Abstract
The outer hair cell is one of the two types of sensory hair cells in the mammalian cochlea. They alter their cell length with the receptor potential to amplify the weak vibration of low-level sound signal. The morphology and electrophysiological property of outer hair cells (OHCs) develop in early postnatal ages. The maturation of outer hair cell may contribute to the development of the auditory system. However, the process of OHCs development is not well studied. This is partly because of the difficulty to measure their function by an electrophysiological approach. With the purpose of developing a simple method to address the above issue, here we describe a step-by-step protocol to study the function of OHCs in acutely dissociated cochlea from postnatal rats. With this method, we can evaluate the cochlear response to pure tone stimuli and examine the expression level and function of the motor protein prestin in OHCs. This method can also be used to investigate the inner hair cells (IHCs).
Keywords: Neuroscience, Issue 135, Auditory brainstem response, organ of Corti, outer hair cell, postnatal rat, whole-cell patch clamp, nonlinear capacitance
Introduction
Two distinct functions of cochlear sensory hair cells are essential for mammalian hearing: mechanoelectric transduction (MET) and electromotility1,2. By MET channels located in the hair bundle, IHCs (IHCs) and OHCs convert sound vibration into membrane potential changes, as well as the electrical signals of innervated spiral ganglion neurons. OHCs change their cell length with the membrane potential and amplify the vibration of low-level sound. This activity termed electromotility is derived by the motor protein prestin located in the lateral wall of OHCs3.
In many species including rodents, the hearing function is immature in early postnatal epoch4,5. No action potential in response to sound signals could be detected in the auditory cortex before the hearing onset6,7. Development of morphology and function of the cochlea has been widely studied in mouse, gerbil, and rat4,5,8. The mechanotransduction and electromotility of hair cells are also developed in the early life epoch5.
In order to evaluate the hearing sensitivity of rats at different postnatal ages, we have developed a method for auditory brainstem response (ABR) recording in rat pups. Whole-cell patch clamp is an ideal technology to investigate the OHCs electrophysiologically. However, compared with the patch clamp performed in neurons and other epithelial cells, the low rate of whole-cell sealing limited the investigation of electromotility of isolated OHCs.
Here we describe a procedure to investigate the OHCs morphologically and electrophysiologically in acutely dissociated cochlea from postnatal rats. This method can be modified to study the molecular mechanisms that regulate inner hair cell development and function.
Protocol
All experimental protocols involving animal subjects were approved by the Animal Ethics Committee of the Southern Medical University.
1. Prepare Solutions for Experiments
Prepare the anesthetic (see Table of Materials): 1.5% pentobarbital sodium dissolved in ddH2O.
Prepare the dissection solution (see Table of Materials): Dissolve one bag of Leiboviz's L-15 powder in 1 L of ddH2O. Adjust pH to 7.3 with 1 M NaOH. Adjust osmolarity to 300-330 mOsm by using an osmometer and 10 mM HEPES before use.
Dissolve 2 mg/mL collagenase IV (see Table of Materials) in dissection solution prepared in step 1.2.
Prepare the immunostaining solutions (see Table of Materials): Dissolve the 4% paraformaldehyde in phosphate buffered saline (PBS). Caution: Paraformaldehyde is toxic; wear appropriate protection.
Dilute the 0.3% permeability agent in PBS. Dilute the 10% normal goat serum in PBS as the blocking buffer. Dilute the prestin antibody 1:200 in blocking buffer. Dilute the Alexa488-conjugated antibody 1:600 in blocking buffer. Dilute the Phalloidin-Tetramethylrhodamine B isothiocyanate 1:200 in PBS.
Prepare the extracellular solution (see Table of Materials): Prepare the Leiboviz's L-15 medium as described above (step 1.2).
Prepare the intracellular solution (see Table of Materials).
2. ABR Recording
NOTE: ABR recording has been previously described in detail in our previous study9.
Anesthetize Sprague Dawley (SD) rats (1-14 day old pups and 2-month old adults, both sexes) with a single injection of 1.5% sodium pentobarbital (22 mg/kg for pups and 30 mg/kg for adults, intraperitoneal (i.p.)).
Place the anesthetized animal in a polyethylene-foam mold to immobilize the animal's body. Place the animal on an anti-vibration table in a sound-attenuating room. Keep the animal's body temperature at 37.5 °C with a heating pad.
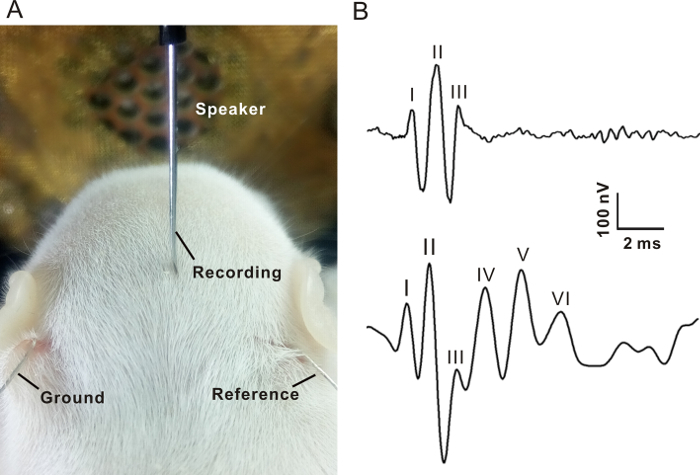
Wipe the head area with 70% (vol/vol) ethanol. By using a fine scissors, make a 1-2 mm incision ventrolateral to the external pinna to place the reference electrode or ground. A subdermal needle electrode (recording electrode) is located over the skull vertex (Figure 1A).
Using the function generator, generate calibrated tone bursts (1 ms rise/fall, 3 ms plateau) of various frequencies (2, 4, 8, 16, 24, and 32 kHz) and intensities (decreased from 85 to 10 dB SPL in 5 dB step). Vary the frequency and amplitude manually or using a computer. Deliver sound stimuli through an electrostatic speaker located 10 cm away toward the head of animal.
Filter (100-1,000 Hz), amplify and average (256 times) the sound elicited potential using a multi-function processor to get the ABRs. The ABR traces is monitored online and stored for offline analysis. It takes about 40 min to complete the ABR recording from one animal. NOTE: Usually, three to seven peaks (wave I to wave VII) with latencies less than 15 ms can be identified (Figure 1B). The ABR threshold is defined as the minimum sound level at which wave I and II could be detected.
Euthanize before the animals awake from anesthesia (with an intraperitoneal injection of 0.3 mL 1.5% sodium pentobarbital).
3. The Organ of Corti (OC) Dissection
Anesthetize rat pups. For rats less than 6 days old (<P6), keep the animal in a 100 mm culture dish, and cover the dish with ice for 10 min. For rats >P6, anesthetize the animal with a single injection of 1.5% sodium pentobarbital (22 mg/kg, i.p.). Decapitate the rat pups after anesthesia.
Sterilize the head by spraying with 70% (vol/vol) ethanol.
Open the skull along the sagittal midline with scissors; remove the brain to expose the inner ear.
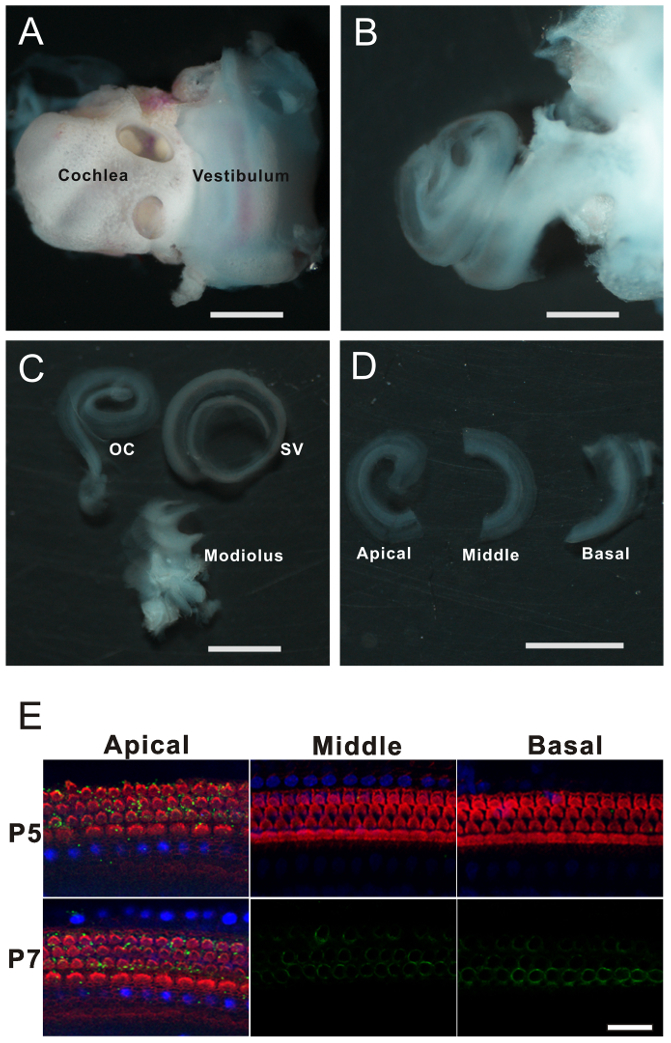
Transfer the inner ear into a 35-mm Petri dish filled with 3 mL of ice-cold L-15 (Figure 2A).
Under the dissection microscope, use fine forceps to remove the bony capsule of the cochlea (Figure 2B).
Unwrap the OC and associated stria vascularis (SV) from the modiolus.
By holding the basal portion of the SV with forceps, separate the OC from the SV completely by unwinding slowly from base-to-apex (Figure 2C).
Cut the OC evenly into three pieces by using fine scissors (Figure 2D).
4. Immunofluorescence Staining
By using a 200 µL pipette tip, transfer the segments of OC (step 3.8) onto a glass slide, and fix with 100 µL of 4% paraformaldehyde for at least 4 h at 4 °C. Caution: Paraformaldehyde is toxic; wear appropriate protection.
Wash the tissue by displacing the paraformaldehyde with 100 µL of fresh PBS. Wash the tissue three times for 10 min.
Incubate the tissue with 0.3% permeability agent in PBS for 30 min at room temperature.
Discard the permeability agent in PBS and wash the tissue three times with PBS.
Block with 10% normal goat serum in PBS for 1 h at room temperature.
Incubate with anti-prestin-C-terminus antibody (1:200) in blocking solution for 2 h at room temperature or at 4 °C overnight.
Wash three times with PBS for 5 min.
Incubate with Alexa488-conjugated secondary antibodies (1:600) in blocking solution for 1 h at room temperature in the dark.
Wash three times with PBS for 5 min in the dark.
Incubate with rhodamine-phalloidin (1:200) for 10 min at room temperature in the dark.
Wash three times with PBS for 5 min in the dark.
Mount on a glass slide with mounting medium (containing DAPI). Image with confocal microscopy by using 405 nm, 488 nm, and 594 nm lasers. Set the laser power at 0.1-0.5 mW and the scan speed at 0.5 frame/s.
5. Patch clamp Recording of Isolated OHCs
Use the pipette puller and micro-forger to make patch pipettes with a tip diameter 2-3 µm. Back-fill the pipettes with intracellular solution. Usually, the initial resistance of patch pipette was 2.5-3.5 MΩ in bath solution.
By using a 200 µL pipette tip, transfer a piece of the OC (from step 3.8) into a 35 mm Petri dish. Digest the tissue with 100 µL of enzymatic digestion medium (collagenase IV, see Table of Materials) for 5 min at room temperature.
Displace the enzymatic digestion medium with 100 µL of L-15. Cut the tissue into small pieces using a micro scalpel to isolate the hair cells.
After gentle pipetting, transfer the cells into a homemade small plastic chamber (diameter ~ 1.5 cm) filled with enzyme-free bath solution (~ 1.5 mL, see Table of Materials).
Place the chamber on the stage of an inverted microscope. Find the healthy-appearing solitary OHCs.
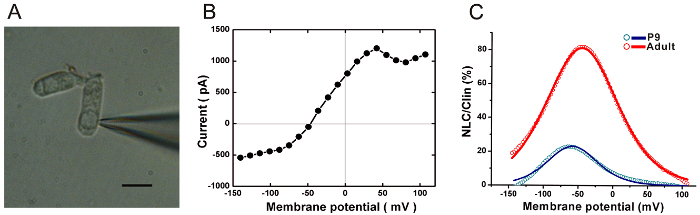
Load the patch pipette into the head stage of a 700B amplifier. Move the patch pipette carefully by a micromanipulator and position it around the bottom of the outer hair cell (Figure 3A).
Whole-cell patch clamp the OHC by sealing the lateral wall of the cell body. Apply light suction until the cell membrane is ruptured. Set the holding potential at -70 mV. Cells with access resistance ranged from 10 to 17 MΩ and membrane resistance ranged from 100 to 500 MΩ, and are considered a successful whole-cell configuration.
Using computer control, apply hyperpolarizing and depolarizing voltage (250 ms duration and ranging from −140 to +94 mV in 13 mV steps) to the cell to elicit whole-cell currents.
Amplify the whole-cell currents, filter (corner frequency of 5 kHz) by a 700B amplifier. Convert the data by a 1440A converter and store for offline analysis.
Measure the membrane capacitance of OHCs using a two sine-wave voltage stimulus protocol controlled by a patch clamp software. Set the stimulate voltage range from -140 to 110 mV. Store the data for offline analysis.
Representative Results
ABR can be elicited from anesthetized rat pups older than postnatal day 7 (P7) using pure tone bursts (Figure 1A). As shown in Figure 1B, the ABR waveforms obtained from rat pups showed only three to four distinct waves with small amplitude. Usually, up to seven peaks were observed in the ABR waveforms of adult animals (Figure 1B).
For the following immunostaining and electrophysiological measurement, rat inner ear was freshly dissected from the temporal bone. We show the consecutive steps of tissue dissection to isolate the OC from SV and the modiolus (Figure 2A-2C). The OC was cut into three pieces evenly with micro-scissors (Figure 2D). To show the morphology of the hair cells, the segments were stained with phalloidin, prestin antibody, and DAPI (Figure 2E). The hair bundles (in red) indicate the apical surface of hair cells, whereas the cell body of the OHCs was marked by prestin (in green). The nucleus was labeled by DAPI (in blue) located in the bottom region of the IHCs and OHCs. As shown in Figure 2E, no prestin was detected in the OHCs of cochlea at P5. Prestin was first observed at P7 in OHCs located in the basal cochlea.
After gentle digestion, the OHCs were isolated from the OC (Figure 3A). Whole-cell voltage-clamp recordings were performed from OHCs acutely isolated from the rat cochlea. A representative example of the whole-cell current recorded from an isolated P9 OHC in response to membrane potential changed from −140 to +107 mV is shown in Figure 3B. Please note the N-shape region of the I-V curve, indicating the presence of KCa current which is a unique response of OHCs. Driven by the membrane voltage, the intracellular Cl- combined with the prestin, appearing as a nonlinear membrane capacitance (NLC) changes under whole-cell patch clamp mode. The typical NLC of a mature OHC is characterized by bell-shaped dependence on membrane potential. The NLC can be fitted with a two-state Boltzmann function and can reflect the electromotility function of OHCs. The Boltzmann function for capacitance fitting is described as:
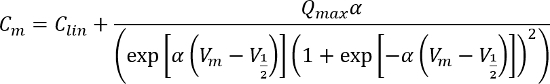
The equation and the parameters were described in our previous paper9. Two representative NLCs are shown in Figure 3C.
Figure 1. Auditory brainstem response recording. (A) The recording electrode was inserted into the scalp between two ears. Ground and reference electrodes were placed under the left and right pinna, respectively. An open field speaker was placed 10 cm away from the nasal tip of the rat. (B) Two representative ABR waveforms in response to 16 kHz, 80 dB SPL tone bursts. Upper trace recorded from a P8 pup. Bottom trace recorded from an adult rat. The numbers indicate the different peaks of waveforms. Please click here to view a larger version of this figure.
Figure 2. The organ of Corti dissection. (A) The inner ear dissected from a P9 rat pup. The cochlea and vestibular region are shown. (B) The structure of cochlea was exposed after removal of the bony wall. (C) The organ of Corti (OC) and stria vascularis (SV) were isolated from the modiolus. (D) The organ of Corti was cut evenly into three pieces. Scale bars in A-D represent 1,000 µm. (E) Confocal images show the hair cells located in different segments (basal, middle, and apical) along the cochlea. Rhodamine-phalloidin (red) represents the hair bundles located on the apical surface of the hair cells. DAPI (blue) represents the nuclei located in the middle-bottom portion of hair cells. The prestin (labeled in green) was only expressed on the lateral wall of the outer hair cells. For middle and basal segments at P7, only the green channel is shown to illustrate the expression of prestin in OHCs. Scale bar represents 20 µm. Please click here to view a larger version of this figure.
Figure 3. Whole-cell patch clamp recording of isolated outer hair cells. (A) An isolated outer hair cell was sealed in whole-cell mode. Scale bar represents 10 µm. (B) A representative I-V curve recorded from a P9 outer hair cell. (C) The nonlinear membrane capacitance (NLC) obtained from two outer hair cells at different ages. The capacitance-voltage responses (open circles) were fitted to the Boltzmann function (shown as the thick lines). The NLC were normalized to the corresponding linear capacitance (Clin). Please click here to view a larger version of this figure.
Discussion
In rats younger than day 11, no action potential in response to sound stimulation could be observed in auditory cortex6,7. Therefore, postnatal day 11 is described as "hearing onset"10. The development of hearing function before hearing onset was not well studied yet. Using the similar method for adult ABR recording, we demonstrate that ABRs could be elicited by pure tone burst from rat pups younger than P11 (Figure 1). However, the ABR waveforms obtained from rat pups showed some different features from those of adult animals. The ABR responses are small in amplitude with relatively high thresholds. This result implies that the developing auditory system has low sensitivity to sound signals. Significant difference was observed in the ABR waveforms recorded from rat pups. Usually, up to seven peaks were observed in adult ABR waveforms9. These peaks with different latency are generated by different brainstem nuclei along the auditory pathway11. Our results indicate that only the I-IV waves were detectable in ABR from rat pups (Figure 1B). The waves I and II represent the responses from the cochlea and the auditory nerve11. Therefore, the results indicate that the higher level auditory nuclei failed to respond to acoustic stimuli during development. These data suggest that cochlea reach functional maturity earlier than does the central auditory system. The method we describe here is important for the study of molecular mechanisms that regulate cochlea development and hair cell function.
High quality dissection of the OC is critical for further experiments including immunostaining, Western blotting, and electrophysiological measurement. These experiments have been performed using adult SD rats and rat pups between 1 and 14 days old. Rat pups are ideal for fine dissection as the cochlear bone is soft and can be easily removed by forceps without damaging the OC. For adult rats, particular caution is required to avoid rupturing the OC while removing the hard-bony capsule. By using fine forceps, the OC and associated SV can be lifted off from the modiolus. The separated OC and SV have different texture (Figure 2C). For rat pups, the OC could be cut into three segments with a length of 800-1200 µm. Please note, all steps should be performed in ice-cold L-15 as fast as possible in order to minimize degradation and tissue deterioration.
Isolated segments of the OC are good preparations for immunostaining, Western blotting, and RNA analysis. Here we show the morphology of sensory hair cells in OC by immunostaining. OHCs have hair bundles on their apical surface whereas the nucleus has them located basally12. The motor protein prestin is expressed on the lateral membrane of OHCs. The confocal imaging data indicated that the prestin was first expressed at P6-P7 and showed a continued increase over the following week (Figure 2E). In further experiments, Western blotting and q-PCR were performed to quantify the observed expression level changes of prestin during development (data not shown). Because the hair cells are the minority in the OC, 6 to 10 cochleae are recommended in a single experiment to achieve robust signals.
For patch clamp recordings of OHCs, mild enzymatic digestion was performed to separate the OHCs. The hair cells are fragile, and special caution is required in this step. Use a 200 µL pipette tip to transfer the tissue and separated cells. The isolated segment of OC was transferred to a drop of collagenase solution (~ 100 µL) in a Petri dish for about 5 min at room temperature. Avoid long digestion times, which will damage the cell membrane of OHCs. Under the microscope, healthy looking OHCs were selected for the patch clamp recording. Cells showing any signs of shrinkage, swelling, damage, or translocation of the nucleus were excluded from next experiment. Healthy appearing solitary OHCs and IHCs are easy to identify by their morphological difference. OHCs show a cylindrical shape and a larger axis-diameter ratio, whereas IHCs are flask-shaped with a tight neck12. Whole-cell patch clamp in OHCs is somewhat difficult from those in neurons and other epithelial cells. For successful whole-cell configuration, the patch pipette was usually located near the nucleus, kept away from the upper lateral membrane containing a high density of prestin particles (Figure 3A). After the membrane was ruptured, a two sine-wave voltage stimulus protocol was applied to the cell in order to obtain the membrane capacitance of OHCs. With the same technique, by using voltage steps, it is possible to elicit whole-cell current.
This method is a good tool to investigate the electromotility function of OHCs in vitro9,13. This method could also be used to investigate the function of ion channels, as well as the pharmacological properties of receptors in OHCs and IHCs14.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by grants from the 973 Program (2014CB943002) and the National Natural Science Foundation of China (11534013, 31500841).
References
- He DZ, Zheng J, Kalinec F, Sacchi Kakehata SSantos-, J Tuning in to the amazing outer hair cell: membrane wizardry with a twist and shout. J Membr Biol. 2006;209:119–134. doi: 10.1007/s00232-005-0833-9. [DOI] [PubMed] [Google Scholar]
- Dallos P. Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol. 2008;18:370–376. doi: 10.1016/j.conb.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, et al. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- Abe T, et al. Developmental expression of the outer hair cell motor prestin in the mouse. J Membr Biol. 2007;215:49–56. doi: 10.1007/s00232-007-9004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguespack J, Salles FT, Kachar B, Ricci AJ. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci. 2007;27:13890–13902. doi: 10.1523/JNEUROSCI.2159-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- De V-SE, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DZ, Evans BN, Dallos P. First appearance and development of electromotility in neonatal gerbil outer hair cells. Hearing Res. 1994;78:77–90. doi: 10.1016/0378-5955(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Hang J, et al. Synchronized Progression of Prestin Expression and Auditory Brainstem Response during Postnatal Development in Rats. Neural Plast. 2016. p. 4545826. [DOI] [PMC free article] [PubMed]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1:179–184. [PubMed] [Google Scholar]
- Møller AR. Hearing:anatomy, physiology, and disorders of the auditory system. Dallas: Academic Press; 2006. [Google Scholar]
- He DZ, Zheng J, Edge R, Dallos P. Isolation of cochlear inner hair cells. Hearing Res. 2000;145:156–160. doi: 10.1016/s0378-5955(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Tang J, Pecka JL, Tan X, Beisel KW, He DZZ. Engineered Pendrin Protein, an Anion Transporter and Molecular Motor. J Biological Chem. 2011;286:31014–31021. doi: 10.1074/jbc.M111.259564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, et al. The Effects of Urethane on Rat Outer Hair Cells. Neural Plast. 2016;2016:1–11. doi: 10.1155/2016/3512098. [DOI] [PMC free article] [PubMed] [Google Scholar]


