Abstract
A 49-year-old male patient, morbidly obese, with a background of Lynch syndrome and subtotal colectomy for colon cancer in 2007, presented with severe abdominal pain in December 2015. Since then, the patient presented multiple times to the emergency department with severe diffuse abdominal pain. After extensive examination, no clear cause for the pain was identified and it was thought to be secondary to adhesions, incisional hernias and psychological. Examinations via radiological imaging were challenging due to body habitus and claustrophobia. In September 2017, the patient was admitted from outpatient clinic with severe abdominal pain, weight loss and anaemia. A CT scan of abdomen and pelvis demonstrated a dilated jejunal loop with a possible tumour. Surgery confirmed a small bowel tumour and, nearly 2 years after the initial presentation, the patient was diagnosed with adenocarcinoma of the jejenum. The patient underwent surgical excision and his symptoms subsided.
Keywords: gastrointestinal surgery, small intestine cancer
Background
Small bowel adenocarcinoma (SBA) is a very rare disease with many surgeons never seeing one. Lynch syndrome is known to predispose to other types of cancer. Small bowel cancer (SBC) is extremely rare even in Lynch syndrome and easily overlooked as a differential diagnosis. Mutation carriers have an estimated lifetime risk of 4% which corresponds to a relative risk of 100 compared with normal population.1
This paper emphasises the importance of investigating patients with Lynch syndrome for multiple types of gastrointestinal cancer in the absence of validated surveillance pathways.
This case also highlights the importance of alternative diagnostic techniques. His radiological investigations were limited by body habitus and claustrophobia, which added further delays in reaching the final diagnosis.
Case presentation
Presentation
A 49-year-old, morbidly obese male (body mass index=55), under consideration for weight loss surgery, presented to the emergency department with severe epigastric pain in December 2015. Over the next 18 months, the patient suffered with worsening epigastric pain that became constant, despite high doses of opiates. There was no change in bowel habit or blood in the stool. Occasionally, the patient had subacute obstructive symptoms. The pain was not associated with food and there were no reflux symptoms, nausea or vomiting. Later on, the patient had weight loss, which was thought to be intentional and microcytic anaemia, for which he had a blood transfusion in May 2017.
The patient has a background of hereditary non-polyposis colorectal carcinoma (HNPCC), subtotal colectomy with ileorectal anastomosis and adjuvant chemotherapy in 2007 for descending colon adenocarcinoma (pT4, pN1, pM0, R0), sleep apnoea and ischaemic heart disease. At the time of admission (September 2017), the patient’s medication was oxycodone, omeprazole, ranitidine, gabapentin, buscopan, aspirin, quinine, iron tablets, and folic acid, and no drug allergies were reported. He was an independent man, living with his partner, smoker and non-drinker. He had five brothers and two sisters, all diagnosed with Lynch syndrome and with various colorectal malignancies.
On examination, the abdomen was obese, but not distended. He was tender in the epigastrium but no signs of peritonism. There were at least three ventral midline incisional hernias, which were all soft, non-tender and reducible. The rest of the physical examination was unremarkable.
Investigations
In March 2016, the blood tests demonstrated mild anaemia (haemoglobin (Hb) 129 g/dL, mean corpuscular haemoglobin (MCH) 26.7 pg, mean corpuscular volume (MCV) 82.0 fL) and CT abdomen and pelvis demonstrated four large anterior abdominal wall midline hernias, a single dilated small bowel loop in the left flank but obvious transition point to suggest acute small bowel obstruction and small volume mesenteric lymphadenopathy.
By June 2016, the patient had developed a worsening anaemia (Hb 110 g/dL, MCH 24.7 pg, MCV 81.0 fL, ferritin 13.3 mcg/L, iron 7.6 µmol/L). A colonoscopy and oesophagogastroduodenoscopy (OGD) were normal.
In May 2017, a repeat CT scan of the abdomen and pelvis demonstrated unchanged small volume lymphadenopathy; however, the blood results demonstrated a much worse microcytic anaemia (Hb 69 g/dL, MCH 20.1 pg, MCV 70.9 fL). The patient had repeated colonoscopy and OGD, which were again normal. In August 2017, an MR enterography followed by a CT enterography were attempted but the patient could not tolerate these investigations due to claustrophobia.
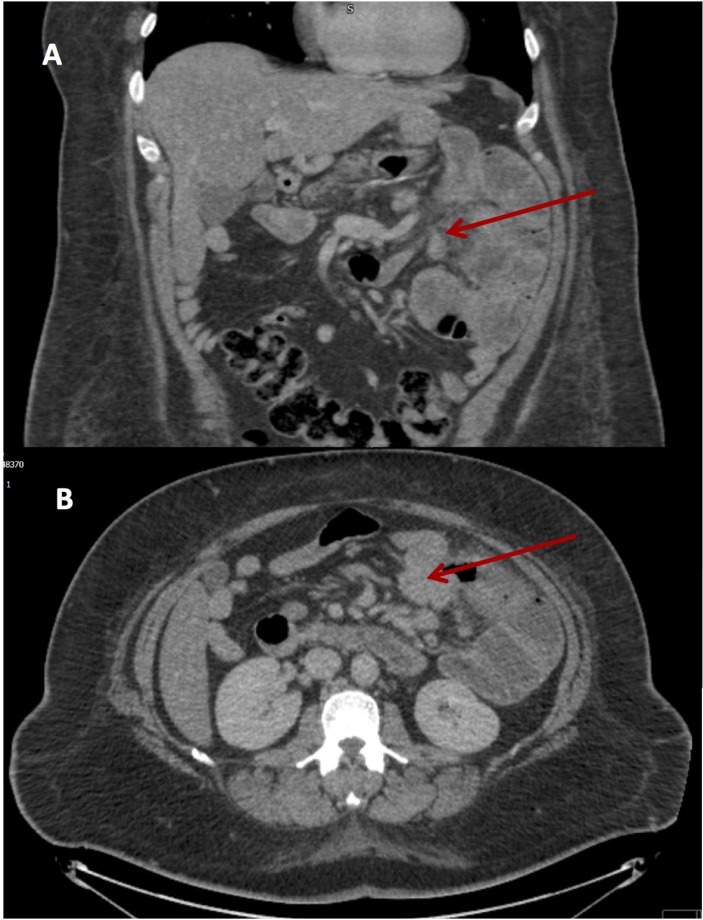
In September 2017, the patient was reviewed in outpatient clinic with severe ongoing abdominal pain, weight loss of over two stones in the preceding year, ongoing anaemia (Hb 78 g/dL). A repeat CT scan of the abdomen and pelvis demonstrated dilatation of the proximal jejunum with haziness of the adjacent fat and suspicion of a small bowel tumour (figure 1). A gastrografin follow through confirmed small bowel dilatation up to 5 cm in left upper quadrant.
Figure 1.
CT abdomen and pelvis, coronal (A) and axial (B) view. The arrow points towards the area of suspected small bowel adenocarcinoma located at the transition point between dilated and collapsed small bowel.
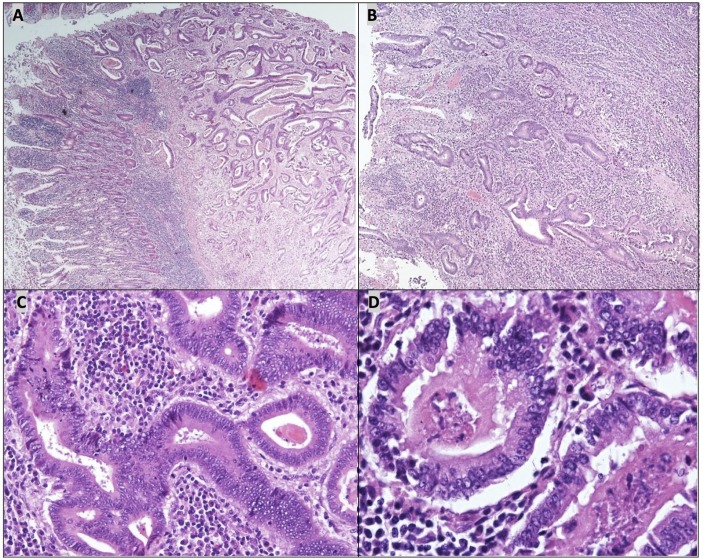
At laparotomy, a jejunal mass was found causing small bowel obstruction. The patient had small bowel resection ‘en block’ with the mass. The histology confirmed moderate to poorly differentiated adenocarcinoma invading the full thickness of small bowel wall and infiltrating into omental fat with CK20+, CDX2+, CK19+ and CK7− genotype and clear resection margins (figure 2). In the view of Lynch syndrome with colectomy for poorly differentiated splenic flexure adenocarcinoma, the appearances were consistent with primary SBA, with pathological stage pT4, pN0, pM0, L0, V0, R0. The Cdx2+ and CK20+ immunohistochemistry results are classic findings for SBA.2
Figure 2.
Small bowel adenocarcinoma (A) with moderate and poorly differentiated areas (B). The better differentiated parts of the tumour comprised infiltrative atypical glands (C) with areas of ‘dirty necrosis’ (D). The tumour cells contained vesicular nuclei with pleomorphism and multiple prominent nucleoli.
Differential diagnosis
Initially, the primary differential diagnosis for the epigastric pain, based on clinical examination and radiological imaging, was adhesional pain as a result of the previous abdominal operations. The patient was also diagnosed with gallstones, but the pain was not typical for gallstones. An OGD ruled out the peptic ulcer disease and gastritis. Another potential diagnosis was strangulation or incarceration of the incisional hernias. However clinical examination and radiological imaging confirmed that these were non-complicated. Subacute bowel obstruction was a possible diagnosis; however, there was no radiological evidence until later in the investigative process. Significant anaemia and weight loss became apparent and a diagnosis of malignancy was raised with further investigations carried out to rule in or rule out this diagnosis.
Treatment
A diagnostic laparotomy (September 2017) confirmed small bowel obstruction as a result of a jejunal tumour with two small bowel (SB) loops and omentum stuck onto it. A 55×30×35 mm tumour was removed ‘en block’ with two SB loops, and two SB anastomosis were performed.
Outcome and follow-up
The immediate postoperative recovery was complicated by an unexpected severe penicillin reaction with anaphylaxis and cardiac arrest. This meant an extended intensive care unit stay above what would have normally been expected. Postoperatively, the patient developed type A surgical site infections which were treated conservatively. The patient was followed up routinely in the colorectal clinic postdischarge.
The patient and his family have found it difficult adjusting back to ‘normal life’ after spending nearly 2 years in and out of hospital but say they are moving in the right direction. However, the follow-up CT scan in December 2017 showed lymph node recurrence around coeliac axis. His pain symptoms returned, and he started chemotherapy.
Discussion
SBC is a rare malignancy that comprises less than 5% of all gastrointestinal malignancies.3 It is most frequently diagnosed among people aged 55–64, with the incidence increasing after 40 years of age.4 SBC has four common histological types: adenocarcinoma, carcinoid, lymphoma and sarcoma whereby adenocarcinoma comprises 30%–40% of these cancers.5 SBAs are most commonly located in the duodenum (73%), while 27% of cases occur in the jejenum/ileum.6 The incidence of SBA in the jejenum is higher (37%) in patients with HNPCC than those with sporadic SBA.7 SBA is associated with a number of predisposing conditions including familial adenomatous polyposis, HNPCC, Peutz-Jeghers syndrome, Crohn’s disease, coeliac disease, previous colorectal malignancy, MUTYH-associated polyposis and cystic fibrosis.8 A number of environmental factors also increase the risk of SBA, and these include alcohol consumption, smoking, high consumption of sugar, red meat and smoked foods.9 10 Typical presenting signs and symptoms of SBA include non-specific abdominal discomfort, such as abdominal pain, nausea, vomiting, intestinal obstruction, abdominal mass, gastrointestinal bleeding, anaemia, weight loss and jaundice. This and the relative inaccessibility of small intestine tumours, the absence of extensive diagnostic studies and misinterpretation of abnormal imaging studies leads to an average delay of 6–10 months in diagnosis.11 Early diagnosis is crucial as the 5-year survival for stage 3 SBA is poor at 30% compared with 70% for stage 1.12 To increase the rates of early diagnosis, improved CT/MRI techniques and endoscopic modalities have been developed to overcome the inaccessibility of the small bowel.13 It is recommended that following inconclusive CT/MRI enteroscopy, as was the case with this patient, capsule endoscopy, double balloon enteroscopy, positron emission tomography scan, endoscopic ultrasound and laparoscopy should be used as additional diagnostic modalities if clinical suspicion persists. A greater awareness of SBA as a possible cause of non-specific abdominal symptoms will also improve the rates of early diagnosis.
Learning points.
Consider small bowel malignancy as differential diagnosis in high-risk patients (Lynch, Crohn, familial adenomatous polyposis, Peutz-Jeghers, coeliac disease, previous colorectal malignancy, cystic fibrosis and MUTYH-associated polyposis.
Never put the cause of a patient’s symptoms down to psychological reasons until all other possible causes have been disproven.
Poor quality imaging can be misleading and diagnostic alternatives should be sought.
Consider all possible causes of disease regardless of the rarity of a condition.
Footnotes
Contributors: JP and EF wrote the first and second draft of the case report. This was proofread by DH and GB-S.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Koornstra JJ, Kleibeuker JH, Vasen HFA. Small-bowel cancer in Lynch syndrome: is it time for surveillance? Lancet Oncol 2008;9:901–5. 10.1016/S1470-2045(08)70232-8 [DOI] [PubMed] [Google Scholar]
- 2.Wong HH, Chu P. Immunohistochemical features of the gastrointestinal tract tumors. J Gastrointest Oncol 2012;3:262–84. 10.3978/j.issn.2078-6891.2012.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small bowel cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control 2005;16:781–7. 10.1007/s10552-005-3635-6 [DOI] [PubMed] [Google Scholar]
- 4.SEER cancer statistics factsheets: small intestine cancer. National Cancer Institute. Bethesda, MD: http://seer.cancer.gov/ststfacts/html/smint.html [Google Scholar]
- 5.Pan SY, Morrison H. Epidemiology of cancer of the small intestine. World J Gastrointest Oncol 2011;3:1–42. 10.4251/wjgo.v3.i3.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakae H, Kanzaki H, Nasu J, et al. The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br J Cancer 2017;117:1607–13. 10.1038/bjc.2017.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koornstra JJ, Kleibeuker JH, Vasen HF, et al. Small-bowel cancer in Lynch syndrome: is it time for surveillance? Lancet Oncol 2008;9:901–5. 10.1016/S1470-2045(08)70232-8 [DOI] [PubMed] [Google Scholar]
- 8.Shenoy S. Genetic risks and familial associations of small bowel carcinoma. World J Gastrointest Oncol 2016;8:509–19. 10.4251/wjgo.v8.i6.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu AH, Yu MC, Mack TM. Smoking, alcohol use, dietary factors and risk of small intestinal adenocarcinoma. Int J Cancer 1997;70:512–7. [DOI] [PubMed] [Google Scholar]
- 10.Chow WH, Linet MS, McLaughlin JK, et al. Risk factors for small intestine cancer. Cancer Causes Control 1993;4:163–9. 10.1007/BF00053158 [DOI] [PubMed] [Google Scholar]
- 11.Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 2004;101:518–26. 10.1002/cncr.20404 [DOI] [PubMed] [Google Scholar]
- 12.Survival Rates of Small Intestine Adenocarcinoma, by Stage. American Cancer Society. https://www.cancer.org/cancer/small-intestine-cancer/detection-diagnosis-staging/survival-rates.html
- 13.Cheung DY, Choi MG. Current advance in small bowel tumors. Clin Endosc 2011;44:13–21. 10.5946/ce.2011.44.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]