Abstract
As the field of tissue engineering has continued to mature, there has been increased interest in a wide range of tissue parameters, including tissue shape. Manipulating tissue shape on the micrometer to centimeter scale can direct cell alignment, alter effective mechanical properties, and address limitations related to nutrient diffusion. In addition, the vessel in which a tissue is prepared can impart mechanical constraints on the tissue, resulting in stress fields that can further influence both the cell and matrix structure. Shaped tissues with highly reproducible dimensions also have utility for in vitro assays in which sample dimensions are critical, such as whole tissue mechanical analysis.
This manuscript describes an alternative fabrication method utilizing negative master molds prepared from laser etched acrylic: these molds perform well with polydimethylsiloxane (PDMS), permit designs with dimensions on the centimeter scale and feature sizes smaller than 25 µm, and can be rapidly designed and fabricated at a low cost and with minimal expertise. The minimal time and cost requirements allow for laser etched molds to be rapidly iterated upon until an optimal design is determined, and to be easily adapted to suit any assay of interest, including those beyond the field of tissue engineering.
Keywords: Bioengineering, Issue 135, Tissue engineering, laser etching, replica molding, contractile mechanics, cardiac regeneration, hydrogels, drug delivery, materials science.
Introduction
Over the past two decades, soft lithography has been used extensively as a fabrication technique to support scientific research, particularly in the fields of microfluidics, materials research, and tissue engineering1,2,3. Replica molding, in which an object with a desired shape is created from a negative master mold, offers a convenient and low-cost method of producing positive PDMS replicates that can be used for casting shaped hydrogels. However, the required negative master molds are typically produced using microfabrication techniques that are expensive, time-consuming, limited in size, and require clean room space and sophisticated equipment. While 3D printing offers a potential alternative, its utility is somewhat limited due to the resolution limits of lower-cost printers and the chemical interactions between common 3D printer polymers and PDMS that can inhibit curing.
Laser cutter systems capable of both cutting and etching materials such as plastic, wood, glass, and metal have recently become drastically less expensive and therefore more accessible for fabricating research tools. Commercial grade laser cutters are capable of fabricating objects on the centimeter scale with minimum features smaller than 25 µm, and further require minimal training, expertise, and time to use. While laser ablation of PDMS has been previously used in the fabrication of microfluidics devices, to our knowledge no manuscript has described a process by which millimeter and centimeter scale molds can be fabricated from laser cut negative master molds4.
We have used this technique primarily to manipulate the shape of engineered tissues in order to improve nutrient diffusion, cellular alignment, and mechanical properties5,6,7. However, versatility of this technique allows for the utilization in any field where molded hydrogels are of interest, such as drug delivery and material science research8. With access to a laser cutter, PDMS mold replicates can be made for nearly any geometry without overhangs (that would inhibit removal without a multi-part mold, which is beyond the scope of this manuscript) and that fits within the dimensions of the laser bed.
Protocol
1. Create the Vector Format Master Mold Designs
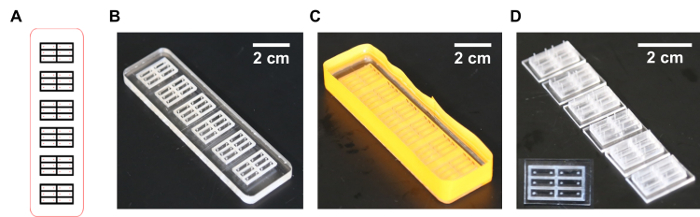
Assemble the desired mold geometry in vector format using a vector graphics program (see Materials, Equipment, and Software Table). Select File| New and create a canvas of appropriate dimensions with RGB color format. Create the desired geometry using the shape tools in the left-hand panel: enter the desired dimensions at the top of the window (click the transform button at the top if not initially visible) to precisely define shape sizes. NOTE: Mold geometries should allow for at least a 6-mm border between the edge of the outermost features and the cutting line to permit trimming of the PDMS meniscus following mold casting. This will allow the finished mold to lay flush with the bottom of a culture dish. Further, mold geometries should take into consideration limitations associated with the laser cutter model that will be used, including kerf width and minimum feature size. The laser used for the work described herein (see Materials, Equipment, and Software Table) featured a 0.2 mm kerf width and a minimum etched feature size of 25 µm (maximum DPI of 1,000). Mold dimensions can be chosen to accommodate a desired culture vessel, such as a 10-cm dish, or 6- or 24-well plates.
- Open the color picker in the top left-hand corner of the window and define new color swatches compatible with the laser cutter software. NOTE: We use red (RGB 255, 0, 0) for cutting and blue (RGB 0, 0, 255) for etching. Additional swatches can be defined depending on requirements for the design (for example, if more than one etching depth is desired).
- Assign these swatch colors to cutting paths by selecting the path and then selecting the cut swatch for the path color and [None] for the fill color from the color picker.
Similarly, assign swatch colors to etching paths by selecting the object and then selecting the etching path for the fill color and [None] for the path color from the color picker.
Save designs as either.ai or.pdf file formats, depending on the vector graphics program and laser cutter compatibility (Figure 1A and Supplementary File).
2. Laser Cut the Acrylic Master Molds
Select a negative master mold material. NOTE: Quarter-inch thick acrylic is well suited to this application due to its compatibility with laser cutting, relatively low cost, and thickness which allows for features up to ~ 5.5 mm in height. However, other materials that satisfy the following requirements may be used as well: (i) Non-reactive with PDMS curing; (ii) Non-porous/strongly adhesive to cured PDMS; (iii) Cuts and etches cleanly with a laser cutter; (iv) Maintains a glassy state up to 60 °C; (v) Of a thickness compatible with the desired maximum feature height.
Prepare the laser cutter for cutting according to the manufacturer's specifications. Ensure that sufficient ventilation is used and that the bed height is properly calibrated to the chosen negative master mold material's thickness.
Open the design file in a vectors graphics program on the computer connected to the laser cutter and click File| Print. Ensure that the laser cutter is set as the printer and that "Do Not Scale" is selected in the Scaling drop down menu to prevent distortion of the design before clicking the Print button at the bottom of the printing dialog.
In the laser cutter printing utility, click the Settings button in the bottom right-hand corner. Assign power, speed, and pulses per inch (PPI) settings to each of the previously chosen colors to set cut/etch parameters for all design features. NOTE: Here, the laser cutter equipped with a 75 W laser (see Materials, Equipment, and Software Table), used settings of the following: 100% power, 1% speed, and 1,000 PPI cuts cleanly through ¼" acrylic; 100% power, 8% speed, and 500 PPI etches to a depth of 2.75 mm in acrylic; and 100% power, 5% speed, 500 PPI etches to a depth of 3.50 mm in acrylic. If unknown for a laser cutter configuration or material, these parameters can be determined empirically through trial and error with a test piece.
Click the large green button in the laser cutter software to laser etch and cut the negative master molds.
Prepare the negative master molds for PDMS casting by removing any residual cutting debris with small brushes and/or compressed air (Figure 1B).
3. Prepare the PDMS Molds for Cell or Tissue Culture
Prepare a PDMS casting mix according to its manufacturer's specifications. Prepare 0.35 mL/cm2 of master mold; this will vary based on the mold features and height.
Degas the prepared PDMS in a vacuum chamber connected to a standard lab vacuum line (<500 mmHg) for 1 h, or until all bubbles have been eliminated.
Tape the outer edge of the mold with vinyl or masking tape (colored label tape works well) such that the tape extends >3 mm above the etched face of the negative master mold. Press the tape firmly against the side of the mold to prevent leaks later on. NOTE: If the acrylic master mold features through-holes, it may be necessary to apply tape to the bottom of the mold as well (Figure 1C).
Pour the degassed PDMS over the etched face of the negative master mold. NOTE: The target PDMS thickness will depend on the application, though thick PDMS mold bottoms can make imaging challenging. A minimum thickness of ~ 1.5 mm strikes a good balance between imaging considerations and ease of mold removal.
Place the PDMS-coated negative master mold into a vacuum chamber and degas again for 1 h, or until all bubbles have been eliminated.
Place the degassed PDMS-coated negative master mold into a 60 °C oven to cure for at least 6 h. Ensure that the oven shelf is level. Alternatively, allow the PDMS to cure at 37 °C overnight, or at ambient temperature for ~ 72 h.
If multiple molds were cast on the same negative master mold, use a razorblade to cut these molds apart while they are still on the negative master mold. Then, peel each PDMS mold off of the negative master mold. Ensure that PDMS molds are collected slowly and carefully to prevent mold feature tearing during collection.
Using a razor blade, trim off regions with the meniscus from casting, which would prevent the mold from lying flat, as well as any excess material or debris (Figure 1D).
If necessary, prepare molds for cell/tissue casting by autoclaving.
4. Cast the Collagen and Fibrin Hydrogel Tissues
NOTE: Use a proper aseptic procedure to maintain sterility.
Adhere the molds to the bottom of the desired vessel. Adhere new molds to the bottom of a non-tissue culture treated plate by firmly pressing the mold against the untreated plastic (due to the hydrophobicity of PDMS). NOTE: However, if tissue-culture treated polycarbonate is to be used, or in the case of re-used molds, which tend to adhere less firmly, a natural or synthetic glue (such as fibrin or silicone sealant cured overnight) can be used to ensure attachment.
Prepare fibrinogen, thrombin, and neutralized collagen casting solutions such that the desired concentration of each will be achieved in the cast tissue. Keep collagen on ice until casting. NOTE: Fibrinogen and thrombin should not be allowed to mix until immediately prior to casting. If necessary, the fibrinogen and thrombin solutions can also be chilled to increase the polymerization time. We have used a final fibrinogen concentration between 0-8 mg/mL, a final thrombin concentration between 10 - 100 U/mL, and a final collagen concentration between 0.8 - 2.0 mg/mL (Figure 2). For engineered cardiac tissues, we often use cardiomyocytes at 12 x 106 cells/mL with 1.6 mg/mL, 4 mg/mL fibrinogen, and 20 U/mL thrombin (thrombin is added immediately prior to casting). Optimal concentrations (even outside of these suggested ranges) should be determined empirically.
Harvest cells following standard protocols and resuspend at a concentration appropriate to achieve the desired initial cell density in the cast tissue. NOTE: Human induced pluripotent stem cell-derived cardiomyocytes were harvested as described in Lian et al.9 Keep the harvested cells on ice. Cell volume should be accounted for when preparing the cell suspension. We have used cell concentrations ranging from 9 - 18 x 106 cells/mL, though optimal ranges are expected to vary with cell population (Figure 2).
Combine the fibrinogen, neutralized collagen, and cell suspension (see step 4.2 for an example) to create a casting mix. Keep the casting mix on ice. NOTE: Fibrinogen and thrombin temperatures and concentrations will determine polymerization kinetics; in order to prevent polymerization during casting, it may be necessary to prepare separate batches of the casting mix depending on the number and volume of the constructs that will be cast.
- Treat the surfaces of the mold that will be exposed to the casting mix to mitigate PDMS hydrophobicity (PDMS hydrophobicity will increase protein adsorption and make casting difficult).
- Achieve hydrophilic surface modification by treating with oxygen plasma, such as with a handheld high frequency generator (e.g., BD-20A from Litton Engineering). Expose all surfaces of the mold that contact the cell/gel mixture to the plasma treatment for 3 - 5 s, about 5 min before casting.
- Alternatively treat with a surfactant, like Pluronic F-127 (1% w/v)10. Perform the surface treatment as close to the casting time as possible, as the change in polarity will deteriorate over time.
Immediately prior to casting, add the thrombin to the casting mix and mix thoroughly by pipetting up and down without introducing bubbles.
Working quickly, pipette the casting mix into the molds, using care to deposit the mix into all corners and crevices of the mold. Avoid pipette ejection beyond the first stop to prevent bubble formation inside the constructs.
Repeat steps 4.6 and 4.7 as necessary for any additional batches of casting mix.
Place the tissue culture vessel in a 37 °C incubator for 45 min. If the incubator is not well humidified, deposit cell media around the molds prior to incubation to prevent construct dehydration. NOTE: The type of cell media will depend on cell type, but for engineered cardiac tissue constructs we use RPMI 1640 + B27 supplement.
After 45 min, return the constructs to the hood and cover with cell media before returning to the incubator.
Change the cell media every 48 - 72 h as needed for the cell and construct type. NOTE: Media change should be planned according to the recommended instructions provided by the supplier to ensure maximum cell health. Use care when changing the media to avoid disturbing the constructs. We have not experienced problems with constructs floating, but if this occurs, it may be prevented by adding tall posts to the mold design that extend beyond the media level.
After use, wash the molds sequentially with 10% bleach, 70% ethanol, and distilled water. Then air dry, and autoclave for re-use up to ~ 10 times.

5. Analysis Techniques: Tissue Compaction
NOTE: Compaction resulting from matrix remodeling is an indicator of tissue viability and development that can be easily measured through optical microscopy and image analysis.
Collect optical microscopy images of the constructs in the molds at time increments ranging from 2 h to 96 h after casting. NOTE: Due to the low degree of magnification required, a dissection microscope with a camera mount is well suited to this application.
Trace the visible construct area in an image analysis suite such as ImageJ (open source, imagej.nih.gov/ij/).
Analyze the rate and final degree of compaction (Figure 2).
6. Analysis Techniques: Tensile Testing
Note: Both active mechanics (forces or strains generated by an engineered tissue because of cell activity) and passive mechanics (forces or strains generated in response to applied strains or forces) are critical functional characteristics of many engineered tissues, and this is particularly true for engineered cardiac tissues. The micromechanical analyzer used for the analyses is described in the Table of Materials. Other mechanical testing apparatuses could be similarly applied assuming they allow for hydrated testing and are capable of length control and force measurements over ranges and resolutions relevant for the tissue. For tissues with cross-sectional areas on the order of single square millimeters and stiffnesses on the order of tens of kPa, a 5 mN load cell is a good fit. Larger and stiffer materials would require a larger load cell. Prior to testing, ensure that both the force transducer and length controller are properly calibrated.
Turn on the length controller, force transducer, pulse generator, and temperature controller.
Fill the mechanical testing trough with Tyrode's solution (1.8 mM calcium chloride, 1.0 mM magnesium chloride, 5.4 mM potassium chloride, 140 mM sodium chloride, 0.33 mM sodium phosphate, 10 mM HEPES, and 5 mM glucose in ddH2O, pH adjusted to 7.4) for engineered cardiac tissues. Adjust the thermocouple such that a bath temperature of 37 °C is achieved.
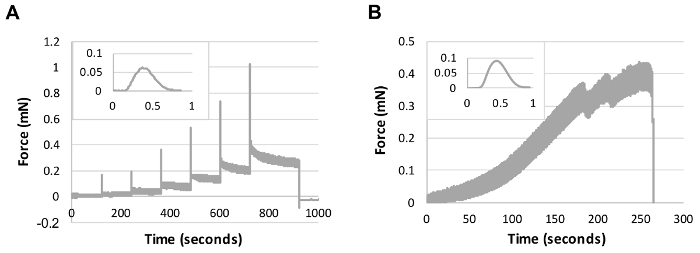
Load a mechanical testing protocol for collecting active contraction data. NOTE: We have evaluated active contractile mechanics using a length step protocol in which length steps in 5% strain increments up to 30% are held for 120 s to evaluate the impact of strain on contraction force (Figure 3A). Additionally, we have evaluated passive mechanical properties through a pull-to-break protocol at a constant strain rate of 10% strain/min (Figure 3B). Both of these protocols may serve as good starting points for active and passive mechanical analysis.
- Under a dissection scope, carefully detach the engineered tissue construct from the PDMS mold, such that it is floating freely. NOTE: Cellularized constructs that have undergone tissue compaction will likely already be detached from the interior mold surfaces, and in those cases minimal manipulation is required.
- If compaction has not caused the construct to release, use forceps to gently separate the construct from the sides and bottom of the mold to avoid damaging the tissue.
Using forceps, gently pick up the construct and transfer it to the mechanical testing bath.
Viewing the construct through a dissection microscope, mount either end of the construct to the hooks attached to the force transducer and lever arm.
Adjust the lever arm position using the micromanipulator until the construct is positioned without applied strain. Zero the length controller and force controller.
Image the construct through the dissection scope so that the dimensions of the construct can be measured via image analysis.
Initiate the active force protocol and save the collected data once the protocol has been completed (Figure 3).
If passive mechanical data are required as well, adjust the lever arm again until there is zero applied strain. Zero the length and force controllers and image the construct a second time before loading and initiating the passive mechanics protocol (Figure 3). Save the collected data.
7. Analysis Technique: Paraffin Histology and Immunohistochemistry
Note: We have had the greatest success in imaging engineered tissue sections using paraffin blocks so that tissue morphology is best preserved. All steps of the process must be carefully considered and tailored to the engineered tissue, including processing samples without vacuum or pressure, empirically determining the appropriate antigen retrieval methods, and titrating the primary antibody concentration. Other techniques, such as using frozen blocks for preparing slides, may require less time and expense while yielding sufficient results depending on the intended application.
Under a dissection scope, carefully detach the engineered tissue construct from the PDMS mold using forceps slid between the construct and PDMS to gently separate it from the PDMS, such that it is floating freely. Transfer these constructs to a microcentrifuge tube for fixation. NOTE: Fragile constructs or those to be fixed under the static stress condition provided by the mold itself may be fixed in the mold by changing solutions in the culture well and removing the construct from the mold after fixation.
Immediately fix the engineered tissues by submerging in 4% paraformaldehyde (PF) for 10 min at room temperature. Estimate no more than 1 h per 1 mm of tissue thickness; do not over-fix.
Rinse the tissues with phosphate buffered saline (PBS).
Keeping the tissue wet, place a drop of eosin on it to stain it pink for 10 - 30 s, and then rinse with 70% ethanol. NOTE: The pink color helps to identify it in the paraffin block for sectioning later and will be washed away during deparaffinization.
Wrap the tissue in lens paper and place in a histology cassette. Use foam pads in the cassette as needed to keep the tissue flat.
Submerge the cassette in 70% EtOH and store at 4 °C until ready for paraffin processing.
Process the tissue by dehydrating it in increasing concentrations of ethanol (2 x 30 min each of 70, 95, and 100%). Then bathe samples in three sequential xylene baths for 30 min each.
Submerge the samples in three sequential paraffin baths for 30 min each.
Warm up the cassettes, carefully unfold and remove the eosin-stained tissue from the lens paper, and embed the paraffin-infiltrated tissue in a paraffin block. Be careful to lay the sample flat on the bottom of the mold to enable easier sectioning.
Section the tissues using a microtome as desired (5 - 8 µm thick) and stain using standard procedures optimized for the engineered tissue (Figure 4). NOTE: An alternative to paraffin sections is to use frozen blocks, although morphology of the sample may be compromised. Place the fixed constructs in 30% sucrose in PBS for 3 h or until the specimen is equilibrated (e.g., overnight). Exchange the solution with 50/50 v/v 30% sucrose and optimal cutting temperature (OCT) freezing medium for 1 h. Place the constructs into frozen blocks with OCT freezing medium using a 70% EtOH with dry ice slurry for rapid block freezing in plastic trays. Section the blocks with a cryostat to 10 - 50 µm thick sections.
8. Analysis Technique: Cell Alignment
Note: Manipulating the tissue shape and internal stress fields can modulate cell alignment, a defining feature of many native tissues.
Prepare the engineered tissue constructs in PDMS molds with geometries of interest.
At the end of the culture period, wash the constructs in PBS, fix in 4% PF (see step 7.2) and harvest constructs to prepare for imaging by sectioning and histology (see Section 7) or whole mount staining. NOTE: Whole mount staining follows similar steps to histology/immunohistochemistry but requires longer incubation periods, and the penetration depth of dyes, the antibodies, and any imaging techniques (such as light penetration depth into the constructs) will need to be empirically determined for the construct composition.
Orient the tissue sections in the horizontal plane (parallel to the plane of the mold bottom) and stain for a marker for the cell of interest. Use an f-actin label, such as phalloidin, to mark the actin stress fibers of most cell types. Alternatively, use a cell specific marker. NOTE: In the case of engineered cardiac tissues, orientation was determined by sarcomeres stained with α-actinin.
Assess the major axis of all cells or nuclei in the region of interest manually or using an analysis tool (e.g., ImageJ, MATLAB). NOTE: It may be useful to categorize different regions of the same construct, depending on the geometry (such as "node" and "bundle" regions in a mesh-like geometry, or "core" and "edge" in a circular geometry).
Representative Results
The optics of the laser cutter will cause etched areas to have very slightly decreased dimensions as etching depth increases, and results in mold walls with a very subtle bevel, due to tapering of the laser beam. This will help facilitate the removal of the cast PDMS molds, but should be carefully considered if very deeply etched negative master molds (>6 mm) are required (Figure 1).
Over time in culture, cellularized constructs will compact due to matrix remodeling, though the rate and extent to which this occurs will depend on the scaffold composition, cell load, and culturing conditions.
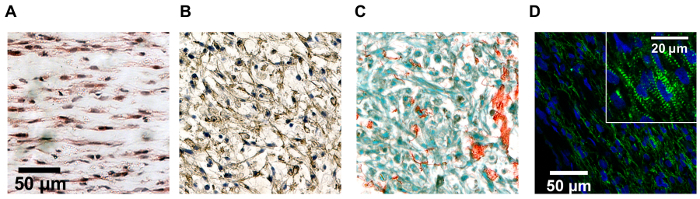
Matrix remodeling can occur through both reorganization and degradation of the surrounding matrix (as well as deposition of new matrix), but is typically associated with an increase in mechanical stiffness due to the decrease in cross-sectional area. With collagen-only constructs composed of 1.6 mg/mL collagen and 12 x 106 cardiomyocytes/mL, we see constructs compact to 19.7 ± 2.8% of their initial width over the four days following casting (Figure 2) via the compaction assay. While this assay yields a representative 2D approximation of a 3D process, the ease of data collection and non-destructive nature make it a powerful tool for studying the construct development process. Note that under cell culture conditions, even in the absence of cells, collagen mechanics can change over time due to both self-assembly and cross-linking11. Fibrin can be rapidly degraded by fibrinolysis both in vivo and in vitro if not in the presence of an antifibrinolytic, such as aprotinin or aminocaproic acid12. Therefore, the impact of scaffold components on long term tissue development, and not just tissue formation, should be considered when selecting a construct formulation. If final tissue size is important for a specific application, compaction must also be considered in mold design and empirically determined based on the cell type(s) and matrix composition. Note that tissue compaction can also induce stress fields within the tissue, which can be manipulated in cellularized constructs to encourage cell alignment (Figure 4).
A wide range of scaffold polymer concentrations and initial cell seeding densities have been used to create engineered tissues in the literature, and this can be attributed primarily to differing requirements for various cell types, cell lines, and applications. Based on our own work with hiPSC-derived cardiomyocytes, we believe that a collagen concentration of 1.25 mg/mL and a seeding density of ~ 15 million cells/mL is a good starting point13. Alternatively, fibrin is widely used as a cardiac tissue scaffold material as well, typically in the range of 3 - 4 mg/mL14. Cell seeding density may be selected based on a number of factors depending on the application, but the cell densities of native tissues provide a good reference point. Also consider that highly concentrated cell solutions can become challenging to work with, especially for small volumes. For a given cell population, the scaffold formulation can be tuned; generally by increasing the polymer concentration when tissues are too fragile or break upon compaction, and increasing the polymer concentration when tissues are too stiff or fail to compact15.
Prior to performing passive mechanical analysis at any time point during culture, it may be appropriate to mechanically precondition the construct sample. Preconditioning of natural polymer hydrogels and engineered tissues will increase the reproducibility of the testing result due to material viscoelasticity and provide a better indication of the properties that the construct will exhibit in a clinical application. We use 8 cycles of 10% strain in a triangular waveform at a rate of 10% strain/min prior to starting mechanical assessment (Figure 3).
Tissue and cell-type specific morphology can be assessed through histology and immunohistochemistry with traditional methods. However, we have found that optimization of nearly all steps of the paraffin processing, embedding, sectioning, antigen retrieval, and staining have been necessary for the engineered cardiac tissue compared to plated cells or sectioned native tissue (Figure 4).
Figure 1: Outline of the process for designing and preparing PDMS molds from laser cut acrylic masters. (A) Mold design prepared in vector graphics format. (B) Cleaned laser etched acrylic negative master. (C) PDMS cast on the surface of the taped acrylic master mold. (D) Resulting PDMS mold ready for sterilization prior to tissue culture. Inset: top view, same scale. Please click here to view a larger version of this figure.
Figure 2: Construct compaction over time in culture. (A) Images of rectangular constructs prepared in triplicate compacting over time in culture. Green overlays represent masks used to calculate visible construct area for image analysis. (B) Plot of construct area (a two-dimensional metric of construct compaction) over time. Horizontal lines represent the mean values and error bars indicate the standard deviation. For all groups, n = 3 and * indicates p <0.05 as evaluated by ANOVA. Please click here to view a larger version of this figure.
Figure 3: Raw traces for mechanical characterization of engineered cardiac tissues. Insets display a single representative twitch contraction trace (same axes as main plot). (A) Active mechanical response resulting from rapid steps followed by holds at 5% strain increments. (B) Passive mechanical response resulting from a pull-to-break test at a rate of 10% strain/min. All samples were analyzed in a 37 °C bath of Tyrode's solution. Please click here to view a larger version of this figure.
Figure 4:Paraffin block histology images for engineered cardiac tissue constructs of various designs. Paraffin block histology images for engineered cardiac tissue constructs of various designs stained with (A) hematoxylin and eosin, (B) diaminobenzidine (anti-cardiac troponin T, brown), and hematoxylin nuclear counterstain, (C) picrosirius red stain for collagen with fast green cytoplasmic counterstain, and (D) mouse anti-α-actinin antibody (green) and 4',6-diamidino-2-phenylindole (DAPI) nuclear counterstain (blue). Cell alignment differs as a result of the construct design and tissue compaction. Scale bar in A applies to B and C as well. Inset in D shows striated cardiomyocytes. Please click here to view a larger version of this figure.
Discussion
Customized PDMS mold geometries that are compatible with tissue culture have great utility in tuning important engineered tissue properties, such as cell alignment, diffusion rate, and effective stiffness. Additionally, these molds are very useful for preparing tissues for analysis applications in which geometry is important, such as mechanical testing16,17. Preparing these devices from laser cut negative master molds offers a rapid, facile, and low-cost method of utilizing these tools, especially when compared to the time and cost associated with traditional microfabrication. Laser cutting also permits a larger maximum mold size, limited only by the bed size of the cutter. We have successfully used these versatile molds to execute a wide variety of studies with engineered cardiac tissues, including optical mapping of action potential propagation, assessment of force production and passive mechanical properties, and implantation in a rat model of myocardial infarction13,18. We recognize that beyond the research niche of cardiovascular regenerative engineering, the applications for the fields tissue engineering, drug delivery, and materials research are vast.
While there are few technically challenging steps in the fabrication of the molds themselves, there are a number of critical steps involved in creating functional tissues. If constructs fail to compact the surrounding matrix after 24 h, first confirm the cell viability through viability staining of the engineered tissues. If cell viability is high, consider altering the construct composition for the next set of tissues. While outcomes will vary greatly depending on the cell population, we have observed increased compaction associated with higher seeding densities and lower collagen concentrations. Finally, it may also be useful to supplement the seeded cell population with a cell type well suited for matrix remodeling, such as fibroblasts, to encourage compaction.
One limitation of these molds is the potential for PDMS to adsorb small hydrophobic molecules. While for our applications this has not been problematic, it may be of concern in assays very sensitive to the loss of these molecules. In these cases, PDMS protein adsorption can be mitigated through treatment with an antifouling agent such as polyhydrophilic or polyzwitterionic materials19. Alternatively, a sterilized PDMS mold could be prepared as a negative master (from a laser-etched positive mold) for a culture mold to be cast in another, non-adsorbent material, such as agarose.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors acknowledge funding from NIH R00 HL115123 and Brown University School of Engineering. They are also grateful to the Brown Design Workshop and Chris Bull for training and support with the laser cutter.
References
- Qin D, Xia Y, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010;5:491. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- Rogers JA, Nuzzo RG. Recent progress in soft lithography. Mater. Today. 2005;8:50–56. [Google Scholar]
- Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft Lithography in Biology and Biochemistry. Annu. Rev. Biomed. Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- Isiksacan Z, Guler MT, Aydogdu B, Bilican I, Elbuken C. Rapid fabrication of microfluidic PDMS devices from reusable PDMS molds using laser ablation. J. Micromechanics Microengineering. 2016;26:035008. [Google Scholar]
- Lee KY, Mooney DJ. Hydrogels for Tissue Engineering. Chem. Rev. 2001;101:1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Kloxin A, Kloxin C, Bowman C, Anseth K. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv. Mater. Deerfield Beach Fla. 2010;22:3484–3494. doi: 10.1002/adma.200904179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin H, et al. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010;31:6941–6951. doi: 10.1016/j.biomaterials.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal MK, et al. Vacancy-Driven Gelation Using Defect-Rich Nanoassemblies of 2D Transition Metal Dichalcogenides and Polymeric Binder for Biomedical Applications. Adv. Mater. 2017;29 doi: 10.1002/adma.201702037. [DOI] [PubMed] [Google Scholar]
- Lian X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxshall K, et al. Simple surface treatments to modify protein adsorption and cell attachment properties within a poly(dimethylsiloxane) micro-bioreactor. Surf. Interface Anal. 2006;38:198–201. [Google Scholar]
- Pins GD, Christiansen DL, Patel R, Silver FH. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys. J. 1997;73:2164–2172. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipan CM, et al. Effects of antifibrinolytic agents on the life span of fibrin sealant. J. Surg. Res. 1992;53:402–407. doi: 10.1016/0022-4804(92)90068-b. [DOI] [PubMed] [Google Scholar]
- Roberts MA, et al. Stromal Cells in Dense Collagen Promote Cardiomyocyte and Microvascular Patterning in Engineered Human Heart Tissue. Tissue Eng. Part A. 2016;22:633–644. doi: 10.1089/ten.tea.2015.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye KY, Sullivan KE, Black LD. Encapsulation of Cardiomyocytes in a Fibrin Hydrogel for Cardiac Tissue Engineering. JoVE. 2011. [DOI] [PMC free article] [PubMed]
- Zimmermann WH, et al. Tissue Engineering of a Differentiated Cardiac Muscle Construct. Circ. Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- McCain ML, Agarwal A, Nesmith HW, Nesmith AP, Parker KK. Micromolded Gelatin Hydrogels for Extended Culture of Engineered Cardiac Tissues. Biomaterials. 2014;35:5462–5471. doi: 10.1016/j.biomaterials.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JJ, Chen GW, Liu YC, Hsu SS. Influence of Specimen Geometry on the Estimation of the Planar Biaxial Mechanical Properties of Cruciform Specimens. Exp. Mech. 2014;54:615–631. [Google Scholar]
- Munarin F, Kaiser NJ, Kim TY, Choi BR, Coulombe KLK. Laser-Etched Designs for Molding Hydrogel-Based Engineered Tissues. Tissue Eng. Part C Methods. 2017;23:311–321. doi: 10.1089/ten.tec.2017.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chiao M. Anti-fouling Coatings of Poly(dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015;35:143–155. doi: 10.1007/s40846-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


