Abstract
A 22-year-old man underwent mediastinal metastasectomy for a testicular germ cell tumour via median sternotomy. Intraoperatively, the tumour was massive, measuring 88 mm in anterior-posterior (AP) diameter. It was densely adherent to the trachea and aggressive debulking resulted in tracheal injury. Therefore, the patient was kept nil by mouth for 3 days postoperatively and was discharged uneventfully. He represented only 2 days later with a large right-sided chylothorax. His chylothorax was managed conservatively with insertion of an intercostal catheter (ICC) and a low-fat diet. Over the course of 9 days, the ICC drained approximately 5 L of fluid. His admission was further complicated by severe gastroparesis requiring feeding Nasojejunal (NJ) tube placement. The delayed feeding in this case resulted in late detection of the occult thoracic duct injury. This case illustrates that conservative and multidisciplinary management of a postoperative chylothorax from a suspected thoracic duct injury achieves favourable outcomes avoiding further surgical intervention.
Keywords: urological cancer, cardiothoracic surgery, stomach and duodenum, diet, nutritional support
Background
Non-seminomatous germ cell testicular tumour is the most common tumour in men aged less than 35 years.1 Although several patients respond well to cisplatin-based chemotherapy, it is estimated that 10%–30% of patients will be left with extraretroperitoneal disease requiring surgical intervention.2 3 The pattern of disease spread is usually stepwise, starting from the retroperitoneal lymphatics to the mediastinum, supraclavicular nodes and chest.4 A large multicentre international study found that the common sites of spread after chemotherapy were the lung (27%) and mediastinum (15%).4 In patients with supradiaphragmatic metastases, 10%–20% will require at least one thoracic surgical procedure.5 Once mediastinal metastases are confirmed, the 5-year survival is 48%.5
Thoracic surgery is associated with a risk of disruption to lymphatic drainage which can result in a postoperative chylothorax. The thoracic duct carries approximately 60%–70% of ingested fat from the gastrointestinal system to the circulatory system.6 On average, 2–2.5 L of chyle is transported through the thoracic duct every day, and injury to the duct can result in expeditious build up of pleural fluid.7 8 Further complications of such an injury include nutritional deficiency, immunosuppression, respiratory compromise and dehydration.7
Case presentation
A 22-year-old Caucasian man was referred to cardiothoracic surgery for debulking of a mediastinal metastasis from a right testicular germ cell tumour (GCT). Of note, the patient had previously undergone bleomycin, etoposide and platinum chemotherapy, right orchidectomy and retroperitoneal lymph node dissection. The patient had a medical history of asthma and previous intravenous drug use.
He underwent aggressive surgical debulking via median sternotomy. The large right-sided mediastinal mass was encased around the innominate artery, vein, superior vena cava and was adherent to the trachea and oesophagus. The right phrenic nerve was preserved, and right vagus nerve was sacrificed. In debulking the tumour from the various encased mediastinal structures, there was injury to the membranous trachea of approximately 2.5 cm in length as well as the serosal layer of the oesophagus. The trachea was repaired with an interrupted 4/0 polydioxanone sutures. The operation proceeded without further complications, and good homeostasis was achieved. An apical and a basal intercostal catheter (ICC) were inserted at the completion of the procedure to monitor postoperative bleeding.
The patient had a routine admission to intensive care unit postoperatively and was extubated on day 2 postoperatively. He was then transferred to the ward for ongoing care. The apical and basal ICCs were removed as drain outputs were minimal. He was kept nil by mouth for 3 days postoperatively to rest his oesophagus, and a soft diet was introduced on day 4 with removal of chest drains. He was upgraded to a normal diet on day 5 and was discharged home.
The patient represented to the emergency department on day 7 postoperatively with nausea, vomiting, rigours and chest pain. A CT chest demonstrated a large right-sided pleural effusion with right lower lobe collapse. Given the low density of the fluid and the rapid progression over 3 days, a thoracic duct injury was suspected. A right-sided 16Fr ICC was inserted and immediately drained 1 L of turbid discharge which was sent for analysis. The results showed presence of chylomicrons with a triglyceride level of 11.5 mmol/L and cholesterol level of 1.7 mmol/L. The patient was commenced on a low-fat diet guided by dietetics. The following day, a further 2 L had drained from the ICC. Over the course of the next few days, he drained a total of 5 L of pleural fluid. As the drain volumes reached 50 mL/day and the consistency became clear pleural fluid, the ICC was removed, 9 days after insertion. He experienced ongoing nausea, vomiting and food intolerance despite prokinetics and antiemetics which was investigated with a gastric emptying study demonstrating severe gastroparesis. He required total parenteral nutrition (TPN) and eventually went on to have a feeding NJ tube placed.
Investigations
CT chest
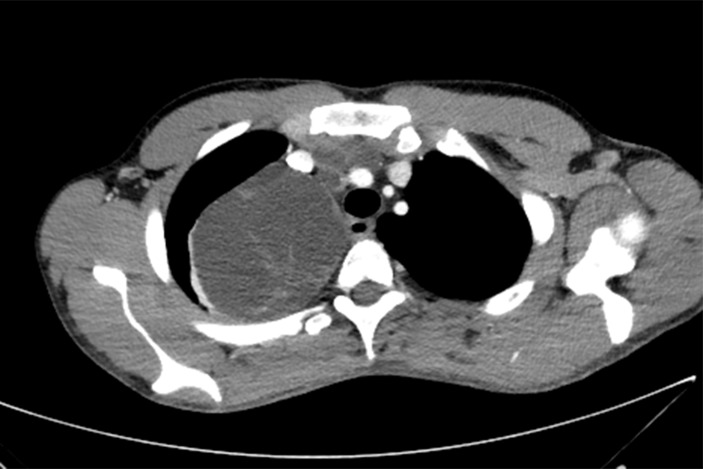
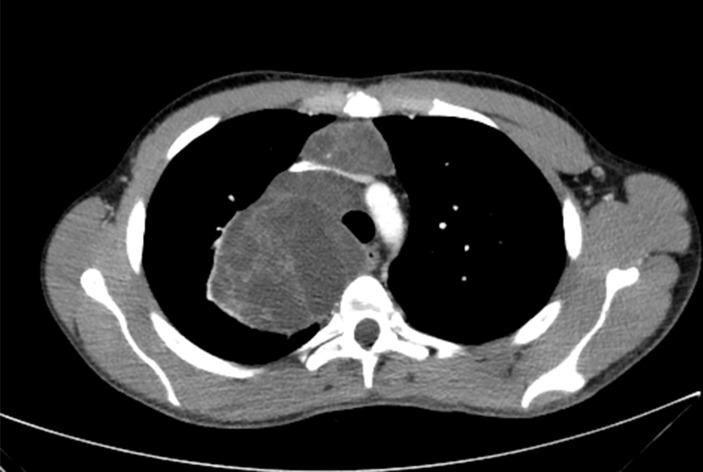
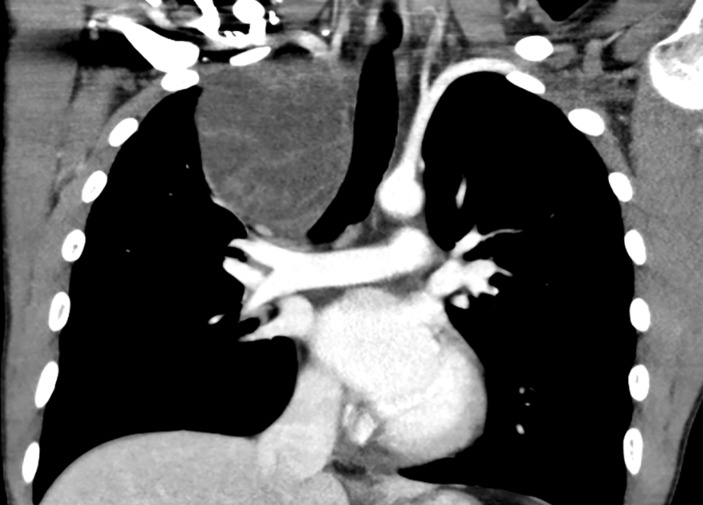
Multiple anterior mediastinal metastases, the largest in the right paratracheal region and right thoracic apex measuring 88 mm AP diameter (figure 1). Two smaller metastases in the prevascular and right paratracheal stations compress the superior vena cava (SVC) and left brachiocephalic vein which remain patent (figure 2). The largest mass minimally displaces the trachea and oesophagus medially and compresses the adjacent right upper lobe (figure 3).
Figure 1.
CT transverse section of the chest showing its maximal AP diameter of 88 mm.
Figure 2.
CT transverse section of the chest. Two smaller metastases in the prevascular and right paratracheal stations compress the SVC and left brachiocephalic vein which remain patent.
Figure 3.
CT coronal section of the chest. Two smaller metastases in the prevascular and right paratracheal stations compress the SVC and left brachiocephalic vein which remain patent.
Histopathology of anterior mediastinal mass
Metastatic teratoma, 105 mm, abutting but not involving benign thymus. Excision appears to be complete.
Pleural fluid analysis
Microscopy, culture and sensitivities:
No bacterial growth. Particles present indicative of chylomicrons.
Cholesterol=1.7 mmol/L
Triglyceride=11.5 mmol/L
Outcome and follow-up
He was discharged home with ongoing follow-up with cardiothoracic surgery, dietetics, gastroenterology and medical oncology. His discharge plan included a low-fat diet for a further 6 weeks. In his most recent 6-month postoperative follow-up, the patient’s chylothorax had completely resolved, however, we was unfortunately having ongoing gastroparesis. This was not responding to antiemetics and prokinetics such as metoclopramide and domperidone. He was therefore referred to general surgery for consideration of gastric pacing. His CT staging scan 6 months postoperatively did not show any new tumours.
Discussion
Surgical resection of extraretroperitoneal metastases is advised in suitable patients as 50% of patients will have an underlying teratoma or viable GCT.9 According to the International Germ Cell Cancer Collaborative Group, the 5-year survival rate with mediastinal metastases is 48%. Therefore, we opted for aggressive debulking to improve survival. Median sternotomy provides adequate exposure to the superior mediastinum, anterior mediastinum, lungs and the pulmonary hilum. For a tumour, as large as our patient’s, this was the ideal surgical approach.
The thoracic duct runs along the vertebral column in the posterior mediastinum and this position usually protects it from injury. A major complication from thoracic surgery is trauma to the thoracic duct resulting in a chylothorax, with a mortality of 50% if left untreated.10 Treatment of a chylothorax involves insertion of intercostal chest catheters and nutrient replacement. Administration of low-fat medium chain triglycerides or nil by mouth settles 50% of traumatic chylothoraces.11 Medium chain triglycerides do not get absorbed into the lymphatic system, they are directly absorbed into the portal circulation. This allows the thoracic duct to heal and prevents exacerbation of the chylothorax.12 Other treatment modalities include surgical management when conservative management fails. This is advocated for in situations where conservative management has failed with drain outputs greater than 1 L/day for 5 days.13 Two surgical techniques most commonly used include direct wound ligature or upstream ligature.14 Other surgical treatments include pleurodesis or placement of a pleuroperitoneal shunt which are used in select cases.14
Due to our patient having an intraoperative complication of laceration to the serosal layer of the oesophagus, he was kept nil by mouth for 3 days. The delayed feeding resulted in delayed recognition of the underlying thoracic duct injury. He therefore represented on day 7 postoperatively with a large right-sided chylothorax. He underwent insertion of a chest drain which drained approximately 5 L of pleural fluid. He was initially placed on a low-fat diet, however, due to his severe postoperative gastroparesis, he was initiated on TPN. It is possible that the severe gastroparesis was secondary to the vagus nerve being sacrificed intraoperatively. The administration of TPN and a low-fat diet allowed for decreased drainage of chyle and subsequent removal of chest drains. Given the large burden of tumour that was resected, we suspect the thoracic duct injury to be large, taking several weeks to heal. The patient was therefore discharged on low-fat diet for a further 6 weeks.
There are no randomised control trials to provide strong evidence for a specific management approach for postoperative chylothorax. However, several retrospective studies have shown that aggressive conservative management yields positive outcomes.
An important learning point from this case is that patients who experience intraoperative complications to vital structures such as the trachea, oesophagus or thoracic duct, should be monitored in hospital for several days prior to discharge. The case illustrates the importance of multidisciplinary approach with the involvement of oncologists for initiating chemotherapy, urologists for removal of the testicular tumour and retroperitoneal lymph node dissection and cardiothoracic surgeons for the resection of the mediastinal mass. In addition, the input from dietitians, speech pathologists and physiotherapists allowed for effective management of the patient’s complications.
Learning points.
The management of metastatic non-seminomatous germ cell testicular tumour (NSGCT) involves a multidisciplinary approach.
Surgical resection of mediastinal metastases from primary testicular NSGCT is favourable in carefully selected patients.
Delayed feeding postcardiothoracic surgery can result in delayed recognition of occult thoracic duct injury. Such patients should be monitored in hospital for longer periods prior to discharge.
Postoperative chylothorax is a rare complication with severe consequences including nutritional and immune deficiency.
A conservative management approach towards large thoracic duct injury results in favourable outcomes without the necessity of further surgical intervention.
Footnotes
Contributors: RL was the consultant surgeon operating on the patient. EW and UA were involved in the postoperative care of the patient. UA was the primary writer for the case report. EW and RL reviewed and finalised the report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med 1997;337:242–54. 10.1056/NEJM199707243370406 [DOI] [PubMed] [Google Scholar]
- 2.Hu B, Daneshmand S. Role of extraretroperitoneal surgery in patients with metastatic germ cell tumors. Urol Clin North Am 2015;42:369–80. 10.1016/j.ucl.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 3.Gerl A, Clemm C, Schmeller N, et al. Sequential resection of residual abdominal and thoracic masses after chemotherapy for metastatic non-seminomatous germ cell tumours. Br J Cancer 1994;70:960–5. 10.1038/bjc.1994.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fizazi K, Dunant A, Oldenburg J, et al. Viable malignant cells after primary chemotherapy for disseminated non-seminomatous germ-cell tumors (NSGCT): An international validation study. Journal of Clinical Oncology 2005;23:4521–S. 10.1200/jco.2005.23.16_suppl.4521 [DOI] [Google Scholar]
- 5.Kesler KA, Donohue JP. Combined Urologic and Thoracic Approaches for Advanced or Disseminated Testis Cancer. Atlas of the Urologic Clinics 1999;7:79–94. 10.1016/S1063-5777(05)70130-4 [DOI] [Google Scholar]
- 6.Zilversmit DB. The composition and structure of lymph chylomicrons in dog, rat, and man. J Clin Invest 1965;44:1610–22. 10.1172/JCI105267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wemyss-Holden SA, Launois B, Maddern GJ. Management of thoracic duct injuries after oesophagectomy. Br J Surg 2001;88:1442–8. 10.1046/j.0007-1323.2001.01896.x [DOI] [PubMed] [Google Scholar]
- 8.Macfarlane JR, Holman CW. Chylothorax. Am Rev Respir Dis 1972;105:287–91. 10.1164/arrd.1972.105.2.287 [DOI] [PubMed] [Google Scholar]
- 9.Carver BS, Sheinfeld J. Management of post-chemotherapy extra-retroperitoneal residual masses. World J Urol 2009;27:489–92. 10.1007/s00345-009-0412-2 [DOI] [PubMed] [Google Scholar]
- 10.McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med 2010;104:1–8. 10.1016/j.rmed.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 11.Alvarez J, Kalache K, Grauel E. Management of Spontaneous Congenital Chylothorax: Oral Medium-Chain Triglycerides Versus Total Parenteral Nutrition. Am J Perinatol 1999;16:0415–20. 10.1055/s-1999-6816 [DOI] [PubMed] [Google Scholar]
- 12.de Beer HG, Mol MJ, Janssen JP. Chylothorax. Neth J Med 2000;56:25–31. 10.1016/S0300-2977(99)00114-X [DOI] [PubMed] [Google Scholar]
- 13.Marts BC, Naunheim KS, Fiore AC, et al. Conservative versus surgical management of chylothorax. Am J Surg 1992;164:532–5. 10.1016/S0002-9610(05)81195-X [DOI] [PubMed] [Google Scholar]
- 14.Fernández Alvarez JR, Kalache KD, Graŭel EL. Management of spontaneous congenital chylothorax: oral medium-chain triglycerides versus total parenteral nutrition. Am J Perinatol 1999;16:0415–20. 10.1055/s-1999-6816 [DOI] [PubMed] [Google Scholar]