Abstract
Extracellular vesicles (EVs) are membranous vesicles released by a variety of cells into the extracellular microenvironment. EVs represent a population of heterogeneous vesicles, whose size range between 40 and 1,000 nm. Accumulated evidence indicated that EVs play important regulatory roles in pathogen-host interactions. A deep understanding of schistosome EVs should provide insights into the mechanisms underlying schistosome-host interactions, enabling development of novel strategies against schistosomiasis. Here, we aim to further study EVs functions in schistosomes by presenting a protocol for the isolation and characterization of EVs from adult Schistosoma japonicum (S. japonicum). EVs were isolated from in vitro culture medium using centrifugation combined with a commercial exosome isolation kit. The isolated S. japonicum EVs (SjEVs) typically possess a diameter of 100 - 400 nm, and are characterized by transmission electronic microscopy and western blotting. The usage of PKH67 dye-labeled SjEVs has demonstrated that SjEVs are internalized by the recipient cells. Overall, our protocol provides an alternative method for isolating EVs from adult schistosomes; the isolated SjEVs may be suitable for functional analysis.
Keywords: Immunology and Infection, Issue 135, Extracellular vesicles, Isolation, Schistosomes, Characterization, In vitro culture, Diagnosis
Introduction
Extracellular vesicles (EVs) are a population of small membrane-bound vesicles encapsulated with various proteins, lipids, and nucleic acids. Recent studies demonstrated that EVs play a crucial role in cell-cell communication, and are involved in the regulation of numerous physiological processes, including cell development, immune regulation, angiogenesis, and cell migration2,3,4,5. Accumulating evidence indicates that EVs, circulating exosomes, and their miRNA cargo represents potential biomarkers of certain diseases6.
Several protozoa such as Trichomonas vaginalis, Trypanosoma cruzi, and Leishmania spp., have been shown to be able to secrete EVs; helminths have additionally been found to secrete EVs into living hosts7. Parasitic EVs have been shown to be involved in the maintenance of infection, pathogenicity8, and immune regulation9. Recent studies in schistosomes both Schistosoma mansoni (S. mansoni) 10,11 and S. japonicum12 have indicated that adult schistosomes secrete exosome-like vesicles that may be involved in the functional regulations of specific biological processes.
To date, several methods have been used to isolate extracellular vesicles, such as ultracentrifugation,ultrafiltration13, the use of polymer-based reagents, size-exclusion chromatography, and immunoaffinity isolation. These different methods possess their own advantages and limitations14. Generally, ultracentrifugation is considered the gold standard method for vesicle isolation. However, this method suffers from the limitation of potential EV aggregation14.
In schistosomes, a couple of methods have been reported for EV isolation: these include ultracentrifugation11,12,10 and the use of commercial EV isolation kit16 for several stages (eggs16, schistosomula11, adult schistosomes10,12,17). Given that microvesicles are of a wide range of size from several hundred nanometers to thousand nanometers, we developed an alternative method that combines with the use of bench centrifuge, ultrafiltration, and a commercial EV isolation kit to isolate the EVs from adult Schistosoma japonicum. The isolated EVs typically possessed a diameter of 100 - 400 nm and were successfully internalized by the recipient cells.
Protocol
All animal experiments were approved by the local ethics committee of Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Permit Number: SHVRIAU-14-0101).
1. In Vitro Culture of Schistosomes
Note: Schistosomes represent a biohazard. Workers should wear latex gloves at all times when handling worms, schistosomal suspensions, or other related biological materials. New Zealand rabbits were infected with ~2,000 cercariae via abdominal exposure. Schistosomes at the liver stage were collected from rabbits infected with S. japonicum cercariae at 28 days post-infection by perfusion of liver and mesenteric veins. The procedures of worm collection have been referenced in previous publications reporting studies in mice18.
Collect parasites in a 500-mL beaker. Wash them thoroughly and gently 3x with 200 mL of preheated (37 °C) PBS (pH 7.4).
Separate ~200 worm pairs for each 90 mm Petri dish. Then wash the parasites thoroughly and gently 2x with 50 mL of preheated (37 °C) RPMI-1640 culture medium containing 100 U of penicillin and 100 mg/mL of streptomycin.
Maintain parasites in 40 mL of preheated RPMI-1640 culture medium without antibiotics at 37 °C under 5% CO2 at a density of ~5 worm pairs /mL for 2 h in 90 mm Petri dish. Notes: The RPMI 1640 medium should not contain serum, otherwise exogenous extracellular vesicle will be introduced.
2. Pre-treated Condition Media
Collect the culture medium and then centrifuge the medium at 4 °C at 2,000 × g for 30 min to remove eggs and worm tegmental debris.
Transfer the supernatant to a fresh tube and then filter the solution using a 0.22 µm syringe filter.
Collect the filtered solution in a dialyzed bag (molecular weight cut-off: 3.5kD) and then dialyze the solution using 8% PEG8000 solution at 4 °C overnight. Notes: This step is important to enrich SjEVs for increasing the yield of SjEVs isolation.
3. Isolation of SjEVs
Transfer the dialyzed solution to a new tube and then add 0.5 volumes of the total exosome isolation reagent.
Mix the solution well by vortexing or pipetting up and down to ensure homogeneity of the solution.
Incubate the mixture at 2 °C to 8 °C overnight.
Centrifuge the mixture at 15,000 × g for 1 h at 2 °C to 8 °C.
Aspirate and discard the supernatant. Notes: EVs are contained in the pellet at the bottom of the tube (not visible in most of the cases).
Resuspend the EVs pellet in 2 mL of PBS and then store the EVs solution at -80 °C until further analysis.
4. Characterization of EVs by Western Blotting
Determine the protein concentration of the SjEVs sample, e.g., by BCA assay.
Prepare 10 - 20 µg of EVs in 22.5 µL RIPA buffer. Then add 7.5 µL of 4x loading buffer and heat for 5 min at 95 °C.
Separate SjEVs proteins by 12% SDS-PAGE gel.
Transfer the proteins to PVDF membrane and then probe with anti-CD63 antibodies (1:1,000 dilution), followed by probing with anti-rabbit IgG-HRP (1:5,000 dilution).
Use ECL detection reagent for membrane development and detect the signals by a chemiluminescence imaging system.
5. Characterization of EVs by Transmission Electron Microscopy
Add 2 µL of isolated SjEVs to 200 mesh formvar-coated grids and then allow it to dry at room temperature.
Stain with 2% Phosphotungstic Acid for 1 min and then allow it to dry at room temperature.
Load the grids onto the sample holder of the transmission electron microscope and exposed to an 80 kV electron beam for image capture.
6. SjEVs Label with PKH67 and Cell Uptake Assay
Notes: All subsequent steps were performed at ambient temperature (20 - 25 °C), unless otherwise indicated.
Transfer 5 µg SjEVs solution into a conical bottom polypropylene tube and adjust the volume to 12 mL by adding RPMI 1640 medium.
Centrifuge the SjEVs at 110,000 × g for 90 min at 4 °C to pellet SjEVs.
After centrifugation, carefully aspirate the supernatant. Note: The PKH67 ethanolic dye should not be added directly to the SjEV pellet, as this would result in heterogeneous staining and reduced cell viability.
Prepare a 2x EV Suspension solution by adding 1 mL of Diluent C to the SjEV pellet; to ensure complete dispersion, resuspend with gentle pipetting.
Just before staining in Diluent C, prepare a fresh 2x Dye Solution by adding 2 µL of the PKH67 ethanolic dye solution to 1 mL of Diluent C in a polypropylene centrifuge tube and mixing well to disperse.
Rapidly add the 1 mL of 2x EV Suspension (Step 6.4) to 1 mL of 2x Dye Solution (Step 6.5) and immediately mix the sample by pipetting.
Incubate the mixture at room temperature for 4 min.
Stop the staining by adding 4 mL of FBS and then incubate for 1 min.
Add RPMI1640 medium to adjust the volume to 12 mL and then centrifuge at 110,000 × g at 4 °C for 90 min.
Remove the supernatant and add RPMI1640 medium to resuspend the PKH67 labeled SjEV pellets.
Centrifuge at 110,000 × g at 4 °C for 90 min to wash once and then add PBS to resuspend the SjEVs pellets.
7. SjEVs Cell Uptake Assay
Seed mammalian cells such as NCTC clone 1469 cells or HEK293T cells in 6-well plates (2× 105 cells per well) and culture the cells overnight.
Add 5 µg PKH67-labelled SjEVs and then culture cells at 37 °C for 2 - 4 h.
After incubation, remove the medium and wash the cells with PBS twice.
Fix the cells with 4% formaldehyde solution for 15 min and washed twice more with PBS.
Stain cell nuclei with 4′,6-diamidino-2-phenylindole (DAPI).
Observe the cells using fluorescence microscopy.
Representative Results
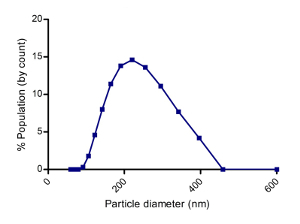
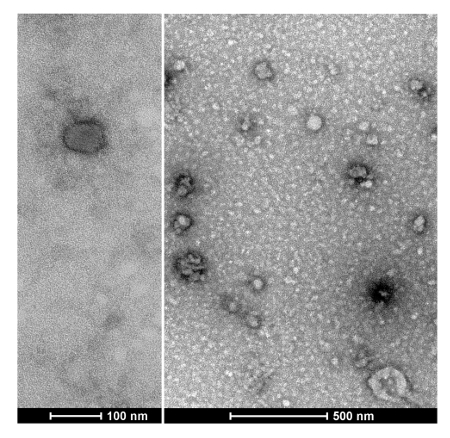
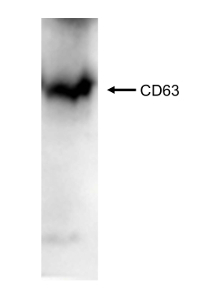
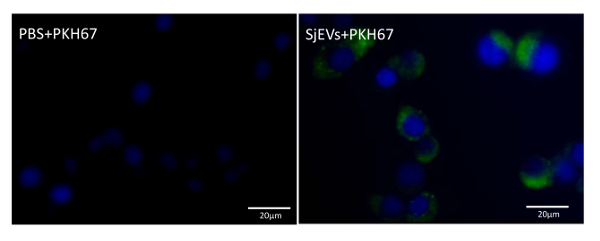
To quantify the yield of isolated SjEVs using the described protocol, we used BCA protein assay to access the protein concentration of the isolated SjEVs from 28-day adult schistosomes. As shown in Table 1, the SjEV protein concentration ranged from 208 µg to 250 µg per 100 mL of medium (Table 1).The particle size, as determined by Malvern nanoparticle analysis, indicated that the isolated SjEVs ranged from 100 nm to 400 nm, with the highest population around 220 nm in size (Figure 1). Further characterization of the SjEVs by transmission electron microscopy revealed EVs to be round vesicles of approximately 100 - 200 nm in diameter (Figure 2). Western blotting analysis indicated that the isolated SjEVs were recognized using CD63 antibodies, which is a typical EV marker (Figure 3). Fluorescence microscopy observation indicated that the PKH67-labeled SjEVs were internalized by the NCTC clone 1,469 cells and that the SjEVs were mainly distributed in the cytoplasm of recipient cells (Figure 4).
| Samples | EVs/100ml culture medium |
| #1 | 250 µg |
| #2 | 208 µg |
| #3 | 220 µg |
Table 1: SjEVs protein obtained from culture medium; the amount of SjEVs accessed by BCA assay per 100 mL of culture medium, containing ~500 worm pairs, is shown.
Figure 1: Size distribution of SjEVs Isolated from cultured medium. 20 µL of SjEVs solution was diluted with 1000 µL PBS; then, the mixture was analyzed using a high performance two angle particle and molecular size analyzer. The result shown is representative of the data from three independent isolations. Please click here to view a larger version of this figure.
Figure 2 : Characterization of SjEVs by transmission electron microscopy. Please click here to view a larger version of this figure.
Figure 3 : Western blotting analysis of the isolated SjEVs against CD63 antibodies. Please click here to view a larger version of this figure.
Figure 4: Uptake of SjEVs by mammalian cells. The PKH labeled SjEVs are shown in green, while the cell nuclei are shown in blue. Please click here to view a larger version of this figure.
Discussion
Recent studies on EVs have demonstrated that schistosome EVs play an important role in host-pathogen interactions3,9,12,16. To further address their regulatory functions, it is essential to isolate EVs from schistosomes. Here, we describe an alternative method for SjEV isolation. This method yields a wide range size of SjEVs, from 100 nm to 400 nm, in adult S. japonicum. The following cell uptake assay indicated that EVs isolated under the described protocol were successfully internalized by the recipient cells and that their associated miRNAs potentially play regulatory roles17.
EVs, which may be secreted by many different types of cells, are also present in various kinds of body fluids19. Consequently, it is important to avoid to artificially introducing exogenous EVs during the process of SjEV isolation. For example, the culture medium should not contain serum. The worm density in the in vitro culture should be reasonable to minimize the potential stress response and lower the mortality rates of worms. In addition, it should be noted that different stages of schistosomes or worms from different hosts may present different EVs. Consequently, it is imperative to maintain the parasites in in vitro environments that closely resemble the host environment, and the conditions under which SjEV isolation is performed must be consistent. Additionally, we are unable to rule out the possibility of variance related to SjEVs caused by culture stress.
Here, we present a protocol for the isolation of SjEVs from 2 h in vitro culture media for adult schistosomes. An advantage of this protocol is that short culture time minimizes the stress to which parasites are exposed, which may result in non-physiological EV secretion. In addition, we found that the SjEVs isolated as described in this protocol were internalized by the recipient cells, suggesting that these SjEVs may be biologically active17. Overall, the present protocol allows the efficient isolation of SjEVs from adult worms using standard laboratory equipment, and enables their characterization using western blotting and cell uptake assay. The isolated EVs may be used to further characterize host-pathogen interactions.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This study was, in part or in whole, supported by National Natural Science Foundation of China (31472187 and 31672550) and The Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences.
References
- Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44(1):11–19. doi: 10.1007/s12020-012-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36(3):301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz F, Cheng G. Exosome-like vesicles of helminths: implication of pathogenesis and vaccine development. Ann Transl Med. 2017;5(7):175. doi: 10.21037/atm.2017.03.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17(5):959–964. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcilla A, et al. Extracellular vesicles in parasitic diseases. J Extracell Vesicles. 2014;3:25040. doi: 10.3402/jev.v3.25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwiklinski K, et al. The Extracellular Vesicles of the Helminth Pathogen, Fasciola hepatica: Biogenesis Pathways and Cargo Molecules Involved in Parasite Pathogenesis. Mol Cell Proteomics. 2015;14(12):3258–3273. doi: 10.1074/mcp.M115.053934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck AH, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. 2014;5:5488. doi: 10.1038/ncomms6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo J, et al. Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int J Parasitol. 2016;46(1):1–5. doi: 10.1016/j.ijpara.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Nowacki FC, et al. Protein and small non-coding RNA-enriched extracellular vesicles are released by the pathogenic blood fluke Schistosoma mansoni. J Extracell Vesicles. 2015;4:28665. doi: 10.3402/jev.v4.28665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. Exosome-like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune- activity of macrophage. Parasitol Res. 2015;114(5):1865–1873. doi: 10.1007/s00436-015-4373-7. [DOI] [PubMed] [Google Scholar]
- Koh YQ, Almughlliq FB, Vaswani K, Peiris HN, Mitchell MD. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front Biosci (Landmark Ed) 2018;23:865–874. doi: 10.2741/4621. [DOI] [PubMed] [Google Scholar]
- Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016;12(9):504–517. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- Livshits MA, et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319. doi: 10.1038/srep17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, et al. Release of extracellular vesicles containing small RNAs from the eggs of Schistosoma japonicum. Parasit Vectors. 2016;9(1):574. doi: 10.1186/s13071-016-1845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, et al. Molecular characterization of S. japonicum exosome-like vesicles reveals their regulatory roles in parasite-host interactions. Sci Rep. 2016;6:25885. doi: 10.1038/srep25885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis F. Schistosomiasis. Curr Protoc Immunol. 2001. p. 11. Chapter 19 Unit 19. [DOI] [PMC free article] [PubMed]
- Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev. 2015;81:75–93. doi: 10.1016/j.addr.2014.09.001. [DOI] [PubMed] [Google Scholar]


