Abstract
In guard cells, membrane hyperpolarization in response to a blue light (BL) stimulus is achieved by the activation of a plasma membrane H+-ATPase. Using the patch clamp technique on broad bean (Vicia faba) guard cells we demonstrate that both steady-state- and BL-induced pump currents require ATP and are blocked by vanadate perfused into the guard cell during patch clamp recording. Background-pump current and BL-activated currents are voltage independent over a wide range of membrane potentials. During BL-activated responses significant hyperpolarization is achieved that is sufficient to promote K+ uptake. BL activation of pump current becomes desensitized by three or four pulses of 30 s × 100 μmol m−2 s−1 BL. This desensitization is not a result of pump inhibition as maximal responses to fusicoccin are observed after full BL desensitization. BL treatments prior to whole cell recording show that BL desensitization is not due to washout of a secondary messenger by whole cell perfusion, but appears to be an important feature of the BL-stimulated pump response. We found no evidence for an electrogenic BL-stimulated redox chain in the plasma membrane of guard cells as no steady-state- or BL-activated currents are detected with NADH or NADPH added to the cytosol in the absence of ATP. Steady-state- nor BL-activated currents are affected by the inclusion along with ATP of 1 mm NADH in the pipette under saturating red light or by including NADPH in the pipette under darkness or saturating red light. These data suggest that reduced products of photosynthesis do not significantly modulate plasma membrane pump currents and are unlikely to be critical regulators in BL-stimulation of the plasma membrane H+-ATPase in guard cells.
Stomatal movements in response to light are engendered by changes in turgor as a consequence of K+ uptake (Humble and Hsiao, 1969; MacRobbie, 1987), Cl− uptake (MacRobbie, 1982), and organic solute production (Talbott and Zeiger, 1996). Solute uptake is driven by light-activated extrusion of H+ across the plasma membrane. The resulting hyperpolarization of the plasma membrane opens voltage sensitive inward K+ channels (Schroeder et al., 1987), and K+ ions enter the guard cell down their electrochemical gradient.
There are both red light (RL) and blue light (BL) components within the action spectrum of stomatal opening (Sharkey and Raschke, 1981). Responses to RL match the absorption spectrum for chlorophyll and RL stimulates photosynthetic activity within the chloroplast, thereby providing an energy source for H+ extrusion (Serrano et al., 1988; Serrano and Zeiger, 1989). In patch clamp experiments RL evokes outward currents that require ATP, are inhibited by vanadate applied to the cytosolic side of the membrane, and are dissipated by the addition of the protonophore carbonylcyanide m-chlorophenylhydrazone (Serrano et al., 1988), thus providing convincing evidence that a plasma membrane H+-ATPase is activated by RL in guard cells.
BL also drives guard cell photosynthesis (Wu and Assmann, 1993), and is thought to also act in guard cells via a cryptochrome or BL receptor (Zeiger, 1990). Similarity of the action spectra for chloroplastic zeaxanthin and the spectra for BL responses in guard cells suggests this pigment may play a significant role in the BL sensitivity of guard cells (Quiñones et al., 1996). Resolution of BL responses without interference from photosynthetic excitation has been achieved using the so-called “dual beam” protocol (Iino et al., 1985). Guard cell protoplasts are challenged with a BL pulse over a background of high fluence rate RL, which saturates photosynthetic responses. In experiments measuring H+ extrusion in response to BL (by monitoring medium acidification), it is necessary to pre-illuminate the suspension of guard cell protoplasts with RL for at least 30 min to obtain maximal responses. This indicates that photosynthetic products are required for BL-dependent H+ extrusion (Shimazaki et al., 1986; Gautier et al., 1992; Mawson, 1993). When treated with the photosynthesis inhibitor 3-(3, 4-dichlorophenyl)-1,1-dimethylurea, guard cell suspensions show a marked decrease in BL-mediated H+ extrusion (Shimazaki et al., 1986; Gautier et al., 1992; Mawson, 1993). When incubated with the respiratory inhibitor oligomycin or under anoxic conditions, the level of ATP in guard cell protoplasts is reduced by 60% with a corresponding inhibition of BL-stimulated H+ extrusion and protoplast swelling (Mawson, 1993). These results imply that products of photosynthesis and oxidative phosphorylation contribute to energizing BL-stimulated H+ extrusion across the plasma membrane (Schwartz and Zeiger, 1984).
Two possible mechanisms have been put forth by which protons may be extruded and the plasma membrane hyperpolarized in response to BL: (a) Activation of an H+-ATPase (Shimazaki et al., 1986; Assmann et al., 1985; Mawson, 1993), or (b) a (presumably) electrogenic plasma membrane redox chain (Møller and Crane, 1990; Gautier et al., 1992). The relative contribution of each mechanism to stomatal responses to BL is still an area of debate (Raghavendra, 1990). A requirement for ATP in evoking BL-stimulated outward currents has been established using patch clamp techniques (Assmann et al., 1985). Attempts to inhibit BL-mediated H+ extrusion by guard cell suspensions by using vanadate and other ATPase inhibitors have been inconclusive (Shimazaki et al., 1986; Gautier et al., 1990). However, studies using chloride-free extracellular solutions demonstrate that vanadate inhibits BL-induced guard cell protoplast swelling (Amodeo et al., 1992) and stomatal opening in epidermal peels (Schwartz et al., 1991) and it is suggested that Cl− uptake competes with vanadate uptake. This phenomenon may account for the negative results obtained in some vanadate experiments. Patch clamp experiments where vanadate is included in the pipette solution demonstrate the sensitivity of RL-induced pump currents to this compound (Serrano et al., 1988). The vanadate sensitivity of resting and BL-activated currents has not been investigated.
Medium acidification by guard cell protoplasts in response to a BL pulse is enhanced by the exogenous application of NADH and is inhibited by exogenous ferricyanide, an impermeant artificial electron acceptor, leading to the suggestion that a plasma membrane redox chain is responsible for mediating H+ extrusion in response to BL (Gautier et al., 1992). However, it remains unclear whether activation of a redox chain underlies electrogenic H+ extrusion per se or whether such a redox chain acts to enhance H+-ATPase activity by some other mechanism.
To clarify some of the issues surrounding BL-activated plasma membrane currents, patch clamp experiments were carried out to further characterize these currents with respect to H+-pump activity.
RESULTS
Steady-State- and BL-Activated Pump Currents
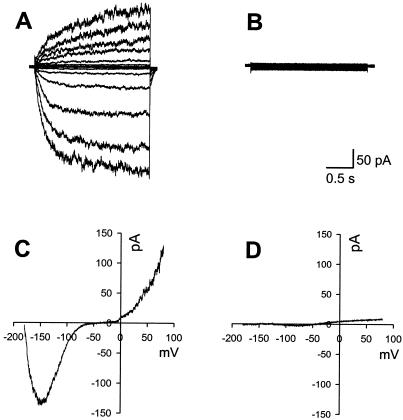
All K+ currents were eliminated by substitution of K+ with N-methyl-glucamine and addition of the K+ channel blocker tetraethylammonium. Other ions were symmetrical except Ca2+. Figure 1A illustrates control inward and outward K+ currents recorded with K+-Glu pipette and bath solutions. Figure 1B shows the block of voltage activated currents by including blockers in the internal and bath solutions. Figure 1C shows control K+ currents in response to a voltage ramp and Figure 1D shows the blocked ramp-evoked currents. The block of voltage-activated currents allowed for the resolution of electrogenic pump current. Zero mV is the equilibrium potential for all permeant ions except Ca2+, which would produce large inward currents if Ca2+-conducting channels were open and no current if the channels were closed. Pump currents were therefore identified as the positive outward current at 0 mV. The slope of the ramp current/voltage (I/V) ramp represents the input resistance of the whole cell recording.
Figure 1.
Resolution of H+-ATPase current. A, Typical K+ currents from a guard cell with membrane voltage stepped from −192 to 68 mV (corrected for liquid junction potential) in 20 mV steps. B, The trace shows that when solutions described in “Materials and Methods” are used, the pipette K+ currents evoked by the same step pulses as A are locked. C, The same cell as in A was subjected to a 3-s voltage ramp shortly after the step I/V in A was completed. Note that the biphasic shape of the current response at negative potentials is due to incomplete activation of inward K+ channels during this rapid I/V sweep. D, Currents evoked by an I/V sweep illustrated in C are blocked by the pump-recording solutions.
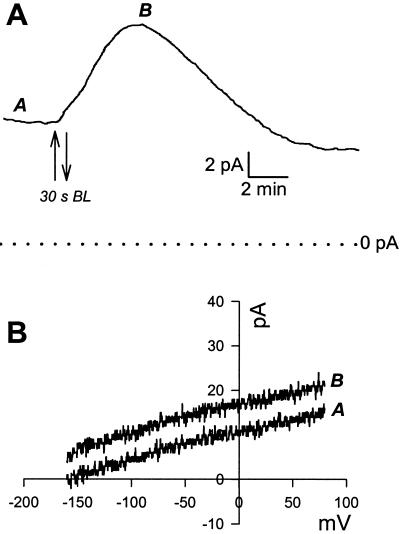
Immediately upon going whole cell, under saturating RL or darkness, many cells exhibited an oscillation in membrane current (Fig. 2). This oscillation is most likely due to the equilibration of pipette and cell contents. The steady-state pump current, which we define as the stable baseline current under saturating RL illumination (800 μmol m−2 s−1), was 6.38 pA (±0.1 n = 75). The I/V relationship for the pump current was linear over the voltage range of the ramp (Fig. 3). Once pump current had stabilized under saturating RL, BL was tested. I/V ramps sampled before and at the peak of the BL response showed a clear increase in pump current seen as a parallel positive shunt in whole-cell current (Fig. 3B). In experiments performed on cells allowed to equilibrate in the dark, no significant increase in pump current was detected upon application of continuous saturating RL (800 μmol m−2 s−1, n = 70, Fig. 4). The BL response was variable in frequency of occurrence and in size. Over all, 61% (total n = 80) of cells responded to BL with an increase in pump current. However, in some cases up to 90% of cells in a batch of protoplasts responded to BL and in other batches no responses were observed. Because of this variability we only compared steady-state- and BL-stimulated currents from the same batches of protoplasts for the treatments described below. Of the cells sensitive to BL, the average peak of BL-stimulated current in response to a single 30-s pulse of BL was 2.84 pA (±0.3, n = 49), with the highest peak response of 8.9 pA and the lowest of 0.9 pA. There was no increase in current when a pulse of RL (30 s, 200 μmol m−2 s−1) was applied under the same conditions i.e. in the presence of background RL (n = 8, data not shown), confirming that the response was specific to BL.
Figure 2.
H+-ATPase activation by a pulse of BL. Saturating RL background illumination was switched on before the beginning of the trace. A, Once stable baseline current is achieved a pulse of BL causes a transient increase in pump current. B, I/V ramps conducted before (A) and at the peak (B) of the response in A show the parallel shunt in pump current.
Figure 3.
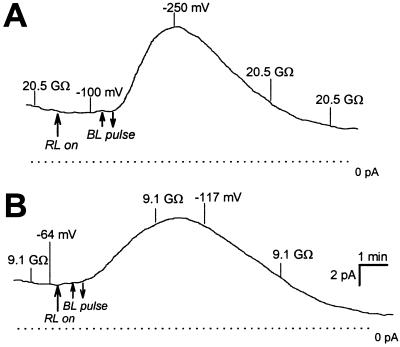
The effect of plasma membrane H+-ATPase currents on membrane potential. The two traces show the pump current measured with ATP in the pipette. Membrane potential and input resistance are indicated on the traces at steady state and during BL-activated stimulation of pump current. Note the insensitivity to saturating RL illumination.
Figure 4.
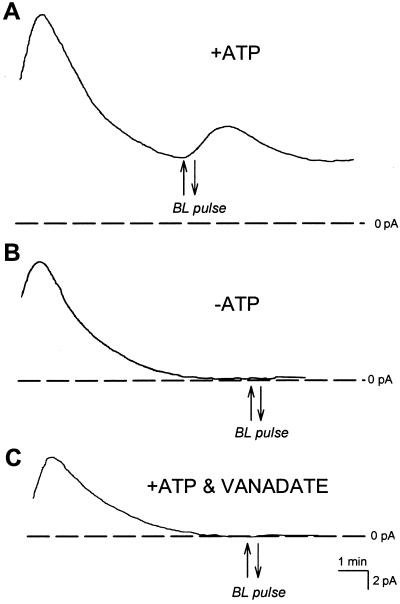
Steady-state- and BL-stimulated pump currents require ATP and are inhibited by vanadate. A, A typical recording with 5 mm ATP in the pipette under saturating RL. The cell responded to a 30-s pulse of BL with a typical transient increase in pump current. B, When ATP is absent from the pipette, cell currents quickly decay to 0 pA under saturating RL and are unresponsive to a pulse of BL. C, Inclusion of ATP and 20 μm vanadate in the pipette causes inhibition of pump current. All cells where pump current was inhibited by vanadate were unresponsive to BL pulses.
Membrane potential measured when current was clamped to 0 pA (Fig. 4) was in agreement with that predicted by the product of the steady-state pump current and input resistance of the cell. Thus the mean whole-cell steady-state current (6.38 pA) was sufficient to generate an average membrane potential of −90 mV based on the high input resistance of 15 GΩ (±1.4, n = 75) induced by the K+ channel blocking solutions in the pipette and bath. Membrane potential was then further hyperpolarized as a result of the BL-stimulated currents (Fig. 4).
Steady-State- and BL-Activated Currents Require ATP
Both steady-state- and BL-activated currents required ATP and were completely blocked by vanadate. Figure 2A shows a typical record of a whole-cell current in the presence of ATP in the pipette. When ATP was absent from the pipette all currents rapidly declined to zero as pipette and cell contents equilibrate and no outward pump currents were then detected (Fig. 2B, n = 20). When ATP and vanadate (20 or 50 μm) were included in the pipette, outward currents also declined to zero (n = 8, Fig. 2C). BL responses could not be elicited either in the absence of ATP (n = 19, Fig. 2B), or in the presence of ATP and vanadate together (n = 8, Fig. 2C).
BL Response Desensitization
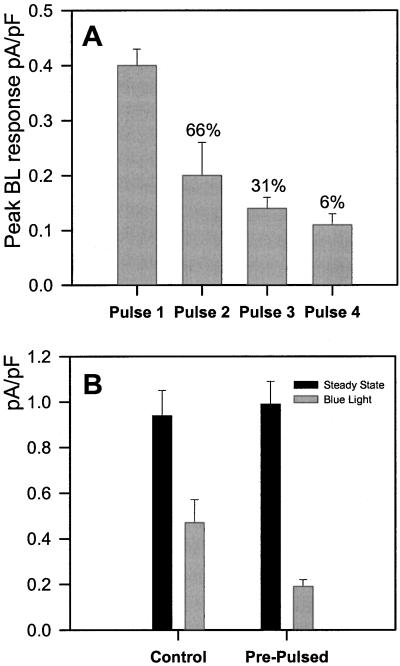
Peak BL-induced pump currents progressively became smaller with each pulse of BL and 95% of cells were unresponsive after the fourth pulse (n = 21, Fig. 5A). Recovery from BL desensitization was not observed after up to 30 min (n = 4, not shown). Because desensitization could be due to washout of a signaling factor in the whole-cell configuration, some cells were pretreated with a single pulse of BL immediately prior to whole-cell recording. The same cell was then tested with a second BL pulse after gaining a stable whole cell-pump current recording. Pre-pulsed cells showed no significant difference in steady-state current, but a significant reduction in BL-induced pump current (P < 0.01, Student's unpaired t test, Fig. 5B). The desensitization of BL pre-treated cells to the second BL pulse (40% of peak amplitude in control response, n = 6, see Fig. 5B) was comparable with the desensitization observed when cells were challenged with both pulses during whole cell recording (50% of peak in first response, n = 21, see Fig. 5A).
Figure 5.
A, BL responses desensitize. Mean (± se) BL-activated pump currents in response to a sequence of BL pulses are plotted against pulse number. Only responsive cells are included with each mean, and the percentage of cells responding to each pulse are indicated above each bar. Peak BL-stimulated pump responses become progressively smaller with each pulse. B, Prepulsing a cell before going whole cell and then applying a second pulse while recording pump current causes a level of desensitization to BL that is comparable with the desensitization observed in cells where both pulses were delivered during whole cell recording (see “pulse 2” in 4A). Only responsive cells are included with each mean.
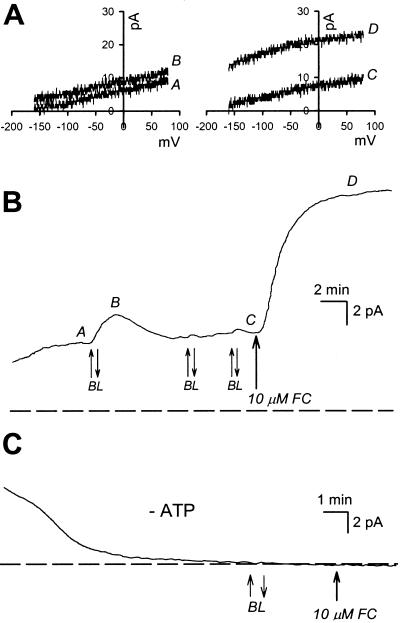
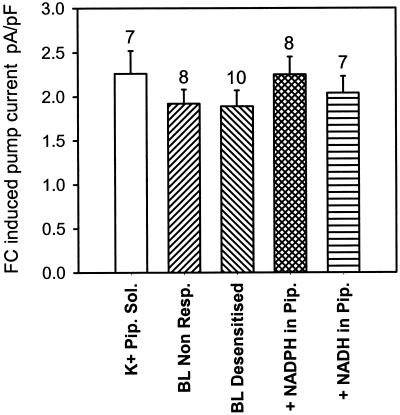
Further experiments were devised to explore the possibility that the basis of BL desensitization is due to inhibition of a proportion of the H+-ATPase molecules sensitive to BL. Cells that had desensitized completely to BL were treated with fusicoccin (FC) added to the bath (final concentration of 10 μm). In the presence of ATP in the pipette, every cell responded to FC with a rapid and sustained increase in pump current (n = 33, Fig. 6, A and B). In the absence of ATP in the pipette no FC response was detected (n = 6, Fig. 6C), and additions of ethanol alone (final concentration 0.15%) had no effect (n = 8, data not shown). The FC-induced pump increase was not significantly different between cells that were BL desensitized, cells that were unresponsive to BL, and cells that had not been subject to BL stimulation (Fig. 7).
Figure 6.
BL-stimulated pump responses were evoked in the cell until desensitization occurred (in this case on the second pulse). A, The ramp I/V (from −160 to 75 mV) curves above the current trace (B) show the small transient positive shunt in current at 0 mV in response to the BL pulse (A and B) and the much larger sustained increase in pump current when FC was added to the bath (C and D). C, In the absence of ATP no steady-state-, BL-, or FC-stimulated currents are detected.
Figure 7.
The change in pump current at 0 mV induced by 10 μm FC was calculated from cells under a variety of conditions. The FC induced pump current (means ± se) was the same regardless of pipette solution or BL responsiveness. Sample sizes for each treatment are indicated above each bar.
Redox Pools and H+-ATPase Pump Currents
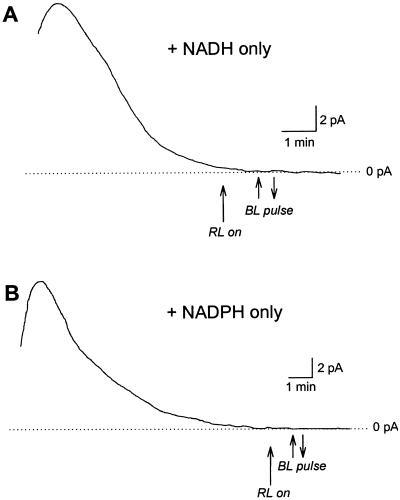
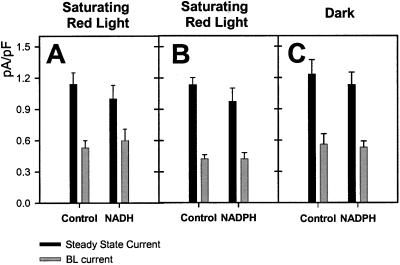
Neither steady-state- nor BL-stimulated currents were observed when 1 mm NADH or NADPH (n = 9 and 13, respectively) were supplied in the pipette as a redox source in the absence of ATP (Fig. 8). In the presence of ATP, neither 1 mm NADH or NADPH (Fig. 9) had a significant effect on steady-state- or BL-stimulated current. Since a residual pool of reductant may be generated by the saturating RL background, comparisons were made with cells subjected to a BL pulse under a dark background with NADPH present or absent from the pipette solution (Fig. 9). No significant differences were detected in steady-state- or BL-stimulated current regardless of background illumination or pipette NADPH levels.
Figure 8.
No steady-state- or BL-activated H+-ATPase current is detected when either 1 mm NADH (A) or 1 mm NADPH (B) is included in the pipette in the absence of ATP.
Figure 9.
Steady-state- and BL-activated H+-ATPase currents in the presence of reduced pyridine nucleotides. A, Pump currents were not significantly affected by inclusion of 1 mm NADH in the pipette (n = 13, means ± se). When NADPH was included in the patch pipette, no significant difference in steady-state- or BL-induced pump current was observed either in B, the dark (n = 7, means ± se) or C, under saturating RL (n = 22, means ± se).
DISCUSSION
Pump currents were measured in these experiments by using symmetrical ion concentrations and by suppressing any residual ion currents by substitution with impermeant ions and using the K+ channel blocker tetraethylammonium. Electrogenic pump activity was observed as an outward current at 0 mV, which was the equilibrium potential for all permeant ions except Ca2+, which would produce large inward currents if channels were active. The currents required the presence of ATP and were against the concentration gradient for H+ ions, confirming the presence of active transport.
The lack of response to saturating RL here and reported previously (Assmann et al., 1985) is not in agreement with a previous patch clamp study (Serrano et al., 1988), where illumination with 1,000 μmol m−2 s−1 of RL caused a small (<1.5 pA) increase in outward pump current. This RL enhancement of pump current was blocked by 3-(3, 4-dichlorophenyl)-1,1-dimethylurea, inhibited by vanadate, and augmented by PO4− included in the patch pipette. The authors argue that a product of photosynthetic phosphorylation other than ATP must be acting to stimulate pump currents in response to RL. It is interesting to note that although a protonophore (carbonylcyanide m-chlorophenylhydrazone) abolished RL- and FC-induced pump currents, it also reveals that there was no baseline current in the dark with up to 2.5 mm ATP in the pipette. This lack of baseline pump current in the presence of ATP is inconsistent with our data and those of Lohse and Hedrich (1992). In our study the average response to FC with K+-based solutions is 3-fold higher than those reported by Serrano et al. (1988). Although Serrano et al. do not show I/V data of their RL stimulated outward current or Vm data in response to RL illumination, one can calculate that RL-stimulated currents of 1.5 pA would generate a maximum resting membrane potential of only −20 mV for a guard cell with 13 GΩ input resistance. The average baseline current recorded in our study would generate a −88 mV resting potential in the dark with peak hyperpolarization to −172 mV generated by an average pulse of BL for the same cell input resistance. The baseline and BL evoked currents we recorded correlate well with the intact physiology of the guard cell.
The pump currents reported here and elsewhere (Assmann et al., 1985; Blatt, 1987; Schroeder, 1988) are sufficient to hyperpolarize the guard cell membrane and promote solute uptake through voltage-regulated channels. The absence of response to a pulse of RL over a continuous background RL illumination confirms that the BL-stimulated H+-ATPase current arises as a consequence of a direct and specific BL signal transduction pathway. The dependence on ATP and the inhibition by vanadate confirm that the H+-ATPase generates the electrogenic response to BL. The steady-state and BL-activated currents are voltage independent over the physiological range of membrane potentials and this is in agreement with previous reports of guard cell plasma membrane H+-ATPase activity (Lohse and Hedrich 1992). The variation in BL-activated H+-ATPase currents among cells within a batch of protoplasts isolated from whole leaves may be accounted for, in part, by the different photosensitivities of guard cells originating from adaxial and abaxial leaf epidermis. Such differences are detected in a wide range of species (Pemadasa, 1979). More recently Goh et al. (1995) have shown that although adaxial guard cells from broad bean have the same H+ pumping capacity as abaxial guard cells, their sensitivity to BL is significantly lower.
The consistent nature of the BL desensitization of the pump response suggests a physiological relevance to this behavior. Stomatal desensitization to BL has been demonstrated in measurements of stomatal conductance in whole leaves (Iino et al., 1985), where a full conductance response to a second pulse of BL requires an interval of at least 20 to 30 min. A recovery period of greater than 30 min is required for the complete restoration of H+ pumping in guard cell protoplast suspensions in response to a second pulse of BL (Goh et al., 1995). In the present single-cell patch clamp study we show that, without exception, the guard cell BL-stimulated pump response desensitizes after the first pulse, although the level of desensitization to the second and subsequent pulses of BL varies from cell to cell. Recovery from BL desensitization in the long term (i.e. ≥30 min) was not tested, as the majority of recordings did not last an adequate length of time.
By using BL pre-treatments of intact cells prior to patch clamping we show that desensitization of the BL signal transduction pathway was not an artifact of whole-cell patch clamping, and presumably corresponds to the desensitization phenomena observed in guard cell suspensions and in stomatal opening in epidermal peels and whole leaves. Desensitization due to a separate H+-ATPase isoform only responsive to BL is unlikely, as the two plasma membrane H+-ATPase isoforms expressed in guard cells from broad bean, VHA1, and VHA2, are also expressed throughout the plant (Hentzen et al., 1996). We therefore argue that modulation of guard cell H+-ATPase in response to BL most likely occurs through a cell-specific signal transduction pathway, and that this pathway rapidly becomes desensitized to the light signal. The robust response to FC by BL-desensitized cells further supports the contention that BL desensitization is occurring at the BL photoreceptor or at an intermediate step in the BL signaling pathway, rather than at the H+-ATPase itself.
The desensitization kinetics of the BL stomatal response in intact leaves has been interpreted in terms of two interconvertible forms of the BL photoreceptor (Iino et al., 1985). The observation that pulses of green light can reversibly inhibit BL induced increases in stomatal aperture in epidermal peels (Frechilla et al., 2000) supports the photoreceptor cycling model. Thus photobiological properties of the BL photoreceptor may underlie the desensitization phenomenon observed in this study, although desensitization of the intermediate BL-signaling elements cannot be ruled out.
Photoreceptor desensitization may contribute to a shift in the osmoregulatory pathways that control guard cell turgor. Osmoregulation in intact guard cells shows two phases: the first in the early or morning phase (0–3 h) of the day light cycle, is associated with rapid solute (K+ and counter anion) uptake and BL-stimulated starch breakdown (Tallman and Zeiger, 1988); the second in the afternoon phase is dominated by RL-stimulated photosynthetic sugar production and net K+ loss (Talbott and Zeiger, 1996). BL, but not RL stimulates cation uptake in guard cells at low light intensities (10 μmol m−2 s−1) and RL only stimulates cation uptake at higher light intensities (Hsiao et al., 1973). These data imply that stomatal opening is initially stimulated by BL-activation of H+-ATPase and rapid solute uptake (and starch breakdown) in the early part of the day, and stomatal aperture is subsequently maintained through photosynthetic sugar production driven by RL.
Although redox activity associated with H+ extrusion has been demonstrated in the guard cell plasma membrane (Pantoja and Willmer, 1991; Gautier et al., 1992), the mechanism and electrogenicity have yet to be determined. Gautier et al. (1992) proposed a model whereby BL stimulates a plasma membrane redox chain resulting in electrogenic pumping of H+ out of the guard cell. Our data showing the absence of detectable current in the absence of ATP and the presence of cytoplasmic redox pools (NADH and NADPH) argue against a redox chain directly driving electrogenic transport across the guard cell plasma membrane, constitutively or in response to BL. Based on the maximum rate of BL-stimulated medium acidification of Comellina communis guard cell suspensions, 100 nmol H+ h−1 (106 cells) −1 (Gautier et al., 1992), we calculate an equivalent membrane current of approximately 3 pA per cell. The peak BL H+-ATPase currents of 3 to 4 pA in whole cell recordings of broad bean can account for the typical rate of medium acidification stimulated by a pulse of BL in guard cell suspension preparations. These observations argue against a plasma membrane redox chain significantly contributing to H+ efflux in the guard cell response to BL.
It is possible that a less direct link between redox activity and H+ pumping exists, for example by modulating of cytosolic pyridine nucleotide levels (Vavasseur et al., 1995). Redox regulation of H+-ATPase activity by increased pools of reductant generated by RL and BL was tested in this study by comparing cells where NADH or NADPH were included in the pipette solution. Our data show that neither reductant has an effect on steady-state- or BL-stimulated currents, indicating that elevated levels of cytosolic pyridine nucleotides are not a significant contributory factor to BL-stimulated pump currents of guard cells.
The mechanism of H+-ATPase activation in response to a BL pulse remains to be completely elucidated. However, the phosphorylation state of Ser-Thr residues on the C-terminal domain of the H+-ATPase is critical in determining pump activity and these domains are targets for a variety of regulatory signals (Sze et al., 1999). BL has been shown to stimulate phosphorylation of Ser and Thr residues of the H+-ATPase of broad bean guard cells and this phosphorylation corresponds to BL-stimulated ATP hydrolytic activity and H+ pumping (Kinoshita and Shimazaki 1999). These observations strongly support the contention that the final step in BL pump signal transduction in the guard cell is a phosphorylation event at the C terminus of the H+-ATPase.
An open question remains as to the physiological role of the BL-activated plasma membrane reductase (Pantoja and Willmer, 1991; Gautier et al., 1992; Vavasseur et al., 1995) if this reductase does not alter the membrane potential, as our data suggest. We propose two possibilities: (a) Trans-plasma membrane redox activity modifies cell wall elasticity required for stomatal swelling and (b) the BL-activated reductase is a component of the signal transduction chain that can regulate the H+-ATPase (see above). Trans-plasma membrane redox activity in plant cells has been most frequently been associated with growth and cell expansion in shoot and root tissues (Barr, 1991), and cell wall structure is modified by redox processes (Bradley et al., 1992; Fry, 1998). Although there is no information on the regulation of guard cell wall elasticity, it is notable that expansins, widely distributed cell wall proteins that induce reversible cell wall relaxation (Cosgrove, 1998), are specifically expressed in guard cells (Cosgrove, 2000). These proteins require low pH and reductants for optimal activity in vitro (McQueen-Mason et al., 1992). If transient guard cell wall loosening occurs during normal stomatal opening, then BL-activated H+-ATPase and reductase activity may play a role in modulating the guard cell wall elasticity necessary to accommodate large increases in cell volume.
A second possible role for BL-activated redox activity in the guard cell plasma membrane may be more directly related to light signal transduction. Redox sensing is now recognized as a key element in several light-signal transduction cascades in plant cells (Huala et al., 1997; Vener et al., 1998). The protein encoded by NPH1 in Arabidopsis is a Ser-Thr kinase essential for phototrophic responses. The kinase activity of NPH1 is dependent on BL (Christie et al., 1998) and arises as a result of BL-induced redox changes of the non-covalent bound flavin mononucleotide chromophore (Huala et al., 1997). Stomatal conductance responses of BL photoreceptor mutants nph1, cry1, and cry2 show that these receptors do not play a role in the guard cell BL response (Lascève et al., 1999), whereas recent reports indicate a role for zeaxanthin in guard cell BL photoreception (Zeiger and Zhu, 1998; Eisinger et al., 2000). Nevertheless, it is tempting to speculate that a BL-activated reductase associated with a flavin (Lüthje et al., 1995) could influence the activity of the plasma membrane H+-ATPase via a redox sensitive phosphorylation cascade. Because flavin mononucleotide and flavin adenine dinucleotide are the prosthetic groups associated with redox sensitive kinases, it will be of great interest to see how these compounds affect BL signal transduction in whole-cell recordings.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Broad beans (Vicia faba) were grown from seeds in controlled environmental chambers with a 10-h photoperiod of white light (150 μmol m−2 sec−1) and day and night temperatures of 19°C and 17°C, respectively.
Isolation of Guard Cells and Measurement of Pump Current
Guard cell protoplasts were isolated from the youngest expanded leaves of 2- to 4-week-old plants as previously described (Miedema and Assmann, 1996). Prior to patch clamp experiments, protoplasts were kept on ice in the dark in the isolation medium consisting of: 0.5 mm CaCl2, MgCl2, 10 μm KH2PO4, 5 mm MES [2-(N-morpholino)-ethanesulfonic acid[, and 450 mm mannitol.
Patch electrodes were fabricated from Kimax 51 glass capillaries and filled with an internal recording solution consisting of 50 mm N-methylglucamine Glu, 2 mm MgCl2, 10 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], 0.1 mm EGTA, 1 mm K H2PO4, 10 mm Suc, 10 mm Glc, 10 mm malate, and 5 mm Mg-ATP, pH 6.8 with tetraethylammonium hydroxide solution (final concentration of approximately 15 mm). Osmolality was raised to 480 to 500 mmol kg−1 with d-mannitol. For consistent pump currents we found it was necessary to make fresh Mg-ATP-Tris [tris(hydroxymethyl)-aminomethane] stocks immediately upon delivery from the supplier, which were kept at −20°C and used within 1 week. In some experiments 1 mm NADH or NADPH was added to the pipette solution. Pyridine nucleotides were obtained in sealed vials and added to pipette solution immediately prior to experiments. Solutions were kept on ice and used within 4 h. To test for stability, pyridine nucleotide A340 was monitored in a spectrophotometer. Less than 4% oxidation occurred over an 8-h period in pipette solution kept on ice. All chemicals were obtained from Sigma (St. Louis).
The bath solution consisted of 50 mm N-methylglucamine, 30 mm glutamatic acid, 10 mm Ca-Glu, 2 mm MgCl2, 10 mm HEPES, 10 mm Suc, 10 mm Glc, and 10 mm malate, pH 6.8 with tetraethylammonium hydroxide solution (final concentration of approximately 15 mm). Osmolality was raised to 480 to 500 mmol kg−1 with d-mannitol. Solutions were kept on ice until use.
For measuring K+ currents, K-Glu-based whole-cell recording solutions were used. The pipette solution was 80 mm K-Glu, 20 mm KCl, 2 mm MgCl2, 2 mm EGTA, and 10 mm HEPES made up to 480 mmol kg−1 with mannitol, pH 7.2 with 1 n KOH. The bath solution comprised 10 mm KCl, 10 mm CaCl2, and 2 mm MgCl2 made up to 450 mmol kg−1 with mannitol and pH 5.5 with 1 n HCl.
Patch clamping was performed under dim RL (50 μmol m−2 sec−1). Currents were recorded using either an Axopatch 1D or an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) connected to a chart recorder (Hitachi Instruments, San Jose, CA). Figures were prepared by scanning the chart recording and tracing the current to generate a bitmap (Corel Draw, Corel, Ontario). Discontinuous (step) and I/V curves were generated using a PC interfaced (Axon Instruments, Digidata) with the amplifier. The I/V ramp protocol consisted of a 3-s sweep usually from −180 mV to 80 mV. All currents were sampled at 3 KHz and filtered at 1 KHz. Once a whole-cell recording was achieved, guard cell membrane potential was clamped to 0 mV, and a period of between 5 and 10 min was allowed for complete equilibrium to be achieved between the pipette solution and the cytoplasm. This equilibration period was either conducted under saturating RL or darkness depending on the experiment type. I/V ramps were performed and measurements of seal resistance, membrane capacitance, and membrane potential were taken at regular intervals throughout each experiment. Membrane capacitance for each cell was used to normalize cell pump currents for any variability in cell size and membrane surface area. Student's unpaired t test was applied to groups of pump current data to determine statistically significant effects of treatments.
Saturating (800 μmol m−2 sec−1) RL was obtained by inserting a gelatin filter (no. 29, Kodak, Rochester, NY; 50% cut off at 590 nm) and a heat filter (700 nm low pass) above the condenser of the microscope's halogen light source. BL pulses (100 μmol m−2 sec−1) were obtained by filtering the output of a fiber optic light source, mounted close to the microscope stage, with a blue Plexiglas filter (peak transmittance 480 nm, bandwidth 30 nm). Fluence rates were measured with a quantum sensor (Li-Cor, Lincoln, NE).
Footnotes
This work was funded by the U.S. Department of Agriculture (grant no. 94–37304–1003 to S.M.A.) and by the Binational Agricultural Research and Development Fund (grant no. US–2595–95 to S.M.A. and Eva J. Pell) and by a Marine Biological Association and Leverhulme Trust Special Research Fellowship (to A.R.T.).
LITERATURE CITED
- Amodeo G, Srivastava A, Zeiger E. Vanadate inhibits blue light-stimulated swelling of Vicia guard cell protoplasts. Plant Physiol. 1992;100:1567–1570. doi: 10.1104/pp.100.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Simoncini L, Schroeder JI. Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature. 1985;318:285–287. [Google Scholar]
- Barr R. The possible role of redox-associated protons on growth of plant cells. J Bioenerg Biomembr. 1991;23:443–467. doi: 10.1007/BF00771014. [DOI] [PubMed] [Google Scholar]
- Blatt MR. Electrical characteristics of stomatal guard cells: the contribution of ATP-dependent, “electrogenic” transport revealed by current-voltage and difference-current-voltage analysis. J Membr Biol. 1987;98:257–274. [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas A, Liscum E, Briggs WR. Arabidopsis NHP1: a flavoprotein with the properties of a photoreceptor for phototropism. Science. 1998;282:1698–1701. doi: 10.1126/science.282.5394.1698. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Cell wall loosening by expansins. Plant Physiol. 1998;118:333–339. doi: 10.1104/pp.118.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. New genes and new biological roles for expansins. Curr Opin Plant Biol. 2000;3:73–78. doi: 10.1016/s1369-5266(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Eisinger W, Swartz TE, Bogomolni RA, Tiaz L. The ultraviolet action spectrum for stomatal opening in broad bean. Plant Physiol. 2000;122:99–105. doi: 10.1104/pp.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechilla S, Talbott LD, Bogomolni RA, Zeiger E. Reversal of blue light-stimulated stomatal opening by green light. Plant Cell Physiol. 2000;41:171–176. doi: 10.1093/pcp/41.2.171. [DOI] [PubMed] [Google Scholar]
- Fry SC. Oxidative scission of plant cell wall polysaccharides by ascarbate-induced hydroxyl radicals. Biochem J. 1998;332:507–515. doi: 10.1042/bj3320507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier H, Vavasseur A, Lascève G, Boudet AM. Redox processes in the blue light response of guard cell protoplasts of Commelina communis L. Plant Physiol. 1992;98:34–38. doi: 10.1104/pp.98.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh C-H, Oku T, Shimazaki K. Properties of proton pumping in response to blue light and fusicoccin in guard cell protoplasts isolated from adaxial epidermis of Vicia leaves. Plant Physiol. 1995;109:187–194. doi: 10.1104/pp.109.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzen AE, Smart LB, Wimmers LE, Fang HH, Schroeder JI, Bennet AB. Two plasma membrane H+-ATPase genes expressed in guard cells of Vicia faba are also expressed throughout the plant. Plant Cell Physiol. 1996;37:650–659. doi: 10.1093/oxfordjournals.pcp.a028994. [DOI] [PubMed] [Google Scholar]
- Hsiao TC, Allway WG, Evans LT. Action spectra for guard cell Rb+ uptake and stomatal opening. Plant Physiol. 1973;51:82–88. doi: 10.1104/pp.51.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han I-S, Larsen E, Briggs WR. Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- Humble GD, Hsiao TC. Specific requirement of potassium for light-activated opening of stomata in epidermal strips. Plant Physiol. 1969;44:230–234. doi: 10.1104/pp.44.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Ogawa T, Zeiger E. Kinetic properties of the blue-light response of stomata. Proc Natl Acad Sci USA. 1985;82:8019–8023. doi: 10.1073/pnas.82.23.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K. Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C terminus in stomatal guard cells. EMBO J. 1999;18:5548–5558. doi: 10.1093/emboj/18.20.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascève G, Leymarie J, Olney MA, Liscum E, Christie JM, Vavasseur A, Briggs WR. Arabidopsis contains at least four independent blue-light-activated signal transduction pathways. Plant Physiol. 1999;120:605–614. doi: 10.1104/pp.120.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse G, Hedrich R. Characterization of the plasma membrane H+-ATPase from Vicia faba guard cells. Planta. 1992;188:206–214. doi: 10.1007/BF00216815. [DOI] [PubMed] [Google Scholar]
- Lüthje S, Döring O, Heuer S, Lüthen H, Böttger M. Oxydoreductases in plant plasma membranes. Biochim Biophys Acta. 1995;1331:81–102. doi: 10.1016/s0304-4157(96)00016-0. [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC. Chloride transport in stomatal guard cells. Phil Trans R Soc Lond B. 1982;299:469–481. [Google Scholar]
- MacRobbie EAC. Ionic relation in guard cells. In: Zeiger E, Farquar GD, Cowan IR, editors. Stomatal Function. Stanford, CA: Stanford University Press; 1987. pp. 125–137. [Google Scholar]
- Mawson BT. Regulation of blue-light-induced proton pumping by Vicia faba L. guard-cell protoplasts: energetic contributions by chloroplastic and mitochondrial activities. Planta. 1993;191:293–301. [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ. Endogenous proteins that induce cell wall expansion in plants. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedema H, Assmann SM. A membrane-delimited effect of internal pH on the K+ outward rectifier of Vicia faba guard cells. J Membr Biol. 1996;154:227–237. doi: 10.1007/s002329900147. [DOI] [PubMed] [Google Scholar]
- Møller IM, Crane FL. Redox processes in the plasma membrane. In: Larsson C, Møller IM, editors. The Plant Plasma Membrane. Berlin: Springer-Verlag; 1990. pp. 112–116. [Google Scholar]
- Pantoja O, Willmer CM. Ferricyanide reduction by guard cell protoplasts. J Exp Bot. 1991;42:323–329. [Google Scholar]
- Pemadasa MA. Movements of abaxial and adaxial stomata. New Phytol. 1979;82:69–80. [Google Scholar]
- Quiñones MA, Zhenmin L, Zeiger E. Close correspondence between the action spectra for the blue light responses of the guard cell and coleoptile chloroplasts, and the spectra for blue light-dependent stomatal opening and coleoptile phototropism. Proc Natl Acad Sci USA. 1996;93:2224–2228. doi: 10.1073/pnas.93.5.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra AS. Blue light effects on stomata are mediated by the guard cell plasma membrane redox system distinct from the proton translocating ATPase. Plant Cell Environ. 1990;13:105–110. [Google Scholar]
- Schroeder JI. K+ transport properties of K+ channels in guard cells. J Gen Physiol. 1988;92:667–683. doi: 10.1085/jgp.92.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E. Voltage dependence of K+ channels in guard cell protoplasts. Proc Natl Acad Sci USA. 1987;84:4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Ilan N, Assmann SM. Vanadate inhibition of stomatal opening in epidermal peels of Commelina communis. Planta. 1991;183:590–596. doi: 10.1007/BF00194281. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Zeiger E. Metabolic energy for stomatal opening: roles of photophosphorylation and oxidative-phosphorylation. Planta. 1984;161:129–136. doi: 10.1007/BF00395472. [DOI] [PubMed] [Google Scholar]
- Serrano EE, Zeiger EE. Sensory transduction and electrical signaling in guard cells. Plant Physiol. 1989;91:795–799. doi: 10.1104/pp.91.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano EE, Zeiger E, Hagiwara S. Red light stimulates an electrogenic proton pump in Vicia guard cell protoplasts. Proc Natl Acad Sci USA. 1988;85:436–440. doi: 10.1073/pnas.85.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Raschke K. Effect of light quality on stomatal opening in leaves of Xanthium strumaruium L. Plant Physiol. 1981;68:1170–1174. doi: 10.1104/pp.68.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Iino M, Zeiger E. Blue light dependent proton extrusion by guard cell protoplasts of Vicia faba. Nature. 1986;319:324–326. [Google Scholar]
- Sze H, Li X, Palmgren MG. Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell. 1999;11:677–689. doi: 10.1105/tpc.11.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. Central roles for potassium and sucrose in guard-cell osmoregulation. Plant Physiol. 1996;111:1051–1057. doi: 10.1104/pp.111.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman G, Zeiger E. Light quality and osmoregulation in Vicia guard cells. Plant Physiol. 1988;88:887–895. doi: 10.1104/pp.88.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavasseur A, Lascève G, Cousson A. Guard cell responses to potassium ferricyanide. Physiol Plant. 1995;93:253–258. [Google Scholar]
- Vener AV, Ohad I, Andersson B. Protein phosphorylation and redox sensing in chloroplast thylakoids. Curr Opin Plant Biol. 1998;1:217–223. doi: 10.1016/s1369-5266(98)80107-6. [DOI] [PubMed] [Google Scholar]
- Wu W-H, Assmann SM. Photosynthesis by guard cell chloroplasts of Vicia faba L.: effects of factors associated with stomatal movement. Plant Cell Physiol. 1993;34:1015–1022. [Google Scholar]
- Zeiger E. Light perception in guard cells. Plant Cell Environ. 1990;13:739–744. [Google Scholar]
- Zeiger E, Zhu JX. Role of zeaxanthin in blue light photoreception and modulation of light-CO2 interactions in guard cells. J Exp Bot. 1998;49:433–442. [Google Scholar]