Abstract
Alterations to body composition (fat or lean mass), metabolic parameters such as whole-body oxygen consumption, energy expenditure, and substrate utilization, and behaviors such as food intake and physical activity can provide important information regarding the underlying mechanisms of disease. Given the importance of body composition and metabolism to the development of obesity and its subsequent sequelae, it is necessary to make accurate measures of these parameters in the pre-clinical research setting. Advances in technology over the past few decades have made it possible to derive these measures in rodent models in a non-invasive and longitudinal fashion. Consequently, these metabolic measures have proven useful when assessing the response of genetic manipulations (for example knockout or transgenic mice, viral knock-down or overexpression of genes), experimental drug/compound screening and dietary, behavioral or physical activity interventions. Herein, we describe the protocols used to measure body composition and metabolic parameters using an animal monitoring system in chow-fed and high fat diet-fed mice.
Keywords: Medicine, Issue 135, High fat diet, obesity, diabetes, metabolism, insulin resistance, metabolic caging, body composition, fat mass, lean mass, oxygen consumption, food intake, physical activity
Introduction
Metabolism underpins many aspects of normal cellular, organ, and whole-body physiology. Consequently, in the setting of various pathologies, alterations to metabolism may directly contribute to the underlying condition or may be adversely impacted as a side-effect of the pathology. Traditionally, metabolic research and studies into energy balance have been concentrated on the field of obesity and related conditions such as insulin resistance, pre-diabetes, glucose intolerance, cardiovascular disease, and diabetes. This research is warranted given the escalating prevalence of such conditions worldwide and the individual, societal, and economic costs these conditions inflict. As such, the development of prevention strategies and new therapeutics to target obesity is a continuing goal in research laboratories around the world and preclinical mouse models are heavily relied upon for these studies.
While weighing mice provides a reliable assessment of weight gain or loss, it does not provide a breakdown of the different components that make up whole-body composition (fat mass, lean mass, free water as well as other components such as fur and claws). The weighing of fat pads at the completion of studies once the mouse is deceased provides an accurate measure of different fat depots but can only provide data for a single time point. As a consequence, it is often necessary to enroll multiple cohorts to investigate the development of obesity over time, significantly increasing animal numbers, time, and costs. The use of dual-energy X-ray absorptiometry (DEXA) provides an approach to assess body fat and lean tissue contents and allows the researcher to obtain data in a longitudinal fashion. However, the procedure requires mice to be anesthetized1, and repeated bouts of anesthesia may impact the accumulation of adipose tissue or impact other aspects of metabolic regulation. EchoMRI utilizes nuclear magnetic resonance relaxometry to measure fat and lean mass, free water, and total water content. This is achievable due to the creation of contrast between the different tissue components, with differences in the duration, amplitude and spatial distribution of generated radio frequencies allowing the delineation and quantification of each tissue type. This technique is advantageous as it is non-invasive, quick, simple, requires no anesthesia or radiation, and, importantly, has been positively validated against chemical analysis2.
A key consideration of obesity and related research is the energy balance equation. While fat accumulation is more complicated than purely energy in (food intake) versus energy out (energy expenditure), they are vital factors to be able to measure. Daily energy expenditure is the total of four different components: (1) basal energy expenditure (resting metabolic rate); (2) the energy expenditure due to the thermic effect of food consumption; (3) the energy required for thermoregulation; and (4) the energy spent on physical activity. As energy expenditure generates heat, measuring heat production by an animal (known as direct calorimetry) can be used to assess energy expenditure. Alternatively, measurement of inspired and expired concentrations of O2 and CO2, allowing for determination of whole-body O2 consumption and CO2 production, can be utilized as a way to indirectly measure (indirect calorimetry) heat production and consequently calculate energy expenditure. An increase in food intake or a decrease in energy expenditure will predispose mice to weight gain and observations of changes in these parameters can provide useful information of likely mechanisms of action in particular models of obesity. A related metabolic parameter of interest is the respiratory exchange ratio (RER), an indicator of the proportion of substrate/fuel (i.e., carbohydrate or fat) that is undergoing metabolism and being utilized to produce energy. Consequently, measurement of food intake (energy consumed) combined with physical activity levels, O2 consumption, RER, and energy expenditure can provide a broad understanding of an organism's metabolic profile. One method to gather such data is to use a comprehensive laboratory animal monitoring system (CLAMS), which is based on the indirect calorimetry method to measure energy expenditure and has the added capabilities of determining physical activity levels (beam breaks) and food intake via scales incorporated into the measurement chamber.
In this protocol we provide a straight-forward description of the use of a body composition analyzer to assess body composition in mice and a metabolic animal monitoring system to measure aspects of metabolism. Considerations and limitations for these techniques will be discussed as well as suggested methods of analysis, interpretation, and data representation.
Protocol
All experiments described were approved by the Alfred Medical Research Education Precinct Animal Ethics Committee (AMREP AEC) and mice were provided humane care in line with the National Health and Medical Research Council (NHMRC) of Australia Guidelines on Animal experimentation. Animals were administered their prescribed diet and water ad libitum and housed in a temperature-controlled environment (~21 - 22 °C) with a 12 h light and 12 h-dark cycle. Seven week old male mice (on a C57Bl/6J background) were fed either regular normal chow diet (energy content 14.3 MJ/kg, consisting of 76% of kJ from carbohydrate, 5% fat, 19% protein; see Table of Materials) or for the high fat-feeding group, a high fat diet (HFD) (energy content 19 MJ/kg, consisting of 36% of kJ from carbohydrate, 43% fat, 21% protein, Specialty Feeds) for 3 weeks. Body weight and body composition measurements using an EchoMRI machine were made weekly while the metabolic monitoring analysis took place in a CLAMS after 3 weeks of the diet.
1. Body Composition Analyzer Procedure
Note: To function optimally, the EchoMRI 4-in-1 used in this protocol should be contained within a room where the air temperature is stable and does not fluctuate. Ideally this should be constantly monitored. Moving of the machine and interruptions to power should also be avoided if possible. If the power supply has been interrupted and the system has to be restarted, allow at least 2 - 3 h for the machine to warm up before using it again. Before starting, ensure that you are wearing correct personal protective equipment.
- Prior to scanning mice, perform a system test on the body composition analyzer machine. This involves using a calibration standard (referred to as a canola oil system test sample (COSTS)) to test the precision of the instrument and to ensure there has been no drift in its accuracy.
- Open the system software, then click the System Test toolbar button or pressing "Alt + Y" simultaneously.
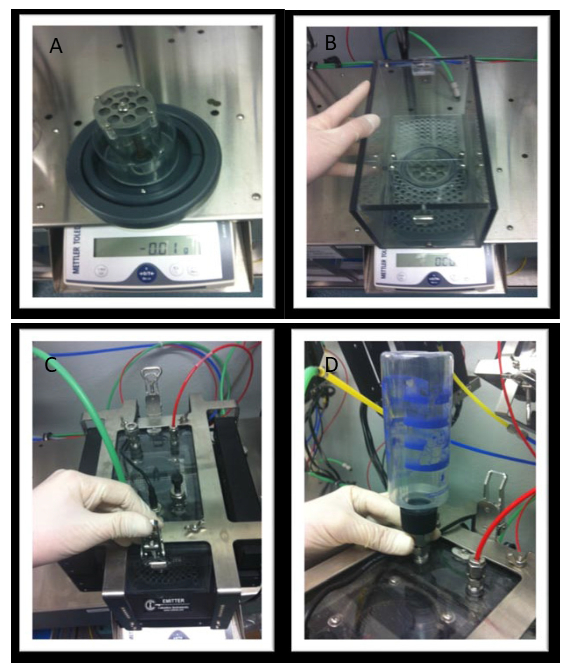
- Before the system test is carried out by the computer, wait for a reminder to verify that the correct COSTS (in this case the mouse-specific COSTS) has been placed inside the gantry of the system ( Figure 1). Once confirmed that this is indeed the case, accept to proceed with the test, which will take few minutes to complete.
- Once the system test has been passed, continue forward with scanning.
- If the system test fails, repeat the system test.
- If the machine continues to be out of range (indicating a deviation has occurred), calibration may be necessary to rectify the situation. Complete this by following the prompts or as described in the user manual provided at time of purchase. If the problem persists, check the manual3 or report the issue to the manufacturer's support team and seek further instruction.
Place the mice in a small animal specimen holder (long cylinder) to keep them contained while in the machine. To do this, place the holder horizontally, pick up the mouse and insert it into the opening of the cylinder head first. Slowly and carefully bring the holder to the vertical position so that the mouse is at the bottom of the cylinder and ready for analysis.
Once within the holder, insert a delimiter to limit the movement of the mouse during the measurement period. In some circumstances, with extremely active mice, it may be necessary to hold the delimiter in place with your fingertip. NOTE: Familiarize the mice with placement in the specimen holders prior to their initial analysis to reduce stress. The use of a red colored animal specimen holder can also reduce the potential stress response, as the mice feel that they are in the dark.
Within the software, select a folder (folder toolbar) to save the data to and create a file name.
If necessary, reduce the amount of random noise in the fat and lean measurements by increasing the number of primary accumulations of the scan. Once the software is initiated, the primary accumulations is set to a recommended default value for general everyday use; unless there is a specific reason to change these parameters, the default settings will give the necessary level of precision to users.
If not interested in obtaining data for free water and total water, turn off the water stage by selecting the tab to say no. Doing so will reduce the scan duration significantly and improve throughput.
Initiate the scan by selecting "start scan" or by pressing F5 on the keyboard. Enter all relevant data about the animal (e.g., animal ID, body mass, etc.) and press "ok" or F5 to commence the scan, which will take approximately 1 min.
After data have been obtained, remove the animal holder containing the mouse from the machine and place the animal back in its cage. Once all animals have been scanned, export the data for further analysis and collation.
Before and after use, thoroughly clean the animal holders as per the instructions of the manufacturer. As these holders are constructed from acrylic plastic, isopropyl alcohol and ethyl alcohol should be avoided as they can cause cracking of the holders and/or rapid deterioration of the holder, thereby increasing the likelihood of breakage. Instead, either use warm dishwashing water solution, or, if further disinfectant is required, use F10 (at a 1:125 dilution) or other disinfectant or cleaning sprays (see Table of Materials) and then wipe off.

2. Metabolic Animal Monitoring System Procedure
NOTE: The system requires ~2 h to warm up and stabilize. If the machine has been turned off, it must be switched on to allow the Zirconia cell to be heated to 725 °C. Also we generally place mice in the body composition analyzer a day prior to entering the animal monitoring system to avoid any issues with restraint stress.
Ensure the computer attached to the animal monitoring system is turned on and open the control program. Select the "Oxymax Utility" option from the tool menu to initiate the pumps.
Fill water bottles with appropriate water, weigh and inspect the health of the mice, and organize food. If measuring food intake in the system, consider powdering the food. Fill the food hoppers by depressing the spring-loaded platform and tip food into the hopper. Ensure that the food hopper and water bottle are completely full to ensure that there is enough food and water to last the allotted experimental time.
Check the status of the drierite/desiccant; if using a color indicator, it should be blue and therefore dry, but if it is pink/purple, it has had significant moisture absorbance and should be replaced or topped up.
Check the condition of the ammonia trap and soda lime and replace if required. If the ammonia trap is connected two at a time, when the second trap displays signs of a color change, replace the first one. An increase in the CO2 offset can also signify the need to replace the soda lime. NOTE: Desiccant can be dried in an oven and reused, however we follow the recommendations from the manufacturer of the system to use fresh each time.
Assemble the chambers. To do this, place the food hopper on the balance, then place the chamber on top with the perforated platform that becomes the floor of the chamber inserted. Carefully place the mouse in the chamber and attach the lid of the system with the front and back clips and secure before positioning the water bottle and fastening. As a precaution, re-check all chamber lids, mice, and water (Figure 2A-D). NOTE: Depending on the size of the mice being examined, it may be necessary to adjust the height of the spaces above the food hopper so that the mice have access to the food but not enough space that they can sleep directly on top of the feeder.
- As it is recommended that the gas sensors be calibrated before each experiment, calibrate the system.
- Use a gas of known composition (0.5% CO2, 20.5% O2, balance nitrogen). Connect the calibration gas tank to the system via a regulator and hose. Turn on and ensure the tank output pressure is reading 5 - 10 psi. Note: Some systems will have a second tank, hose and regulator for use of pure Nitrogen as an "offset" gas. The system we operate instead utilizes soda lime to generate CO2 free air.
- Follow procedures to calibrate both the O2 and CO2 sensors. Select "calibration" from the tools menu and sequentially calibrate both the O2 and CO2. Before calibrating ensure that 1) sample and reference flows are 0.400LPM, 2) the Zirconia O2 sensor temperature is 725 °C (± 1 °C), 3) the sample and reference drier and air pumps are on, and 4) the calibration gas is attached and turned on.
- If necessary, when calibrating the O2 sensor, slightly adjust the offset control on the front of the zirconia oxygen sensor to achieve an O2 ratio value of 1.0000 (± 0.0002). This is to ensure it is within acceptable limits (highlighted in green font in the software display on the computer screen).
- After successful O2 and CO2 sensor calibration, turn off the calibration gas cylinder and disconnect the hose from the regulator. After calibration, O2 for reference air (atmospheric) should read 20.92 (± 00.02). If calibration is out of tolerance, repeat, and refer to trouble shooting guides from the manufacturer. Failing this, contact the manufacturer for further instructions.
Proceed with experimental set-up. Select "experimental file open" from the experiment menu. Select the appropriate template (e.g., mouse). Under "setup" in the experiment menu define the parameters of the experiment that should be recorded (e.g., mouse ID, weight, group, etc.) De-select any chambers not in use and select the location for the experiment to be saved.
Ensure the scales have been tared if measuring food intake and start the capturing of data by selecting "run" in the experiment menu. Data is captured for different lengths of time depending on the phenotype, institutional guidelines on animal isolation, and system usage. NOTE: In our hands, the experiment is routinely run for 48 h, with the first 24 h used as acclimatisation to the new environment and the second 24 h used for data analysis. The data collection period is based on how long the investigator wishes to keep their mice singly housed and dependent on animal ethics approval. Alternatively, if provisions exist, mice may be acclimatised in the chambers prior to being placed into the system and connected. Each chamber is measured approximately once every 13 min when a 12 chamber system is in use.
Regularly check and monitor the results that are obtained while the mice are in the system to ensure animal welfare and that appropriate data is being collected. Any issue may be able to be identified at this stage and rectified. Check on each mouse every morning and evening when they are in the system.
Check the metabolic tab at the top of the data file page for the data collected in real time for each mouse in regards to the oxygen consumption, RER, and energy expenditure. Meanwhile, beam breaks and food consumption data can be located in the activity and feeding tabs, respectively. Check that the "O2 In" is reading around 20.90 - 20.94, the "CO2 In" is around 0.040 - 0.050, the RER is between 0.7 and 1, and the flow rate is constant at 0.5 - 0.6 L/min.
At regular intervals, check that the mice have access to food and water and that they are consuming each. Ensure they are not demonstrating any signs of distress such as digging at the perforated flooring. Also, monitor the results which are displayed.
At the completion of the allocated experimental time, select "stop" from the experiment menu and export the results (as CSV files, File>Export>Generate Subject CSV's) for analysis.
- Inspect the health of the mice, weigh them and then return to their home cages.
- Mice may be hostile towards each other after separation, so monitor once they are housed together again.
- Disassemble the cages, remove excess food from hoppers, and tip out any feces, urine, and food from the cages. Submerge bottles and sippers in diluted T-bac solution, soak, and clean the other components in diluted bleach solution. Rinse with clean water and leave to air dry.
Calculate metabolic parameters with the software. The software utilizes a number of equations to provide the final data output4. For calculation of oxygen consumption and carbon dioxide production: Oxygen consumption: VO2 (LPM)= ViO2i - VoO2o; Carbon dioxide production: VCO2 (LPM)=VoCO2o-ViCO2i Where: Vi = the input ventilation rate (LPM), Vo = the output ventilation rate (LPM), O2i = the O2 concentration at input, O2o = the O2 concentration at output, CO2i = the CO2 concentration at input, CO2o = the CO2 concentration at output. For calculation of RER: RER = VCO2 / VO2. Note that protein oxidation was not measured and therefore the RER was not adjusted for this. For calculation of energy expenditure: Energy Expenditure: CV = 3.815 + 1.232* RER Heat (Kcal/h)) = CV * VO2. Where: CV is the calorific value (the relationship between heat and the volume of oxygen consumption). This is derived from "The Elements of the Science of Nutrition" referred to as the Lusk Table, comprised by Graham Lusk.
Representative Results
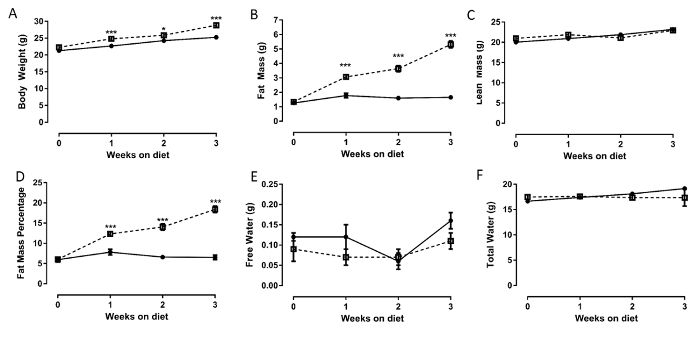
The results seen in Figure 3 display a typical change in body composition parameters upon high fat feeding, as measured via EchoMRI. At baseline there was no difference in any parameter measured (Figure 3A-F). However, after just 1 week of high fat feeding, there was a significant increase in body weight, fat mass, and fat mass percentage in the HFD group (Figure 3A,B,D). The magnitude of the differences between the two groups for these measures continued to increase over the 3 week dietary intervention. Lean mass, free water, and total water content (Figure 3C,E,F) did not differ between the groups at any time point. It can also be seen that the chow fed mice continued to put on weight over the study period (Figure 3A) and that this was due to an increase in lean mass (Figure 3C) rather than a fat mass increase (Figure 3B).
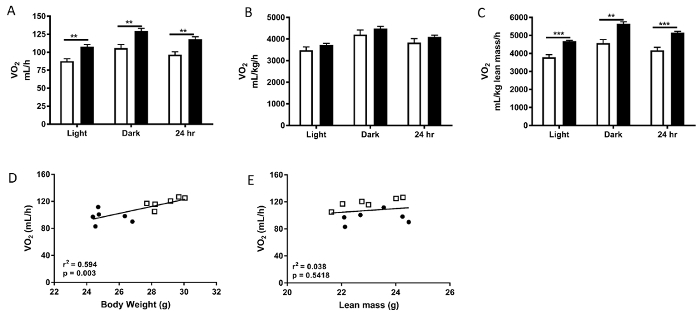
As can be seen in Figure 4, three weeks of high fat feeding led to a number of changes as detected in the metabolic animal monitoring system. VO2 when not adjusted for body weight was significantly higher in the heavier high fat fed mice (Figure 4A). Notably, normalization of VO2 via two different factors resulted in two different outcomes. Normalization to total body weight led to no difference in VO2 between the standard chow fed and high fat fed mice, while normalization to lean mass produced a significant difference (Figure 4B,C). These results demonstrate that normalization of VO2 data by dividing by mass variables significantly affected the results, and caution should be exercised when interpreting VO2 data when it is expressed in such a way. For a detailed discussion of how to express VO2 data and the effects of normalizing to different parameters see the excellent discussion in Tschop, et al.5 In their guide to the analysis of mouse energy metabolism, Tschop and colleagues suggest the use of analysis of co-variance (ANCOVA) to statistically interrogate the effects of body weight or body composition on energy expenditure and food intake data. In this case, performing an ANCOVA on the data shown in Figure 4A, using body weight as the covariate, reveals that no statistically significant difference exists between normal chow and HFD, thus indicating that once accounting for body weight, there is no difference in oxygen consumption between the groups. This result can be easily visualized when plotting VO2 against body weight as a scatterplot as shown in Figure 4D. Plotting VO2 against body weight (Figure 4D) demonstrates that the VO2 data lie on a common line in relation to body weight, with the heavier animals consuming more oxygen. Of note, plotting VO2 against lean mass demonstrates that the VO2 data lie on two distinct lines in relation to lean mass (Figure 4E).
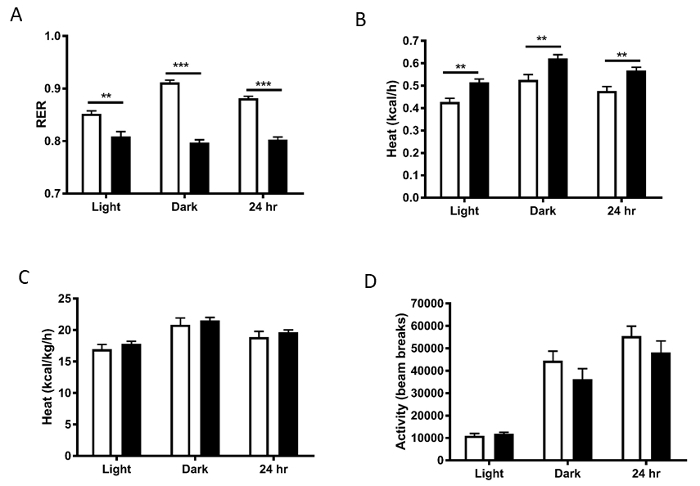
RER was significantly lower in the high fat fed mice, indicating fat utilization over carbohydrate utilization when fed the high fat diet (Figure 5A). Energy expenditure (heat) without normalization was increased in the heavier animals, likely due to the animals having more metabolically active tissue (Figure 5B), with this difference being lost once normalized to body weight (Figure 5C). Also note the increases in VO2, RER, and energy expenditure in the dark cycle as compared to the light cycle when the mice are more active. These differences represent the classical daily alterations in metabolism that occur in mice. While in this example, we have divided the data into 12 h blocks, division of the data further into smaller time epochs can also be useful. Physical activity levels are also a factor that contribute to energy expenditure. These were not different between groups, suggesting that a decrease in movement was not the driver of the obese phenotype in the high fat fed mice (Figure 5D).
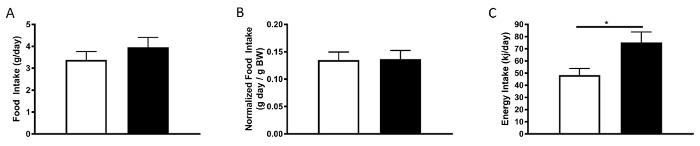
The other side of the energy balance equation is the amount of energy that is consumed and enters the body. To look at this aspect of metabolism we analyzed the amount of food that the mice consumed while in the metabolic animal monitoring system. As can be observed in Figure 6A, the mice ate the same quantity of food as measured by weight or when normalized to body weight (Figure 6B). (ANCOVA can again be used to assess the impact of body weight on food intake.) Normalization of food intake to body weight may be an important step to consider if energy expenditure has also been normalized to weight, thus keeping each side of the energy equation in balance. While the mice ate the same quantity of food, it is important to account for the energy density of each of the diets used. When taking this factor into account, we observe the mice on the HFD consuming more energy (Figure 6C) and from these experiments it is likely that this is driving the obese phenotype. That is, since the mice are taking in more energy, but they are not proportionally expending more energy, their obesity can be attributed to energy storage.
Statistics
All data in this paper are presented as mean ± standard error of the mean (SEM). Statistical significance was set at p <0.05. * indicates p <0.05, ** indicates p <0.01, *** indicates p <0.001, and n = 6 per group unless indicated. Investigators were unable to be blinded to the dietary group intervention due to a difference in color of the diets. The mice were randomly chosen as to which diet they were given.
Figure 1: Correct placement of mouse COSTS and small animal specimen holder containing mice within the body composition analyzer. To perform a system test using a calibration standard (COSTS) or for scanning of mice within the small animal specimen holder, place each inside the gantry of system. The red arrows indicate the cylinder in which the mice will be contained entering the gantry of the machine.
Figure 2: Assembly of individual chambers. A) Place the food hopper in the center of the balance. B) Insert the platform into each chamber and place chamber over the hopper. C) Place mice in the chambers individually and secure lid. D) Position the water bottle and fasten.
Figure 3: Body composition analysis over 3 weeks of a high fat diet. A) Body weight, B) fat mass, C) lean mass, D) fat mass percentage, E) free water content, and F) total water content. Circles represent normal chow diet, squares represent HFD. Please click here to view a larger version of this figure.
Figure 4: Metabolic parameters obtained from metabolic animal monitoring system experiments after 3 weeks of the respective diets. Mice were housed in the chambers for 48 h with the first 24 h acting as familiarization. The data obtained from the second 24 h was analyzed and presented in these figures. A) Raw VO2 rates, B) VO2 normalized to body weight C) VO2 normalized to lean mass, D) scatterplot for unadjusted VO2 (total 24h period) to body weigh,t and E) unadjusted VO2 to lean mass. A-C White bars represent normal chow diet, black bars represent high fat diet. D-E Circles represent normal chow diet, squares represent HFD. Please click here to view a larger version of this figure.
Figure 5: A) Respiratory exchange ratio (RER), B) heat (energy expenditure), and C) heat normalized to body weight. D) Activity levels calculated as the sum of the ambulatory X and Y beam breaks and Z beam breaks. White bars represent normal chow diet; black bars represent HFD.
Figure 6: Food intake data obtained in the system for the final 24 h. A) Food intake in grams, B) food intake normalized to body weight and C) calculated energy intake. n = 4 - 5 (3 mice were excluded due to making a large mess with their food). White bars represent normal chow diet; black bars represent HFD. Please click here to view a larger version of this figure.
Discussion
Critical steps
The protocols described herein provide an example of ways in which to measure body composition and various metabolic parameters in mice using a body composition analyzer and a metabolic animal monitoring system. For both techniques, it is critically important to ensure that the machines are working optimally, and to do this, it is imperative that the researcher performs a system test for the body composition analyzer and calibrates to a known gas composition for the metabolic animal monitoring system prior to use of the equipment. This will ensure greater consistency of results and the opportunity to detect any potential issues with the machinery.
The way in which data is normalized for the metabolic animal monitoring experiments is also vitally important to ensure the validity of the results obtained from the technique. As indicated in our representative results (Figure 4A-E) VO2 can be reported in a number of different ways: its absolute rate (L/min), relative to the body mass of the mouse (mL/kg*min), or relative to lean body mass (mL/kgLBM*min) if that data is available (for example obtained from a body composition analyzer). Depending on the phenotype, it may be more appropriate to normalize the values a particular way to rule out any potential bias. For example, if an animal has increased body mass, they have more tissue that is available and able to consume oxygen and naturally their energy expenditure is higher. Normalizing to total body mass may not be the best option as it will bias towards the observation of a decrease in oxygen consumption per unit of mass, even though the oxygen consumption of the tissues may not be different. As an alternative to normalizing to body weight, one can normalize to the lean body mass of the mouse. As lean tissue mass is primarily responsible for oxygen consumption, and lean mass is typically unaltered or only modestly different between experimental groups, normalization in this manner may be a more representative way of expressing VO2 data. It should be noted that the lean mass compartment is comprised of many different tissues, all with different metabolic rates, and consequently normalization in this manner may not be appropriate or provide any insight into which lean mass component is driving the change. Also, it rules out the contribution of the fat mass component on metabolism.
Given these issues, an alternative statistically-based method has also been proposed5,6. Analysis of covariance (ANCOVA)is a statistical test that allows the comparison of a variable (e.g., energy expenditure) across multiple groups while correcting for other factors or variables termed covariates. In this manner factors such as body weight, fat mass and lean mass can be included as variables that influence energy expenditure, but even this method has its own specific assumptions6, including the fact that using multiple variables in ANCOVA is likely to invalidate it unless the variables are independent of each other. Given there seems to be no perfect or universally agreed single way to normalize and present VO2 or energy expenditure data, it may be appropriate to display and present the data in a number of ways to give the clearest picture of the phenotype to the reader. Physical activity levels can increase oxygen consumption, and so in animals which have activity phenotypes (an increase or decrease), it may also be necessary to account/normalize for changes in movement to determine if this can account completely or partially account for any change in VO2.
Modifications and troubleshooting
The representative results displayed in this protocol were obtained from experiments conducted at a room temperature of 21 - 22 °C. The thermoneutral zone of a mouse is approximately 30 °C, so in a traditional animal house with its temperature set to 20 - 22 °C for human comfort, a mouse is put under thermal stress. To counter this, non-shivering thermogenesis is activated at these colder temperatures, resulting in an up to a 2-fold increase in energy expenditure between mice housed at 20 °C compared with those housed at 30 °C7. The environmental housing of mice is an important consideration for these experiments as it has been shown that housing of mice at thermoneutrality can potentiate the development of some conditions such as atherosclerosis8 and high fat diet-induced non-alcoholic fatty liver disease (NAFLD) pathogenesis9. Environmental temperature is therefore also an important consideration when conducting experiments in a metabolic animal monitoring system, as a phenotype can be present at certain temperatures but not at others, which could point to a potential mechanism of action. One such scenario could be a phenotype that involves the activation of recruited beige fat whereby a greater quantity of this tissue allows for a larger increase in thermogenesis under cooler conditions10. Thus, it may be necessary to modify the environmental set up that was described in these current experiments and to conduct experiments under multiple environmental temperatures to get an accurate depiction of the true metabolic status of the model. For troubleshooting due to technical errors, it may be necessary to contact the manufacturers directly for instruction. If there are issues with this type of body composition analyzer it is recommended to perform a Repeat Scans test, which runs 25 scans against the COST. The company will need this information for diagnostics. Similarly with the metabolic animal monitoring system, if issues arise, collect the data files from the last time the system worked well and the files from when the issues arose so that support can make a likely diagnosis.
Limitations
While the body composition analyzer provides excellent data on whole-body fat accumulation, it doesn't allow for the determination of regional adipose depots. This is important in the field of obesity research, as not all fat is the same, with the location that the fat has accumulated and its functional properties being particularly important. Indeed, the protective effects of subcutaneous fat depots (or metabolically healthy fat) have been described11. Micro-computed tomography (micro-CT) can discriminate between subcutaneous and visceral fat12, as can magnetic resonance imaging (MRI) analysis13. Use of these techniques can provide further information on the site of adipose accumulation. The metabolic animal monitoring system also has its limitations. While total daily energy expenditure can be measured, the system is not capable of discerning between the different components that make up energy expenditure. A further limitation of the system is that it is possible that obesity can develop without a measurable decrease in energy expenditure detected via these types of systems, even independently of food/energy intake alterations. Studies have shown that small decreases in energy expenditure, which are substantial enough to cause significant weight gain over the long term, cannot be robustly detected in such metabolic systems over the short term14,15,16. While we have used an n of 6 per group in the current study to demonstrate this methodology as an example study, to detect small differences in energy expenditure that could contribute to obesity likely requires many more mice5. Advancements in the resolution of detection in these systems and the ability to perform these types of studies over a longer time period will aid in the ability to detect these smaller but significant changes. With regards to the measurement of food intake, we have typically observed that 24 h food intake in mice housed within the metabolic animal monitoring system is lower than would be observed in the home cage, likely due to the reasons discussed above. Therefore, in addition to monitoring food intake in this system, we additionally assess food intake in the home cages of mice. While this can only be done in a situation where mice from particular experimental groups are housed separately, it has the advantage of allowing near continuous daily assessment. The investigator simply weighs the amount of food in the hopper at a specified time of day, always accounting for food scattered throughout the cage, and then divides this total amount of food consumed by the number of mice present in the cage.
Future applications
While within this review we have used obesity acquired via high fat feeding as an example of a disease state where measuring body composition and metabolic parameters are useful, the use of this equipment is far from confined to this research field. The use of these techniques is also valuable when studying diseases such as diabetes, cardiovascular disease, age-related sarcopenia, frailty, cancer-cachexia, muscular dystrophies, and lipodystrophy. While the initial costs of purchasing such infrastructure is considerable, the ability to use the equipment across multiple and diverse fields of medical research mitigates this initial cost. Furthermore, ongoing reagent and consumable costs are minimal for these machines; however, preventative maintenance and servicing must be considered and budgeted for.
Just as lean mass obtained via body composition analysis may be an important normalization factor for oxygen consumption derived from the metabolic animal monitoring system, lean mass determination can also be used to normalize drug/test dosages. For instance, in metabolic studies, it is commonplace to perform an intraperitoneal or oral glucose tolerance test (GTT), or an intraperitoneal insulin tolerance test (ITT). These tests examine the ability of a mouse to dispose of a glucose load or respond to insulin. Alterations in blood glucose levels in response to these tests provides information on the level of whole-body glucose and insulin tolerance in the model. Traditionally, the glucose and insulin bolus administered in these tests is dosed as per the body weight of the mouse. However, as models of obesity accumulate fat mass over lean mass, dosing per body weight could bias the heavier model towards glucose intolerance in a GTT as they receive more glucose. This is due to the fact that the liver, skeletal muscle, and brain, organs that dispose of the majority of glucose in the post-prandial state17, are components of the lean mass measurement and rarely or mildly change in most models. Conversely, in an ITT when dosed to body weight, a heavier model which would receive more insulin may appear more sensitive to the glucose lowering effects of insulin purely because it has received a greater quantity. Therefore, if the investigator has access to body composition data, the lean mass may be the most appropriate measure, as opposed to the whole-body mass, for such dosage calculations18. Taking this further, lean mass data obtained from body composition analysis could also be used to dose experimental drugs if the need arose to account for lean mass at the exclusion of fat mass. Another application of the metabolic animal monitoring system that has not been discussed or demonstrated in this manuscript is attaching an enclosed motorized treadmill to the system so that the metabolic parameters discussed herein can also be measured during exercise.
The procedures described in this review can be used to characterize body composition and various metabolic parameters in mice. These measures are applicable to a wide-range of research fields and can provide important information for the characterization of a phenotype. Data derived from these methods can also provide evidence towards underlying mechanisms driving a particular metabolic phenotype. Further development and refinement of these technologies will enable researchers to advance their findings towards therapeutic outcomes.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank the staff from the Alfred Medical Research and Education Precinct Animal Services (AMREP AS) team for their assistance and care of the mice used in this study and for the support of the Operational Infrastructure Support scheme of the Victorian State Government.
References
- Chen W, Wilson JL, Khaksari M, Cowley MA, Enriori PJ. Abdominal fat analyzed by DEXA scan reflects visceral body fat and improves the phenotype description and the assessment of metabolic risk in mice. Am J Physiol Endocrinol Metab. 2012;303(5):E635–E643. doi: 10.1152/ajpendo.00078.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovner I, Taicher GZ, Mitchell AD. Calibration and validation of EchoMRI whole body composition analysis based on chemical analysis of piglets, in comparison with the same for DXA. Int J Body Compos Res. 2010;8(1):17–29. [PMC free article] [PubMed] [Google Scholar]
- EchoMRI. Software User Manual: Whole body composition analyzer. 2016.
- Columbus Instruments. Oxymax for Windows User Manual. 2014. September.
- Tschop MH, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9(1):57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR. Measuring energy metabolism in the mouse - theoretical, practical, and analytical considerations. Front Physiol. 2013;4 doi: 10.3389/fphys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, et al. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol. 2008;294(4):H1581–H1588. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- Tian XY, et al. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab. 2016;23(1):165–178. doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles DA, et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med. 2017;23(7):829–838. doi: 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160(1-2):74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusminski CM, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18(10):1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judex S, et al. Quantification of adiposity in small rodents using micro-CT. Methods. 2010;50(1):14–19. doi: 10.1016/j.ymeth.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia B, et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 2016;24(6):820–834. doi: 10.1016/j.cmet.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Matthews VB, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53(11):2431–2441. doi: 10.1007/s00125-010-1865-y. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Garcia MC, et al. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55(5):1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- Kowalski GM, Bruce CR. The regulation of glucose metabolism: Implications and considerations for the assessment of glucose homeostasis in rodents. Am J Physiol Endocrinol Metab. 2014;307(10):E859–E871. doi: 10.1152/ajpendo.00165.2014. [DOI] [PubMed] [Google Scholar]
- McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab. 2009;297(4):E849–E855. doi: 10.1152/ajpendo.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


