 h dark conditions, at room temperature, substantially decreases their life expectancy to 2-3 months. Maintaining E. tenax in our artificial hibernation cycle for over a year is significantly longer than previous attempts using different protocols (77 days33, 4 months9, 18 weeks30). The main factor influencing this increased longevity is likely the use of artificial hibernation at 8-10 °C. By cyclically breaking the hibernation, every 3-4 days (Step 2, Figure 1), we allow hoverflies to both feed and self-groom, thus maintaining the nutritional state and wellbeing of the hoverflies, as evidenced by an observed increase in weight (Figure 4) and no change in locomotor activity even after long periods in captivity (Figure 3, and see39). Indeed, reports in the literature of unsuccessful attempts at artificial hibernation do not break the hibernation cycle, thus leading to increased mortality and the presence of mold9.
Additionally, there is some controversy throughout the literature as to the provision of pollen as a food source. Several papers state that bee pollen is not sufficient, specifically for oviposition, and only the provision of dry or indeed fresh pollen is suitable9,29. Our findings indicate that by complementing bee pollen with honey and water, we see both longevity and oviposition, even after long periods of captivity, with an increase in weight seen in both sexes (Figure 4) and oviposition still occurring in females after more than 5 months in captivity39. This increased longevity allows us to study the behaviors of hoverflies at all life stages.
In the field, female hoverflies are fertilized prior to seasonal hibernation and remain in reproductive diapause, where sperm is stored and oocytes remain underdeveloped, until spring28. Given that a typical female is capable of laying 3000 eggs in 60 days29, rearing of these eggs is therefore a quick and efficient way to increase our captive population. However, our current understanding of the factors that lead to oocyte development after a period of hibernation are limited. Temperature, humidity, light intensity and nutritional state have all been suggested as playing a role in controlling reproductive diapause28,40. Experimental manipulation of such factors may lead to a greater governance of oviposition timing and rates.
Similarly, we have successfully delayed the development of larvae, as well as the eclosion of pupae, by storage in darkness at 8-10 °C for 2 weeks, although viability may be much longer. Indeed, Heal30 reported a pupal duration increase of up to 37 days when the pupal temperature was dropped from 25 °C to 10 °C. Employing these strategies and delaying the production of eggs and/or the development of pupae would allow for a greater manipulation of the demographics of the captive population.
While temporal consistency of supply is of much greater importance for our requirements than large yield, this may be more important for other uses, such as pollination in greenhouses. We found that when using our technique with rabbit feces, we got 163 ± 34 eclosed hoverflies from each clutch of eggs (N=3). Given that a typical female lays up to 200 eggs40, we might be able to increase this yield by either decreasing overcrowding and food competition, or by adjusting the temperature, as these have been implicated as significantly affecting larval growth9,31,40,41. However, there is no indication that the basis of the media greatly influences yield32. In addition, as opposed to feces from other vertebrates29,30,31,42, rabbit feces is relatively odor free, allowing the colony to be kept under normal laboratory conditions without the need for additional ventilation. Decreasing the density of larvae in the media, or adding nutritional supplements such as yeast, as well as keeping a constant temperature between 20-25 °C, is probably sufficient to fully optimise yield31,32,40.
The practicalities of collecting sufficient numbers of freely flying hoverflies, or sustaining a genetically diverse captive population, are both impractical and time restrictive for small scale research projects. Therefore, rearing the offspring of wild mated females, and supplementing supplies by harvesting mature larvae7, allow the most practical options for year-round use of E. tenax in a research setting. As these methods are limited by the seasons in which collection can occur, there is a need for both ensuring the longevity of adult hoverflies and rearing any eggs laid by captured gravid females.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The research in our lab is currently funded by the Australian Research Council (ARC, DP170100008 and DP180100144), the US Air Force Office of Scientific Research (AFOSR, FA9550-15-1-0188), and Stiftelsen Olle Engkvist Byggmästare (2016/348). We thank past members of the lab that have contributed to developing hoverfly stocks, Cederholms Lantbruk and C M & T L Green & Son for access to cow dung and hoverflies on their farms, and the Adelaide and Uppsala Botanic Gardens for collection permits and ongoing support.
References
- Goulard R, Julien-Laferriere A, Fleuriet J, Vercher JL, Viollet S. Behavioural evidence for a visual and proprioceptive control of head roll in hoverflies (Episyrphus balteatus) Journal of Experimental Biology. 2015;218(Pt 23):3777–3787. doi: 10.1242/jeb.127043. [DOI] [PubMed] [Google Scholar]
- Dyakova O, Lee YJ, Longden KD, Kiselev VG, Nordström K. A higher order visual neuron tuned to the spatial amplitude spectra of natural scenes. Nature Communications. 2015;6:8522. doi: 10.1038/ncomms9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauker F, Bondarenko B, Becker HC, Steffan-Dewenter I. Pollination efficiency of wild bees and hoverflies provided to oilseed rape. Agricultural and Forest Entomology. 2012;14(1):81–87. [Google Scholar]
- Gladis T. Bees versus flies? Rearing methods and effectiveness of pollinators in crop germplasm regeneration. ActaHortic. 1997. pp. 235–238.
- Ssymank A, Kearns CA, Pape T, Thompson FC. Pollinating flies (Diptera): A major contribution to plant diversity and agricultural production. Biodiversity (Ottawa) 2008;9(1-2):86–89. [Google Scholar]
- Nordström K, et al. In situ modeling of multimodal floral cues attracting wild pollinators across environments. Proceedings of the National Academy of Sciences U S A. 2017;114(50):13218–13223. doi: 10.1073/pnas.1714414114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altincicek B, Vilcinskas A. Analysis of the immune-inducible transcriptome from microbial stress resistant, rat-tailed maggots of the drone fly Eristalis tenax. BMC Genomics. 2007;8:326. doi: 10.1186/1471-2164-8-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker H, Gallant P. Getting started : an overview on raising and handling Drosophila. Methods in Molecular Biology. 2008;420:27–44. doi: 10.1007/978-1-59745-583-1_2. [DOI] [PubMed] [Google Scholar]
- Gladis T. Establishment and utilization of a mass rearing of Eristalis tenax (Diptera, Syrphidae) in the Gatersleben genebank. Insecta Berlin. 1994;1:287–294. [Google Scholar]
- Francuski L, et al. Shift in phenotypic variation coupled with rapid loss of genetic diversity in captive populations of Eristalis tenax (Diptera: Syrphidae): consequences for rearing and potential commercial use. Journal of Economic Entomology. 2014;107(2):821–832. doi: 10.1603/ec13243. [DOI] [PubMed] [Google Scholar]
- Pérez-Bañón C, Hurtado P, García-Gras E, Rojo S. SEM studies on immature stages of the drone flies (diptera, syrphidae): Eristalis similis (Fallen 1817) and Eristalis tenax (Linnaeus, 1758) Microscopy Research and Technique. 2013;76:853–861. doi: 10.1002/jemt.22239. [DOI] [PubMed] [Google Scholar]
- Fruend J, Linsenmair KE, Bluethgen N. Pollinator diversity and specialization in relation to flower diversity. Oikos. 2010;119(10):1581–1590. [Google Scholar]
- Biesmeijer JC, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313(5785):351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Pape T, Evenhuis NL. Systema Dipterorum, Version 1.5. 2013.
- Miranda GFG, et al. Key to the genera of Nearctic Syrphidae. Canadian Journal of Arthropod Identification. 2013;23:1–351. [Google Scholar]
- Nationalnyckeln. Nationalnyckeln till Sveriges flora och fauna. Tvåvingar: Blomflugor: Syprhidae: Syrphinae. Artdatabanken: SLU; 2009. [Google Scholar]
- Mitra B, Roy S, Imam I, Ghosh M. A review of the hover flies (Syrphidae: Diptera) from India. International Journal of Fauna and Biological Studies. 2015;2(3):61–73. [Google Scholar]
- Sengupta J, et al. An updated distributional account of indian hover flies (Insecta: Diptera: Syrphidae) Journal of Entomology and Zoology Studies. 2016;4(6):381–396. [Google Scholar]
- Shah GM, Jan U, Wachkoo AA. A checklist of hoverflies (Diptera: Syrphidae) in the western Himalaya, India. Acta Zool Acad Scient Hung. 2014;60(4):283–305. [Google Scholar]
- Francuski L, Djurakic M, LUDOŠKI J, MILANKOV V. Landscape genetics and spatial pattern of phenotypic variation of Eristalis tenax across Europe. Journal of Zoological Systematics and Evolutionary Research. 2013;51(3):227–238. [Google Scholar]
- Thomson FC. Revision of the Eristalis flower flies (Diptera: Syrphidae) of the Americas south of the United States. Proceedings of the Entomological Society of Washington. 1997;99(2):209–237. [Google Scholar]
- Hull FM. A Review of the Genus Eristalis Latreille in North America. Part II. Ohio Journal of Science. 1925;25(6):285–312. [Google Scholar]
- Osburn RC. Studies in Syrphidæ-IV. Species of Eristalis New to America, with Notes on Others. Journal of the New York Entomological Society. 1915;23(2):139–145. [Google Scholar]
- Bankowska R. Notes on syrphid flies (Diptera, Syrphidae) of Japan. Fragmenta Faunistica. 2000;43(16):203–207. [Google Scholar]
- Brower J, Brower L. Experimental studies of mimicry. 8. Further investigations of honeybees (Apis mellifera) and their dronefly mimics (Eristalis spp) The American Naturalist. 1965;99:173–187. [Google Scholar]
- Gilbert FS. Diurnal activity patterns in hoverfies (Diptera, Syphidae) Ecological Entomology. 1985;10:385–392. [Google Scholar]
- Ottenheim MM. Annual and diurnal rhythms of Eristalis species (Diptera: Syrphidae) Proceedings of the Section Experimental and Applied Entomology of the Netherlands Entomological Society (N.E.V.) 2000;11:169–174. [Google Scholar]
- Kendall DA, Stradling DJ. Some observations on over wintering of the drone fly Eristalis tenax Syrphidae. Entomologist. 1972;105(1311):229–230. [Google Scholar]
- Dolley JW, Hassett C, Bowen W, Phillies G. Needham JG, editor. Culture methods for invertebrate animals. 1937.
- Heal JR. Variation and seasonal changes in hoverfly species: interactions between temperature, age and genotype. Biological Journal of the Linnean Society. 1989;36:251–269. [Google Scholar]
- Ottenheim MM, Holloway GJ. The effect of diet and light and larval and pupal development of laboratory-reared Eristalis arbustorum (Diptera: Syprhidae) Netherlands Journal of Zoolog. 1995. pp. 305–314.
- Hurtado P. Estudio del ciclo de vida de sírfidos eristalinos (Diptera, Syrphidae) y bases para su cría artificial. Spain: Universidad de Alicante; 2013. [Google Scholar]
- Dolley WL, Jr, White JD. The effect of illuminance on the reversal temperature in the drone fly, Eristalis tenax. Biological Bulletin. 1951;100(2):84–89. doi: 10.2307/1538679. [DOI] [PubMed] [Google Scholar]
- Wellington W, Fitzpatrick S. Territoriality in the drone fly, Eristalis tenax (Diptera, Syrphidae) Canadian Entomologist. 1981;113(6):695–704. [Google Scholar]
- Nordström K, Barnett PD, Moyer de Miguel IM, Brinkworth RSA, O'Carroll DC. Sexual dimorphism in the hoverfly motion vision pathway. Current Biology. 2008;18(9):661–667. doi: 10.1016/j.cub.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Collett TS, Land MF. Visual control of flight behaviour in the hoverfly, Syritta pipiens L. Journal of Comparative Physiology A. 1975;99:1–66. [Google Scholar]
- Pitnick S, Miller G, Reagan J, Holland B. Males' evolutionary responses to experimental removal of sexual selection. Proceedings of the Royal Society of London. Series B, Biological Sciences. 2001;268:1071–1080. doi: 10.1098/rspb.2001.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B, Rice W. Experimental removal of sexual selection reverses intersexual antagonists coevolution and removes a reproductive load. Proceedings of the National Academy of Sciences USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyselius M, Nordström K. Hoverfly locomotor activity is resilient to external influence and intrinsic factors. Journal of Comparative Physiology A. 2016;202(1):45–54. doi: 10.1007/s00359-015-1051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal J. Colour patterns of Syrphidae: 1. Genetic Variation in the dronefly Eristalis tenax. Heredity. 1979;42(2):223–236. [Google Scholar]
- Ireland S, Turner B. The effects of larval crowding and food type on the size and development of the blowfly, Calliphora vomitoria. Forensic Science International. 2006;159:175–181. doi: 10.1016/j.forsciint.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Dolley WL, Golden LH., Jr The effect of sex and age on the temperature at which reversal in reaction to light in Eristalis tenax occurs. Biology Bulletin. 1947;92(3):178–186. [PubMed] [Google Scholar]


