Abstract
It is increasingly evident that the brain is not truly an immune privileged site and that cells of the central nervous system are sensitive to the inflammation generated when the brain is fighting off infection. Among the many microorganisms that have access to the brain, the apicomplexan protozoan Toxoplasma gondii has been one of the most studied. This parasite has been associated with many neuropsychiatric disorders including schizophrenia. This article provides a comprehensive review of the status of Toxoplasma research in schizophrenia. Areas of interest include (1) the limitations and improvements of immune-based assays to detect these infections in humans, (2) recent discoveries concerning the schizophrenia–Toxoplasma association, (3) findings of Toxoplasma neuropathology in animal models related to schizophrenia pathogenesis, (4) interactions of Toxoplasma with the host genome, (5) gastrointestinal effects of Toxoplasma infections, and (6) therapeutic intervention of Toxoplasma infections.
Keywords: microorganism, psychiatric disorders, pathogenesis
Introduction
Converging evidence points to a central role of the immune system in the etiopathogenesis of schizophrenia and related psychiatric disorders. Involvement of the immune system is supported by genetic, neuropathological, neuroimaging, and metabolic analyses of multiple populations.1,2 The brain has been generally considered an immune-privileged environment due to the perceived exclusion of immune components and the blood–brain barrier. However, recent discoveries indicate that the brain is not truly immune privileged, and inflammatory events will occur when the brain is fighting off infection, potentially leading to profound structural and functional changes. The inflammatory response is recognized as a causal factor in the pathology and chronic course of many central nervous system (CNS) diseases.3 Causes of inflammation have been related to a variety of cues, including infection, traumatic brain injury, toxic metabolites, or autoimmunity.4
Biological and Epidemiological Characteristics of Microbial Agents Likely to Be Associated With Schizophrenia
Schizophrenia is a persistent brain disease that is usually first evident in late adolescence or early adulthood and proceeds along a lifelong course. If a microbial agent contributes to schizophrenia pathogenesis, there are a number of biological and epidemiological requirements that the agent must fulfill to be a plausible candidate organism. For example, it might be expected that the candidate infectious agent or group of agents demonstrate a worldwide distribution as is similar for schizophrenia. Furthermore, given the schizophrenia is likely a disorder of neurodevelopment, the timing of exposure to a putative infectious agent is likely to occur during time periods important for neurodevelopment.5,6 The candidate agent must likewise persist in the brain, as numerous studies indicate some degree of immune dysregulation and inflammation in the brains of many individuals with schizophrenia.7 Since neurological destruction may not be present early in the course of schizophrenia, it is also likely that such organisms can persist in a nonreplicating (latent) or very slowly replicating (inactive) state for extended periods of time. Thus, the reactivation of a latent or slowly replicating infection would be consistent with early exposure and development of symptoms later in life. Causes of such reactivation might include normal endocrine and other developmental processes associated with adolescence as well as exposure to infectious agents more commonly infecting individuals in this time period. In addition, although there are seasonal variations, the rates of occurrence of schizophrenia are relatively constant from year to year and do not differ greatly across geographic areas.8,9 With respect to schizophrenia, 2 models for endemic infections may be relevant. One scenario involves infectious agents with high prevalence that are spread person to person with inapparent symptoms on exposure. Another potential source of endemic agents would be ones transmitted through the environment by common sources such as food, water, or soil. In both cases the exposures are relatively constant and would show low rates of epidemic variation. Finally, family studies indicate a strong association of shared risk of schizophrenia within families10 and genes modulating the immune response are identified as significant components of the genetic risk for schizophrenia. It is thus likely that infectious agents associated with schizophrenia are ones that interact with host genetic factors. The interaction with genetic factors would also serve to explain how endemic infectious agents with relatively high prevalence might be associated with disorders that are less prevalent, because clinically apparent disease would be most likely to result from exposure to the infectious agent by genetically susceptible individuals. It is of note that these familial factors need not be limited to Mendelian inherited genes but would also include epigenetic modifications and microbial genes carried by a host as part of the inherited microbiome.
Seroepidemiological Studies of Toxoplasma and Psychiatric Disorders
Although there are several pathogenic agents that meet these criteria, the one which has been best studied in the context of schizophrenia is the apicomplexan protozoan Toxoplasma gondii. Toxoplasma is one of the most pervasive pathogens that infects approximately a billion people worldwide. Although initial infection with Toxoplasma is associated with few symptoms in immune-competent individuals, the host is often left with lifelong persistence of tissue cysts in the brain, retina, and muscles. When the immune response wanes, tissue cysts can become reactivated and invade any nucleated cell, as noted in immunocompromised patients. The parasite has a complex life cycle involving sexual replication in members of the cat family (Felidae) and asexual propagation in a wide variety of warm-blooded hosts.11 There are 3 infectious stages in the life cycle of Toxoplasma: tachyzoites, which facilitate expansion during acute infection; bradyzoites (in tissue cysts), which maintain chronic infection; and sporozoites (in oocysts), which are disseminated in the environment.12 For the purposes of this article, we will provide a comprehensive review of the status of Toxoplasma research in schizophrenia.
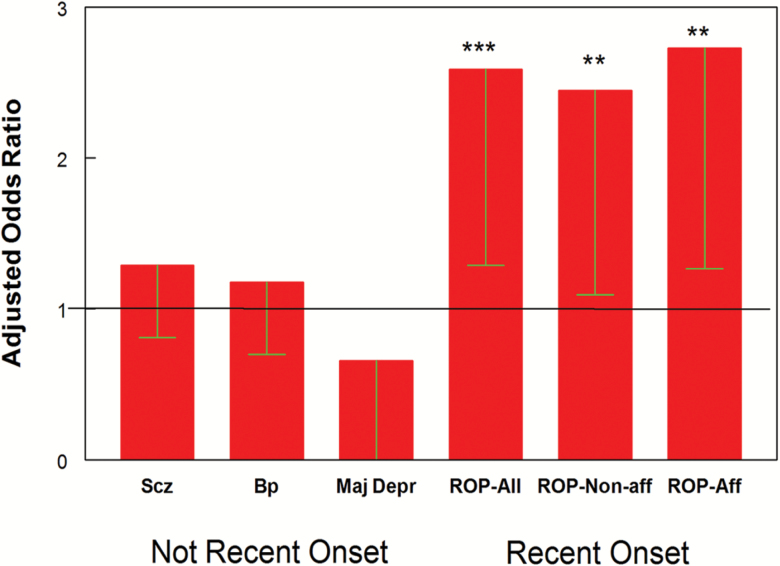
Multiple epidemiological studies and meta-analyses have documented that individuals with psychiatric disorders have increased odds of Toxoplasma exposure.13 However, there have also been studies that have not found this association. Many of the negative studies were performed in individuals with established schizophrenia living in populations with relatively low prevalence rates of Toxoplasma infection. This discrepancy was largely resolved by a recent study indicating that, in a low prevalence population, Toxoplasma exposure was associated with an increased risk in individuals with the recent onset of psychosis but not in individuals with established schizophrenia (figure 1).14 The difference is likely related to the timing of Toxoplasma exposure relative to the onset of symptoms. It is also possible that the antiparasitic effects of some of the medications commonly used to treat schizophrenia affect the levels of antibodies.15 Consistent with this possibility is the recent report of increased levels of Toxoplasma antibodies in individuals with treatment resistant forms of schizophrenia.16
Fig. 1.
Odds ratios associated with Toxoplasma exposure by clinical diagnosis. Bars represent odds ratios (Mean-95% confidence interval) associated with the indicated clinical diagnosis calculated by logistic regression as described in the text with age, gender, race, maternal education, and place of birth as covariates. The abbreviations used are as follows: Scz = nonrecent schizophrenia; Bp = nonrecent bipolar disorder; Maj Depr = major depressive disorder without recent onset psychosis; ROP-All = recent onset psychosis, all cases; ROP-Non-aff = recent onset psychosis, individuals with nonaffective psychosis; ROP-Aff = recent onset psychosis, individuals with affective psychosis. *** P < .003, **P < .03. Not recent onset refers to individuals who did not have recent onset of psychosis. Reprinted from PLoS Negl Trop Dis. 2017 Nov; 11(11): e0006040.
Although most studies of Toxoplasma have examined associations with schizophrenia, increased rates of exposure to Toxoplasma have been found in a range of other psychiatric disorders including psychotic-like symptoms,17 bipolar disorder,18,19 self-directed violence20 and suicide attempts,21 general anxiety disorders,22 mixed anxiety and depressive disorder,23 obsessive-compulsive disorder,24 Autism,25 and depression during pregnancy.26 It is worth mentioning that there are some controversies about the role of Toxoplasma in major depression.27Toxoplasma seropositivity has also been associated with alterations in cognitive functioning and overall cognitive decline in the elderly.28,29 At the other end of the age spectrum, exposure to Toxoplasma has been associated with decreased reading skills and memory capacity in school-aged children.30 In some cases, psychiatric disorders such as suicide attempts were associated with specific levels of antibodies rather than just seroprevalence as in the case of suicide attempts31 and personality disorders.32 Moreover, studies have shown that latent Toxoplasma infections could have serious impacts on human health.33 These findings suggest that exposure to Toxoplasma is not only a risk factor for schizophrenia but for a range of other disorders as well. These findings are consistent with a recently increased understanding of the shared genetic susceptibilities among psychiatric disorders and the overlapping of clinical phenotypes.
Limitations and Improvements of Immune-Based Assays for Characterization of Toxoplasma Infection
Because Toxoplasma persists largely in the form of tissue cysts in the brain, it is often not possible to directly detect organisms in infected individuals. Most studies of human infection rely almost entirely on the measurement of anti-Toxoplasma antibodies. The IgG class antibodies to Toxoplasma provide a marker of past exposure to the organism. However, with an increased understanding the biology of the parasite, information related to the timing, the strain type, the infectious stage, the existence, or persistence of infection have become increasingly important.34–36
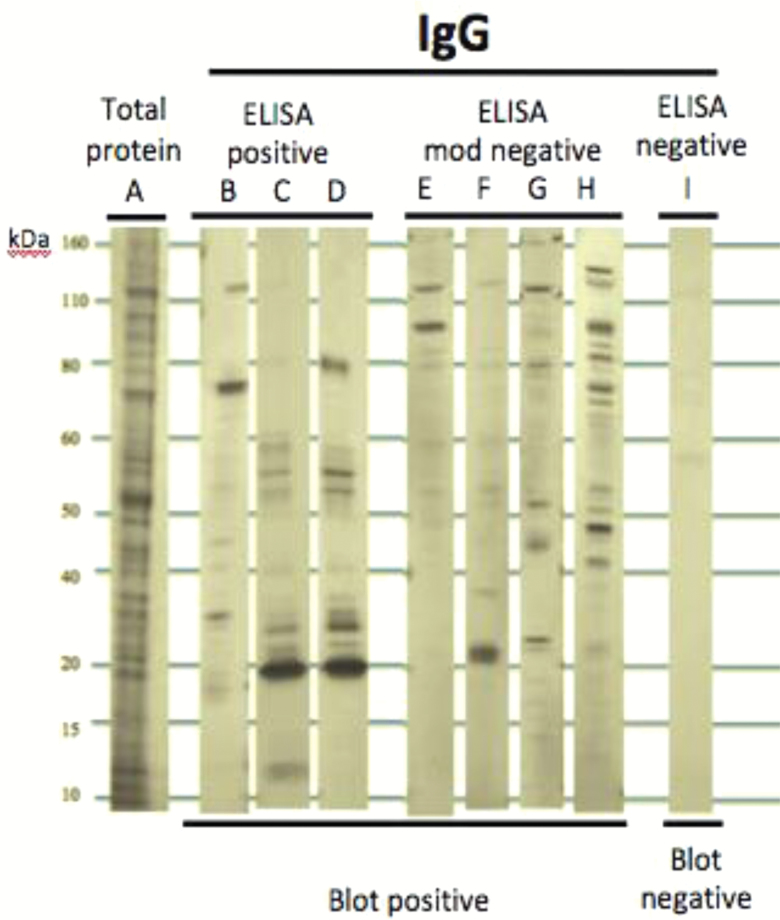
Recent studies have revealed that Toxoplasma populations are geographically structured and have genetic compositions that vary among strains.34 However, currently available solid phase immunoassays were developed in the 1970s to detect strains that were circulating at that time.37 This raises concern whether these immunoassays have sufficient sensitivity to detect contemporary strains in all populations. We have found that many individuals who are “seronegative” using standard enzyme-linked immunosorbent assay (ELISA) nevertheless have evidence of reactivity to Toxoplasma proteins visualized by Western blotting techniques. For example, in a pilot study of 34 individuals, 25 of whom had psychiatric disorders (bipolar disorder, schizophrenia, recent onset psychosis) only 3 individuals (8.2 %) were IgG seropositive to Toxoplasma as defined by a commercially available assay (obtained from IBL America). On the other hand, 12 individuals (35.3%) showed clear reactivity to different Toxoplasma proteins by Western blot (figure 2). These findings indicate that standard assays may substantially underestimate the prevalence of Toxoplasma infection in a population and its effect on health and disease. These findings are also of interest in light of studies purporting decreasing levels of antibodies to Toxoplasma in individuals living in the United States38 and Europe39 because such studies might be reflective of antigenic variation rather than truly decreased prevalence. Recently, much progress has been made in immune-based assays to characterize Toxoplasma infection.
Fig. 2.
Underestimates of Toxoplasma seropositivity revealed by immunoblotting. We performed a pilot study of 34 individuals, 25 of whom had psychiatric disorders. Cellular lysate from Toxoplasma gondii strain RH were homogenized and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blotting using the human sera characterized in ELISAs. A total protein stain of the cellular lysate is shown in the first lane. Standards listed in the far left of the diagram refer to kilodaltons. ELISA mod negative refers to an absorbance value that was slightly elevated from a true negative value. ELISA positive refers to an absorbance value that was slightly below the positivity cutoff value. Twelve of 34 individuals were positive for IgG via immunoblotting compared to 3 who were positive based on ELISAs.
Serological Determination of Cyst Burden
Because, as noted earlier, tissue cysts are predominantly located in the brain, measurement of parasite burden is difficult to achieve in living humans and animals. To overcome this limitation, we have developed a noninvasive, highly sensitive, and specific method for detecting tissue cysts in any disease state. This assay measures humoral immune response against a Toxoplasma matrix antigen called MAG1 using synthetic peptides.40,41 MAG1 is abundantly expressed within the cyst and in the cyst wall surrounding the bradyzoites.42 Tissue cysts of Toxoplasma were found in brains of all MAG1-seropositive mice, whereas no cysts were detected in MAG1-seronegative mice. We have shown that MAG1 antibody level is strongly correlated with the number of brain cysts in the infected host (r = 0.82, P = 0.0021). For mice that remained MAG1 seronegative after exposure, we performed a bioassay to examine the possibility of latent infection below the detection limit. One month after the feeding of brains of MAG1 seronegative mice, sera from recipient mice were tested for the presence of Toxoplasma antibodies. None of mice (n = 5) generated the specific antibody confirming that MAG1 seronegative mice did not become latently infected and that they were free from brain cysts. These data suggest that MAG1 antibody can serve as a serologic marker for evidence of chronic infection and for cyst burden.
Serological Detection of Infecting Serotype and Form
Given Toxoplasma strains vary in their pathogenic potential, we and others have developed peptide-based assays to distinguish infection with the 3 main recognized types of Toxoplasma.43,44 These assays are capable of distinguishing the type of Toxoplasma infections in many individuals. We used this assay to identify serotypes of Toxoplasma in 219 pregnant women whose children developed schizophrenia and psychotic illnesses in adult life and 618 matched unaffected control mothers. We found that the offspring of mothers with virulent strain of Toxoplasma infection were at significantly increased risk for the development of psychoses as compared with the matched unaffected control mothers.44 However, there were also a substantial number of cases who were cross-reactive or indeterminate, probably as a result of infection with more than one type or from a type variant from one of the recognized strains. The development and widespread application of assays capable of accurately identifying infecting type, as well as ones capable of distinguish infections caused by oocysts as opposed to tissue cysts, would represent major tools for an improved understanding of Toxoplasma biology and epidemiology.
Heterogeneity in Infection State and Behavioral Effect
There is marked variation in the human response to Toxoplasma infection. Apart from a human genetic component, heterogeneity of host response is likely to arise through differences in parasite genotype/serotype,45 the infection state,41 the infectious form at exposure,46 and the timing of first exposure. The present review focuses on the necessity to distinguish infection states between persistent infection with brain-dwelling tissue cysts and resolved infection with specific anti-Toxoplasma antibodies (failed to progress to chronic stage). In a study reported from the Sapolsky laboratory,47 changes in behavior were found to be associated with cyst presence. In their study, all Toxoplasma-exposed rats tested positive for serum anti-Toxoplasma IgG, but only a subset of Toxoplasma-exposed rats developed chronic infection with cysts in the forebrain. The presence of behavioral changes including predator odor aversion and anxiety-related behavior were all linked with cyst presence. In another study, Afonso et al.48 demonstrated that cysts, as well as manifestations of infection, were only evident in a subpopulation of infected C57BL/6J mice. The mice with brain cysts, versus those without cysts, displayed a plethora of behavioral alterations.
Toxoplasma type I strains do not readily develop tissue cysts or latent infection in laboratory mice. Recent studies from our group, which utilized a chronic model of type I infection, further addresses why abnormalities occur in some mice, but not in others. In our study,40 we found that outbred mice following exposure to the virulent type I strain (GT1) displayed variable outcomes ranging from aborted to severe infections, characterized by antibody profiles, alterations in cytokine and gene expression, and behavior changes. The extent of most changes was directly correlated with levels of cyst burden determined by MAG1 antibody. We found that mice with high levels of MAG1 antibody (MAG1 > 0.5) exhibited reduced locomotor and exploratory activity, impaired object recognition memory, and lack of response to amphetamine induced activity. These changes were not found in mice with a lower level of cyst burden (MAG1 < 0.5) or mice that were acutely but not chronically infected (lack of MAG1 antibodies).
These variable phenotypes displayed in the rodent models may be analogous to the diversity of human responses. Previously, we measured MAG1 antibody responses in serum samples from 22 patients with clinical toxoplasmosis and from 26 patients with serological evidence of past exposure to Toxoplasma (more than 1year infection).41 We found that not all Toxoplasma seropositive individuals develop MAG1 antibodies, and MAG1 antibody level can differentiate active from inactive human toxoplasmosis. The finding of tissue cysts and altered behavior in only a subset of infected mice is consistent with several human studies. For example, Luft and Remington49 reported that 50% of Toxoplasma-seropositive HIV/AIDS patients showed no symptoms of toxoplasmic encephalitis despite being highly immune compromised.
Neuropathogenesis Is Crucial to Explain the Behavioral Changes
Most studies on rodents have shown that Toxoplasma cysts have a widespread distribution within the brain.50 In vivo studies have revealed that these cysts are located within neurons but not other cell types of the CNS.51,52 To analyze which parts of neurons are infected by cysts, Haroon et al.53 performed a detailed immunohistochemical investigation. They found that parasitic antigens are not sequestered in cysts but reside in all major parts of neurons including the neuronal soma, dendrites, and axons. Moreover, recent analysis of cyst biology reveals a dynamic and replicating bradyzoite population.54 This is in contrast to the usual view that bradyzoites are dormant, poorly replicating, or nonreplicating entities. These findings implicate that tissue cysts have extraordinary potential to alter the structure of neurons and their connections, thereby affecting behavior.
Persistence of tissue cysts requires a continuous immune response provided by resident CNS and/or infiltrating peripheral immune cells to prevent parasite reactivation and encephalitis. Previous data demonstrate that a low-grade inflammation is often triggered throughout the brain. For example, activated microglia and astrocytes often physically encircle parasitic cysts, complement factor C1q locates in the vicinity of tissue cysts, and brain magnetic resonance imaging showed mild to moderate ventricular dilatation.47,55–57 This inflammatory milieu may influence the function and morphology of neurons, leading to brain activity and connectivity changes.
The Morphology of Neurons is Altered by Tissue Cysts
In a study focused on the position of Toxoplasma-induced lesions, Parlog et al.58 reveal impaired local connectivity evidenced by altered fiber density, loss of fiber continuity, and reduced levels of synaptic proteins PSD95 and synaptophysin. In a study to characterize CNS pathology with chronic infection, David et al.59 reported alterations in neuronal morphology (a reduction in dendritic spines) and network activity. To address the effect of latent Toxoplasma infection in schizophrenia, Horacek et al.60 studied whole-brain voxel-based morphometry of gray and white matter. They found a significant reduction of gray matter volume in Toxoplasma positive compared with Toxoplasma-negative patients.
Toxoplasma Infection and Neurotransmission
Alterations in brain connectivity can have potential downstream effects in the pathways of neurotransmitters. Previous studies have reported several disrupted neurotransmitter pathways including dopamine, gamma-aminobutyric acid (GABA), and glutamate.59,61
Glutamate is arguably the most important neurotransmitter in the brain and implicated in the pathogenesis of schizophrenia. Converging evidence support a significant disruption in the glutamate signaling following infection. First, David et al.59 found a significant reduction in astrocyte glutamate transporter (GLT-1). One of the major roles of astrocytes is to remove extracellular glutamate to prevent neuroexcitotoxicity. As expected, downregulation of GLT-1 is linked to an increase in extracellular glutamate in infected animals. Second, Toxoplasma is a tryptophan auxotroph. Interferon gamma (IFNγ) suppresses the growth of the parasite through acceleration of tryptophan degradation.62 IFNγ has the ability to regulate the critical step of the kynurenine pathway, the major pathway of tryptophan metabolism.62 Metabolism further along this pathway produces kynurenic acid (KYNA), an antagonist of the N-methyl-d-aspartate receptor (NMDAR). Elevated levels of KYNA can block the glutamate recognition site of the NMDAR. As rodents infected with Toxoplasma and patients with schizophrenia have increased KYNA levels in brain,63 KYNA has been hypothesized to be a pathogenic link between Toxoplasma infection and schizophrenia. Third, glutamate signaling could potentially be disrupted by GABAergic signaling. The distribution of glutamic acid decarboxylase 67 (GAD67), the enzyme responsible for converting glutamate to GABA, exhibited profound changes in Toxoplasma infected animals.61 Finally, recent studies from our group reported that NMDAR autoantibodies are elevated in chronic murine toxoplasmosis.56,64 NMDARs are heteromers of GluN1 subunits that bind glycine and GluN2 subunits that bind glutamate. NMDAR autoantibodies are reported to decrease the availability of NMDAR binding sites through the process of internalization,65 potentially leading to increase in glutamate levels, as noted by David et al.59
In the context of schizophrenia, there has been the most interest in the effects of infection on levels of dopamine. The Toxoplasma genome contains 2 aromatic amino acid hydroxylase genes (AAH1 and AAH2) that encode proteins that can produce l-DOPA, the direct precursor of dopamine.66 Therefore, an intriguing hypothesis has been put forth that Toxoplasma might influence dopamine production and hence dopamine synaptic transmission. As has been reviewed recently in detail,67 many studies report changes in the dopamine pathway in both acute and chronic infection. For example, McConkey’s group reported increased levels of dopamine in vitro and in vivo.68 In human studies, the role of dopamine was suggested by decreased novelty seeking in Toxoplasma-infected patients and by a high level of positive symptoms in Toxoplasma-seropositive patients with schizophrenia.69 Here, we focus on recent studies that evaluate the impact of AAH gene deficiency on biochemical and behavior changes. A study from the Sibley group reported that neither overexpression nor deletion of AAH2 from parasites altered dopamine levels in vitro or in vivo.70 We and others found no effects of AAH2 deletion on the host’s exploratory behaviors as well as dopamine-dependent behavioral alterations.71,72 Recently, studies found that the AAH genes appear to be critically involved in the sexual cycle of the parasite in the gut of the definitive feline host where they likely play a role in oocyst wall formation.73 Although studies with Δaah2 parasites suggest that AAH2 is not required for biochemical and behavioral abnormalities observed in chronically infected mice, dopamine remains an important question relating to Toxoplasma pathogenesis.
Interaction of Toxoplasma With the Host Genome
There is an increasing realization that susceptibility to common infectious agents can be heritable and that a significant portion of this heritability is based on genetic polymorphisms. In the case of Toxoplasma, studies of congenital infection have implicated a number of toll-like receptors and other immune response genes relating to increased risk.74,75 In regards to infection in later life, a population study of 1227 individuals from a Mexican American community in Texas found that seropositivity to Toxoplasma was heritable with a heritability factor (H2) of 0.21 ± 0.05 (P < .001). However, specific polymorphisms significantly associated with Toxoplasma seropositivity were not identified. A population study of Ashkenazi Jews found that the Toxoplasma seropositivity in individuals with schizophrenia was associated with polymorphisms located near genes encoding glucocorticoid-inducible kinase 1 (SGK1) and solute carrier family 2 member 12 (SLC2A12), although these associations did not achieve statistical significance on a genome-wide level.76
CD8+ T cells and IFNγ are significant effectors mediating resistance to chronic Toxoplasma infection. However, recent studies found that chronic Toxoplasma infection results in CD8± T cell exhaustion,77 a state in which these cells lose their functional ability defined by their capacity to proliferate and secrete effector cytokines. As patients with schizophrenia have decreased levels of CD8 immunity,78–80 attenuated CD8 T cells have been proposed to play a role in mediating schizophrenia in Toxoplasma-seropositive patients.81 This hypothesis posits that the decreased CD8 functionality might result in reactivation of some of the quiescent Toxoplasma parasites, leading to focal necrosis and localized inflammation.
Nurr1 is an orphan nuclear receptor that is essential for the development and continued survival of mesencephalic dopamine neurons. Because rare mutations in Nurr1 have been reported in schizophrenia patients82,83 and were associated with attention deficits in patients with schizophrenia,84 this receptor is implicated as a potential contributor to the development of schizophrenia. Eells et al.85 tested how Toxoplasma infection interacts with the heterozygous deletion of the Nurr1 gene. Their results suggest that reduced Nurr1 function, as found in the Nurr1 +/− genotype, makes mice more susceptible to Toxoplasma-induced alterations in the dopamine-related behaviors (elevated open field activity and a trend toward disrupted sensorimotor gating). The data suggest that combination of the genetic mutations that predispose individuals to schizophrenia and Toxoplasma infection are capable of causing neurological effects.
Gastrointestinal Effects of Toxoplasma Infections
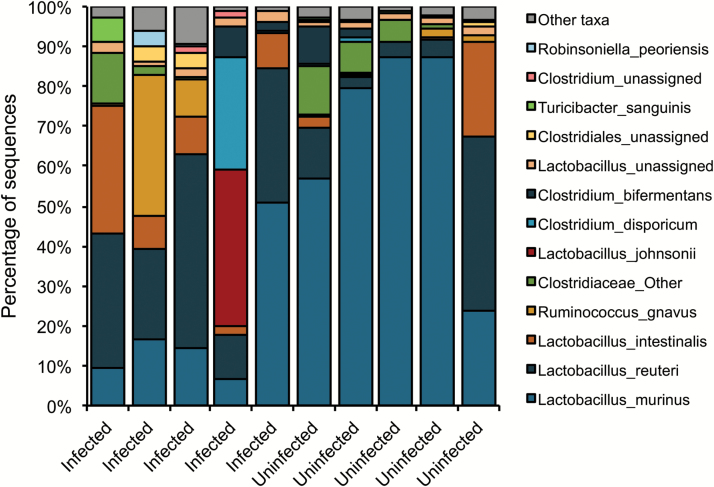
Although the main effects of Toxoplasma relating to schizophrenia are thought to be due to the development of an immune response and pathology within the brain, it is important to note that Toxoplasma generally infects humans through the gastrointestinal tract and, in doing so, can alter gastrointestinal structure and functioning.86 On ingestion of the parasite by the host, Toxoplasma organisms migrate to the surface epithelium and lamina propria of the small intestine.87 From here, organisms penetrate the blood–gut endothelial barrier and gain access to the circulatory system and host organs. Recent studies indicate that alterations in gastrointestinal functioning are common in schizophrenia and other serious psychiatric disorders and that some of the manifestations of these disorders may be related intestinal abnormalities through aberrations in the gut–brain axis.88 In particular, primary studies have shown measures of intestinal inflammation correlate with exposure indices to the parasite in individuals with psychiatric disorders compared with controls.89 Another way in which the gut–brain axis is affected is through changes in the composition of the intestinal microbiome because the intestinal microbiome has been found to be altered in individuals with recent onset psychosis.90 It is thus of interest that acute infection with Toxoplasma has been shown to alter the intestinal microbiome in mice.91 We have also found that chronic Toxoplasma infection alters the composition of the intestinal microbiome in mice as long as 5 months following infection (figure 3). Changes in the host bacterial microflora in the upper intestinal tract may potentially contribute to behavior changes during chronic infection.
Fig. 3.
Individual differences at the species level in chronic Toxoplasma infected and uninfected mice. Female CD-1 mice were infected intraperitoneally with 500 GT1 tachyzoite, whereas uninfected controls received PBS only (5 per group). To establish a chronic infection, both control and infected mice were treated with sulfadiazine sodium (400 mg/L) from day 5 to 30. Mice were killed at 5 months postinfection. 16S rDNA libraries were sequenced using the MiSeq Illumina sequencing platform. Sequences were analyzed utilizing QIIME 1 focusing on v3–v4 region. The graph shows the relative bacterial composition of the intestinal microflora in each mouse. Species that has higher than 100 average counts are depicted individually, lower counts are combined in the “Other” taxa.
Therapeutic Intervention of Toxoplasma Infections Relevant to Psychiatric Disorders
Although there is a great deal of information pointing to a role for Toxoplasma infections in the etiopathogenesis of schizophrenia, the ultimate documentation of this association would be the demonstration that anti-Toxoplasma medications alter the clinical symptoms or course of psychiatric disorders in infected individuals. This goal is hampered by the lack of effective anti-Toxoplasma therapy particularly for the tissue cyst form of the organism. Barriers to effective therapy of tissue cysts include a low level of replication and metabolism, making these forms relatively resistant to medications directed at folate metabolism and other pathways employed by rapidly replicating organisms. The need for a therapeutic medication to cross the blood–brain barrier constitutes another potential barrier. There have been 4 studies evaluating the therapeutic efficacy of adjunctive antiparasitics agents in patients with schizophrenia, as recently reviewed by Chorlton.92 No trials have demonstrated a change in psychopathology with adjunctive treatment. However, as noted earlier, possible reasons for treatment failure include selected drugs without evidence against chronic infection or used them at doses too low to reduce brain cyst burden. Our recent study showed, using a mouse model of chronic Toxoplasma infection, that abrogation of the inhibitory PD-1 signaling pathway that maintains T cells in an exhausted state significantly diminished brain cyst burden (77% lower).93 The effect is likely mediated by brain leukocyte infiltration (CD3+ T cells, CD8+ T cells, and CD11b+ cells) through pathways connected to the cerebrospinal fluid-filled compartments such as ventricles and subarachnoid space. Our study provides proof of concept for blockade of immune checkpoint inhibitors as a therapy for chronic toxoplasmosis. Clinical trials will be necessary to document efficacy in humans and the potential role of this immunological approach in the treatment of psychiatric disorders.
Evolutionary Implications of Toxoplasma Infection
An etiological role for a zoonotic agent such as Toxoplasma can also provide an explanation for the persistence of psychiatric disorders such as schizophrenia within a human population. In the mouse model of Toxoplasma infection, the alterations in cognitive functioning and fear response result in behavioral changes associated with the likelihood of increased predation by felines, presumably, to facilitate the spread of the parasite to its definitive host. Although such behavior is detrimental to the host, it is beneficial to the organism in terms of sexual reproduction and the completion of the life cycle. Although vestigial in humans, who are unlikely to be consumed by felines, the biological properties of the organism relating to altered cognition and fear response would be expected to be functioning regardless of the host. In fact, studies in humans have reported increased rates of risk assessment94 (manifested as decreased levels of accident avoidance) and cognitive control95 in some populations.
Conclusion
Numerous studies link exposure to Toxoplasma as a risk factor for schizophrenia and a range of other psychiatric disorders. The multifaceted effects of Toxoplasma infection on the gastrointestinal tract, neuroinflammation, neurodegeneration, and behavior are just starting to be understood. As a gastrointestinal pathogen, Toxoplasma might affect the brain through local and systemic inflammation or through changes in the intestinal microbiome. As a neurotropic pathogen, Toxoplasma might affect information processing within a wide variety of brain functional systems. Findings from epidemiology and psychiatric genetics have suggested a significant role of gene × Toxoplasma interaction in psychiatric disorders. Experimental Toxoplasma infection provides a relevant model by which we can understand the complexity of human neurological diseases. Recent advances using human neurons derived from cellular reprogramming methods offer the opportunity to develop better models to study Toxoplasma in human neurons.96 These studies may point the way to novel methods for the prevention and treatment of schizophrenia and other devastating psychiatric disorders.
Funding
This work was supported by the Stanley Medical Research Institute.
Conflict of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Leboyer M, Berk M, Yolken RH, Tamouza R, Kupfer D, Groc L. Immuno-psychiatry: an agenda for clinical practice and innovative research. BMC Med. 2016;14:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. [DOI] [PubMed] [Google Scholar]
- 4. Gendelman HE. Neural immunity: friend or foe?J Neurovirol. 2002;8:474–479. [DOI] [PubMed] [Google Scholar]
- 5. Fineberg AM, Ellman LM, Buka S, Yolken R, Cannon TD. Decreased birth weight in psychosis: influence of prenatal exposure to serologically determined influenza and hypoxia. Schizophr Bull. 2013;39:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Severance EG, Gressitt KL, Buka SL, Cannon TD, Yolken RH. Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophr Res. 2014;159:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volk DW, Chitrapu A, Edelson JR, Roman KM, Moroco AE, Lewis DA. Molecular mechanisms and timing of cortical immune activation in schizophrenia. Am J Psychiatry. 2015;172:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28:1–38. [DOI] [PubMed] [Google Scholar]
- 9. Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R. An evaluation of variation in published estimates of schizophrenia prevalence from 1990 horizontal line 2013: a systematic literature review. BMC Psychiatry 2015;15:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sørensen HJ, Nielsen PR, Pedersen CB, Benros ME, Nordentoft M, Mortensen PB. Population impact of familial and environmental risk factors for schizophrenia: a nationwide study. Schizophr Res. 2014;153:214–219. [DOI] [PubMed] [Google Scholar]
- 11. Dubey JP. The history of Toxoplasma gondii–the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. [DOI] [PubMed] [Google Scholar]
- 12. Sibley LD, Khan A, Ajioka JW, Rosenthal BM. Genetic diversity of Toxoplasma gondii in animals and humans. Philos Trans R Soc Lond B Biol Sci. 2009;364:2749–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38:642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yolken R, Torrey EF, Dickerson F. Evidence of increased exposure to Toxoplasma gondii in individuals with recent onset psychosis but not with established schizophrenia. PLoS Negl Trop Dis. 2017;11:e0006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62:237–244. [DOI] [PubMed] [Google Scholar]
- 16. Vlatkovic S, Sagud M, Svob Strac D, et al. . Increased prevalence of Toxoplasma gondii seropositivity in patients with treatment-resistant schizophrenia. Schizophr Res. 2018;193:480–481. [DOI] [PubMed] [Google Scholar]
- 17. Lindgren M, Torniainen-Holm M, Härkänen T, Dickerson F, Yolken RH, Suvisaari J. The association between toxoplasma and the psychosis continuum in a general population setting. Schizophr Res. 2018;193:329–335. [DOI] [PubMed] [Google Scholar]
- 18. Dickerson F, Stallings C, Origoni A, et al. . Antibodies to Toxoplasma gondii in individuals with mania. Bipolar Disord. 2014;16:129–136. [DOI] [PubMed] [Google Scholar]
- 19. Hamdani N, Daban-Huard C, Lajnef M, et al. . Relationship between Toxoplasma gondii infection and bipolar disorder in a French sample. J Affect Disord. 2013;148:444–448. [DOI] [PubMed] [Google Scholar]
- 20. Pedersen MG, Mortensen PB, Norgaard-Pedersen B, Postolache TT. Toxoplasma gondii infection and self-directed violence in mothers. Arch Gen Psychiatry. 2012;69:1123–1130. [DOI] [PubMed] [Google Scholar]
- 21. Okusaga O, Langenberg P, Sleemi A, et al. . Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophr Res. 2011;133:150–155. [DOI] [PubMed] [Google Scholar]
- 22. Markovitz AA, Simanek AM, Yolken RH, et al. . Toxoplasma gondii and anxiety disorders in a community-based sample. Brain Behav Immun. 2015;43:192–197. [DOI] [PubMed] [Google Scholar]
- 23. Alvarado-Esquivel C, Sanchez-Anguiano LF, Hernandez-Tinoco J, et al. . Toxoplasma gondii infection and mixed anxiety and depressive disorder: a case-control seroprevalence study in Durango, Mexico. J Clin Med Res. 2016;8:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flegr J, Horáček J. Toxoplasma-infected subjects report an obsessive-compulsive disorder diagnosis more often and score higher in obsessive-compulsive inventory. Eur Psychiatry. 2017;40:82–87. [DOI] [PubMed] [Google Scholar]
- 25. Flegr J, Horacek J. Toxoplasmosis, but not borreliosis, is associated with psychiatric disorders and symptoms. Schizophr Res 2018. [DOI] [PubMed] [Google Scholar]
- 26. Groer MW, Yolken RH, Xiao JC, et al. . Prenatal depression and anxiety in Toxoplasma gondii-positive women. Am J Obstet Gynecol 2011;204:433 e431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gale SD, Berrett AN, Brown B, Erickson LD, Hedges DW. No association between current depression and latent toxoplasmosis in adults. Folia Parasitol (Praha) 2016;63:32–37. [DOI] [PubMed] [Google Scholar]
- 28. Beste C, Getzmann S, Gajewski PD, Golka K, Falkenstein M. Latent Toxoplasma gondii infection leads to deficits in goal-directed behavior in healthy elderly. Neurobiol Aging. 2014;35:1037–1044. [DOI] [PubMed] [Google Scholar]
- 29. Nimgaonkar VL, Yolken RH, Wang T, et al. . Temporal cognitive decline associated with exposure to infectious agents in a population-based, aging cohort. Alzheimer Dis Assoc Disord. 2016;30:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mendy A, Vieira ER, Albatineh AN, Gasana J. Toxoplasma gondii seropositivity and cognitive functions in school-aged children. Parasitology. 2015;142:1221–1227. [DOI] [PubMed] [Google Scholar]
- 31. Alvarado-Esquivel C, Sánchez-Anguiano LF, Arnaud-Gil CA, et al. . Toxoplasma gondii infection and suicide attempts: a case-control study in psychiatric outpatients. J Nerv Ment Dis. 2013;201:948–952. [DOI] [PubMed] [Google Scholar]
- 32. Hinze-Selch D, Däubener W, Erdag S, Wilms S. The diagnosis of a personality disorder increases the likelihood for seropositivity to Toxoplasma gondii in psychiatric patients. Folia Parasitol (Praha). 2010;57:129–135. [DOI] [PubMed] [Google Scholar]
- 33. Flegr J, Escudero DQ. Impaired health status and increased incidence of diseases in Toxoplasma-seropositive subjects: an explorative cross-sectional study. Parasitology. 2016;143:1974–1989. [DOI] [PubMed] [Google Scholar]
- 34. Su C, Khan A, Zhou P, et al. . Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci U S A. 2012;109:5844–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiao J, Jones-Brando L, Talbot CC Jr, Yolken RH. Differential effects of three canonical Toxoplasma strains on gene expression in human neuroepithelial cells. Infect Immun. 2011;79:1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saeij JP, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005;21:476–481. [DOI] [PubMed] [Google Scholar]
- 37. Voller A, Bidwell DE, Bartlett A, Fleck DG, Perkins M, Oladehin B. A microplate enzyme-immunoassay for toxoplasma antibody. J Clin Pathol. 1976;29:150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones JL, Kruszon-Moran D, Rivera HN, Price C, Wilkins PP. Toxoplasma gondii seroprevalence in the United States 2009–2010 and comparison with the past two decades. Am J Trop Med Hyg 2014;90:1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nogareda F, Le Strat Y, Villena I, De Valk H, Goulet V. Incidence and prevalence of Toxoplasma gondii infection in women in France, 1980–2020: model-based estimation. Epidemiol Infect 2014;142:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao J, Li Y, Prandovszky E, et al. . Behavioral abnormalities in a mouse model of chronic toxoplasmosis are associated with MAG1 antibody levels and cyst burden. PLoS Negl Trop Dis. 2016;10:e0004674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiao J, Viscidi RP, Kannan G, et al. . The Toxoplasma MAG1 peptides induce sex-based humoral immune response in mice and distinguish active from chronic human infection. Microbes Infect. 2013;15:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferguson DJ, Parmley SF. Toxoplasma gondii MAG1 protein expression. Trends Parasitol. 2002;18:482. [DOI] [PubMed] [Google Scholar]
- 43. Kong JT, Grigg ME, Uyetake L, Parmley S, Boothroyd JC. Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J Infect Dis. 2003;187:1484–1495. [DOI] [PubMed] [Google Scholar]
- 44. Xiao J, Buka SL, Cannon TD, et al. . Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect. 2009;11:1011–1018. [DOI] [PubMed] [Google Scholar]
- 45. Xiao J, Yolken RH. Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta Physiol (Oxf). 2015;213:828–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boyer K, Hill D, Mui E, et al. ; Toxoplasmosis Study Group Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin Infect Dis. 2011;53:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Evans AK, Strassmann PS, Lee IP, Sapolsky RM. Patterns of Toxoplasma gondii cyst distribution in the forebrain associate with individual variation in predator odor avoidance and anxiety-related behavior in male Long-Evans rats. Brain Behav Immun. 2014;37:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Afonso C, Paixão VB, Costa RM. Chronic Toxoplasma infection modifies the structure and the risk of host behavior. PLoS One. 2012;7:e32489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. [DOI] [PubMed] [Google Scholar]
- 50. Berenreiterová M, Flegr J, Kuběna AA, Němec P. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS One. 2011;6:e28925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Melzer TC, Cranston HJ, Weiss LM, Halonen SK. Host cell preference of Toxoplasma gondii cysts in murine brain: a confocal study. J Neuroparasitology 2010;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferguson DJ, Hutchison WM. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73:483–491. [DOI] [PubMed] [Google Scholar]
- 53. Haroon F, Händel U, Angenstein F, et al. . Toxoplasma gondii actively inhibits neuronal function in chronically infected mice. PLoS One. 2012;7:e35516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watts E, Zhao Y, Dhara A, Eller B, Patwardhan A, Sinai AP. Novel approaches reveal that Toxoplasma gondii bradyzoites within tissue cysts are dynamic and replicating entities in vivo. MBio. 2015;6:e01155–e01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao J, Li Y, Gressitt KL, et al. . Cerebral complement C1q activation in chronic Toxoplasma infection. Brain Behav Immun. 2016;58:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kannan G, Crawford JA, Yang C, et al. . Anti-NMDA receptor autoantibodies and associated neurobehavioral pathology in mice are dependent on age of first exposure to Toxoplasma gondii. Neurobiol Dis. 2016;91:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hermes G, Ajioka JW, Kelly KA, et al. . Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parlog A, Harsan LA, Zagrebelsky M, et al. . Chronic murine toxoplasmosis is defined by subtle changes in neuronal connectivity. Dis Model Mech. 2014;7:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. David CN, Frias ES, Szu JI, et al. . GLT-1-dependent disruption of CNS glutamate homeostasis and neuronal function by the protozoan parasite Toxoplasma gondii. PLoS Pathog. 2016;12:e1005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Horacek J, Flegr J, Tintera J, et al. . Latent toxoplasmosis reduces gray matter density in schizophrenia but not in controls: voxel-based-morphometry (VBM) study. World J Biol Psychiatry. 2012;13:501–509. [DOI] [PubMed] [Google Scholar]
- 61. Brooks JM, Carrillo GL, Su J, Lindsay DS, Fox MA, Blader IJ. Toxoplasma gondii infections alter GABAergic synapses and signaling in the central nervous system. MBio. 2015;6:e01428–e01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984;81:908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schwarcz R, Hunter CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid?Schizophr Bull. 2007;33:652–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kannan G, Gressitt KL, Yang S, et al. . Pathogen-mediated NMDA receptor autoimmunity and cellular barrier dysfunction in schizophrenia. Transl Psychiatry. 2017;7:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hughes EG, Peng X, Gleichman AJ, et al. . Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4:e4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tedford E, McConkey G. Neurophysiological changes induced by chronic Toxoplasma gondii infection. Pathogens 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6:e23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Flegr J. Schizophrenia and Toxoplasma gondii: an undervalued association?Expert Rev Anti Infect Ther. 2015;13:817–820. [DOI] [PubMed] [Google Scholar]
- 70. Wang ZT, Harmon S, O’Malley KL, Sibley LD. Reassessment of the role of aromatic amino acid hydroxylases and the effect of infection by Toxoplasma gondii on host dopamine. Infect Immun. 2015;83:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Afonso C, Paixão VB, Klaus A, et al. . Toxoplasma-induced changes in host risk behaviour are independent of parasite-derived AaaH2 tyrosine hydroxylase. Sci Rep. 2017;7:13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McFarland R, Wang ZT, Jouroukhin Y, et al. . AAH2 gene is not required for dopamine-dependent neurochemical and behavioral abnormalities produced by Toxoplasma infection in mouse. Behav Brain Res. 2018;347:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang ZT, Verma SK, Dubey JP, Sibley LD. The aromatic amino acid hydroxylase genes AAH1 and AAH2 in Toxoplasma gondii contribute to transmission in the cat. PLoS Pathog. 2017;13:e1006272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wujcicka W, Gaj Z, Wilczyński J, Nowakowska D. Possible role of TLR4 and TLR9 SNPs in protection against congenital toxoplasmosis. Eur J Clin Microbiol Infect Dis. 2015;34:2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shimokawa PT, Targa LS, Yamamoto L, Rodrigues JC, Kanunfre KA, Okay TS. HLA-DQA1/B1 alleles as putative susceptibility markers in congenital toxoplasmosis. Virulence. 2016;7:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Avramopoulos D, Pearce BD, McGrath J, et al. . Infection and inflammation in schizophrenia and bipolar disorder: a genome wide study for interactions with genetic variation. PLoS One. 2015;10:e0116696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci U S A. 2011;108:9196–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Steiner J, Jacobs R, Panteli B, et al. . Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260:509–518. [DOI] [PubMed] [Google Scholar]
- 79. Craddock RM, Lockstone HE, Rider DA, et al. . Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS One. 2007;2:e692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Riedel M, Spellmann I, Schwarz MJ, et al. . Decreased T cellular immune response in schizophrenic patients. J Psychiatr Res. 2007;41:3–7. [DOI] [PubMed] [Google Scholar]
- 81. Bhadra R, Cobb DA, Weiss LM, Khan IA. Psychiatric disorders in toxoplasma seropositive patients–the CD8 connection. Schizophr Bull. 2013;39:485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Buervenich S, Carmine A, Arvidsson M, et al. . NURR1 mutations in cases of schizophrenia and manic-depressive disorder. Am J Med Genet. 2000;96:808–813. [DOI] [PubMed] [Google Scholar]
- 83. Chen YH, Tsai MT, Shaw CK, Chen CH. Mutation analysis of the human NR4A2 gene, an essential gene for midbrain dopaminergic neurogenesis, in schizophrenic patients. Am J Med Genet. 2001;105:753–757. [DOI] [PubMed] [Google Scholar]
- 84. Ancín I, Cabranes JA, Vázquez-Álvarez B, et al. . NR4A2: effects of an “orphan” receptor on sustained attention in a schizophrenic population. Schizophr Bull. 2013;39:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Eells JB, Varela-Stokes A, Guo-Ross SX, et al. . Chronic Toxoplasma gondii in Nurr1-null heterozygous mice exacerbates elevated open field activity. PLoS One. 2015;10:e0119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Severance EG, Xiao J, Jones-Brando L, et al. . Toxoplasma gondii-a gastrointestinal pathogen associated with human brain diseases. Int Rev Neurobiol. 2016;131:143–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dubey JP. Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii. J Eukaryot Microbiol. 1997;44:592–602. [DOI] [PubMed] [Google Scholar]
- 88. Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. 2015;17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Severance EG, Alaedini A, Yang S, et al. . Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012;138:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schwarz E, Maukonen J, Hyytiäinen T, et al. . Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398–403. [DOI] [PubMed] [Google Scholar]
- 91. von Klitzing E, Ekmekciu I, Kühl AA, Bereswill S, Heimesaat MM. Intestinal, extra-intestinal and systemic sequelae of Toxoplasma gondii induced acute ileitis in mice harboring a human gut microbiota. PLoS One. 2017;12:e0176144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chorlton SD. Toxoplasma gondii and schizophrenia: a review of published RCTs. Parasitol Res. 2017;116:1793–1799. [DOI] [PubMed] [Google Scholar]
- 93. Xiao J, Li Y, Yolken RH, Viscidi RP. PD-1 immune checkpoint blockade promotes brain leukocyte infiltration and diminishes cyst burden in a mouse model of Toxoplasma infection. J Neuroimmunol. 2018;319:55–62. [DOI] [PubMed] [Google Scholar]
- 94. Flegr J. How and why Toxoplasma makes us crazy. Trends Parasitol. 2013;29:156–163. [DOI] [PubMed] [Google Scholar]
- 95. Stock AK, Dajkic D, Köhling HL, von Heinegg EH, Fiedler M, Beste C. Humans with latent toxoplasmosis display altered reward modulation of cognitive control. Sci Rep. 2017;7:10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Halonen SK. Use of human neurons derived via cellular reprogramming methods to study host-parasite interactions of Toxoplasma gondii in neurons. Cells 2017;6:32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]