Abstract
The onset of schizophrenia occurs during a period critical for development of social relationships and functional independence. As such, interventions that target the early course of illness have the potential to stave off functional decline and restore functioning to pre-illness levels. In this entirely remote study, people with recent-onset schizophrenia spectrum disorders (SSDs) participated in a 12-week randomized controlled trial to determine the efficacy of PRIME (personalized real-time intervention for motivational enhancement), a mobile-based digital health intervention designed to improve motivation and quality of life. Participants were randomized into the PRIME (n = 22) or treatment-as-usual/waitlist (TAU/WL) condition (n = 21) and completed assessments at baseline, post-trial (12 wk), and for people in the PRIME condition, 3 months after the end of the trial. After 12-weeks, WL participants received PRIME, resulting in a total sample of 38 participants completing PRIME. In PRIME, participants worked towards self-identified goals with the support of a virtual community of age-matched peers with schizophrenia-spectrum disorders as well as motivation coaches. Compared to the WL condition, people in the PRIME condition had significantly greater improvements in self-reported depression, defeatist beliefs, self-efficacy, and a trend towards motivation/pleasure negative symptoms post-trial, and these improvements were maintained 3 months after the end of trial. We also found that people in the PRIME condition had significantly greater improvements in components of social motivation post-trial (anticipated pleasure and effort expenditure). Our results suggest that PRIME has the potential to be an effective mobile-based intervention for improving aspects of mood and motivation in young people with SSDs.
Keywords: randomized control trial, recent-onset schizophrenia, reward learning
Introduction
Schizophrenia is a serious and disabling disorder, but with targeted early interventions, individuals may experience functional outcomes equivalent to those living without the disorder.1–4 An increasing body of evidence suggests that motivational deficits play a critical role in determining functional outcomes in schizophrenia spectrum disorders (SSDs).5,6 These deficits in adaptive goal-directed behavior encompass a range of underlying component processes, including difficulty learning from rewarding outcomes,6,7 diminished anticipation of pleasure for rewarding outcomes,8 as well as a reduction in effort expended to obtain rewarding outcomes.9,10 These impairments have been observed to be less severe early in the course of illness, suggesting it might be an ideal time to intervene in order to stave off further decline and disruptions to functioning during a critical period of development.
Utilizing technology, such as smartphone apps and web-based platforms, to deliver behavioral interventions is a promising area of research and has been found to be a feasible and effective approach to early intervention in psychosis.11–14 In addition to using technology platforms to deliver interventions, digital tools have been successfully adopted with adherence rates typically high (~80%) as well as useful for measuring precise phenotypic features of psychosis, which has significantly enhanced our understanding of individuals living with psychosis.15,16 Deploying interventions using ubiquitous technology may help make care more accessible and is a particularly important methodology given that individuals with schizophrenia typically experience motivational deficits that may disrupt engagement in traditional delivery systems of care, such as weekly psychotherapy visits.17,18 In addition to harnessing technology to deliver interventions, it is possible to utilize tech-enabled platforms to remotely conduct clinical trials—an approach that may make participation in clinical trials more accessible. Equally important to addressing engagement and access is ensuring that digital interventions are rigorously evaluated for their effectiveness and specific indications. While some digital interventions may be sufficient as a stand-alone care option (ie, self-management tools for depression), in schizophrenia, digital interventions will likely be considered adjunctive to existing care. Digital interventions may be particularly well suited to either augment existing approaches or target domains of the illness (ie, cognitive and motivational deficits), which may be difficult to treat using traditional approaches.
The purpose of this study was to test the efficacy of a new mobile intervention called PRIME (personalized real-time intervention for motivational enhancement), which was designed to improve motivational impairments early in the course of schizophrenia. PRIME is a mobile app intervention that includes a peer community, goal and achievement tracking, and cognitive behavioral therapy (CBT) based coaching. The intervention was designed to target the motivational system by utilizing social reinforcement to engage and sustain goal-directed behavior. The targeting of motivated behavior was hypothesized to require successful engagement of the various component process of reward processing, known to be disrupted in psychosis spectrum disorders. This study was conducted after our team demonstrated the feasibility and acceptability of the intervention in a pilot sample, involving 10 participants in a trial.11 In this randomized controlled trial, the delivery of PRIME over a 12-week period was compared to a treatment-as-usual/waitlist (TAU/WL) control group. We hypothesized that participants in the PRIME condition would experience significantly greater improvements in self-reported and task-based motivational impairments, relative to the TAU/WL condition. A secondary aim of the study was to test the feasibility of conducting an entirely remote clinical trial for individuals with schizophrenia.
Methods
Recruitment
Participants were recruited remotely using Craigslist, online message boards, and flyers posted in clinics and doctor’s offices. Study investigators also listed the study on the UCSF School of Medicine Clinical Trials website and the lab website, and directly contacted other research labs. Interested participants contacted the study team and were screened and enrolled entirely remotely from 13 states across the United States (California: n = 23, Texas: n = 3, Tennessee: n = 2, New York: n = 2, Nevada: n = 1, Idaho: n = 1, Arkansas: n = 1, Maryland: n = 1, Virginia: n = 1, Washington: n = 1, North Carolina: n = 1, South Carolina: n = 1, Colorado: n = 1), as well as 2 countries outside of the United States (Canada: n = 3, Australia: n = 1). Inclusion and exclusion criteria for participants were: (1) meeting DSM-IV-TR criteria for a diagnosis of schizophrenia, schizophreniform, or schizoaffective disorder, (2) being in the early course of illness (defined as being within the first 5 y of formal diagnosis), (3) being between the ages of 16 and 36, (4) not meeting DSM-IV-TR criteria for substance dependence within the 6 months prior to starting the study, (5) being clinically stable (no changes in outpatient status or medication) for at least 1 month prior to starting the study, (6) being able to provide informed consent, (7) not having history of neurological disorders or serious head trauma, (8) being fluent in English, and (9) having an estimated IQ > 70 as measured by the Wechsler Test of Adult Reading (WTAR).19 Demographic information, such as age, diagnoses, and years of education, as well as utilization of treatment resources (eg, therapy, psychiatric services), can be found in table 1.
Table 1.
Demographic and Clinical Characteristics
| PRIME (n = 22) Mean (SD) | Waitlist (n = 21) Mean (SD) | t or x2 (P) | |
|---|---|---|---|
| Age (y) | 24.32 (2.6) | 23.79 (4.5) | .68 |
| % Male | 60% | 65% | .70 |
| Education (y) | 14.08 (2.3) | 13.37 (1.8) | .24 |
| SSD diagnoses | .93 | ||
| Schizophrenia | 12 (55%) | 11 (52%) | |
| Schizoaffective | 8 (36%) | 8 (38%) | |
| Schizophreniform | 2 (9%) | 2 (10%) | |
| Duration of illness (y) | 2.32 (1.4) | 2.73 (1.6) | .32 |
| Racial background (%) | |||
| Caucasian | 50% | 55% | .86 |
| Asian | 15% | 20% | |
| African American | 12.5% | 5% | |
| Other | 22.5% | 20% | |
| % Seeing therapist | 70% | 70% | .95 |
| % Seeing psychiatrist | 57.5% | 65% | .62 |
| WTAR FSIQ | 111.89 (8.9) | 113.77 (10.0) | .48 |
| PANSS | |||
| Positive symptoms | 7.17 (3.8) | 8.10 (4.6) | .40 |
| Negative symptoms | 12.54 (5.4) | 12.45 (5.1) | .95 |
| CPZ equivalents | 273.03 (295.4) | 298.50 (389.1) | .81 |
Note: PRIME group is combined sample of both conditions. PRIME, personalized real-time intervention for motivational enhancement; WTAR FSIQ, wechsler test of adult reading full scale IQ; PANSS, Positive and Negative Syndrome Scale; CPZ, Chlorpromazine; SSD, schizophrenia spectrum disorder.
Remote Data Collection Procedures
For all participants, informed consent documents were built and sent via Qualtrics Insight Platform (Provo, UT). Participants were guided through the informed consent process over the phone with a trained research assistant and provided their informed consent by checking a box and typing their name in the online document. Self-report measures and behavioral tasks were also administered via Qualtrics Insight Platform. All interview-based clinical assessments were conducted via FaceTime or Skype.
Study Design
This 12-week randomized control trial (RCT) tested the efficacy of the second iteration of PRIME, including modifications informed by the results of our pilot study. Participants were randomized to either receive PRIME or a TAU/WL control condition. Participants were compensated for their time to complete study-related assessments ($20/h) but were not paid for their participation in the intervention. Study evaluators were blind to treatment condition.
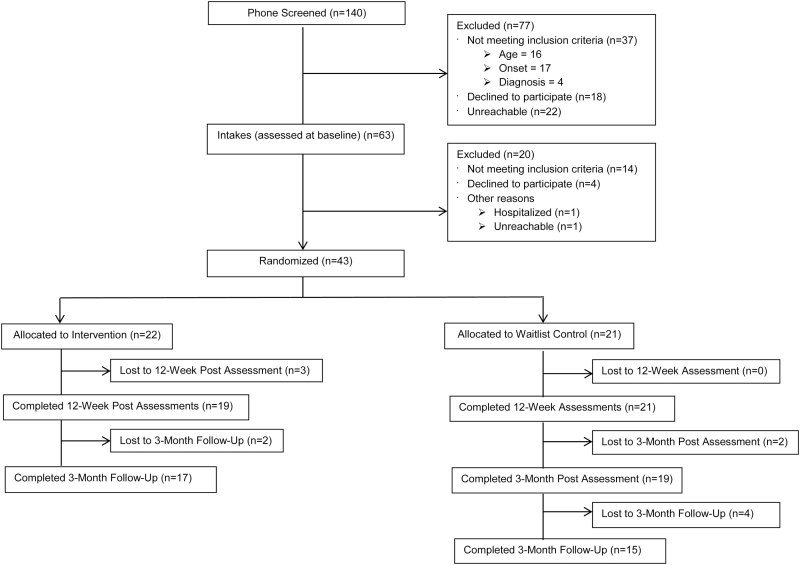
Study Sample
Of the participants who completed an initial phone screen of eligibility (n = 140), 77 were excluded. The remaining 63 potentially eligible participants then completed a thorough intake assessment to confirm eligibility, with a total of 43 participants being randomized to PRIME (n = 22) or a WL condition (n = 21). Participants in the WL condition were offered the opportunity to receive PRIME after 12 weeks (see figure 1 for CONSORT diagram); 20 out of 21 waitlisted participants opted to receive PRIME after these 12 weeks. The assessments completed at this time doubled as final WL condition scores and as baseline scores prior to entry into PRIME. All participants met DSM-IV-TR20 criteria for a SSD, evaluated using the Structured Clinical Interview for DSM-IV-TR Disorders.21 Participants were given the option of borrowing a smartphone from the researchers, and 12 of the 43 participants exercised this option. Of the 43 participants randomized to the PRIME or WL conditions, 37 (86%) were taking an antipsychotic medication at the time of the study. In order to compare the antipsychotic burden across people with an SSD, we calculated chlorpromazine (CPZ) equivalents using a standardized conversion table.22 Clinical and demographic characteristics for both conditions may be found in table 1.
Fig. 1.
CONSORT diagram for the randomized controlled trial.
PRIME Description
PRIME was designed in collaboration with IDEO, a global design, and innovation firm, to implement a human-centered design process and make the platform appealing to users and address their needs. With PRIME, participants joined a supportive online environment where they selected and documented progress on small, self-determined goals in the domains of health/wellness, social relationships, creativity, and productivity. When participants set up a PRIME account, they selected long-term goals from a 36-item list, which included goals such as “deepen my relationship with my family” and “feel more relaxed.” A feature was developed later in the study permitting users to add to and modify the goals they selected during account set up. When using the goal feature later, participants chose one of the long-term goals they indicated interest in during setup. This triggered a display of brief challenges (able to be completed in a day) that contributed to that goal, such as “offer to help a family member with a chore, like shopping or cleaning!” or “invite a family member to do something fun with you.” Each long-term goal contained more than 15 suggested challenges on average. Participants sequentially viewed these suggested challenges and had the ability to create a custom challenge, which they could manually enter. Participants viewed the suggested challenges one at a time and, as a participant completed several challenges for the same goal, the sequence adapted to display more ambitious options. Suggestions laddered from challenges like “listen to relaxing music for five minutes…” to “go to a yoga class…” but participants were always able to view and select the easier-to-accomplish challenges at any time. Participants were given automatic reminders of the challenges and indicated when they completed the challenges they selected. Completion prompted participants with an opportunity to post a quick “accomplishment moment” which they shared with their coach and the PRIME community.
PRIME provides users with motivation coaches; masters-level clinicians who use evidence-based interventions drawn from CBT, behavioral activation, mindfulness, and psychoeducation to help participants overcome the daily obstacles that hinder goal progress and improve health outcomes. Additionally, the PRIME community provides a platform for users to interact with one another. Users may send messages directly to each other and can also capture and share positive, spontaneous moments in their daily life with the whole PRIME community.
When first-time participants signed in to the app, a research assistant guided them through the process of creating a user profile. Participants created a username, uploaded a profile picture, selected their interests, goals, and symptoms, and wrote a short bio. Once a user was registered to the app, an assigned motivation coach sent the new participant a welcome message and an offer to support the participant in achieving his/her goals. The coach informed his/her assigned participants that he/she would be available to message with them “most days” per week, but would modify the frequency depending on their preference, clinical issues, and overall progress towards goal achievement. When possible, messaging between coaches and participants was synchronous (ie, in real-time) to facilitate intervention development and implementation. Participants could also request to speak with coaches on the phone or via FaceTime. Goal achievement was measured by the number of challenges completed in each goal domain. Participants in the PRIME condition were encouraged to use the app daily, whether it be to message with coaches and/or peers or complete challenges. However, the minimum expectation for participation in this intervention was logging into PRIME at least 1×/wk over the 12-week period.
PRIME Outcome Measure
The primary outcomes for this trial were changes in components of motivated behavior from baseline to 12-weeks using a modified version of the Trust Task.23–25 In line with recent models of motivation impairment in people with schizophrenia8,26 the Trust Task was designed to assess 3 components of motivation: reward learning, anticipated pleasure, and effort expenditure. The initiation and execution of motivated behavior involves each of these components, and with PRIME participants receive support in their pursuit of goal-directed behavior as well as feedback about their performance. As such, the Trust Task, which assesses these specific motivation components in a social context, can provide an objective index of how engagement with PRIME generalizes to improvements in motivation for social interaction more broadly.
During this task, participants interacted with 4 simulated social partners identified by name and a dynamic video of them expressing a facial display. Participants indicated their anticipated pleasure from the outcome of the interaction (1 to 7 scale). To measure reward learning, participants decided how many points to send to a social partner (between 0 and 10) using the keyboard. The amount of points sent by the participant was then quadrupled, and social partners returned a percentage of the quadrupled amount (0% to 100%), with both the participant and social partner’s percentage shown on the screen. Thus, the key variables related to trust for each trial were the amount of points sent by a participant (represents how much he/she trusted a social partner) and the percentage of points returned by the social partner (represents the extent to which participant trust was reciprocated). Intact reward learning would mean giving more points to trustworthy and fewer points to untrustworthy social partners. Finally, participants could influence the likelihood of interacting with this social partner again in the future by expending effort in the form of repeated key presses. Participants could repeatedly press a specific key to increase the likelihood, a different key to decrease the likelihood, or simply choose to do nothing for the duration of the 6-second response window if they did not have a preference. We averaged the number of key presses across the response window to create an index of the number of key presses per second per participant. Social partner behavior was predetermined so that interactions with 2 partners resulted in positive outcomes (average return double the amount sent) while interactions with the other 2 social partners resulted in negative outcomes (average return half the amount sent). Participants interacted with each social partner 8 times for a total of 32 trials. In previous studies, both the average amount of trust placed24 and the average amount anticipated pleasure23 during interactions with trustworthy social partners has been shown to be positively associated with social functioning in people with schizophrenia.
PRIME Outcome Assessment: Secondary
In addition to our primary outcome, we assessed self-reported defeatist beliefs and change in motivation using the Motivation and Pleasure-Self Report scale (MAP-SR),27 which is a self-report version of the Motivation and Pleasure scale from the Clinical Assessment Interview for Negative Symptoms.28 Second, we assessed real-world functioning in independent living, work, family, and social domains using the interview-based Role Functioning Scale (RFS).29 In addition, we assessed quality of life in social and vocational domains using the interview-based Quality of Life Scale – Abbreviated (QOL-A).30 We also assessed defeatist beliefs about successfully performing goal-directed behavior using the 15-item subscale31 of the Dysfunctional Attitudes Scale,32 depression symptom severity with the Beck Depression Inventory, Second Edition (BDI),33 and self-efficacy with the Revised Self-Efficacy Scale (R-SES).34 All self-report measures had acceptable internal consistency (α > .80). We also assessed positive and negative symptoms using the Positive and Negative Syndrome Scale (PANSS).35
The same remote assessment schedule was used for participants in both conditions and included clinical evaluations at baseline and 12-weeks. Because participants in the WL condition were given the option to join the PRIME condition immediately after the 12-week time point, we did not conduct a 3-month post-study assessment with these participants. Since our primary outcome was changes in motivated behavior between study conditions, and we did not conduct a 3-month assessment for WL participants, the Trust Task was not administered at this time point. Outcome evaluators in the RCT were blind to condition.
PRIME Acceptability
We assessed PRIME acceptability during an exit interview at the 12-week time point (post-trial) where participants rated their satisfaction with the specific features of PRIME, such as the ability to interact with peers and the different goal categories, on a 1 (not at all) to 10 (very much) scale. We also assessed retention in the trial as a measure of acceptability.
PRIME Feasibility
To evaluate feasibility, we assessed the following use metrics: login frequency (average number of days logged in per week), average number of challenges completed (both overall and by individual challenge category), challenge completion percentage, and the average number of peer and coach interactions. Interactions included direct messaging on PRIME as well as commenting on and liking content posted to the community moments feed. To further understand how participants were engaging with the PRIME platform, we evaluated “active use rate.”11 To do this, we added together the average number of challenges completed, peer, and coach interactions and divided this total by the number of weeks the participant had access to PRIME. Thus, a value of 2.3 would mean that a participant was active on PRIME 2.3 times/wk. Passive use was defined as logging into the app, but not posting a moment, completing a challenge or interacting with peers or coaches. Thus, a participant may log in to the app 4 days per week but actively engage with the features of the app 2 times/wk.
Data Analysis Plan
We used an intent-to-treat analysis, and thus all participants who completed baseline assessments were randomized and included in the analyses. All analyses involving the PRIME condition included both participants initially randomized into this condition as well as WL condition participants who received PRIME after 12-weeks. First, we examined whether any demographic variables were related to baseline motivation (MAP-SR, Trust Task components) or functioning (RFS, QOL-A) and whether any demographic variables, baseline motivation, and functioning were related to PRIME utilization using correlations. To determine PRIME acceptability, we examined the average ratings from the PRIME satisfaction survey administered at the 12-week time point for overall satisfaction as well as the most and least popular PRIME features. Furthermore, we also reviewed qualitative feedback from the PRIME exit interview. To investigate PRIME feasibility, we examined descriptive statistics for the following PRIME metrics: login frequency, challenges completed, spontaneous and goal achievement moments, peer and coach interactions, and active use rate.
To investigate the effect that PRIME had on our primary and secondary outcomes, we conducted ANCOVAs comparing changes from baseline to the 12-week time point for participants in the PRIME and WL conditions. ANCOVA allowed us to examine group differences in our outcomes of interest at the 12-week time point while controlling for baseline scores. For participants in the WL condition, the 12-week time point data also served as their baseline time point data for when they entered the PRIME condition.
To test whether changes in our primary and secondary outcomes persisted at the 3-month time point, we conducted paired samples t-tests between 12-week minus baseline and 3-month minus baseline change scores, with the exception of the Trust Task, which was only administered at the baseline and 12-week time points. To explore the degree to which specific aspects of PRIME use were associated with improvements in our primary and secondary outcomes, we computed correlations between change scores in our outcome measures (12-wk time point minus baseline) and PRIME login percentage (passive use), PRIME active use rate, total number of coach interactions, total number of peer interactions, and total goals completed.
Results
Five participants in both the PRIME and WL conditions dropped out while using PRIME or were unreachable for post-intervention assessments, and an additional 6 were unreachable or otherwise did not complete follow-up assessments 3 months later (figure 1). Since participants in the WL condition were able to use PRIME after 12-weeks, analyses comparing changes at the 12-week time point have n = 38 in the PRIME condition (19 originally randomized into PRIME plus an additional 19 WL participants who received PRIME after 12-wk) and n = 21 in the WL condition. The sample size for analyses looking at the 3-month time point in the PRIME condition is n = 32, which means that 74% (32/43) were retained for the duration of the trial and follow-up period.
Participant demographics were not related to baseline clinical symptoms or PRIME use metrics. Further, there were no differences in demographic or baseline symptoms between participants randomized into either condition. Both PRIME use metrics (login percentage, interactions) and the effort expenditure component of the modified Trust Task were not normally distributed, so we conducted a root transformation on these data.
The Effect of PRIME on Our Primary Outcome: Motivated Behavior
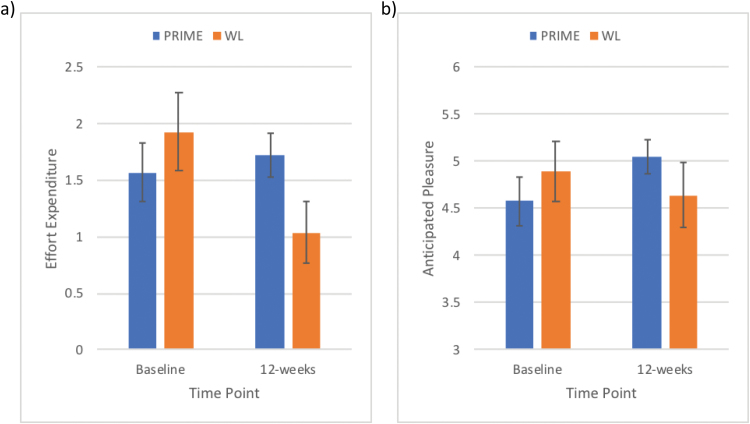
To investigate the effects of PRIME on our primary outcome, we compared the PRIME and WL conditions on changes in the Trust Task from baseline to post-trial (12-wk time point). We found a significant difference between conditions in anticipated pleasure during the modified Trust Task, F(1,56) = 4.75, P = .03, with participants in the PRIME condition showing a greater increase from baseline to 12 weeks compared to WL, t(55) = -2.39, P = .02, d = 0.64 (figure 2a). Similarly, we found a significant difference between conditions in effort expended to increase the likelihood of future social interactions with positive outcomes, F(1,56) = 4.66, P = .04, with participants in the PRIME condition showing a greater increase from baseline to 12 weeks compared to WL, t(55) = −2.17, P = .03, d = 0.58 (figure 2b). Furthermore, we found a trend towards significant improvement in learning from positive outcomes during the modified Trust Task, F(1,56) = 3.53, P = .07. There were no significant differences in effort expended to decrease the likelihood of future interactions with positive outcomes, nor in changes in components of motivation for interactions with negative outcomes (Ps > .20).
Fig. 2.
Improvements in our primary outcomes. Compared to the waitlist (WL) condition, participants in the PRIME (personalized real-time intervention for motivational enhancement) condition increased their (a) expenditure of effort to increase the likelihood of interactions and (b) anticipated pleasure on the modified Trust Task from baseline to the 12-week time point.
The Effect of PRIME on Secondary Outcomes: Self-reported and Clinically Assessed Motivation, Symptoms, and Functioning
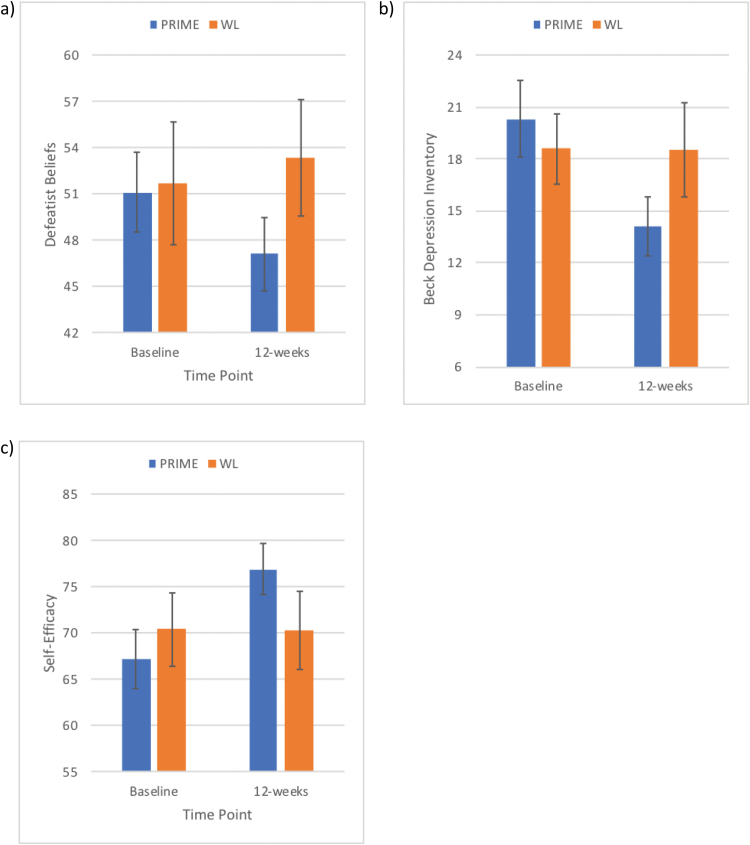
Next, we investigated the effects of PRIME on our secondary outcomes: self-reported defeatist beliefs, motivation, depression, self-efficacy, and clinically assessed positive and negative symptoms and functioning. We found significant differences between conditions for defeatist beliefs, F(1,57) = 5.58, P = .02, with participants in the PRIME condition showing a greater decrease from baseline to 12 weeks compared to WL, t(56) = 2.22, P = .03, d = 0.59 (figure 3a). We found similar effects for depression symptoms, F(1,56) = 7.06, P = .01, and self-efficacy, F(1,55) = 5.76, P = .02, with the PRIME participants showing greater improvements from baseline to 12 weeks, t(53) = −2.30, P = .03, d = 0.63 (figure 3b), and t(56) = −2.39, P = .02, d = 0.64 (figure 3c), respectively. Importantly, comparing changes from baseline to 12-weeks and baseline to 3-months, we found no differences, suggesting that these gains were maintained 3 months post-trial. We also found a trend towards significant improvement on the MAP-SR, F(1,57) = 3.79, P = .06. There were no group differences in changes in positive or negative symptoms (PANSS), quality of life (QOL-A), or functioning (RFS) from baseline to the 12-week time point, nor the 12-week to the 3-month time point (Ps > .28).
Fig. 3.
Improvements in our secondary outcomes. Compared to the waitlist (WL) condition, participants in the PRIME (personalized real-time intervention for motivational enhancement) condition had significant decreases in (a) defeatist beliefs and (b) depression and significant increases in (c) self-efficacy.
As an exploratory follow-up, we examined whether the same pattern of findings was true for only participants in the WL condition who then received PRIME. Generally consistent with our primary outcomes findings for the combined sample (PRIME first and WL first), we found participants who were randomized to the WL condition had significantly greater learning from positive outcomes following 12 weeks of PRIME, F(1,37) = 4.53, P = .04. We also found trends toward greater anticipated pleasure, F(1,37) = 3.64, P = .07, and effort expended to increase the likelihood of future social interactions with positive outcomes following 12 weeks of PRIME, F(1,37) = 3.28, P = .08. Our secondary outcome findings were less consistent with the combined sample as participants who were randomized to the WL condition had significantly greater improvements in depression, F(1,38) = 3.98, P = .05, and defeatist beliefs, F(1,38) = 4.39, P = .04, but not the MAP-SR (P = .41) or self-efficacy (P = .10).
Exploring Whether PRIME Use is Related to Changes in Primary and Secondary Outcomes
To explore how PRIME improved symptoms and behavior, we explored correlations between PRIME metrics (PRIME login percentage, PRIME active use rate, total number of coach interactions, total number of peer interactions, and total goals completed) and changes in our primary and secondary outcomes from baseline to post-trial (12-wk). We did not find any significant correlations between PRIME metrics and change in our outcomes measures (Ps > .10).
PRIME Acceptability
Mean overall satisfaction with PRIME for the entire sample, as rated during the exit interview administered at the 12-week post-assessment, was 8.21 (SD: 1.9). Some of the comments made by participants when asked about how PRIME impacted their lives included: “(PRIME) helped me see that ‘you’re not the only one’ by seeing others do well and be able to get better”, and “It was good to have coaches to speak to when I needed it. Working with my coach really helped me work on testing some paranoid beliefs and teaching me how to test those on my own as well. …Helped reduce suicidality primarily through instilling some level of hope, which came from feeling connected to a larger group and resource. This connection felt like a solid foundation. Decreased helplessness too.”. The most popular PRIME feature was the ability to directly message coaches (M: 8.38, SD: 2.5), and the least popular PRIME feature was the ability to track your mood (mean: 6.33, SD: 2.4).
PRIME Feasibility
Average PRIME use data per participant (login frequency, challenge completion, interactions) can be found in table 2 and are presented separately for participants randomized to receive PRIME first or after 12-weeks in the WL condition. On average, participants logged in a little over 4 d/wk. Over a 12-week period, participants were highly engaged in the platform, with 5152 direct messages sent from participants to coaches. In terms of peer-to-peer interactions, participants initiated interactions with each other a total of 497 times. Participants initiated about 10 interactions with coaches for every initiated peer interaction. All 38 participants initiated at least 1 message to a coach and 13 (33%) initiated more than the average of 128.8 coach interactions. However, there was a considerable amount of variability in coach messaging, with the range for initiated coach interactions being 12 to 574. Participants completed an average of 1.5 challenges per week. Health/wellness challenges were the most popular at about 1 challenge completed every 2 weeks, followed by creativity, social challenges, and productivity challenges of which participants completed approximately 1 every 3 to 4 weeks. Challenge completion percentage was high (88%), suggesting that participants had little difficulty completing the challenges that they set.
Table 2.
PRIME Utilization Data
| PRIME Use Metric (Range) | PRIME First (n = 19) | Waitlist First (n = 19) |
|---|---|---|
| Average logins per week (1.2–7 d/wk) | 4.03 (1.4) | 4.10 (1.5) |
| Challenge completion rate (50%–100%) | 91.47 (12.2) | 83.58 (21.0) |
| Average number of user-initiated peer interactions | ||
| Comments (0–29) | 4.54 (7.0) | 4.58 (5.5) |
| Likes (0–74) | 8.91 (20.2) | 11.05 (18.4) |
| Messages (0–131) | 9.91 (16.5) | 14.95 (30.4) |
| Total (0–174) | 23.36 (39.2) | 30.58 (37.0) |
| Average number of user-initiated coach interactions | ||
| Comments (0–39) | 6.50 (11.6) | 8.63 (10.5) |
| Likes (0–50) | 6.95 (14.6) | 5.84 (9.7) |
| Messages (12–574) | 150.68 (142.0) | 100.74 (85.0) |
| Total (17–581) | 164.14 (141.0) | 115.21 (95.0) |
| Challenges completed | ||
| Overall (1 to 52) | 14.91 (13.1) | 18.11 (15.4) |
| Health/wellness (0 to 27) | 4.68 (6.3) | 6.74 (6.2) |
| Social (0 to 13) | 3.00 (3.2) | 3.79 (4.3) |
| Creativity (0 to 20) | 3.77 (4.15) | 4.79 (6.1) |
| Productivity (0 to 12) | 3.45 (3.5) | 2.79 (3.1) |
| Active use rate (0.27 to 4.97) | 1.76 (1.3) | 1.94 (1.4) |
Note: PRIME, personalized real-time intervention for motivational enhancement.
PRIME activity, defined as both the number of challenges completed and number of messages sent, was highest during the first month. However, engagement with the PRIME features dropped after the first month of the trial before leveling out over the second and third months. To illustrate this point, participants initiated an average of 10.3 challenges in the first month compared to 4.1 and 3.0 in the second and third months. A similar pattern was observed for messaging with coaches, with participants initiating an average of 53.9 messages in the first month compared to 34.2 and 31.8 messages in the second and third months. Three participants discontinued PRIME use right before or during the third month of the trial, which could have contributed to this pattern of decreased engagement over time. Taken together, the relative maintenance of PRIME use over the course of the second and third months may reflect a more stable, long-term engagement with the application.
Discussion
This study demonstrated that PRIME is a feasible, acceptable, and efficacious intervention for improving mood and motivation in young people with an SSD. The overall 74% retention rate for the treatment (and 88% retention post-intervention), demonstrated that this intervention was very well tolerated. Participants rated their overall satisfaction with PRIME highly, which was demonstrated by the degree of engagement observed in the app. Participants, on average, used PRIME a little over 4 d/wk and sent over 5000 messages to coaches and approximately 500 to their peers. Many participants noted that it was the first time they had seen or interacted with other young people with an SSD, and they particularly appreciated being able to have on-demand coaching, as demonstrated by the qualitative feedback and this feature being rated as the most satisfying.
This preliminary examination of efficacy found that participants in PRIME experienced significant improvements in depression, defeatist beliefs, self-efficacy and several important components of motivation, such as reward learning, anticipated pleasure, and effort expenditure. As such, PRIME appeared to function as a behavioral activation intervention. The improvements in the domains of motivation were a specific focus in the initial design of PRIME,11 which emphasized engagement in goal-directed behavior, capturing images and cataloging positive experiences, and sustaining engagement (encouraged by social reinforcement) in sharing experiences in the PRIME community. Furthermore, we showed that PRIME specifically targets and engages components of motivation that work together to produce motivated behavior. Indeed, our findings show that PRIME increased learning from positive outcomes, anticipated pleasure for positive outcomes, and expenditure of effort to obtain future positive outcomes, which are components of motivation not typically improved by medications nor in-person psychotherapy.36,37 As such, PRIME may act as an important adjunctive intervention to treatment approaches that are usually more focused on treating the positive psychotic symptoms, and offer a more holistic approach to improving outcomes for people with an SSD.
There were several limitations to this study, including a relatively small sample size that may not have been representative of the population with SSD or adequately powered to determine whether deploying PRIME would be successful in improving other important clinical outcomes, such as role or social functioning. Secondly, the use of a TAU/WL control condition for this study did not allow for an understanding of the relative effect of PRIME compared to other types of mobile interventions or treatment approaches. And lastly, the relatively short follow up period (3 mo) may not have been long enough to conclude whether the effects of PRIME would translate into longer term and clinically meaningful outcomes. Further research is needed to improve our knowledge about the moderators of outcomes as well as refine our understanding of who may benefit more or less from this intervention approach.
To our knowledge, this is the first study to demonstrate that a mobile intervention may improve critical domains of impairment in SSD. Further, this study was conducted entirely remotely and successfully recruited, enrolled, and engaged young people all over the United States, Canada, and Australia. By using this methodology, clinical trials may be conducted more efficiently and potentially recruit an even more diverse sample than in academic settings. Lastly, by implementing a human-centered design process, we ensured that PRIME was able to pair a scientific foundation with an approach that resonated with the needs of young people with an SSD.
Funding
This work was supported by the National Center for Research Resources at the National Institutes of Health (R34 MH100399).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Chien WT, Leung SF, Yeung FK, Wong WK. Current approaches to treatments for schizophrenia spectrum disorders, part II: psychosocial interventions and patient-focused perspectives in psychiatric care. Neuropsychiatr Dis Treat. 2013;9:1463–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry. 2008;7:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Austin SF, Mors O, Secher RG, et al. Predictors of recovery in first episode psychosis: the OPUS cohort at 10 year follow-up. Schizophr Res. 2013;150:163–168. [DOI] [PubMed] [Google Scholar]
- 4. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-Year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173:362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(Suppl. 2):S107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol. 2014;24:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res. 2016;170:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161:382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schlosser D, Campellone T, Kim D, et al. Feasibility of PRIME: a cognitive neuroscience-informed mobile app intervention to enhance motivated behavior and improve quality of life in recent onset schizophrenia. JMIR Res Protoc. 2016;5:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biagianti B, Schlosser D, Nahum M, Woolley J, Vinogradov S. Creating live interactions to mitigate barriers (CLIMB): a mobile intervention to improve social functioning in people with chronic psychotic disorders. JMIR Ment Health. 2016;3:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ben-Zeev D, Brenner CJ, Begale M, Duffecy J, Mohr DC, Mueser KT. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alvarez-Jimenez M, Alcazar-Corcoles MA, González-Blanch C, Bendall S, McGorry PD, Gleeson JF. Online, social media and mobile technologies for psychosis treatment: a systematic review on novel user-led interventions. Schizophr Res. 2014;156:96–106. [DOI] [PubMed] [Google Scholar]
- 15. Granholm E, Ben-Zeev D, Fulford D, Swendsen J. Ecological momentary assessment of social functioning in schizophrenia: impact of performance appraisals and affect on social interactions. Schizophr Res. 2013;145:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bell IH, Lim MH, Rossell SL, Thomas N. Ecological momentary assessment and intervention in the treatment of psychotic disorders: a systematic review. Psychiatr Serv. 2017;68:1172–1181. [DOI] [PubMed] [Google Scholar]
- 17. Chien WT, Mui J, Gray R, Cheung E. Adherence therapy versus routine psychiatric care for people with schizophrenia spectrum disorders: a randomised controlled trial. BMC Psychiatry. 2016;16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wechsler D. Wechsler Test of Adult Reading (WTAR). New York, NY: Pearson Education, Inc; 2001. [Google Scholar]
- 20. Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 21. First MB, Spitzer RL, Gibbon M, Williams J.. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version, Patient Edition. (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 22. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campellone TR, Kring AM. Anticipated pleasure for positive and negative social interaction outcomes in schizophrenia. Psychiatry Res. 2018;259:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campellone TR, Fisher AJ, Kring AM. Using social outcomes to inform decision-making in schizophrenia: relationships with symptoms and functioning. . 2016;125:310–321. [DOI] [PubMed] [Google Scholar]
- 25. Campellone TR, Truong B, Gard D, Schlosser DA. Social motivation in people with recent-onset schizophrenia spectrum disorders. J Psychiatr Res. 2018;99:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Llerena K, Park SG, McCarthy JM, Couture SM, Bennett ME, Blanchard JJ. The Motivation and pleasure scale-self-report (MAP-SR): reliability and validity of a self-report measure of negative symptoms. Compr Psychiatry. 2013;54:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McPheeters HL. Statewide mental health outcome evaluation: a perspective of two southern states. Community Ment Health J. 1984;20:44–55. [DOI] [PubMed] [Google Scholar]
- 30. Bilker WB, Brensinger C, Kurtz MM, et al. Development of an abbreviated schizophrenia quality of life scale using a new method. Neuropsychopharmacology. 2003;28:773–777. [DOI] [PubMed] [Google Scholar]
- 31. Cane DB, Olinger LJ, Gotlib IH, Kuiper NA. Factor structure of the dysfunctional attitude scale in a student population. J Clin Psychol. 1986;42:307–309. [Google Scholar]
- 32. Weissman AN, Beck AT. Development and validation of the dysfunctional attitude scale: a preliminary investigation. Paper presented at: American Educational Research Association 1978 Annual Meeting; 27–31 March 1978; Toronto, Ontario, Canada: http://eric.ed.gov/ERICWebPortal/recordDetail?accno=ED167619. Accessed June 11, 2018. [Google Scholar]
- 33. Beck A, Steer R, Brown G.. Beck Depression Inventory II Manual. San Antonio, TX: Psychological Corporation;1996. [Google Scholar]
- 34. McDermott BE. Development of an instrument for assessing self-efficacy in schizophrenic spectrum disorders. J Clin Psychol. 1995;51:320–331. [PubMed] [Google Scholar]
- 35. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 36. Lieberman JA, Stroup TS, McEvoy JP, et al. ; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. [DOI] [PubMed] [Google Scholar]
- 37. Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]