Abstract
Introduction
Several characteristics associated with increased risk for Parkinson’s disease (PD) have been identified, including specific genotypes and various non-motor symptoms. Characterizing non-motor features, such as cognitive abilities, among individuals considered at-risk for PD is essential to improving prediction of future neurodegeneration.
Methods
Participants belonging to the following cohorts of the Parkinson Progression Markers Initiative (PPMI) study were included: de novo PD with dopamine transporter binding deficit (n = 423), idiopathic REM sleep behavior disorder (RBD, n = 39), hyposmia (n = 26) and non-PD mutation carrier (NMC; Leucine-rich repeat kinase 2 (LRRK2) G2019S (n = 88) and glucocerebrosidase (GBA) gene (n = 38) mutations)). Inclusion criteria enriched the RBD and hyposmia cohorts, but not the NMC cohort, with individuals with dopamine transporter binding deficit. Baseline neuropsychological performance was compared, and analyses were adjusted for age, sex, education, and depression.
Results
The RBD cohort performed significantly worse than the hyposmia and NMC cohorts on Symbol Digit Modality Test (mean (SD) 32.4 (9.16) vs. 41.8 (9.98), p = 0.002 and vs. 45.2 (10.9), p<0.001) and Judgment of Line Orientation (11.3 (2.36) vs.12.9 (1.87), p = 0.004 and vs. 12.9 (1.87), p<0.001). The RBD cohort also performed worse than the hyposmia cohort on the Montreal Cognitive Assessment (25.5 (4.13) vs. 27.3 (1.71), p = 0.02). Hyposmics did not differ from PD or NMC cohorts on any cognitive test score.
Conclusion
Among individuals across a spectrum of risk for PD, cognitive function is worse among those with the characteristic most strongly associated with future risk of PD or dementia with Lewy bodies, namely RBD.
Introduction
In Parkinson’s disease (PD), the second most common neurodegenerative disorder, motor symptoms constitute the core diagnostic criteria[1]. However, the pathophysiological changes of PD begin years to decades before clear-cut motor symptoms manifest[2]. These manifestations include a cluster of at-risk characteristics or prodromal manifestations. Therefore, the definition of PD has been extended to include individuals considered at-risk for PD. These fall along a broad spectrum of risk: asymptomatic carriers of mutations associated with PD, as well as individuals with prodromal non-motor clinical signs/symptoms, biomarker findings, or genetic polymorphisms that alone or in combination predict increased risk for PD to varying degrees[3].
In many cases among individuals at-risk for PD, the course/progression to the motor manifestations of PD aligns well, both anatomically and temporally, with the neuropathological staging system proposed by Braak[4,5], as follows: (1) In Braak stage I, involvement of the olfactory tubercle and medulla manifests clinically with hyposmia (i.e., impaired olfaction), reduced heart rate variability, and other manifestations of autonomic dysfunction; (2) In Braak stage II, there is involvement of more rostral brainstem structures, including the serotonergic dorsal raphe nuclei, which clinically may manifest with anxiety and depression, and the glutamatergic peri-locus coeruleus, which has been hypothesized to lead to REM sleep behavior disorder [RBD]. Involvement of norepinephrine-producing neurons in the locus coeruleus at this stage may also mediate subtle abnormalities in cognition [e.g., attention and working memory] reported in the prodromal PD state[6].
Data on cognition in individuals at-risk for PD are limited, and cognitive changes in subgroups across the at-risk spectrum have not been well described. In addition to enrolling a cohort of de novo PD patients, the Parkinson Progression Markers Initiative (PPMI) study also enrolled individuals without a diagnosis of PD but who are considered at-risk for PD based on the presence of one of the following characteristics: genetic profile (i.e., carriers of Leucine-rich repeat kinase 2 (LRRK2) G2019S or glucocerebrosidase (GBA) gene mutations), hyposmia, or a diagnosis of RBD. This cohort thus represents a mixture of individuals, some who are at-risk for PD but who will never develop it, as well as individuals that may be in the PD prodrome, presumed to be manifesting the earliest signs of neurodegeneration. For brevity, in this manuscript this cohort will hereto forth be referred to as the “at-risk PPMI cohort”. The at-risk PPMI cohort provides a unique opportunity to investigate differences in cognition among at-risk subgroups. Based on Braak staging, we hypothesized a “gradient of prodromalness” in which the RBD cohort would have worse cognition than the hyposmia cohort, which in turn would have worse cognition than the non-PD mutation carrier (NMC cohort). In this study, we investigated this hypothesis in the at-risk PPMI cohort.
Methods
Study participants
PPMI is a multicenter, international, longitudinal cohort study. Study aims, methodology, and details of study assessments have been published elsewhere[7] and are available on the PPMI website (http://www.ppmi-info.org/study-design). PPMI includes several study cohorts. Inclusion criteria vary based on the cohort, as detailed below. Exclusion criteria applying to all cohorts included in this analysis were: (i) dementia based on the site investigator’s clinical assessment and (ii) any medical conditions precluding participation at the discretion of the investigator.
PPMI includes 4 cohorts of participants included in this analysis:
PD cohort (n = 423): newly diagnosed, untreated at enrollment. PD patients were required, at baseline, to have been diagnosed within two years of study enrollment, have dopamine transporter (DAT) binding deficit based on visual interpretation of DaTscan SPECT (as described in the supporting information), and be untreated for PD.
RBD cohort (n = 39). RBD was diagnosed by the site principal investigator (based on clinical history along with polysomnographic findings, where available). Exclusion criteria for this cohort included motor signs that meet criteria for a diagnosable parkinsonian syndrome based on the opinion of the investigator. In order to enrich this cohort with individuals presumed to have incipient motor PD[8], they underwent DAT imaging. All those who had DAT binding deficit (as defined in S1 File) qualified for inclusion in PPMI. In addition, approximately 10% of those without a DAT binding deficit were also included, with the goal of keeping site investigators blinded to DAT SPECT results.
Hyposmia cohort (n = 26). Olfaction was measured using the University of Pennsylvania Smell Identification Test (UPSIT)[9]. Any individual without a diagnosis of PD was eligible to undergo olfactory testing. Recruitment for this cohort occurred from various sources including the community (via targeted online ads) and PPMI sites’ outpatient clinics. Individuals expressing interest in olfactory testing were mailed an UPSIT, and they mailed completed UPSITs back to a central “olfaction core” which scored the smell tests and contacted individuals meeting criteria for hyposmia. Hyposmia was defined as a score of <10th percentile for age and sex. These individuals were then seen at a PPMI site for a screening visit. In order to enrich this cohort with individuals presumed to have incipient motor PD[10–12]) a DAT SPECT was performed at screening. All those who had a DAT binding deficit qualified for inclusion in PPMI. In addition, approximately 10% of those without a DAT binding deficit were also included, with the goal of keeping site investigators blinded to DAT SPECT results.
Non-manifesting mutation carrier (NMC) cohort (n = 126). These were individuals without a diagnosis of PD who are carriers of the G2019S mutation in the LRRK2 gene (n = 88), or the following GBA mutations (n = 38): 84GG (c.115+1G>A), IVS2+1G>A, c.1226A>G (N370S), c.1448T>C (L444P). These individuals were identified through various sources. For example, any adult who was Ashkenazi Jewish and had a 1st degree relative with PD could be referred for telephone-based genetic counseling and screened for the LRRK2 G2019S and GBA mutation, or individuals with a known mutation (regardless of how it was identified) could have self-referred for participation. PPMI also enrolled carriers of synuclein (SCNA) gene mutations but given the small number enrolled at the time of this analysis (n = 5) this subgroup was not included.
The study protocol was approved by the institutional review board of the University of Rochester. Institution review board approval was also obtained at each PPMI site. Written informed consent was obtained from all study participants.
Assessments
Assessments obtained on the PPMI cohort and considered in these analyses included:
Demographics and handedness: age at baseline, sex, education, and self-reported handedness (because only 2% of the cohort reported mixed handedness these were combined with the right-handed group).
Neuropsychological test battery (the domains tested by the respective test is indicated, preceding the test name): Global cognitive function—Montreal Cognitive Assessment (MoCA)[13], Processing speed/attention—Symbol Digit Modalities Test (SDMT)[14], Executive function/working memory—Semantic fluency[15] (number of words generated for animals, vegetables, fruit) and Letter-Number Sequencing (LNS), Verbal memory—Hopkins Verbal Learning Test-Revised (HVLT-R)[16], immediate and delayed free recall and recognition discrimination, Visuospatial function—Benton Judgment of Line Orientation (JOLO) 15-item (split-half) version[17].
Participants were categorized as having mild cognitive impairment (MCI) if they scored >1.5 SD below the mean on ≥2 detailed neuropsychological test scores, regardless of cognitive domain [18].
Depression assessment—15-item Geriatric Depression Scale (GDS-15) [19]
DAT SPECT—DAT SPECT was performed as previously described[7]. A binary determination of DAT binding deficit was made in the at-risk cohort based on the definition described in supplementary material. The striatal specific binding ratio (SBR) was also considered.
Olfaction—UPSIT scores were used to categorize all participants into olfactory levels of normosmia, hyposmia, and anosmia based on age and sex-specific normative values[9,20],
RBD—REM Sleep Behavior Disorder Questionnaire (RBDSQ)[21]. The cutoff score indicative of possible RBD was ≥6 in the PD cohort[22] and ≥5 in all other cohorts[21].
Statistical analysis
All clinical and biomarker data included in this study were downloaded from the PPMI database on August 1, 2016. Baseline characteristics were summarized using descriptive statistics, and compared across cohorts using generalized linear models assuming a normal distribution for continuous variables and a binomial distribution for categorical variables.
Differences in variables of interest among the 4 cohorts were examined using generalized linear models for continuous variables and logistic regression models for categorical variables. The following variables were examined, each in a separate model: cognitive test scores, presence of MCI, UPSIT, presence of DAT binding reduction, DAT SSBR, and presence of possible RBD based on RBDSQ score. A normal distribution was assumed for continuous variables and a binomial distribution for categorical variables. Age, sex, education and GDS-15 score were included as co-variates. For any variables that showed a significant difference with a p-value = 0.1 or less, pairwise comparisons between all cohort combinations were performed, and values with p<0.05 were regarded as statistically significant.
A sub-group analysis, utilizing the same statistical tests, was performed comparing the GBA and LRRK2 mutation carriers that constitute the NMC cohort.
Adjustments for multiple comparisons were not made given the exploratory nature of this analysis.
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline demographic characteristics are shown in Table 1.
Table 1. Demographics, clinical, and DAT SPECT characteristics in the PD, RBD, Hyposmia, and non-PD mutation carrier groups.
| Variable | PD cohort (N = 423) |
RBD cohort (N = 39) |
Hyposmia cohort (N = 26) |
Non-PD mutation carriers (N = 126) |
Asymptomatic LRRK2 mutation carriers (N = 88) |
Asymptomatic GBA mutation carriers (N = 38) |
p-value* for test of difference between groups) | p-value* for test of difference between LRRK2 and GBA groups only |
|---|---|---|---|---|---|---|---|---|
| Age Mean (SD; range) | 61.6 (9.7; 33–85) | 69.6 (5.5; 59–82) | 68.1 (6.2; 61–83) | 62.2 (7.3; 50–84) | 61.6 (7.1; 50–81) | 63.6 (7.5; 52–84) | < 0.0001 | 0.1578 |
| Sex Male N (%):Female N (%) | 277 (65): 146 (35) | 33 (85): 6 (15) | 18 (69): 8 (31) | 83 (66):43 (34) | 32 (36):56 (64) | 11 (29):27(71) | < 0.0001 | 0.4214 |
| Education | ||||||||
| < 13 yrs N(%): ≥13 years N (%) | 76 (18):347 (82) | 14 (36):25 (64) | 3 (12): 23 (88) | 26 (21):97 (77) | 23 (26):63 (72) | 3 (8):34 (89) | 0.0433 | 0.0341 |
| Number with Missing Data N (%) | 0 | 0 | 0 | 3 (2) | 2 (2) | 1 (3) | ||
| Self-reported handedness | ||||||||
| Right or Mixed N(%):Left N(%) | 385 (91):38 (9) | 39 (100):0 (0) | 23 (88)3 (12) | 104 (83): 18 (14) | 73 (83): 12 (14) | 31 (82): 6 (16) | 0.1261 | 0.8729 |
| Number with Missing Data N (%) | 0 | 0 | 0 | 4 (3) | 3 (3) | 1 (3) | ||
| Geriatric Depression Scale-15 | ||||||||
| Mean (SD; range) | 2.3 (2.4; 0–14) | 2.8 (2.6;0–10) | 1.5 (1.5; 0–6) | 1.7 (2.1; 0–9) | 1.6 (2.0) | 1.9 (2.4; 0–9) | 0.0063 | 0.4126 |
| Number with Missing Data N (%) | 0 | 0 | 0 | 6 (5) | 5 (6) | 1 (3) | ||
| UPSIT (categorical) | ||||||||
| Normosmia N (%) | 39 (9) | 1 (3) | 0 (0) | 44 (35) | 27 (31) | 17 (45) | ||
| Hyposmia N (%) | 237 (56) | 18 (46) | 7 (27) | 80 (63) | 57 (65) | 18 (47) | < 0.0001 | 0.1722 |
| Anosmia N (%) | 147 (35) | 18 (46) | 19 (73) | 3 (2) | 2 (2) | 1 (3) | ||
| Number with Missing Data N (%) | 0 (0) | 2 (5) | 0 (0) | 4 (3) | 2 (2) | 2 (5) | ||
| REM sleep behavior disorder (score ≥ 5)** | ||||||||
| No N (%): Yes N (%) | 312 (74): 108 (26) | 4 (10): 34 (87) | 15 (58): 11 (42) | 92 (73): 23 (18) | 67 (76):16 (18) | 25 (66):7 (18) | < 0.0001 | 0.7104 |
| Number with Missing Data N (%) | 3 (1) | 1 (3) | 0 | 11 (9) | 5 (6) | 6 (16) | ||
| DAT binding deficit | ||||||||
| No N (%):Yes N(%) | 1 (0.2): 413 (98) | 3 (8): 36 (92) | 4 (15): 22 (85) | 83 (66): 18 (14) | 56 (64):16 (18) | 27 (71):2 (5) | < 0.0001 | 0.0332 |
| Number with Missing Data N (%)*** | 4 (1) | 0 | 0 | 25 (20) | 16 (18) | 9 (24) | ||
| Mean striatal specific binding ratio | ||||||||
| Mean (SD) | 1.4 (0.40;0–3) | 1.5 (0.39; 1–3) | 1.9 (0.40; 1–3) | 2.6 (0.50; 1–4) | 2.5 (0.49’ 2–4) | 2.7 (0.50; 1–4) | < 0.0001 | 0.0002 |
| Number with Missing Data N (%)*** | 4 (1) | 0 | 0 | 25 (20) | 16 (18) | 9 (24) |
*Generalized linear models were used to test for differences in continuous variables and a logistic regression model was used to test for differences in categorical variables
** The cutoff score indicative of possible RBD was ≥6 in the PD cohort[21] and ≥5 in all other cohorts[20]. Note that the diagnosis of RBD in the RBD group was based on interview and not necessarily RBDSQ score. Furthermore, it is likely the majority of individuals with RBD in the RBD group were being treated at the time of enrollment in PPMI/completion of this questionnaire.
***1 subject was enrolled but terminated study participation prior to undergoing DaTscan. 3 subjects were enrolled at sites in a country in which DaTscan is not available. These participants underwent AV-133 imaging to determine their eligibility for study participation
Mean age, sex, education level, and GDS-15 were significantly different between at least two of the cohorts. As a result, all subsequent between-group analyses were adjusted for age, sex, education and GDS-15 scores.
Olfaction was significantly more impaired, and RBDSQ score higher, in the PD, RBD, and hyposmia cohorts compared to the NMC cohort. As expected, most subjects in the PD, RBD, and hyposmia cohorts had a DAT binding deficit, whereas less than 20% of the NMC cohort had a DAT binding deficit. The mean striatal SBR was significantly lower in the PD cohort compared to all other cohorts (p<0.0001 for all pairwise comparisons). Mean striatal SBR was significantly lower in the RBD cohort compared to the hyposmia (p = 0.0009) and NMC (p<0.0001) cohorts, and the hyposmia cohort compared to the NMC cohort (p<0.0001).
Mean scores on the neuropsychological test battery in the four cohorts are shown in Table 2.
Table 2. Cognitive performance in the PD, RBD, hyposmic, and NPD-GC arms.
| Cognitive Domain | Measure | PD Cohort (N = 423) |
RBD Cohort (N = 39) |
Hyposmic Cohort (N = 26) |
Non-PD Mutation Carriers (N = 126) |
p-value* for test of difference between groups) |
|---|---|---|---|---|---|---|
| Mild cognitive impairment | 2 or more tests > 1.5 SD below mean | |||||
| yes N(%): no N(%) | 46 (10.9): 373 (88.2) | 11 (28.2): 27 (69.2) | 1 (3.8): 24 (92.3) | 12 (9.5): 85 (67.5) | 0.1477 | |
| Number with Missing Data | 4 (0.9%) | 1 (2.6%) | 1 (3.8%) | 29 (23.0) | ||
| Global cognition | MoCA | |||||
| Mean (SD; range) | 27.1 (2.32; 17–30) | 25.5 (4.13; 11–30) | 27.3 (1.71; 23–30) | 26.9 (2.54; 19–30) | 0.0451 | |
| Number with Missing Data | 3 (1) | 0 | 0 | 3 (2) | ||
| Verbal memory | HVLT Immediate Recall | |||||
| Mean (SD) | 24.4 (4.98; 9–36) | 21.1 (5.12; 9–33) | 22.8 (5.55; 12–33) | 25.5 (5.89; 5–35) | 0.3562 | |
| Number with Missing Data N (%) | 1 (0.2) | 1 (3) | 1 (4) | 4 (3) | ||
| HVLT Delayed Recall | ||||||
| Mean (SD; range) | 8.4 (2.52; 0–12) | 6.5 (3.24; 0–12) | 7.6 (3.37; 0–12) | 9.1 (2.80; 0–12) | 0.1496 | |
| Number with Missing Data N (%) | 1 (0.2) | 1 (3) | 1 (4) | 4 (3) | ||
| HVLT Delayed Recognition | ||||||
| Mean (SD; range) | 11.2 (1.23; 0–12) | 10.5 (1.37; 7–12) | 11.1 (1.39; 6–12) | 11.2 (1.68; 0–12) | 0.6388 | |
| Number with Missing Data N (%) | 2 (0.5) | 1 (3) | 1 (4) | 7 (5) | ||
| Visuospatial function | Benton Judgment of Line Orientation | |||||
| Mean (SD; range) | 12.8 (2.13; 5–15) | 11.3 (2.36; 3–15) | 12.9 (1.87; 8–15) | 12.8 (2.05; 5–15) | 0.001 | |
| Number with Missing Data N (%) | 1 (0.2) | 2 (5) | 1 (4) | 4 (3) | ||
| Processing speed/attention | Symbol Digit Modalities Test | |||||
| Mean (SD; range) | 41.2 (9.73; 7–82) | 32.4 (9.16; 15–56) | 41.8 (9.98; 16–55) | 45.0 (10.9; 0–74) | < 0.0001 | |
| Number with Missing Data N (%) | 1 (0.2) | 2 (5) | 1 (4) | 7 (5) | ||
| Executive function/working memory | Letter-Number Sequencing | |||||
| Mean (SD; range) | 10.6 (2.66; 2–20) | 9.0 (3.33; 3–17) | 10.2 (1.80; 6–14) | 10.7 (2.99; 2–20) | 0.3796 | |
| Number with Missing Data N (%) | 1 (0.2) | 1 (3) | 1 (4) | 4 (3) | ||
| Semantic Fluency total | ||||||
| Mean (SD; range) | 48.7 (11.6; 20–103) | 43.7 (8.74; 27–65) | 47.0 (13.4; 26–75) | 54.1 (13.8; 18–98) | 0.0084 | |
| Number with Missing Data N (%) | 1 (0.2) | 1 (3) | 1 (4) | 4 (3) |
*Analyses are adjusted for age, sex, education and GDS-15 score
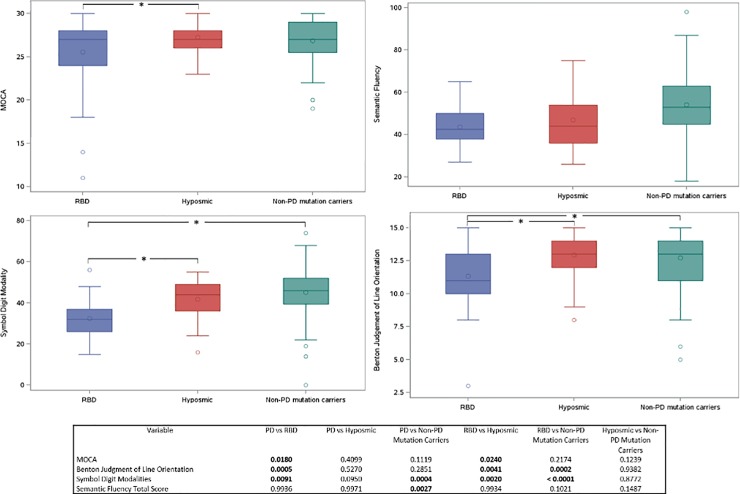
P-values for pairwise comparisons between the different cohorts are shown in Fig 1. The measure of global cognition (MoCA score) was worse in the RBD cohort compared to the PD and hyposmia cohorts. The RBD cohort performed significantly worse on measures of two cognitive domains compared to all other cohorts: processing speed/attention (SDMT) and visuospatial function (JOLO) (Fig 1). Hyposmics did not differ from PD or NMC cohorts in any cognitive domain. The PD cohort performed significantly worse than the NMC on a measure of executive function (semantic fluency) and processing speed/attention (SDMT).
Fig 1. Graphical comparison of select neuropsychological test battery scores in the 3 at-risk groups.
The scores for MoCA, semantic fluency, Symbol Digit Modalities Test (SDMT), and Benton Judgment of Line Orientation are shown for the 3 at-risk groups. Asterisks indicate significant difference in pairwise comparisons between groups.
The RBD cohort had the highest prevalence of MCI compared to the other cohorts; however, none of the differences between groups was significant after adjusting for age, sex, education, and GDS-15 score.
In comparing the LRRK2 and GBA cohorts, the LRRK2 mutation carriers had lower scores on both global cognition (MoCA) and a measure of verbal memory (HVLT immediate free recall) (Table 3).
Table 3. Cognitive performance in the LRRK2 G2019S and GBA mutation carrier groups.
| Cognitive Domain | Measure | Asymptomatic LRRK2 mutation carriers (N = 88) |
Asymptomatic GBA mutation carriers (N = 38) |
p-value* for test of difference between LRRK2 and GBA groups only |
|---|---|---|---|---|
| Global cognition | MoCA | |||
| Mean (SD; range) | 26.5 (2.72; 19–30) | 27.6 (1.86; 20–30) | 0.0427 | |
| Number with Missing Data N (%) | 2 (2) | 1 (3) | ||
| Verbal memory | HVLT Immediate Recall | |||
| Mean (SD; range) | 24.6 (6.03; 5–34) | 27.5 (5.05; 14–35) | 0.0181 | |
| Number with Missing Data N (%) | 2 (2) | 2 (5) | ||
| HVLT Delayed Recall | ||||
| Mean (SD; range) | 8.8 (2.96; 0–12) | 9.7 (2.27; 4–12) | 0.1216 | |
| Number with Missing Data N (%) | 2 (2) | 2 (5) | ||
| HVLT Delayed Recognition | ||||
| Mean (SD; range) | 11.1 (1.89; 0–12) | 11.5 (0.93; 8–12) | 0.3895 | |
| Number with Missing Data N (%) | 3 (3) | 4 (11) | ||
| Visuospatial function | Benton Judgment of Line Orientation | |||
| Mean (SD; range) | 12.7 (2.08; 5–15) | 12.9 (1.90; 8–15) | 0.2660 | |
| Number with Missing Data N (%) | 2 (2) | 2 (5) | ||
| Processing speed/attention | Symbol Digit Modalities Test | |||
| Mean (SD; range) | 44.2 (11.7; 0–74) | 47.0 (8.49; 29–68) | 0.3235 | |
| Number with Missing Data N (%) | 3 (3) | 4 (11) | ||
| Executive function/working memory | Letter-Number Sequencing | |||
| Mean (SD; range) | 10.7 (3.14; 2–20) | 10.9 (2.63; 6–18) | 0.7698 | |
| Number with Missing Data N (%) | 2 (2) | 2 (5) | ||
| Semantic Fluency total | ||||
| Mean (SD; range) | 54.0 (14.6; 18–98) | 54.1 (11.5; 25–78) | 0.7457 | |
| Number with Missing Data N (%) | 2 (2) | 2 (5) |
*Analyses are adjusted for age, sex, education and GDS-15 score
Discussion
In this study, we demonstrate significant differences in cognition among four cohorts presumed to be at-risk for PD, but to varying extents. As hypothesized, the RBD cohort performed worse than the other at-risk cohorts. RBD is thought to reflect a prodromal PD state resulting from neurodegeneration of pontine nuclei, including the glutamatergic peri-locus coeruleus. Involvement of nearby nuclei, including the noradrenergic locus coeruleus as well as the cholinergic pedunculopontine nucleus, could account for some of the cognitive dysfunction seen in RBD cases. Furthermore, the lower mean striatal SBR seen in this cohort compared to the hyposmia and NMC cohort indicates greater nigrostriatal dysfunction which could also help account for the worse cognition in this cohort[23].
Interestingly, and not consistent with our hypothesis, the RBD cohort was also more cognitively impaired than the PD cohort. The RBD cohort was predominantly older, male, and had a lower education level than other cohorts, all risk factors for cognitive impairment. It is likely that approximately half of the RBD cohort will develop dementia with Lewy Bodies [DLB][24] rather than idiopathic PD, in which cognitive dysfunction is mild early on[25]. This may partly explain the worse cognition in this cohort, possibly mediated by concomitant neurodegenerative disease pathology in the cortex and cholinergic nucleus basalis of Meynert, specifically Lewy body disease with or without Alzheimer’s disease pathology. The RBD cohort performed worse compared to all other cohorts in measures of processing speed/attention (SDMT) and visuospatial function (JOLO). This is of note considering that among individuals with RBD, abnormalities in tests of attention (as well as executive function) are predictive of future risk of DLB in RBD[26], and visuospatial dysfunction is a hallmark of DLB[27].
The hyposmia cohort did not differ from the PD cohort or the NMC cohort in any of the cognitive measures, despite significantly lower striatal SBRs. This is in contrast to the Parkinson Associated Risk Syndrome (PARS) cohort, in which individuals with both hyposmia and DAT binding reduction performed significantly worse on measures of global cognition, executive function/working memory, and verbal memory[6] compared to normosmics or hyposmics without DAT binding reduction. This discrepancy may be due to the small sample size (and reduced power) of the hyposmia cohort or to true intrinsic differences between the PPMI and PARS cohorts.
The NMC cohort includes predominantly healthy individuals, and the low prevalence of DAT binding reduction in that cohort suggests that at baseline they are indeed “low” on the spectrum of “prodromalness” (i.e., most of them have a low risk of conversion to motor PD). However, they are genetically heterogeneous and their risk of PD and its manifestations is likely largely influenced by their genotype. GBA mutations confer increased risk of cognitive dysfunction among individuals with PD[28], and this may result, pathophysiologically, from a synergistic effect between glucocerebrosidase dysfunction and alpha-synuclein pathology[29]. There are limited data on cognition in asymptomatic GBA mutation carriers. Similarly, there are limited data on cognition in asymptomatic LRRK2 G2019S carriers, but what data are available suggest that at least a subset of such individuals have worse performance on measures of executive function compared to non-carriers[30]. A study comparing cognitive function among asymptomatic GBA and LRRK2 mutation carriers found no differences between the cohorts[31]. In our cohort, while cognition was overall similar between the two cohorts, there were some differences. LRRK2 cohort participants had a lower mean MoCA and performed worse on a measure of verbal memory. Some of these findings may again be explained by evidence of greater nigrostriatal dysfunction[23] in the LRRK2 cohort. In addition, LRRK2 has higher penetrance for PD compared to GBA mutations [by age 85, estimates are approximately 30% for LRRK2[32] vs. 10% GBA mutations[33,34]]. Therefore, all other things being equal, a greater proportion of individuals at-risk for PD on the basis of LRRK2 mutations would be expected to have some degree of neuronal dysfunction or neurodegeneration [that could potentially manifest with cognitive dysfunction] compared to at-risk GBA mutation carriers.
In PD, GBA mutations associated with more severe phenotypes, such as L444P, are much more strongly associated with risk of dementia compared to other GBA mutations[35]. The sample size of the asymptomatic GBA cohort in PPMI limits genotype-phenotype correlations within this cohort at this time but will be of great interest as the sample size of this cohort increases (recruitment to this cohort is ongoing).
There are several limitations of this study, The Movement Disorders Society (MDS) research criteria for prodromal PD[3] were proposed after the at-risk cohort of PPMI was recruited and thus these criteria were not accounted for in the inclusion criteria. Rather, the at-risk PPMI cohorts were selected based on a range of at-risk or prodromal characteristics narrower than what the MDS criteria encompass, and the RBD cohort was also enriched for individuals with DAT binding deficit. These inclusion criteria likely limit the generalizability of our findings to other at-risk cohorts and the general population of individuals at-risk for PD. The latter, combined with the relatively small numbers in some of the cohorts, as well as missing data, limit conclusions that can be drawn, especially with respect to the hyposmia cohort. In addition, participants in all cohorts of the PPMI study may not be representative of the respective populations from which they are drawn. Comparison to individuals without known risk of PD was not possible as the healthy control cohort of PPMI was recruited with different exclusion criteria specifically in regards to cognition (i.e., individuals with a MoCA score of <27 were excluded from the healthy control cohort of PPMI, whereas this criterion was not applied to the other cohorts). Furthermore, the neuropsychological test battery, while relatively comprehensive in domain coverage, was limited in the number of tests used to examine each cognitive domain. In addition, some cohorts differed in global cognitive performance, and this alone may have influenced the differences in cognitive profile as well. Finally, while the administered cognitive tests preferentially represent specific cognitive domains, there is overlap in the cognitive domains measured, lowering the strength of the conclusions about affected cognitive domains.
Despite these limitations, our findings provide insight into the cognitive profile of individuals at-risk or in a prodromal state for PD. They lend support to the idea that there is a gradient of prodromalness that is consistent with the proposed Braak staging, such that individuals with manifestations presumably resulting from more rostral neurodegeneration, namely the RBD cohort, have worse cognition than hyposmics or asymptomatic carriers of PD-associated genes. Longitudinal follow-up of this cohort will yield additional insights across the spectrum of individuals at risk for PD and other neurodegenerative parkinsonian syndromes.
Supporting information
(DOCX)
Acknowledgments
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
Parkinson’s Progression Markers Initiative Contributors
Lead Author:
Kenneth Marek, MD1 (kmarek@mnimaging.com)
Steering Committee and Study Cores:
Kenneth Marek, MD1 (Principal Investigator); Shirley Lasch, MBA1; Caroline Tanner, MD, PhD9 (Site Investigator);Tanya Simuni, MD3 (Site Investigator); Christopher Coffey, PhD4 (Statistics Core, PI); Karl Kieburtz, MD, MPH5 (Clinical Core, PI); Renee Wilson, MA5; Werner Poewe, MD7 (Site Investigator); Tatiana Foroud, PhD 16 (Genetics Coordination Core, BioRepository PI); Daniel Weintraub, M.D (Cognitive and Behavioral) 13; John Trojanowski, MD, PhD13; Les Shaw, PhD13; Todd Sherer, PhD6; Sohini Chowdhury6; Mark Frasier, PhD6; Catherine Kopil, PhD6; Vanessa Arnedo6
Clinical Coordination Core: Cynthia Casaceli, MBA5
Imaging Core: John Seibyl, MD1(Principal Investigator); Nichole Deagle1; Duygu Tosun-Turgut9 Norbert Schuff, PhD9
Statistics Core: Christopher Coffey, PhD4; Chelsea Caspell 4; Liz Uribe4; Eric Foster 4; Katherine Gloer PhD4; Jon Yankey MS4
Bioinformatics Core: Arthur Toga, PhD10 (Principal Investigator), Karen Crawford, MLIS10
BioRepository: Danielle Elise Smith16; Paola Casalin12; Giulia Malferrari12
Bioanalytics Core: Brit Mollenhauer, MD8 (Principal Investigator/ Site Investigator), Douglas Galasko, MD15(Co-Principal/ Site Investigator)
Genetics Core: Andrew Singleton, PhD14, (Principal Investigator)Genetics Coordination Core: Cheryl Halter 16; Laura Heathers16
Site Investigators:
David Russell, MD, PhD1 Site; Stewart Factor, DO17; Penelope Hogarth, MD18; David Standaert, MD, PhD19; Robert Hauser, MD, MBA20; Joseph Jankovic, MD21; Matthew Stern, MD13; Lana Chahine, MD13; Shu-Ching HU, MD PhD22; Marie Saint-Hilaire MD23; Irene Richard, MD24; Klaus Seppi, MD7; Holly Shill, MD 25; Hubert Fernandez, MD 26; Anwar Ahmed, MD26; Isabel Wurster MD27; Zoltan Mari, MD28; David Brooks, MD29; Nicola Pavese, MD29; Yen Tai, MD29; Paolo Barone, MD, PhD30; Stuart Isaacson, MD31; Alberto Espay, MD, MSc 32; Dominic Rowe, MD, PhD33; Karen Marder35; Jean-Christophe Corvol36; Jose Felix Martí Masso37; Eduardo Tolosa38; Jan O. Aasly39; Nir Giladi40; Leonidas Stefanis41; Salima Brillman2; Susan Bressman42; Kathrin Brockmann27; Javier Ruiz Martinez37
Coordinators:
Debra Smejdir; Julia Pelaggi; Linda Rees, MPH1; Barbara Sommerfeld, RN, MSN17; Cathy Wood-Siverio, MS17; Karen Williams3; Christine Hunter, RN21; Baochan Tran13;; Carly Linder13; Gretchen Todd22; Cathi-Ann Thomas, RN, MS23; Raymond James, RN23; Fabienne Sprenger, MD7; Diana Willeke8; Zoran Obradov25; Jennifer Mule26; Kathleen Comyns9 Deborah Fontaine, BSN, MS, RN, GNP, MS 15; Becky Dunlop28; Susan Ainscough30; Kristy Espay32; Madelaine Ranola33; Helen M. Santana35; Elisabet Rezola37; Bjorg Waro39; Anat Mirlman40; Maria Stamelou41; Paul DeRitis24; Madeline Cresswell18; Erica Stacy28; Courtney Blair19; Farah Kausar9; Samantha Betheil9; Deborah Raymond42; Kelley Park3; Krista Specketer22; Chris Firestone26; Victoria Brown25; Lisbeth Carvajal31; Stephanie Carvahol36; Ella Hilt27; Beatrice Heim7; Katarzyna Wachowicz7; Alilcia Garrido38; Anne Grete Kristiansen39
ISAB (Industry Scientific Advisory Board):
Maurizio Facheris, MD43; Andrew Siderowf, MD, MSCE44; Mark A. Mintun, MD44; Jesse Cedarbaum, MD45; Peggy Taylor, ScD46; Holly Soares, PhD43; Irfan Qureshi, PhD47; Michael Ahlijanian, PhD47; Lawrence Slieker, PhD48; Colin Watson, PhD49; Etienne Montagut, MBA49; Zulfiqar Haider Sheikh49; Marcel van der Brug, PhD50; Remi Forrat51; Pablo Sardi, PhD51; Tanya Fischer, MD, PhD51; Alastair D. Reith, PhD52; Jan Egebjerg, PhD53; Lone Frydelund Larsen53; Nathalie Breysse, PhD53; Paul E.G. Kristijansen, MD, PhD53; Barbara Saba, MD54; Vera Kiyasova, MD, PhD54; Chris Min, MD, PhD55; Thomas McAvoy, PhD55; Robert Umek, PhD56; Eva Jajos-Korcsok, PhD57; Jeremy Edgerton, PhD57; Susan De Santi, PhD58; Christian Czech, PhD59; Frank Boess, PhD59; Jeffrey Sevigny, MD59; Thomas Kremer, PhD59; Igor Grachev, MD, PhD60; Kaplana Merchant, PhD61; Andreja Avbersek, MD62; Pierandrea Muglia, MD62; Alexandra Stewart, MBA63; Rene Prashad, PhD63
1 Institute for Neurodegenerative Disorders, New Haven, CT
2 The Parkinson’s Institute, Sunnyvale, CA
3 Northwestern University, Chicago, IL
4 University of Iowa, Iowa City, IA
5 Clinical Trials Coordination Center, University of Rochester, Rochester, NY
6 The Michael J. Fox Foundation for Parkinson’s Research, New York, NY
7 Innsbruck Medical University, Innsbruck, Austria
8 Paracelsus-Elena Klinik, Kassel, Germany
9 University of California, San Francisco, CA
10 Laboratory of Neuroimaging (LONI), University of Southern California
11 Coriell Institute for Medical Research, Camden, NJ
12 BioRep, Milan, Italy
13 University of Pennsylvania, Philadelphia, PA
14 National Institute on Aging, NIH, Bethesda, MD
15 University of California, San Diego, CA
16 Indiana University, Indianapolis, IN
17 Emory University of Medicine, Atlanta, GA
18 Oregon Health and Science University, Portland, OR
19 University of Alabama at Birmingham, Birmingham, AL
20 University of South Florida, Tampa, FL
21 Baylor College of Medicine, Houston, TX
22 University of Washington, Seattle, WA
23 Boston University, Boston, MA
24 University of Rochester, Rochester, NY
25 Banner Research Institute, Sun City, AZ
26 Cleveland Clinic, Cleveland, OH
27 University of Tuebingen, Tuebingen, German
28 Johns Hopkins University, Baltimore, MD
29 Imperial College of London, London, UK
30 University of Salerno, Salerno, Italy
31 Parkinson’s Disease and Movement Disorders Center, Boca Raton, FL
32 University of Cincinnati, Cincinnati, OH
33 Macquarie University, Sydney Australia
34 Philipps University Marburg, Germany
35 Columbia Medical, New York, NY
36 Pitié-Salpêtrière Hospital, Paris France
37 University of Donostia-Service of Neurology Hospital, San Sebastian, Spain
38 University of Barcelona-Hospital Clinic of Barcelona, Barcelona, Spain
39 Norwegian University of Science and Technology, Trondheim, Norway
40 Tel Aviv Sourasky Medical Center, Tel Aviv, Isreal
41 Foundation for Biomedical research of the Academy of Athens, Athens, Greece
42 Mount Sinai Beth Israel Medical Center
43 Abbvie
44 Avid Radiopharmaceuticals
45 Biogen Idec
46 BioLegend
47 Bristol-Myers Squibb Company
47 Eli Lilly and Company
49 GE Healthcare
50 Genentech
51 Genyzme Sanofi
52 GlaxoSmithKline Pharmaceuticals R&D
53 H. Lundbeck A/S
54 Institut de Recherches Internationales Servier
55 Merck
56 Meso Scale Discovery
57 Pfizer Inc
58 Piramal
59 Roche
60 Teva
61 TransThera Consulting
62 UCB Pharma S.A.
63 Weston Brain Institute
Data Availability
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015. October;30(12):1591–1601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 2.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991. October;114 (Pt 5)(Pt 5):2283–2301. [DOI] [PubMed] [Google Scholar]
- 3.Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord 2015. October;30(12):1600–1611. 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003. Mar-Apr;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 5.Chahine LM, Stern MB. Characterizing Premotor Parkinson's Disease: Clinical Features and Objective Markers. Movement Disorders Clinical Practice 2014;1(4):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chahine LM, Weintraub D, Hawkins KA, Siderowf A, Eberly S, Oakes D, et al. Cognition in individuals at risk for Parkinson's: Parkinson associated risk syndrome (PARS) study findings. Mov Disord 2016. January;31(1):86–94. 10.1002/mds.26373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011. December;95(4):629–635. 10.1016/j.pneurobio.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iranzo A, Valldeoriola F, Lomena F, Molinuevo JL, Serradell M, Salamero M, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol 2011. September;10(9):797–805. 10.1016/S1474-4422(11)70152-1 [DOI] [PubMed] [Google Scholar]
- 9.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984. March;32(3):489–502. [DOI] [PubMed] [Google Scholar]
- 10.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters EC, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 2004. August;56(2):173–181. 10.1002/ana.20160 [DOI] [PubMed] [Google Scholar]
- 11.Ponsen MM, Stoffers D, Twisk JW, Wolters EC, Berendse HW. Hyposmia and executive dysfunction as predictors of future Parkinson's disease: a prospective study. Mov Disord 2009. May 15;24(7):1060–1065. 10.1002/mds.22534 [DOI] [PubMed] [Google Scholar]
- 12.Berendse HW, Ponsen MM. Diagnosing premotor Parkinson's disease using a two-step approach combining olfactory testing and DAT SPECT imaging. Parkinsonism Relat Disord 2009. December;15 Suppl 3:S26–30. [DOI] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005. April;53(4):695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 14.Smith A. Symbol digit modalities test: Manual Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 15.Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment 1999. June;6(2):147–178. 10.1177/107319119900600204 [DOI] [PubMed] [Google Scholar]
- 16.Brandt J, Benedict RHB. The Hopkins Verbal Learning Test-Revised Odessa, FL: Psychological Assessment Reources; 2001. [Google Scholar]
- 17.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A clinical test. Arch Neurol 1978. June;35(6):364–367. [DOI] [PubMed] [Google Scholar]
- 18.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012. March;27(3):349–356. 10.1002/mds.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weintraub D, Oehlberg KA, Katz IR, Stern MB. Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry 2006. February;14(2):169–175. 10.1097/01.JGP.0000192488.66049.4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 1984. February;94(2 Pt 1):176–178. [DOI] [PubMed] [Google Scholar]
- 21.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord 2007. December;22(16):2386–2393. 10.1002/mds.21740 [DOI] [PubMed] [Google Scholar]
- 22.Chahine LM, Daley J, Horn S, Colcher A, Hurtig H, Cantor C, et al. Questionnaire-based diagnosis of REM sleep behavior disorder in Parkinson's disease. Mov Disord 2013. March 20;28(8):1146–1149. 10.1002/mds.25438 [DOI] [PubMed] [Google Scholar]
- 23.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol 2002. May;67(1):53–83. [DOI] [PubMed] [Google Scholar]
- 24.Iranzo A, Fernandez-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, et al. Neurodegenerative Disorder Risk in Idiopathic REM Sleep Behavior Disorder: Study in 174 Patients. PLoS One 2014. February 26;9(2):e89741 10.1371/journal.pone.0089741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Siderowf A, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Mov Disord 2015. March 4;30(7):919–927. 10.1002/mds.26170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchand DG, Montplaisir J, Postuma RB, Rahayel S, Gagnon JF. Detecting the cognitive prodrome of dementia with Lewy bodies: A prospective study of REM sleep behavior disorder. Sleep 2016. September 26. [DOI] [PubMed] [Google Scholar]
- 27.Gurnani AS, Gavett BE. The Differential Effects of Alzheimer's Disease and Lewy Body Pathology on Cognitive Performance: a Meta-analysis. Neuropsychol Rev 2017. March;27(1):1–17. 10.1007/s11065-016-9334-0 [DOI] [PubMed] [Google Scholar]
- 28.Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer’s disease pathology. Neurology (in press) 2012;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher Disease Glucocerebrosidase and alpha-Synuclein Form a Bidirectional Pathogenic Loop in Synucleinopathies. Cell 2011. July 8;146(1):37–52. 10.1016/j.cell.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaler A, Mirelman A, Gurevich T, Simon E, Orr-Urtreger A, Marder K, et al. Lower cognitive performance in healthy G2019S LRRK2 mutation carriers. Neurology 2012. September 4;79(10):1027–1032. 10.1212/WNL.0b013e3182684646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bregman N, Thaler A, Mirelman A, Helmich RC, Gurevich T, Orr-Urtreger A, et al. A cognitive fMRI study in non-manifesting LRRK2 and GBA carriers. Brain Struct Funct 2017. April;222(3):1207–1218. 10.1007/s00429-016-1271-4 [DOI] [PubMed] [Google Scholar]
- 32.Marder K, Wang Y, Alcalay RN, Mejia-Santana H, Tang MX, Lee A, et al. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology 2015. July 7;85(1):89–95. 10.1212/WNL.0000000000001708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rana HQ, Balwani M, Bier L, Alcalay RN. Age-specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genet Med 2013. February;15(2):146–149. 10.1038/gim.2012.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alcalay RN, Dinur T, Quinn T, Sakanaka K, Levy O, Waters C, et al. Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol 2014. June;71(6):752–757. 10.1001/jamaneurol.2014.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, et al. Survival and dementia in GBA-associated Parkinson's disease: The mutation matters. Ann Neurol 2016. November;80(5):662–673. 10.1002/ana.24777 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.