Abstract
Local delivery of therapeutic drugs into the inner ear is a promising therapy for inner ear diseases. Injection through semicircular canals (canalostomy) has been shown to be a useful approach to local drug delivery into the inner ear. The goal of this article is to describe, in detail, the surgical techniques involved in canalostomy in both adult and neonatal mice. As indicated by fast-green dye and adeno-associated virus serotype 8 with the green fluorescent protein gene, the canalostomy facilitated broad distribution of injected reagents in the cochlea and vestibular end-organs with minimal damage to hearing and vestibular function. The surgery was successfully implemented in both adult and neonatal mice; indeed, multiple surgeries could be performed if required. In conclusion, canalostomy is an effective and safe approach to drug delivery into the inner ears of adult and neonatal mice and may be used to treat human inner ear diseases in the future.
Keywords: Genetics, Issue 135, Canalostomy, cochlea, vestibule, hair cell, local drug delivery, semicircular canal, neonatal
Introduction
Sensorineural hearing loss and vestibular dysfunction affect a substantial number of patients and are closely associated with inner ear disorders. Delivery of therapeutic drugs into the inner ear shows promise for the treatment of inner ear disorders. A systemic or local approach can be used to deliver drugs into the inner ear. Some inner ear diseases are successfully treated with systemic drug administration, such as idiopathic sudden hearing loss, which is commonly treated with systemic steroid1. In addition, Lentz et al. showed that systemic administration of antisense oligonucleotide was able to improve hearing and balance functions in the Ush1c mutant mouse model2. However, a large portion of inner ear diseases are not effectively treated by systemic drug administration because of the blood-labyrinth barrier, which limits drug access to the inner ear3,4. In contrast, local drug delivery strategies can treat inner ear disorders more efficiently. Indeed, the inner ear is potentially an ideal target for local drug delivery; it is filled with fluid, which facilitates dissemination of the drug after one-site diffusion or injection, and it is relatively isolated from neighboring organs, which limits side-effects5,6.
Local drug delivery strategies include intratympanic and intralabyrinthine methods. The effectiveness of the intratympanic route largely relies on drug permeability through the round window membrane (RWM) and the residence time of the drug on the RWM3,4,7,8. Thus, it is not suitable for the delivery of drugs or reagents that cannot penetrate the RWM. Intralabyrinthine methods involve inoculation of drugs directly into the inner ear, resulting in a high dose and widespread distribution. However, intralabyrinthine methods require delicate surgeries and are invasive, leading to damage to inner ear function. Currently, the intralabyrinthine injection of drugs is used only in animal studies as it has not been demonstrated to be sufficiently safe for use in humans9. Therefore, surgical procedures must be simplified, and the risk of injury reduced to translate intralabyrinthine approaches into the clinic.
Several intralabyrinthine approaches have been assessed in animals by injection through the RWM5,10,11 and into the scala media12,13,14, the scala tympani15,16, the scala vestibule17, the semicircular canals16,18,19,20, and the endolymphatic sac21. Each of these approaches has advantages and disadvantages6. Delivery through the RWM is atraumatic in neonatal mice5,22. However, a mild hearing loss is observed in adult mice after RWM injection23, possibly due to middle ear effusion after the surgery24. Scala media injection, which involves the injection of the reagent directly into the endolymphatic space containing the sensory epithelium, achieves a high reagent concentration in target end-organs12,14,25,26. However, this approach requires a complex procedure and results in significant elevation of the hearing threshold if performed later than postnatal day 5 (P5)25,27, which limits its application.
Compared to the above-mentioned intralabyrinthine approaches, canalostomy causes minimal damage to the inner ear, especially in adult mice16,18,28,29,30, which is important for the assessment of protective effects and translational aspects. Furthermore, in rodents, the semicircular canals are located beyond the bulla, which facilitates surgical procedures and avoids disturbance of the middle ear during surgery. In the clinic, semicircular canal surgeries are used for intractable benign paroxysmal positional vertigo31,32,33, suggesting the clinical feasibility of canalostomy. Since it was first described by Kawamoto et al.16 in 2001, canalostomy has been used to deliver various reagents, such as viral vectors, siRNA, stem cells, and aminoglycoside, into the murine inner ear18,19,28,29,34,35,36,37. Inoculation of adeno-associated virus (AAV) vectors by canalostomy enable overexpression of exogenous genes in the sensory epithelium and primary neurons of the cochlea and vestibular end-organs18,28,29,30. Whirlin gene therapy by canalostomy restores balance function and improves hearing in a mouse model of human Usher syndrome19, suggesting that canalostomy is useful for studies of gene therapy for genetic cochleovestibular diseases. Transplantation of mesenchymal stem cells by canalostomy results in reorganization of cochlear fibrocytes and hearing recovery in a rat model of acute sensorineural hearing loss35. Additionally, canalostomy can be used to introduce aminoglycosides into the inner ear to establish vestibular lesions18,34,38, and multiple injections can be performed if required18,34.
In the present article, we describe, in detail, canalostomy techniques in adult and neonatal mice. We inoculated various reagents, including fast-green dye and AAV serotype 8 (AAV8), together with the green fluorescent protein (GFP) gene (AAV8-GFP) and streptomycin, into the mouse inner ear to evaluate the immediate and long-term outcomes after canalostomy.
Protocol
All procedures and animal surgeries were conducted according to the guidelines of the Animal Care and Use Committee of the Capital Medical University of China.
1. Device Preparations
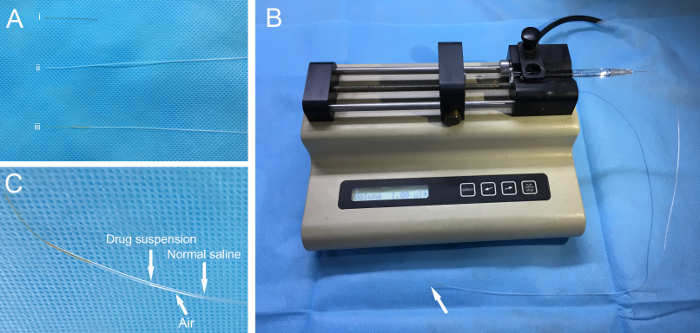
To make the injection cannula (Figure 1A), connect polyimide tubing (inner diameter 114.3 µm, outer diameter 139.7 µm, length ~3 cm) to polyethylene tubing (inner diameter 280 µm, outer diameter 640 µm, length ~40 cm). Using superglue, seal the connection with at least three applications. Sterilize the injection cannula with ethylene oxide. NOTE: When sealing the tubing, prevent the superglue from entering the polyimide tubing, which can result in a blockage of the cannula. Seal the tubing at least three times, because gas sterilization may cause leakage at the connection.
Using a 30 G needle, connect the end of the polyethylene tubing to a 1 cc syringe containing normal saline. Fill the cannula with normal saline by injection with the 1-cc syringe to check for any leakage or blockage at the connection between the polyimide tubing and polyethylene tubing. NOTE: If there is a leakage or a blockage of the connection, the cannula cannot be used in the following procedures.
Evacuate a 10 µL micro-syringe with normal saline and connect it to the aforementioned 30 G needle with the injection cannula. Install the micro-syringe on a microinjection pump (Figure 1B). Set the injection speed to 0.5 µL/min and the volume to 1 µL for fast-green dye and AAV8-GFP, or 2 µL for streptomycin. NOTE: The recommended range of injection speed is 0.1−0.5 µL/min, and the recommended range of injection volume is 0.5−2 µL.
Pull back the micro-syringe to 1 µL and extract the injection reagent. An air gap will form between the normal saline and the injected reagent (Figure 1C).
Optionally label the position of the gas-fluid border on the polyethylene tubing using a marker pen. This can be used to monitor reagent flow during injection.
2. Canalostomy in Adult Mice
Anesthetize an adult mouse (female, FVB/N, 5 to 6-week old) by intraperitoneal injection of ketamine HCl (120 mg/kg) and xylazine HCl (7 mg/kg). Wait for 5−10 min until the animal exhibits no response to painful stimuli (the toe-pinch reflex). Place the animal on a preheated electric pad after anesthesia. An analgesic, Meloxicam (1 mg/kg), was applied subcutaneously before surgery.
Cover the animal's eyes with eye ointment. Shave the left post-auricular region with an electric animal clipper and disinfect the skin three times with 75% ethanol.
Place the animal on a preheated electric pad. Set the temperature of the electric pad to ~37°C. Place the animal in the right lateral position to facilitate surgery on the left ear.
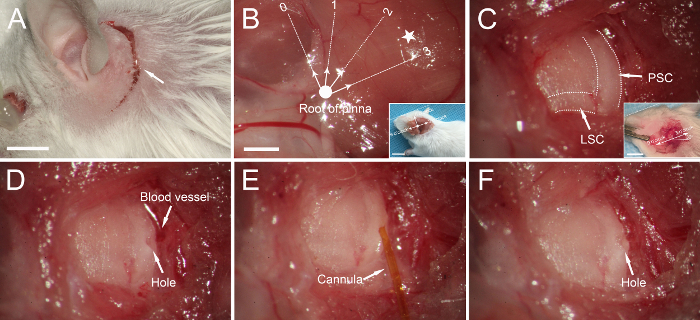
Make a 1-1.5-cm post-auricular incision ~3 mm from the left retroauricular groove (Figure 2A).
Positioning the posterior semicircular canal (PSC) and lateral semicircular canal (LSC): When the root of the pinna (bold dot in Figure 2B) is defined as the origin and the plane parallel to the calvarium as 3 to 9 o'clock, the PSC and the LSC are typically located ~3 mm from the root of the pinna between 2 and 3 o'clock (Figure 2B).
Bluntly dissect the muscle covering the temporal bone with micro-forceps to expose the PSC and LSC, whose margins are clearly visible as dark stripes in the temporal bone (Figure 2C). The LSC is at an approximately 30° angle from the plane parallel to the calvarium, and the PSC is vertical to the LSC (Figure 2C). Collect a small piece of muscle with micro-forceps and let it dry. In the following step, this muscle will be used to seal the hole. NOTE: Avoid excessive tearing of the tissues to minimize injury to muscles. Avoid damaging the adjacent vessel when exposing the PSC (Figure 2D).
Make a small hole in the middle portion of the PSC using a 26 G needle (Figure 2D). Fluid leakage through the hole indicates successful penetration of the bony wall of the PSC. Enlarge the hole to an appropriate size, slightly larger than the diameter of the polyimide tubing. NOTE: Drill and enlarge the hole gently and gradually to avoid fracture of the PSC.
Clean the effusion surrounding the hole of the PSC using a cotton pellet.
Insert the tip of the polyimide tubing gently into the PSC toward the crus commune to a depth of 1−2 mm (Figure 2E). Start the injection by pressing the 'run' button on the pump.
After the injection, wait ~2 min to allow the reagent to spread. Trim the small piece of muscle collected in step 2.6 with micro-scissors. Next, remove the injection cannula and immediately place the muscle into the hole in the PSC. NOTE: Check for any liquid leaking from the hole after plugging to ensure that the hole is completely sealed.
Return the separated muscles and subcutaneous tissues. Suture the incision using a 5-0 suture. Disinfect the incision region with povidone iodine. NOTE: All the above surgical procedures take about 25 min.
Position the animal on an electric pad preheated to ~37 °C. Place the animal in the right lateral position for recovery.
Perform auditory brainstem response (ABR) measurements and swimming tests one week after the surgery18.
If required, perform repeated injections in a different area of the PSC or LSC.
3. Canalostomy in Neonatal Mice
Use hypothermia to induce and maintain sedation in neonatal mice (FVB/N) at postnatal days 1 to 2 (P1-2). Place the pup on a plastic-wrap-covered bed of crushed ice for ~4 min. Next, lay the pup on an ice-filled platform. Disinfect the surgical field three times with 75% ethanol. NOTE: Ensure that the head of the pup is out of the ice. Perform the entire surgery with the pup on an ice-filled platform.
- The following steps differ in the neonate compared to the adult mice.
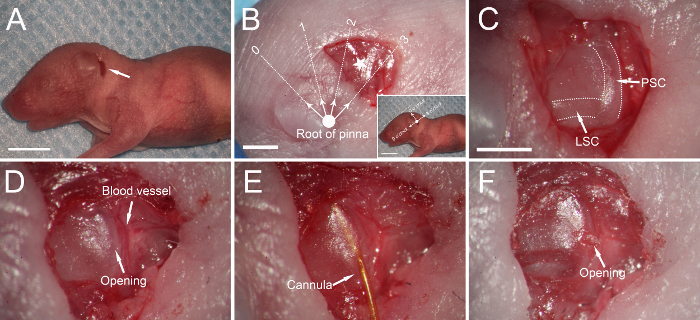
- Make a ~3-mm postauricular incision from ~2 mm posterior to the auricular crease (Figure 3A - B).
- Gently make an opening in the soft PSC using a 26 G needle. Insert the cannula into the PSC without enlarging the opening (Figure 3C - E).
- After injection, use a piece of muscle to cover, rather than plug, the opening as the latter can lead to a fracture of the soft PSC (Figure 3F).
- Close the skin using a 6-0 suture.
- Perform ABR measurements and swimming tests at P3030.
- Parental cannibalism is a common problem after neonatal surgery. The following steps reduce the likelihood of parental cannibalism.
- Clean up the blood surrounding the incision with alcohol wipes after surgery.
- Ensure that the neonate can move freely before returning it to the dam.
- Smear neonates with soiled bedding from the mother's cage, and place the neonates back in the middle of the litter.
- Any that do not undergo surgery should receive a similar postauricular incision and suture.
- Separate the male from the breeding cage.
Representative Results
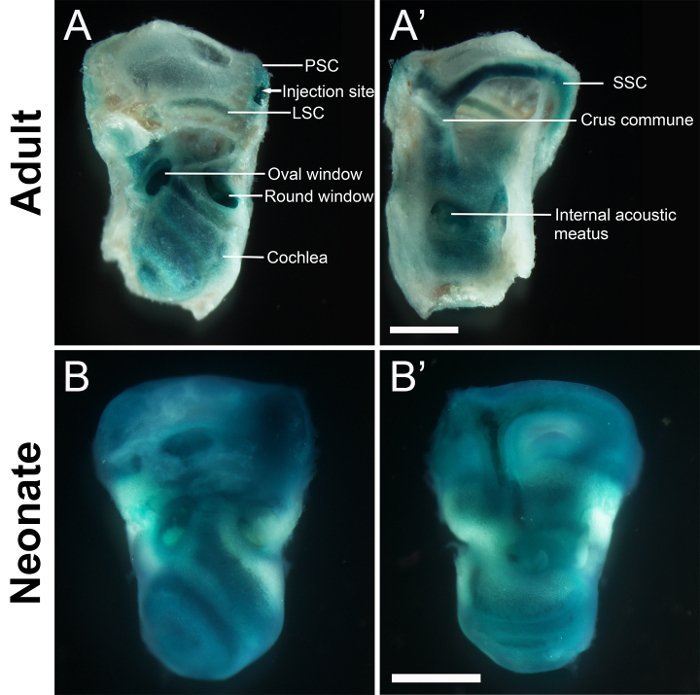
Fast-green dye was injected into the PSC of adult and neonatal mice to evaluate its immediate distribution in the inner ear. The dye was detected throughout the cochlea, vestibule, and semicircular canals immediately after surgery (Figure 4).
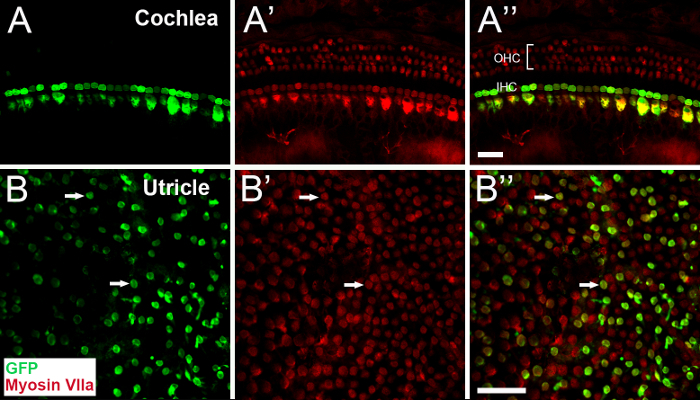
To evaluate the safety and efficiency of canalostomy for inner ear gene delivery, AAV8-GFP was injected into the inner ear of adult and neonatal mice. All animals exhibited normal ABR thresholds and swimming test scores after AAV8-GFP injection18,30. Immunohistology revealed that, in the auditory sensory epithelium, inner hair cells (IHCs) and a few outer hair cells (OHCs) of the basal turn showed robust GFP expression30. In the vestibular sensory epithelium, GFP expression was detected in the utricle, saccule, and ampullae30. Both vestibular hair cells (Figure 5) and supporting cells were transduced with high efficiency18,30.
Infusion of streptomycin by canalostomy induces severe hair cell loss in the utricle18,34,38. To test the feasibility of multiple injections via canalostomy, mice were given streptomycin through the LSC followed 7 days later by AAV8-GFP suspensions through the PSC. This experiment was also designed to evaluate the transduction characteristics of AAV8-GFP in the lesioned utricle, which was useful for gene therapy studies in the damaged inner ear after HC loss18. GFP was distributed throughout the lesioned utricle (Figure 6). Numerous GFP-positive cells were negative for myosin VIIA, indicating that they were transduced SCs. A few HCs (myosin VIIa-positive cells) with immature hair bundles also expressed GFP.
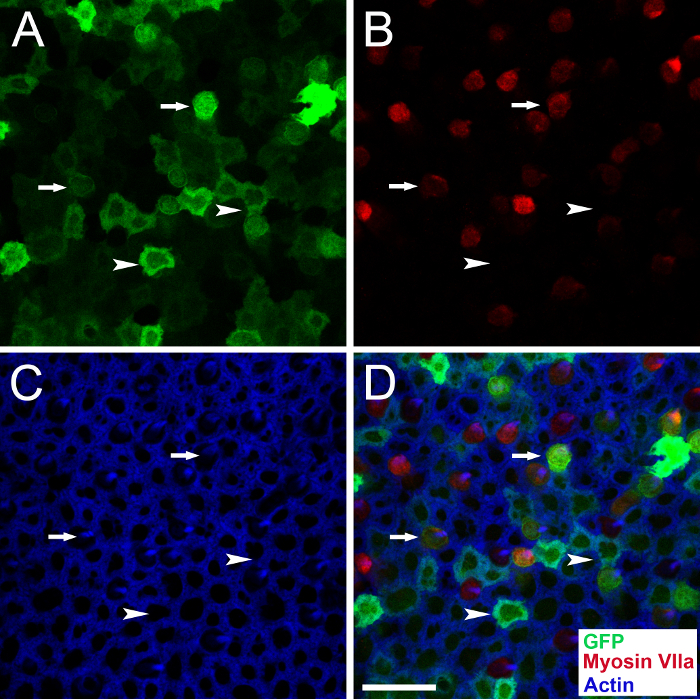
Figure 1: Device preparation. (A) The polyimide tubing (i) and the polyethylene tubing (ii) are sealed to make an injection cannula (iii). (B) The cannula is connected to a 10-µL micro-syringe with a 30 G needle and then installed on a pump. Arrow indicates the cannula tip. (C) Injection reagent and normal saline are separated by an air gap. Please click here to view a larger version of this figure.
Figure 2: Canalostomy in an adult mouse. (A) A post-auricular incision (arrow). (B) Muscles covering the temporal bone are exposed. If we define the root of the pinna (bold dot) as the origin and the plane parallel to the calvarium as 3/9 o'clock, the posterior semicircular canal (PSC) and lateral semicircular canal (LSC) are generally located in the region between 2 and 3 o'clock (pentagram), ~3 mm from the origin. Inset: lower-magnification image of the orientation. The dotted line indicates the calvarium plane. (C) The PSC and LSC are exposed (dotted lines). Inset: The LSC is at an approximately 30° angle from the plane parallel to the calvarium, and the PSC is vertical to the LSC. (D) A small hole is made in the PSC. (E) The tip of the cannula is inserted into the PSC, and the reagent is injected. (F) The hole is sealed with a small piece of muscle. Scale bar in A is 5 mm, that in B is 1 mm (B for B-F), that in the inset to B is 1 cm, and that in the inset to C is 5 mm. Please click here to view a larger version of this figure.
Figure 3: Canalostomy in a neonatal mouse.(A) A post-auricular incision (arrow). (B) Muscles covering the temporal bone are exposed, and the posterior semicircular canal (PSC) is ~2 mm from the root of the pinna (bold dot) at 2-3 o'clock (pentagram). The orientation is identical to that in adult mice (Figure 2B). (C) The PSC and lateral semicircular canal (LSC) are exposed (dotted lines). (D) A small opening is made in the PSC. (E) The cannula is inserted into the PSC. (F) A small piece of muscle is used to cover the opening after injection. Scale bars in A and B inset are 5 mm, that in B is 1 mm, and that in C is 1 mm (C for C-F). Please click here to view a larger version of this figure.
Figure 4: Stereo microscopic images of the inner ears of adult (A-A') and neonatal mice (B-B') administered fast-green dye via canalostomy. Samples were collected immediately after surgery. (A and B) The extracranial surface. (A' and B') The intracranial surface. Fast-green dye distributes throughout the cochlea, vestibule, and semicircular canals. Scale bars are 1 mm (A' for A-A' and B' for B-B'). PSC, posterior semicircular canal. LSC, lateral semicircular canal. SSC, superior semicircular canal. Please click here to view a larger version of this figure.
Figure 5: Representative confocal images of whole mounts of the cochlea (A-A") and utricle (B-B") of adult mice, prepared 30 days after AAV8-GFP injection via canalostomy. Samples are stained with antibodies for GFP (green) and myosin VIIa (red). (A-A") GFP is expressed in most inner hair cells (IHC). (B-B") At the focal plane of the cuticular plate of the utricle, numerous hair cells express GFP (arrows). GFP, green fluorescent protein. OHC, outer hair cells. Scale bars are 25 µm (A" for A-A", B" for B-B"). Please click here to view a larger version of this figure.
Figure 6: Representative confocal images of a traumatized utricle obtained 30 days after AAV8-GFP injection via canalostomy. An adult mouse was injected with streptomycin via the lateral semicircular canal and 7 days later injected with AAV8-GFP via the posterior semicircular canal. GFP (green), myosin VIIa (red), and actin (blue). Arrowheads indicate representative supporting cells transduced with AAV8-GFP (GFP+/myosin VIIa- cells), and arrows indicate representative hair cells transduced with AAV8-GFP (GFP+/myosin VIIa+ cells) with immature hair bundles. Scale bar is 20 µm (D for A-D). Please click here to view a larger version of this figure.
Discussion
In this study, we showed that drug delivery by canalostomy resulted in widespread distribution of the reagent throughout the cochlea and vestibular end-organs. As an inner ear gene delivery method, canalostomy resulted in GFP expression in the inner ears of adult and neonatal mice with minimum damage to hearing and vestibular function. Furthermore, multiple injections can be easily performed in the same animal.
One of the greatest strengths of canalostomy is that it causes minimal damage to inner ear function, especially in adult mice16,18,28,29,30, which is significant for the assessment of protective effects and for translational aspects. Several groups have used canalostomy to deliver various reagents, such as viral vectors, siRNA, stem cells, and aminoglycoside, into the murine inner ear18,19,28,29,34,35,36,37. In the current study, we described step-by-step, detailed surgical techniques of canalostomy in adult and neonatal mice. Compared to previous studies, our study provides additional details on the positioning of the semicircular canals, which is a key procedure for successful surgery. As shown in Figure 2B and C, the PSC and LSC were generally located ~3 mm from the root of the pinna at 2-3 o'clock. The LSC was at an approximately 30° angle from the plane parallel to the calvarium, and the PSC was vertical to the LSC. Furthermore, we simplified the surgical procedures and shortened the surgical time to around 25 min with comparable results29.
During canalostomy, it is imperative to avoid leakage at the injection site and blockage of the cannula. Before switching on the pump, it is important to ensure that the tip of the cannula is inserted in the semicircular canal and that the cannula is not bent or blocked. Dry muscle is recommended to be used when sealing the hole in the semicircular canal of adult mice because, in the presence of semicircular canal fluid, it expands and plugs the hole (see steps 2.6 and 2.10). Because the wall of the semicircular canal of neonatal mice is soft and fragile, the opening should be sealed by covering with autogenous muscle or medical glue30. We also removed the cannula without covering the opening with muscle; the results revealed comparable AAV transduction efficiency in the inner ear (data not shown), indicating that the opening in the PSC of neonatal mice was closed satisfactorily.
Another advantage of canalostomy is the feasibility of multiple procedures, which enables repetitive applications of the same or different reagents (Figure 6). Because fibrotic and granulation tissues are frequently found at previous surgical sites, and the semicircular canal may be obstructed after surgery34, it is recommended to perform injections during repeated surgeries in a different area of the lateral and/or posterior semicircular canals18.
The main limitation of canalostomy is the difficulty in determining whether the injection cannula is inserted into the perilymphatic or endolymphatic space. After administration of adenoviral vectors into the inner ear16 via canalostomy, most transduced cells were located in the perilymphatic space, suggesting that the injection was likely performed in that area. Low molecular weight compounds (e.g., AAV, streptomycin, and siRNA) can pass through the barrier between the perilymph and endolymph and reach the sensory epithelium after injection into the perilymphatic or endolymphatic space18,19,34. However, after injection into the perilymph, reagents may remain in the perilymphatic space if they cannot penetrate the barrier between the perilymph and endolymph16,39,40. Therefore, the permeability of the injected reagents should be considered before using canalostomy.
In conclusion, canalostomy results in a broad distribution of reagents in the cochlea and vestibule with minimal disturbance of hearing and vestibular function. The surgery is easily implemented in adult and neonatal mice, and multiple procedures can be performed if required. Canalostomy is thus an effective and safe approach for drug delivery into the inner ear of rodents and may, in the future, be used clinically to treat human cochleovestibular diseases.
Disclosures
No conflicts of interest are declared.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 81570912, 81771016, 81100717).
References
- Stachler RJ, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 Suppl):S1–S35. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- Lentz JJ, et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat Med. 2013;19(3):345–350. doi: 10.1038/nm.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera T, Sanz L, Camarero G, Varela-Nieto I. Drug delivery to the inner ear: strategies and their therapeutic implications for sensorineural hearing loss. Curr Drug Deliv. 2012;9(3):231–242. doi: 10.2174/156720112800389098. [DOI] [PubMed] [Google Scholar]
- El Kechai N, et al. Recent advances in local drug delivery to the inner ear. Int J Pharm. 2015;494(1):83–101. doi: 10.1016/j.ijpharm.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Akil O, Rouse SL, Chan DK, Lustig LR. Surgical method for virally mediated gene delivery to the mouse inner ear through the round window membrane. J Vis Exp. 2015. p. e52187. [DOI] [PMC free article] [PubMed]
- Ahmed H, Shubina-Oleinik O, Holt JR. Emerging Gene Therapies for Genetic Hearing Loss. J Assoc Res Otolaryngol. 2017;18(5):649–670. doi: 10.1007/s10162-017-0634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Cuesta S, et al. A Comparative Study of Drug Delivery Methods Targeted to the Mouse Inner Ear: Bullostomy Versus Transtympanic Injection. J Vis Exp. 2017. p. e54951. [DOI] [PMC free article] [PubMed]
- Stevens SM, Brown LN, Ezell PC, Lang H. The Mouse Round-window Approach for Ototoxic Agent Delivery: A Rapid and Reliable Technique for Inducing Cochlear Cell Degeneration. J Vis Exp. 2015. p. e53131. [DOI] [PMC free article] [PubMed]
- Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiol Neurootol. 2009;14(6):350–360. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil O, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75(2):283–293. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat Biotechnol. 2017;35(3):264–272. doi: 10.1038/nbt.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, et al. Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene Ther. 2011;18(6):569–578. doi: 10.1038/gt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Chang Q, et al. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol Med. 2015;7(8):1077–1086. doi: 10.15252/emmm.201404929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Mikulec AA, McKenna MJ, Sewell WF, Kujawa SG. A method for intracochlear drug delivery in the mouse. J Neurosci Methods. 2006;150(1):67–73. doi: 10.1016/j.jneumeth.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Oh SH, Kanzaki S, Brown N, Raphael Y. The functional and structural outcome of inner ear gene transfer via the vestibular and cochlear fluids in mice. Mol Ther. 2001;4(6):575–585. doi: 10.1006/mthe.2001.0490. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, et al. Neurotrophin-3 transduction attenuates cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6(1):12–18. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- Wang GP, et al. Adeno-associated virus-mediated gene transfer targeting normal and traumatized mouse utricle. Gene Ther. 2014;21(11):958–966. doi: 10.1038/gt.2014.73. [DOI] [PubMed] [Google Scholar]
- Isgrig K, et al. Therapy Restores Balance and Auditory Functions in a Mouse Model of Usher Syndrome. Mol Ther. 2017;25(3):780–791. doi: 10.1016/j.ymthe.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassner D, Durham D, Pfannenstiel SC, Brough DE, Staecker H. Canalostomy as a surgical approach for cochlear gene therapy in the rat. Anat Rec (Hoboken) 2012;295(11):1830–1836. doi: 10.1002/ar.22593. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Yagi M, Roessler BJ, Miller JM, Raphael Y. Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum Gene Ther. 1999;10(5):769–774. doi: 10.1089/10430349950018526. [DOI] [PubMed] [Google Scholar]
- Xia L, Yin S, Wang J. Inner ear gene transfection in neonatal mice using adeno-associated viral vector: a comparison of two approaches. PLoS One. 2012;7(8):e43218. doi: 10.1371/journal.pone.0043218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien WW, McDougald DS, Roy S, Fitzgerald TS, Cunningham LL. Cochlear gene transfer mediated by adeno-associated virus: Comparison of two surgical approaches. Laryngoscope. 2015;125(11):2557–2564. doi: 10.1002/lary.25317. [DOI] [PubMed] [Google Scholar]
- Zhu BZ, Saleh J, Isgrig KT, Cunningham LL, Chien WW. Hearing Loss after Round Window Surgery in Mice Is due to Middle Ear Effusion. Audiol Neurootol. 2017;21(6):356–364. doi: 10.1159/000449239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Early postnatal virus inoculation into the scala media achieved extensive expression of exogenous green fluorescent protein in the inner ear and preserved auditory brainstem response thresholds. J Gene Med. 2013;15(3-4):123–133. doi: 10.1002/jgm.2701. [DOI] [PubMed] [Google Scholar]
- Lee MY, et al. Survival of human embryonic stem cells implanted in the guinea pig auditory epithelium. Sci Rep. 2017;7:46058. doi: 10.1038/srep46058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto S, Kawamoto K, Kanzaki S, Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear Res. 2002;173(1-2):187–197. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- Okada H, et al. Gene transfer targeting mouse vestibule using adenovirus and adeno-associated virus vectors. Otol Neurotol. 2012;33(4):655–659. doi: 10.1097/MAO.0b013e31825368d1. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Hashimoto K, Xiao R, Vandenberghe LH, Liberman MC. Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci Rep. 2017;7:45524. doi: 10.1038/srep45524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, et al. Cochleovestibular gene transfer in neonatal mice by canalostomy. Neuroreport. 2017;28(11):682–688. doi: 10.1097/WNR.0000000000000827. [DOI] [PubMed] [Google Scholar]
- Beyea JA, Agrawal SK, Parnes LS. Transmastoid semicircular canal occlusion: a safe and highly effective treatment for benign paroxysmal positional vertigo and superior canal dehiscence. Laryngoscope. 2012;122(8):1862–1866. doi: 10.1002/lary.23390. [DOI] [PubMed] [Google Scholar]
- Naples JG, Eisen MD. The History and Evolution of Surgery on the Vestibular Labyrinth. Otolaryngol Head Neck Surg. 2016;155(5):816–819. doi: 10.1177/0194599816665807. [DOI] [PubMed] [Google Scholar]
- Hamilton L, Keh S, Spielmann PM, Hussain SS. How we do it: locating the posterior semicircular canal in occlusion surgery for refractory benign paroxysmal positional vertigo: a cadaveric temporal bone study. Clinical Otolaryngology. 2016;41(2):190–193. doi: 10.1111/coa.12479. [DOI] [PubMed] [Google Scholar]
- Jung JY, et al. siRNA targeting Hes5 augments hair cell regeneration in aminoglycoside-damaged mouse utricle. Mol Ther. 2013;21(4):834–841. doi: 10.1038/mt.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171(1):214–226. doi: 10.2353/ajpath.2007.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenstiel SC, Praetorius M, Plinkert PK, Brough DE, Staecker H. Bcl-2 gene therapy prevents aminoglycoside-induced degeneration of auditory and vestibular hair cells. Audiol Neurootol. 2009;14(4):254–266. doi: 10.1159/000192953. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Izumikawa M, Beyer LA, Atkin GM, Raphael Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res. 2009;247(1):17–26. doi: 10.1016/j.heares.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GP, et al. Notch signaling and Atoh1 expression during hair cell regeneration in the mouse utricle. Hear Res. 2010;267(1-2):61–70. doi: 10.1016/j.heares.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietola L, et al. HOX-GFP and WOX-GFP lentivirus vectors for inner ear gene transfer. Acta Otolaryngol. 2008;128(6):613–620. doi: 10.1080/00016480701663409. [DOI] [PubMed] [Google Scholar]
- Han JJ, et al. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum Gene Ther. 1999;10(11):1867–1873. doi: 10.1089/10430349950017545. [DOI] [PubMed] [Google Scholar]


